Trends in the Incidence of Ovarian Cancer Among Premenopausal and Postmenopausal Women in the United States, 2001 to 2021
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Data
2.2. Statistical Analysis
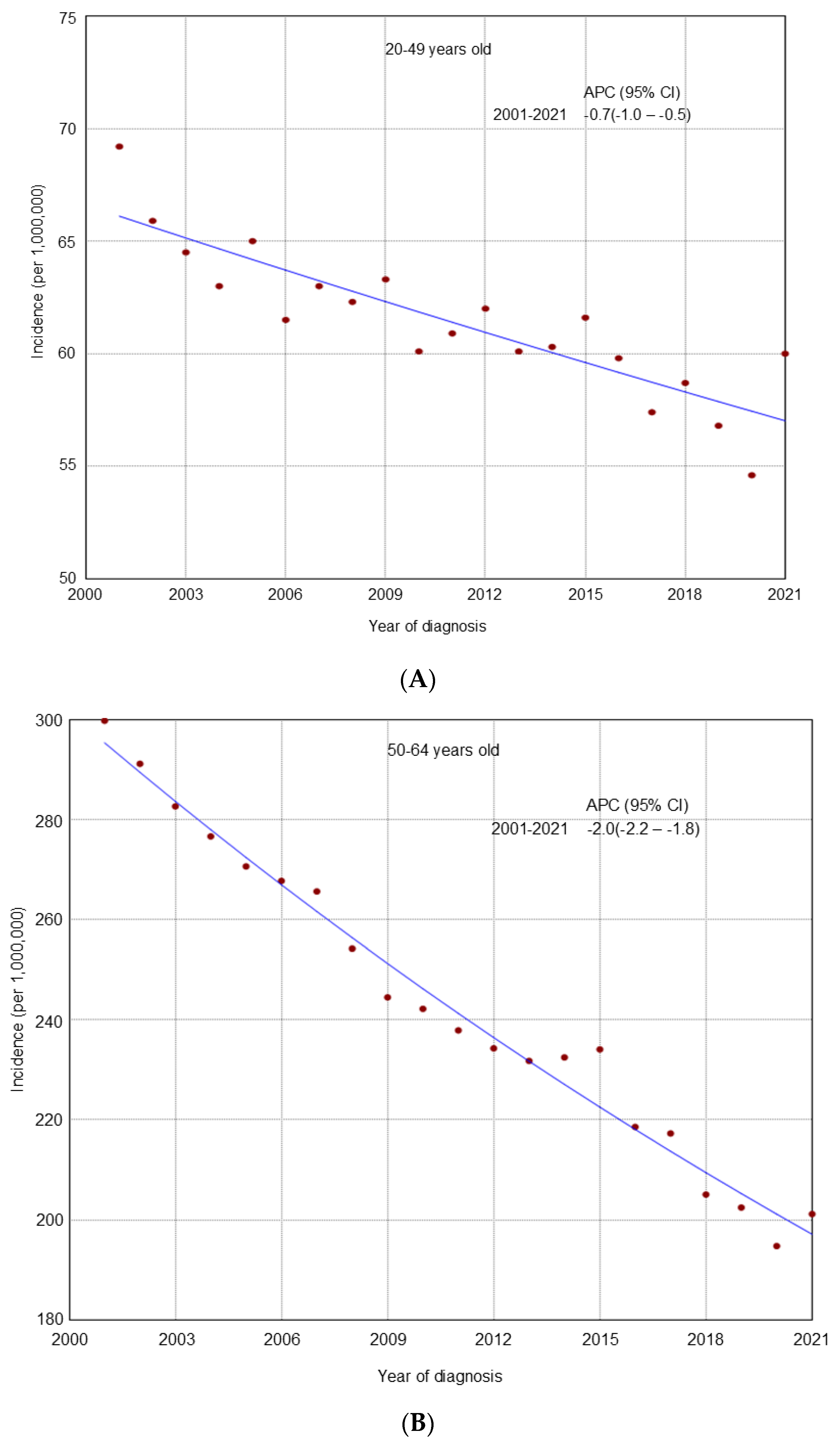
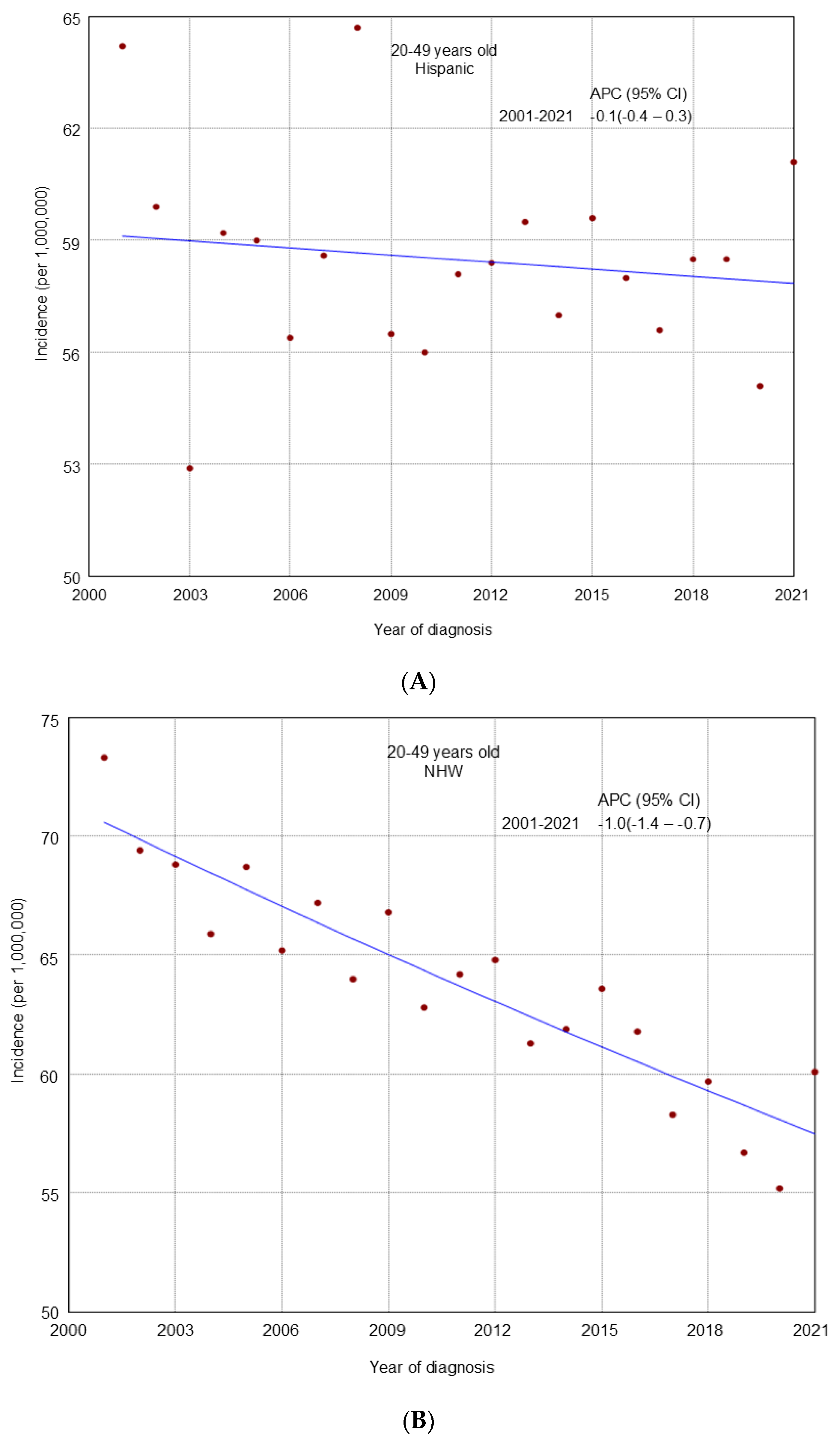
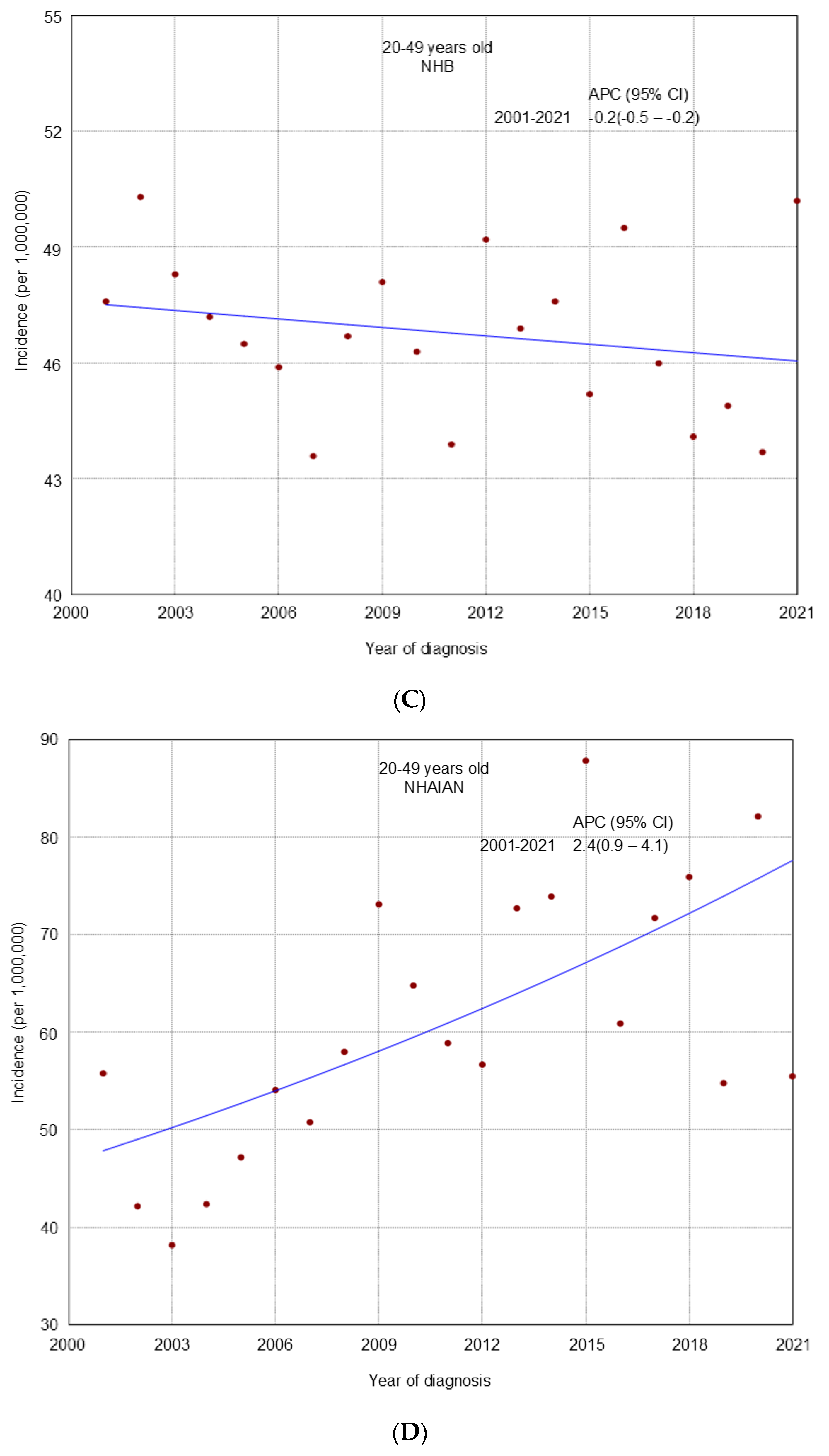
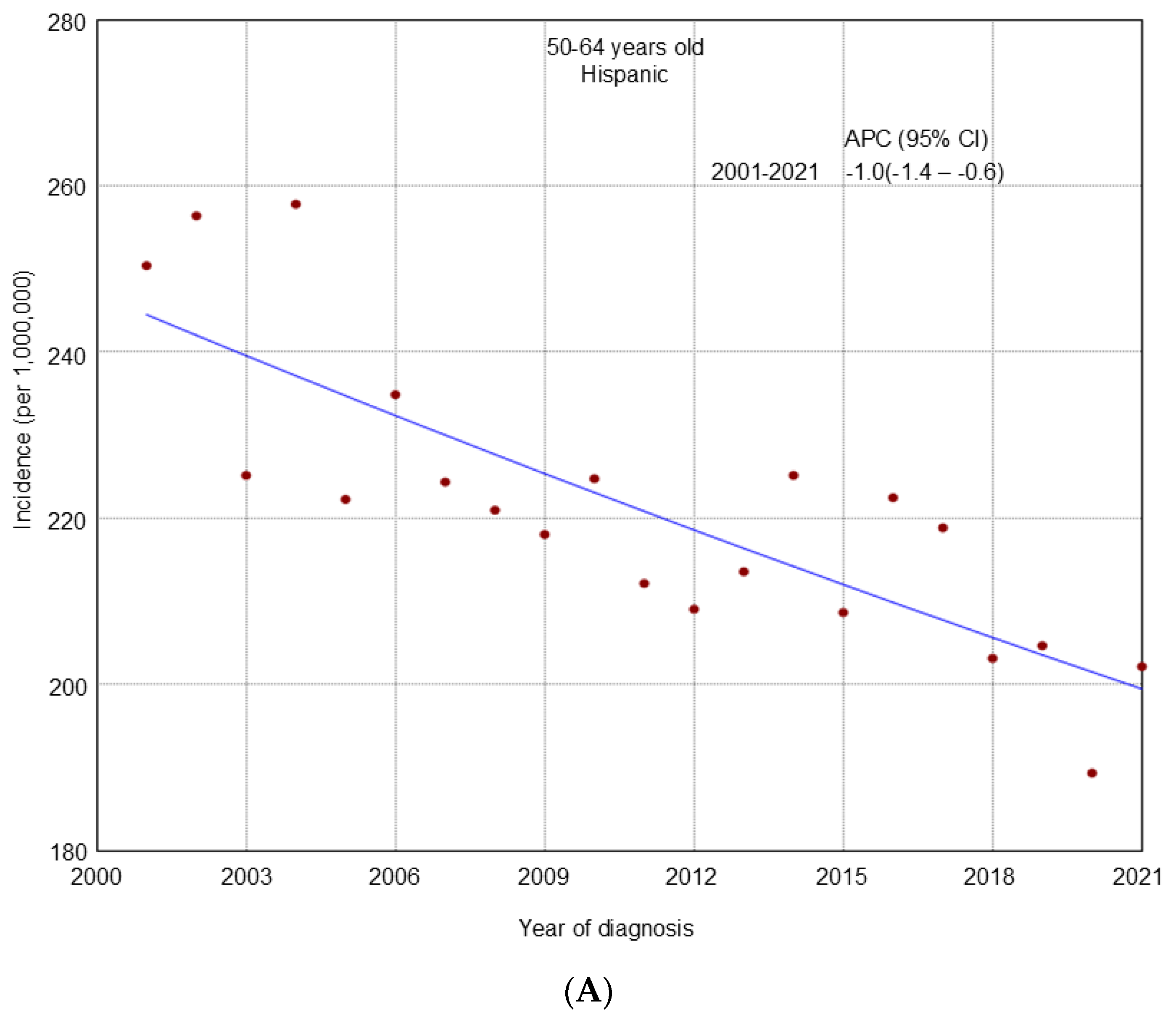
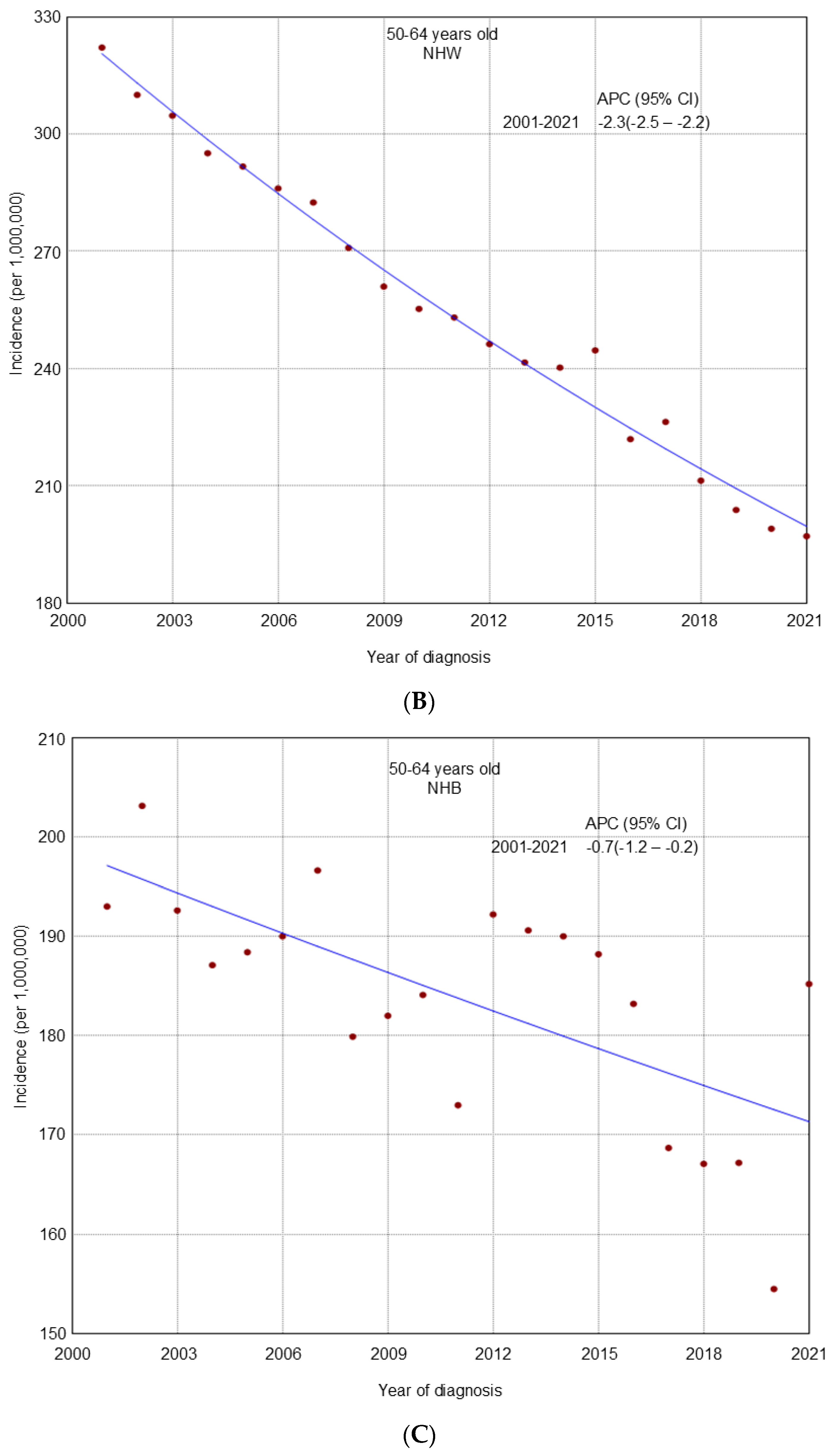
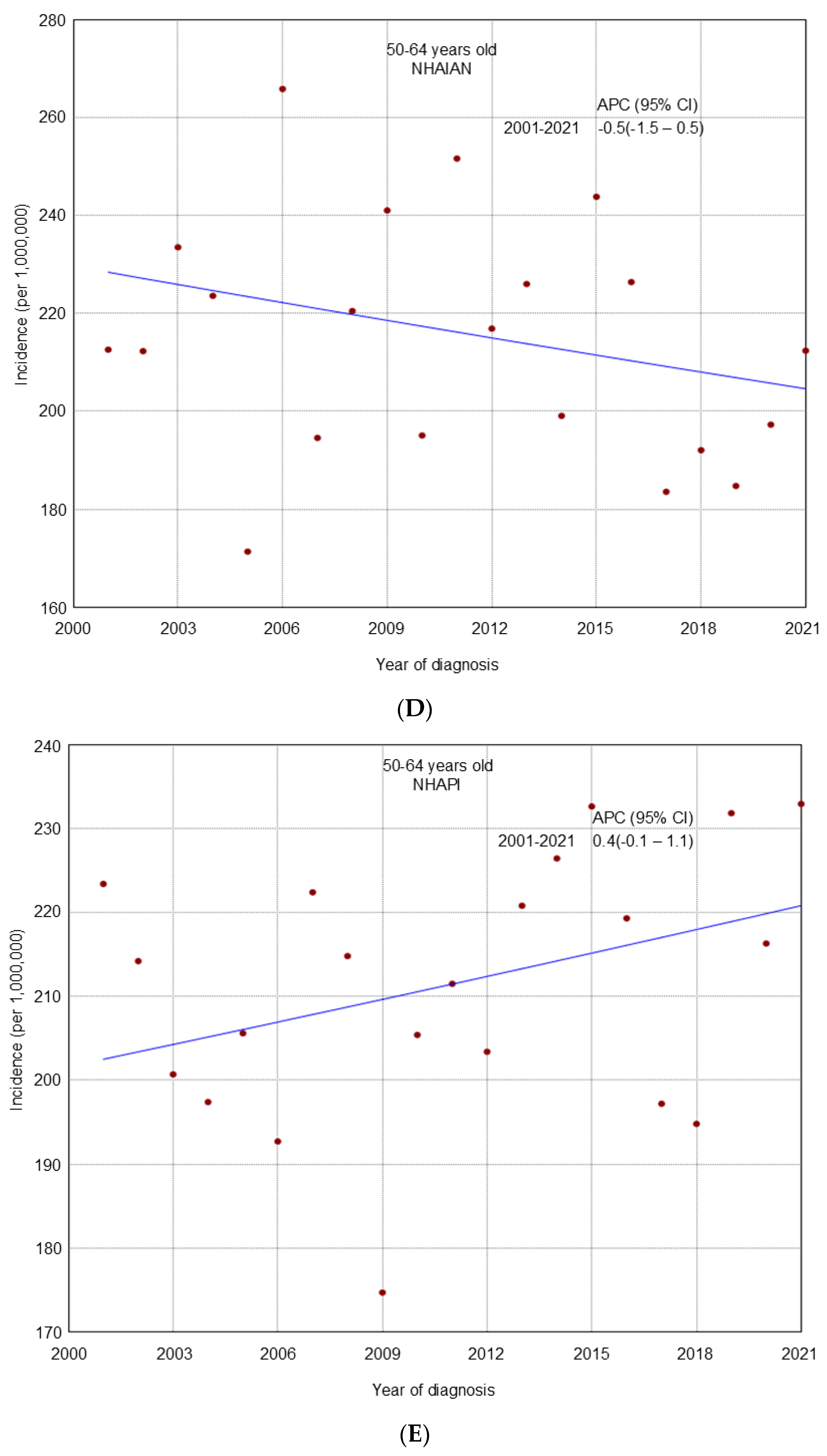
3. Results
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Mazidimoradi, A.; Momenimovahed, Z.; Allahqoli, L.; Tiznobaik, A.; Hajinasab, N.; Salehiniya, H.; Alkatout, I. The global, regional and national epidemiology, incidence, mortality, and burden of ovarian cancer. Health Sci. Rep. 2022, 5, e936. [Google Scholar] [CrossRef]
- He, J.; Hu, Q. Ovarian cancer disease burden decreased in the United States from 1975 to 2018: A joinpoint and age-period-cohort analysis. Medicine 2023, 102, e36029. [Google Scholar] [CrossRef]
- Huang, J.; Chan, W.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.J.; Elcarte, E.; Withers, M.; et al. Worldwide Burden, Risk Factors, and Temporal Trends of Ovarian Cancer: A Global Study. Cancers 2022, 14, 2230. [Google Scholar] [CrossRef]
- Tung, K.H.; Wilkens, L.R.; Wu, A.H.; McDuffie, K.; Nomura, A.M.; Kolonel, L.N.; Terada, K.Y.; Goodman, M.T. Effect of anovulation factors on pre- and postmenopausal ovarian cancer risk: Revisiting the incessant ovulation hypothesis. Am. J. Epidemiol. 2005, 161, 321–329. [Google Scholar] [CrossRef]
- Rosenberg, S.M.; Ruddy, K.J.; Tamimi, R.M.; Gelber, S.; Schapira, L.; Come, S.; Borges, V.F.; Larsen, B.; Garber, J.E.; Partridge, A.H. BRCA1 and BRCA2 Mutation Testing in Young Women with Breast Cancer. JAMA Oncol. 2016, 2, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Petrucelli, N.; Daly, M.B.; Feldman, G.L. Hereditary breast and ovarian cancer due to mutations in BRCA1 and BRCA2. Genet. Med. 2010, 12, 245–259. [Google Scholar] [CrossRef]
- Sung, H.; Jiang, C.; Bandi, P.; Minihan, A.; Fidler-Benaoudia, M.; Islami, F.; Siegel, R.L.; Jemal, A. Differences in cancer rates among adults born between 1920 and 1990 in the USA: An analysis of population-based cancer registry data. Lancet Public Health 2024, 9, e583–e593. [Google Scholar] [CrossRef] [PubMed]
- Roett, M.A.; Evans, P. Ovarian cancer: An overview. Am. Fam. Phys. 2009, 80, 609–616. [Google Scholar]
- Wernli, K.J.; Newcomb, P.A.; Hampton, J.M.; Trentham-Dietz, A.; Egan, K.M. Hormone therapy and ovarian cancer: Incidence and survival. Cancer Causes Control. 2008, 19, 605–613. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, G.; Li, M.; Guo, P.; Xiao, Y.; Ji, H.; Hao, Y. Global patterns and trends in ovarian cancer incidence: Age, period and birth cohort analysis. BMC Cancer 2019, 19, 984. [Google Scholar] [CrossRef] [PubMed]
- Phung, M.T.; Pearce, C.L.; Meza, R.; Jeon, J. Trends of Ovarian Cancer Incidence by Histotype and Race/Ethnicity in the United States 1992-2019. Cancer Res. Commun. 2023, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Park, H.K.; Ruterbusch, J.J.; Cote, M.L. Recent Trends in Ovarian Cancer Incidence and Relative Survival in the United States by Race/Ethnicity and Histologic Subtypes. Cancer Epidemiol. Biomarkers Prev. 2017, 26, 1511–1518. [Google Scholar] [CrossRef]
- NPCR and SEER Incidence—USCS Public Use Databases. Available online: https://www.cdc.gov/cancer/npcr/public-use/index.htm (accessed on 15 October 2017).
- Peres, L.C.; Cushing-Haugen, K.L.; Kobel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J. Natl. Cancer Inst. 2019, 111, 60–68. [Google Scholar] [CrossRef]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Joinpoint Regression Program, Version 4.5.0.1—June 2017; Statistical Methodology and Applications Branch, Surveillance Research Program, National Cancer Institute: Bethesda, MD, USA, 2017.
- Tiwari, R.C.; Clegg, L.X.; Zou, Z. Efficient interval estimation for age-adjusted cancer rates. Stat. Methods Med. Res. 2006, 15, 547–569. [Google Scholar] [CrossRef] [PubMed]
- Kleinbaum, D.G.; Kupper, L.L.; Muller, K.E. Applied Regression Analysis and Other Multivariable Methods, 2nd ed.; PWS-Kent: Boston, MA, USA, 1988. [Google Scholar]
- Somasegar, S.; Reddy, R.A.; Chow, S.; Dorigo, O.; Renz, M.; Karam, A. Trends in ovarian, fallopian tube, and primary peritoneal cancer incidence, mortality, and survival: A 15-year population-based analysis. Gynecol Oncol. 2024, 184, 190–197. [Google Scholar] [CrossRef]
- Dilley, S.E.; Straughn, J.M., Jr.; Leath, C.A., 3rd. The Evolution of and Evidence for Opportunistic Salpingectomy. Obstet. Gynecol. 2017, 130, 814–824. [Google Scholar] [CrossRef]
- Hicks-Courant, K.D. Growth in salpingectomy rates in the United States since 2000. Am. J. Obstet. Gynecol. 2016, 215, 666–667. [Google Scholar] [CrossRef]
- Wentzensen, N.; Poole, E.M.; Trabert, B.; White, E.; Arslan, A.A.; Patel, A.V.; Setiawan, V.W.; Visvanathan, K.; Weiderpass, E.; Adami, H.O.; et al. Ovarian Cancer Risk Factors by Histologic Subtype: An Analysis from the Ovarian Cancer Cohort Consortium. J. Clin. Oncol. 2016, 34, 2888–2898. [Google Scholar] [CrossRef]
- Sopik, V.; Iqbal, J.; Rosen, B.; Narod, S.A. Why have ovarian cancer mortality rates declined? Part I. Incidence. Gynecol. Oncol. 2015, 138, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Beral, V.; Gaitskell, K.; Hermon, C.; Moser, K.; Reeves, G.; Peto, R. Menopausal hormone use and ovarian cancer risk: Individual participant meta-analysis of 52 epidemiological studies. Lancet 2015, 385, 1835–1842. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.W.; Ness, R.B.; Roman, L.D.; Terry, K.L.; Schildkraut, J.M.; Chang-Claude, J.; Doherty, J.A.; Menon, U.; Cramer, D.W.; Gayther, S.A.; et al. Association Between Menopausal Estrogen-Only Therapy and Ovarian Carcinoma Risk. Obstet. Gynecol. 2016, 127, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Barbaglia, G.; Macià, F.; Comas, M.; Sala, M.; del Mar Vernet, M.; Casamitjana, M.; Castells, X. Trends in hormone therapy use before and after publication of the Women’s Health Initiative trial: 10 years of follow-up. Menopause 2009, 16, 1061–1064. [Google Scholar] [CrossRef]
- Yang, L.; Toriola, A.T. Menopausal Hormone Therapy Use Among Postmenopausal Women. JAMA Health Forum 2024, 5, e243128. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results From the Women’s Health Initiative randomized controlled trial. JAMA 2002, 288, 321–333. [Google Scholar] [CrossRef]
- Huang, J.; Lok, V.; Ngai, C.H.; Zhang, L.; Yuan, J.; Lao, X.Q.; Ng, K.; Chong, C.; Zheng, Z.J.; Wong, M.C.S.; et al. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology 2021, 160, 744–754. [Google Scholar] [CrossRef]
- Wong, M.C.S.; Huang, J.; Wang, J.; Chan, P.S.F.; Lok, V.; Chen, X.; Leung, C.; Wang, H.H.X.; Lao, X.Q.; Zheng, Z.J. Global, regional and time-trend prevalence of central obesity: A systematic review and meta-analysis of 13.2 million subjects. Eur. J. Epidemiol. 2020, 35, 673–683. [Google Scholar] [CrossRef]
- Lundberg, F.E.; Iliadou, A.N.; Rodriguez-Wallberg, K.; Gemzell-Danielsson, K.; Johansson, A.L.V. The risk of breast and gynecological cancer in women with a diagnosis of infertility: A nationwide population-based study. Eur. J. Epidemiol. 2019, 34, 499–507. [Google Scholar] [CrossRef]
- Berek, J.S.; Bast, R.C., Jr. Epithelial ovarian cancer. In Holland-Frei Cancer Medicine, 6th ed.; BC Decker: Hamilton, ON, Canada, 2003. [Google Scholar]
- Berek, J.S.; Renz, M.; Friedlander, M.L.; Bast, R.C., Jr. Epithelial Ovarian, Fallopian Tube, and Peritoneal Cancer. Holl.-Frei Cancer Med. 2022, 1, 1–23. [Google Scholar]
- Kim, S.I.; Lim, M.C.; Lim, J.; Won, Y.J.; Seo, S.S.; Kang, S.; Park, S.Y. Incidence of epithelial ovarian cancer according to histologic subtypes in Korea, 1999 to 2012. J. Gynecol. Oncol. 2016, 27, e5. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Han, R.; Huang, X.; Zhou, J.; Yang, J.; Luo, P.; Wu, M. Increase of Incidence and Mortality of Ovarian Cancer during 2003-2012 in Jiangsu Province, China. Front Public Health 2016, 4, 146. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, T.H.; Chung, H.H.; Song, Y.S. Risk and prognosis of ovarian cancer in women with endometriosis: A meta-analysis. Br. J. Cancer 2014, 110, 1878–1890. [Google Scholar] [CrossRef]
- Menon, U.; Gentry-Maharaj, A.; Burnell, M.; Singh, N.; Ryan, A.; Karpinskyj, C.; Carlino, G.; Taylor, J.; Massingham, S.K.; Raikou, M.; et al. Ovarian cancer population screening and mortality after long-term follow-up in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): A randomised controlled trial. Lancet 2021, 397, 2182–2193. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | Premenopausal (20–49) | Postmenopausal (50–64) | Postmenopausal (65+) | |||
|---|---|---|---|---|---|---|
| Count | Rate (95% CI) | Count | Rate (95% CI) | Count | Rate (95% CI) | |
| Race/ethnicity | ||||||
| Hispanic | 749 | 61.1 (56.8–65.7) | 907 | 202.2 (189.3–215.9) | 820 | 299.8 (279.3–321.3) |
| Non-Hispanic White | 2014 | 60.1 (57.5–62.7) | 4225 | 197.2 (191.1–203.5) | 7523 | 337.0 (329.4–344.8) |
| Non-Hispanic Black | 425 | 50.2 (45.5–55.2) | 786 | 185.2 (172.3–198.9) | 919 | 299.4 (279.9–319.8) |
| Non-Hispanic American Indian/Alaska Native | 28 | 55.5 (36.7–80.3) | 55 | 212.4 (158.9–278.2) | 74 | 389.9 (304.5–491.9) |
| Non-Hispanic Asian or Pacific Islander | 331 | 66.7 (59.7–74.3) | 466 | 232.9 (212.1–255.2) | 329 | 206.1 (184.1–230.0) |
| Histologic type | ||||||
| Serous | 1055 | 18 (16.9–19.1) | 2996 | 90.5 (87.2–93.8) | 5054 | 169.7 (165.0–174.5) |
| Endometrioid | 590 | 10.1 (9.3–11.0) | 784 | 25.6 (23.8–27.5) | 497 | 16.5 (15.0–18.0) |
| Clear cell | 285 | 4.9 (4.3–5.5) | 556 | 17.9 (16.4–19.4) | 363 | 11.8 (10.6–13.0) |
| Mucinous | 368 | 6.0 (5.4–6.6) | 360 | 11.6 (10.4–12.8) | 315 | 10.3 (9.2–11.5) |
| Stage | ||||||
| Localized | 1618 | 26.8 (25.5–28.2) | 1819 | 58.3 (55.6–61.1) | 1368 | 45.2 (42.8–47.7) |
| Regional | 772 | 13.0 (12.1–13.9) | 1360 | 42.5 (40.2–44.9) | 1599 | 53.3 (50.7–56.0) |
| Distant | 1029 | 17.5 (16.4–18.6) | 3053 | 92.2 (88.9–95.6) | 5903 | 198.4 (193.3–203.6) |
| Region of Residence | ||||||
| Northeast | 671 | 64.7 (59.9–69.9) | 1236 | 202.9 (191.5–214.8) | 1918 | 338.1 (323.0–353.7) |
| Midwest | 617 | 56.6 (52.2–61.2) | 1204 | 191.1 (180.2–202.6) | 1925 | 329.1 (314.4–344.3) |
| South | 1424 | 60.1 (57–63.3) | 2447 | 193.4 (185.6–201.3) | 3709 | 319.1 (308.9–329.7) |
| West | 871 | 59.3 (55.4–63.4) | 1615 | 221.6 (210.7–232.9) | 2167 | 320.9 (307.4–334.8) |
| Rural/Metro | ||||||
| Metro | 3152 | 60.1 (58.1–62.3) | 5684 | 204.9 (199.5–210.4) | 8265 | 329.4 (322.2–336.6) |
| Rural | 431 | 59.4 (53.9–65.3) | 818 | 179.9 (167.3–193.2) | 1454 | 302.5 (287.0–318.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adekanmbi, V.; Berenson, A.B.; Shakir, B.; Hsu, C.D.; Hoang, T.N.; Sokale, I.O.; Sajobi, T.T.; Guo, F. Trends in the Incidence of Ovarian Cancer Among Premenopausal and Postmenopausal Women in the United States, 2001 to 2021. Cancers 2025, 17, 2119. https://doi.org/10.3390/cancers17132119
Adekanmbi V, Berenson AB, Shakir B, Hsu CD, Hoang TN, Sokale IO, Sajobi TT, Guo F. Trends in the Incidence of Ovarian Cancer Among Premenopausal and Postmenopausal Women in the United States, 2001 to 2021. Cancers. 2025; 17(13):2119. https://doi.org/10.3390/cancers17132119
Chicago/Turabian StyleAdekanmbi, Victor, Abbey B. Berenson, Batul Shakir, Christine D. Hsu, Thao N. Hoang, Itunu O. Sokale, Tolulope T. Sajobi, and Fangjian Guo. 2025. "Trends in the Incidence of Ovarian Cancer Among Premenopausal and Postmenopausal Women in the United States, 2001 to 2021" Cancers 17, no. 13: 2119. https://doi.org/10.3390/cancers17132119
APA StyleAdekanmbi, V., Berenson, A. B., Shakir, B., Hsu, C. D., Hoang, T. N., Sokale, I. O., Sajobi, T. T., & Guo, F. (2025). Trends in the Incidence of Ovarian Cancer Among Premenopausal and Postmenopausal Women in the United States, 2001 to 2021. Cancers, 17(13), 2119. https://doi.org/10.3390/cancers17132119






