Survival and Functional Outcomes Following Surgical Resection of Intramedullary Spinal Cord Tumors: A Series of 253 Patients over 22 Years
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Cohort
2.2. Parameters
2.3. Statistical Analysis
3. Results
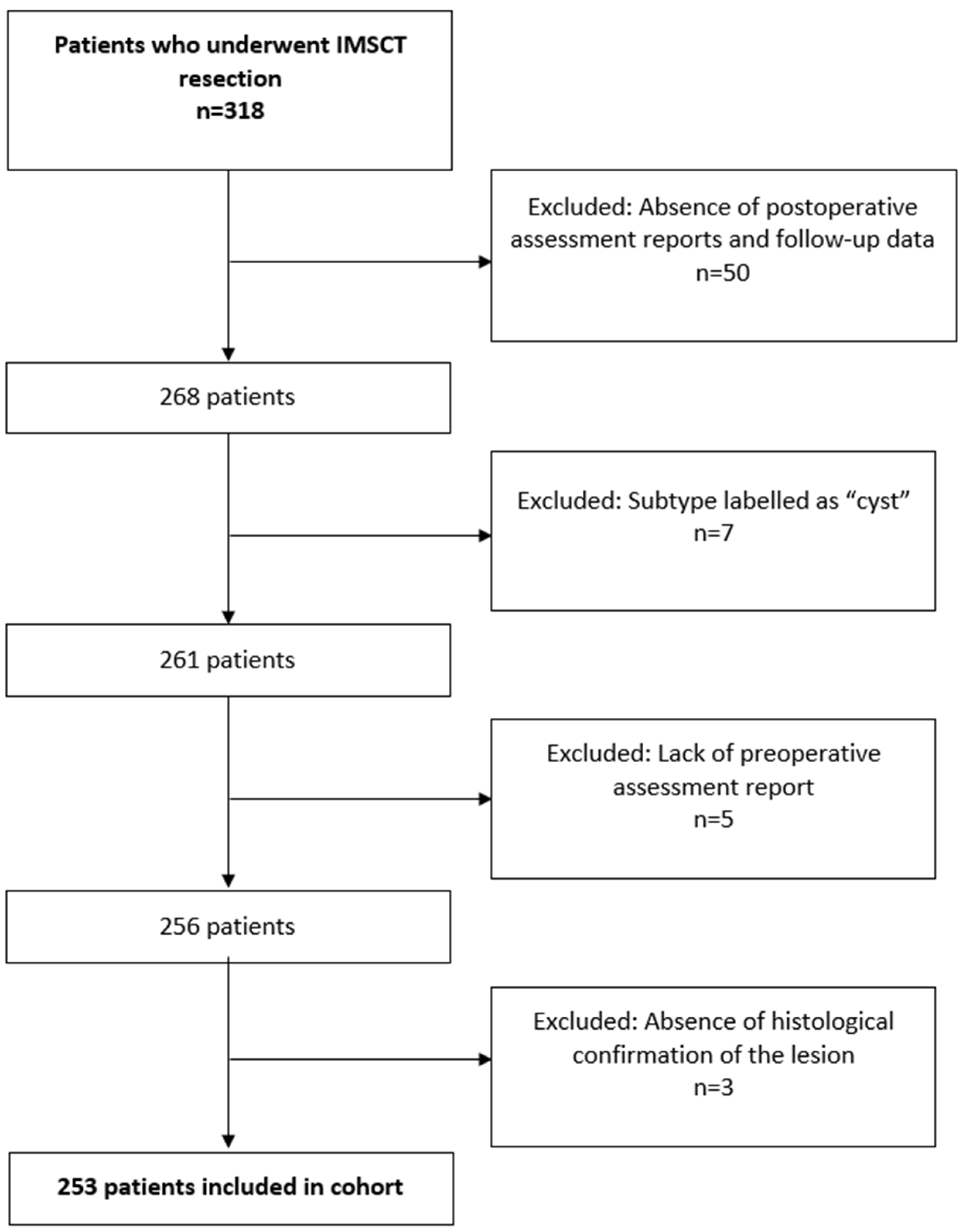
3.1. Patient Population
3.2. Tumor Histology
3.3. Tumor Location
3.4. Tumor Resection and Levels Analysis
3.5. Indications for 30-Day Readmission and 30-Day Reoperation
3.6. Neurological and Functional Status Presentation Stratified by Tumor Location
3.7. Neurological and Functional Status Change
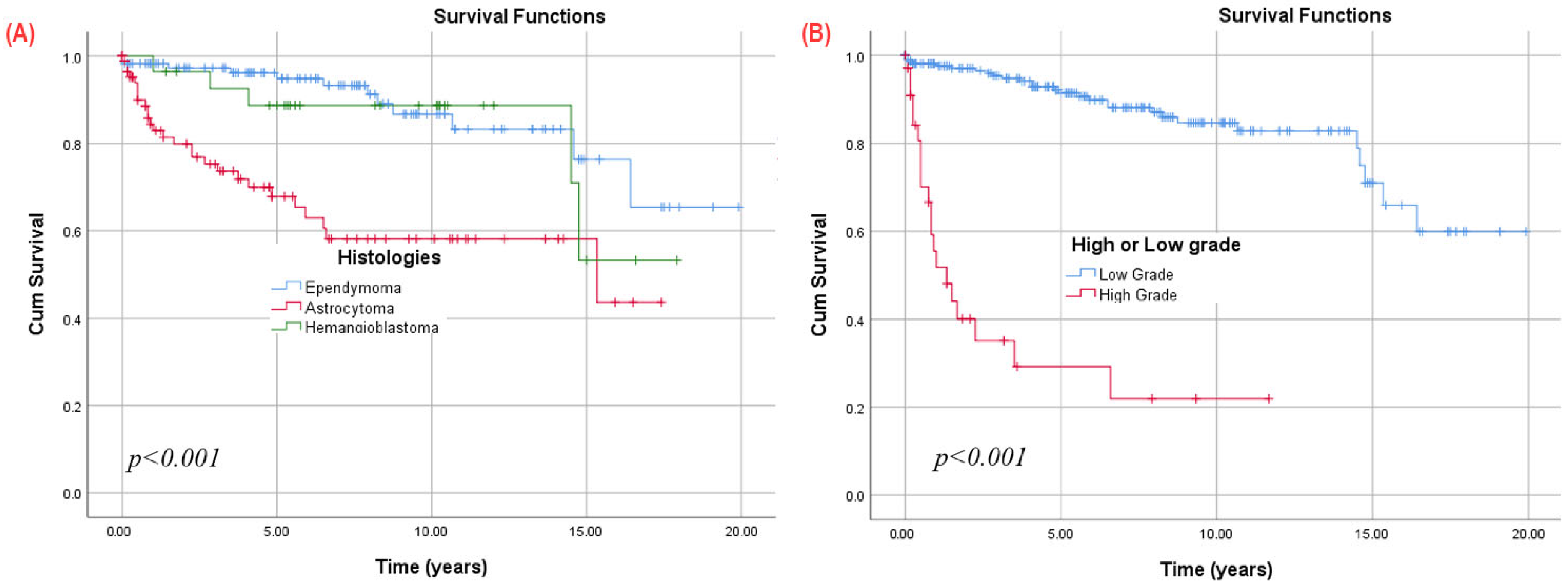
3.8. Survival
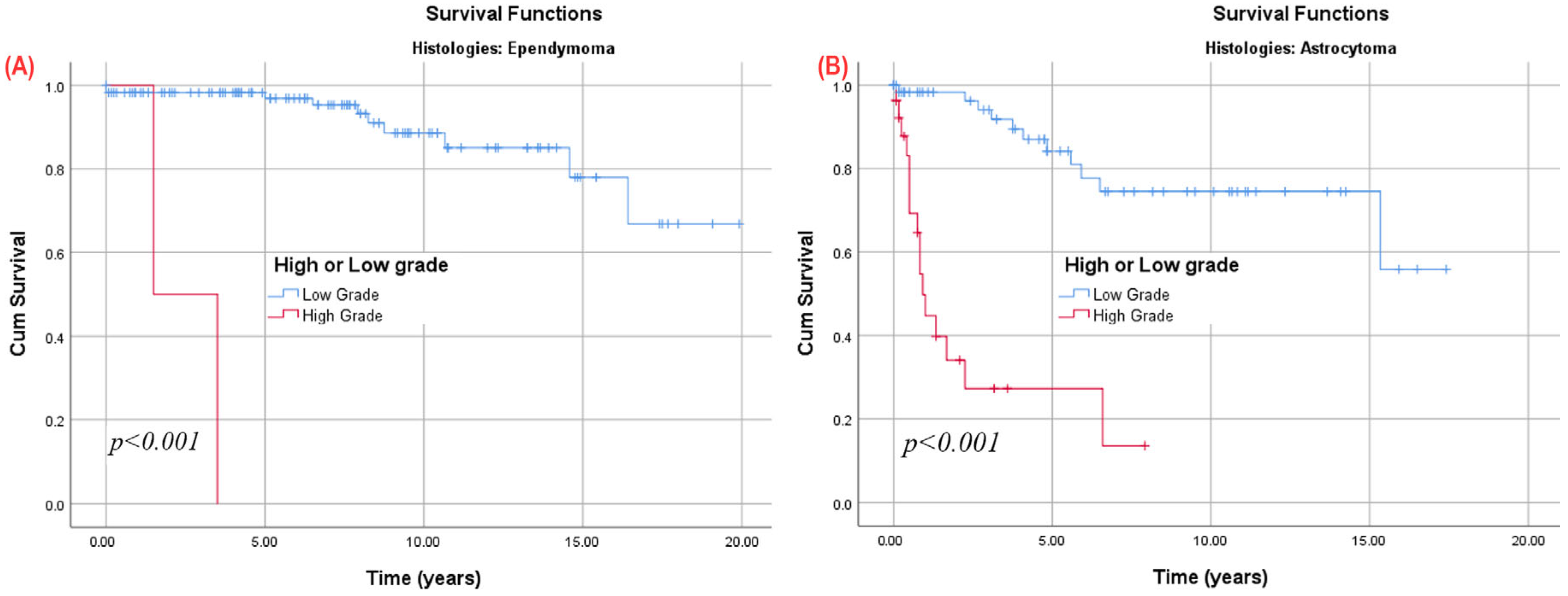
3.9. Subgroup Analysis
3.9.1. Astrocytoma Patients
3.9.2. Patients Stratified by Preoperative Modified McCormick Scale (mMS)
3.9.3. Patients with at Least One Year of Follow-Up, Stratified by Tumor Histology
3.9.4. Patients Stratified by Preoperative Extent of Resection
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IMSCT | intramedullary spinal cord tumor |
| CNS | central nervous system |
| BMI | body mass index |
| CCI | Charlson Comorbidity Index |
| CNS | central nervous system |
| EBL | estimated blood loss |
| GTR | gross total resection |
| STR | sub-total resection |
| LOS | length of stay |
| mMS | modified McCormick Scale |
| OS | overall survival |
| STR | subtotal resection |
| EPND | ependymoma |
| ASTR | astrocytoma |
| HMNG | hemangioblastoma |
| MISC | miscellaneous |
References
- Akinduro, O.O.; Ghaith, A.K.; El-Hajj, V.G.; Ghanem, M.; Soltan, F.; Bon Nieves, A.; Abode-Iyamah, K.; Shin, J.H.; Gokaslan, Z.L.; Quinones-Hinojosa, A.; et al. Effect of race, sex, and socioeconomic factors on overall survival following the resection of intramedullary spinal cord tumors. J. Neurooncol. 2023, 164, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Quattrone, M.; Ostrom, Q.; Ryken, T.C.; Sloan, A.E.; Barnholtz-Sloan, J.S. Incidence patterns for primary malignant spinal cord gliomas: A Surveillance, Epidemiology, and End Results study. J. Neurosurg. Spine 2011, 14, 742–747. [Google Scholar] [CrossRef]
- Das, J.M.; Hoang, S.; Mesfin, F.B. Intramedullary Spinal Cord Tumors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK442031/ (accessed on 18 May 2024).
- Fathy, M.; Keshk, M.; El Sherif, A. Surgical management and outcome of intramedullary spinal cord tumour. Egypt. J. Neurosurg. 2019, 34, 2. [Google Scholar] [CrossRef]
- Zhang, M.; Iyer, R.R.; Azad, T.D.; Wang, Q.; Garzon-Muvdi, T.; Wang, J.; Liu, A.; Burger, P.; Eberhart, C.; Rodriguez, F.J.; et al. Genomic Landscape of Intramedullary Spinal Cord Gliomas. Sci. Rep. 2019, 9, 18722. [Google Scholar] [CrossRef] [PubMed]
- Klekamp, J. Treatment of intramedullary tumors: Analysis of surgical morbidity and long-term results. J. Neurosurg. Spine 2013, 19, 12–26. [Google Scholar] [CrossRef]
- Tufo, T.; Grande, E.; Bevacqua, G.; Di Muccio, I.; Cioni, B.; Meglio, M.; Ciavarro, M. Long-term quality of life and functional outcomes in adults surgically treated for intramedullary spinal cord tumor. Front. Psychol. 2023, 14, 1136223. [Google Scholar] [CrossRef]
- Howell, E.P.; Williamson, T.; Karikari, I.; Abd-El-Barr, M.; Erickson, M.; Goodwin, M.L.; Reynolds, J.; Sciubba, D.M.; Goodwin, C.R. Total en bloc resection of primary and metastatic spine tumors. Ann. Transl. Med. 2019, 7, 226. [Google Scholar] [CrossRef]
- McCormick, P.C.; Stein, B.M. Intramedullary tumors in adults. Neurosurg. Clin. N. Am. 1990, 1, 609–630. [Google Scholar] [CrossRef]
- Jecko, V.; Roblot, P.; Mongardi, L.; Ollivier, M.; Delgado Piccoli, N.; Charleux, T.; Wavasseur, T.; Gimbert, E.; Liguoro, D.; Chotard, G.; et al. Intramedullary Spinal Cord Lesions: A Single-Center Experience. Neurospine 2022, 19, 108–117. [Google Scholar] [CrossRef]
- Harrop, J.S.; Ganju, A.; Groff, M.; Bilsky, M. Primary intramedullary tumors of the spinal cord. Spine 2009, 34 (Suppl. S22), S69–S77. [Google Scholar] [CrossRef]
- Babu, R.; Karikari, I.O.; Owens, T.R.; Bagley, C.A. Spinal cord astrocytomas: A modern 20-year experience at a single institution. Spine 2014, 39, 533–540. [Google Scholar] [CrossRef]
- Nakamura, M.; Chiba, K.; Ishii, K.; Ogawa, Y.; Takaishi, H.; Matsumoto, M.; Toyama, Y. Surgical outcomes of spinal cord astrocytomas. Spinal Cord 2006, 44, 740–745. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Hida, K.; Yano, S.; Aoyama, T.; Koyanagi, I.; Sasamori, T.; Hamauch, S.; Houkin, K. Clinical Factors for Prognosis Treatment Guidance of Spinal Cord Astrocytoma. Asian Spine J. 2016, 10, 748–754. [Google Scholar] [CrossRef]
- Lee, H.K.; Chang, E.L.; Fuller, G.N.; Aldape, K.D.; Atkinson, G.J.; Levy, L.B.; McCutcheon, I.E.; Maor, M.H. The prognostic value of neurologic function in astrocytic spinal cord glioma. Neuro-Oncology 2003, 5, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Patel, J.; Pennington, Z.; Porras, J.L.; Goldsborough, E.; Antar, A.; Elsamadicy, A.A.; Lubelski, D.; Wolinsky, J.P.; Jallo, G.I.; et al. Perioperative outcomes and survival after surgery for intramedullary spinal cord tumors: A single-institution series of 302 patients. J. Neurosurg. Spine 2022, 37, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.; Chamberlain, M.C. Adult primary spinal cord tumors. Expert Rev. Neurother. 2009, 9, 1487–1495. [Google Scholar] [CrossRef]
- Samartzis, D.; Gillis, C.C.; Shih, P.; O’Toole, J.E.; Fessler, R.G. Intramedullary Spinal Cord Tumors: Part I—Epidemiology, Pathophysiology, and Diagnosis. Glob. Spine J. 2015, 5, 425–435. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, J.-H.; Choi, S.H.; Sohn, C.-H.; Yun, T.J.; Kim, C.H.; Chang, K.-H. Differentiation between Intramedullary spinal ependymoma and astrocytoma: Comparative MRI analysis. Clin. Radiol. 2014, 69, 29–35. [Google Scholar] [CrossRef]
- Samartzis, D.; Gillis, C.C.; Shih, P.; O’Toole, J.E.; Fessler, R.G. Intramedullary Spinal Cord Tumors: Part II—Management Options and Outcomes. Glob. Spine J. 2016, 6, 176–185. [Google Scholar] [CrossRef]
- Klekamp, J. Spinal ependymomas. Part 1: Intramedullary ependymomas. Neurosurg. Focus 2015, 39, E6. [Google Scholar] [CrossRef]
- Svoboda, N.; Bradac, O.; de Lacy, P.; Benes, V. Intramedullary ependymoma: Long-term outcome after surgery. Acta Neurochir. 2018, 160, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Chung, C.K.; Kim, C.H.; Yoon, S.H.; Hyun, S.-J.; Kim, K.-J.; Kim, E.-S.; Eoh, W.; Kim, H.-J. Long-term outcomes of surgical resection with or without adjuvant radiation therapy for treatment of spinal ependymoma: A retrospective multicenter study by the Korea Spinal Oncology Research Group. Neuro-Oncology 2013, 15, 921–929. [Google Scholar] [CrossRef]
- Zheng, Y.; Ong, S.H.; Nga, V.D.W.; Vellayappan, B. Adjuvant radiotherapy versus observation after gross total resection of WHO grade II ependymoma: A systematic review and individual-participant data meta-analysis. Chin. Clin. Oncol. 2024, 13, 22. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Huang, C.-I.; Wong, T.-T.; Chen, M.-H.; Shiau, C.-Y.; Wang, L.-W.; Ho, D.M.-T.; Yen, S.-H. Treatment of spinal cord ependymomas by surgery with or without postoperative radiotherapy. J. Neurooncol. 2005, 71, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Chaskis, E.; Bouchaala, M.; David, P.; Parker, F.; Aghakhani, N.; Knafo, S. Long-Term Outcomes after Incomplete Resection of Intramedullary Grade II Ependymomas: Is Adjuvant Radiotherapy Justified? Cancers 2023, 15, 3674. [Google Scholar] [CrossRef]
- Chamberlain, M.C. Salvage chemotherapy for recurrent spinal cord ependymona. Cancer 2002, 95, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Smith, Z.A.; Wong, A.P.; Melkonian, S.; Harris, D.A.; Lam, S. Predictors of survival in patients with spinal ependymoma. Neurol. Res. 2015, 37, 650–655. [Google Scholar] [CrossRef]
- Ogunlade, J.; Wiginton, J.G.; Elia, C.; Odell, T.; Rao, S.C. Primary Spinal Astrocytomas: A Literature Review. Cureus 2019, 11, e5247. [Google Scholar] [CrossRef]
- Kandemirli, S.G.; Reddy, A.; Hitchon, P.; Saini, J.; Bathla, G. Intramedullary tumours and tumour mimics. Clin. Radiol. 2020, 75, 876.e17–876.e32. [Google Scholar] [CrossRef]
- Seaman, S.C.; Bathla, G.; Park, B.J.; Woodroffe, R.W.; Smith, M.; Menezes, A.H.; Noeller, J.; Yamaguchi, S.; Hitchon, P.W. MRI characteristics and resectability in spinal cord glioma. Clin. Neurol. Neurosurg. 2021, 200, 106321. [Google Scholar] [CrossRef]
- Pojskić, M.; Rotim, K.; Splavski, B.; Arnautović, K.I. Microsurgical Management of Low-Grade Spinal Cord Astrocytoma in Adults: A Personal Case Series Report and Brief Literature Review. Acta Clin. Croat. 2020, 59, 505–512. [Google Scholar] [CrossRef]
- AlRaddadi, K.K.; Farrash, F.; Baeesa, S.; Alkhani, A.M. Primary spinal intramedullary astrocytomas; long-term outcomes and literature review. Interdiscip. Neurosurg. 2022, 27, 101401. [Google Scholar] [CrossRef]
- Khalid, S.I.; Kelly, R.; Carlton, A.; Wu, R.; Peta, A.; Melville, P.; Maasarani, S.; Meyer, H.; Adogwa, O. Adult intradural intramedullary astrocytomas: A multicenter analysis. J. Spine Surg. 2019, 5, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Antar, A.; Pennington, Z.; Aygun, N.; Patel, J.; Goldsborough, E.; Porras, J.L.; Elsamadicy, A.A.; Lubelski, D.; Wolinsky, J.P.; et al. Predictors of survival and time to progression following operative management of intramedullary spinal cord astrocytomas. J. Neurooncol. 2022, 158, 117–127. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, R.; Wang, R.; Wang, C.; Chen, C. Outcome predictors in the management of intramedullary classic ependymoma. Medicine 2018, 97, e10870. [Google Scholar] [CrossRef] [PubMed]
- de Paiva, J.L.R.; Sabino, J.V.; Pereira, F.V.; Okuda, P.A.; Villarinho, L.L.; Queiroz, L.S.; França, M.C., Jr.; Reis, F. The Role of MRI in the Diagnosis of Spinal Cord Tumors. Semin. Ultrasound CT MRI 2023, 44, 436–451. [Google Scholar] [CrossRef]
- Jankovic, D.; Hanissian, A.; Rotim, K.; Splavski, B.; Arnautovic, K.I. Novel Clinical Insights into Spinal Hemangioblastoma in Adults: A Systematic Review. World Neurosurg. 2022, 158, 1–10. [Google Scholar] [CrossRef]
- Yousef, A.; Rutkowski, M.J.; Yalcin, C.E.; Eren, O.C.; Caliskan, I.; Tihan, T. Sporadic and Von-Hippel Lindau disease-associated spinal hemangioblastomas: Institutional experience on their similarities and differences. J. Neurooncol. 2019, 143, 547–552. [Google Scholar] [CrossRef]
- Takeshima, Y.; Takami, H.; Endo, T.; Mizuno, M.; Hida, K. Comparison of the Recurrence and Surgical Outcome of Spinal Hemangioblastoma in Sporadic and Von Hippel-Lindau Diseases: A Subanalysis of a Nationwide Study by the Neurospinal Society of Japan. Neurospine 2023, 20, 756–765. [Google Scholar] [CrossRef]
- Prokopienko, M.; Kunert, P.; Podgórska, A.; Marchel, A. Surgical treatment of sporadic and von Hippel-Lindau syndrome-associated intramedullary hemangioblastomas. Neurol. Neurochir. Pol. 2016, 50, 349–355. [Google Scholar] [CrossRef]
- Endo, T.; Inoue, T.; Mizuno, M.; Kurokawa, R.; Ito, K.; Ueda, S.; Takami, T.; Hida, K.; Hoshimaru, M. Current Trends in the Surgical Management of Intramedullary Tumors: A Multicenter Study of 1,033 Patients by the Neurospinal Society of Japan. Neurospine 2022, 19, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chan, L.; Bai, H.X.; Li, X.; Zhang, Z.; Wang, Y.; Cao, Y.; Karakousis, G.; Huang, R.; Xiao, B.; et al. Assessment of care pattern and outcome in hemangioblastoma. Sci. Rep. 2018, 8, 11144. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.K.; Geraghty, J.R.; Engelhard, H.H.; Linninger, A.A.; Mehta, A.I. Intramedullary spinal cord tumors: A review of current and future treatment strategies. Neurosurg. Focus 2015, 39, E14. [Google Scholar] [CrossRef] [PubMed]
- Sandalcioglu, I.E.; Gasser, T.; Asgari, S.; Lazorisak, A.; Engelhorn, T.; Egelhof, T.; Stolke, D.; Wiedemayer, H. Functional outcome after surgical treatment of intramedullary spinal cord tumors: Experience with 78 patients. Spinal Cord 2005, 43, 34–41. [Google Scholar] [CrossRef]
- Gembruch, O.; Chihi, M.; Haarmann, M.; Parlak, A.; Darkwah Oppong, M.; Rauschenbach, L.; Michel, A.; Jabbarli, R.; Ahmadipour, Y.; Sure, U.; et al. Surgical outcome and prognostic factors in spinal cord ependymoma: A single-center, long-term follow-up study. Ther. Adv. Neurol. Disord. 2021, 14, 17562864211055694. [Google Scholar] [CrossRef]
| Total (N = 253) | EPND (N = 114) | ASTR (N = 90) | HMNG (N = 28) | MISC (N = 21) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Without MISC | EPND vs. ASTR | EPND vs. HMNG | ASTR vs. HMNG | ||||||
| Gender, Male | 146.0 (57.7%) | 67.0 (58.8%) | 54.0 (60.0%) | 14.0 (50.0%) | 11.0 (52.4%) | 0.635 1 | 0.859 1 | 0.401 1 | 0.350 1 |
| Age at surgery, Years | 36.2 (±19.1) | 42.0 (±13.6) | 29.7 (±23.5) | 38.5 (±15.7) | 29.3 (±18.4) | <0.001 2 | <0.001 4 | 0.236 4 | 0.066 4 |
| BMI, kg/m2 | 27.0 (±10.6) | 28.1 (±6.3) | 26.4 (±15.8) | 26.6 (±7.1) | 23.3 (±4.9) | 0.614 2 | 0.374 4 | 0.315 4 | 0.973 4 |
| Smoking status | 0.009 1 | 0.013 1 | 0.428 1 | 0.001 1 | |||||
| Never smoker | 166.0 (75.5%) | 72.0 (72.0%) | 67.0 (89.3%) | 16.0 (59.3%) | 11.0 (61.1%) | ||||
| Current smoker | 14.0 (6.4%) | 9.0 (9.0%) | 1.0 (1.3%) | 4.0 (14.8%) | 0.0 (0.0%) | ||||
| Former smoker | 40.0 (18.2%) | 19.0 (19.0%) | 7.0 (9.3%) | 7.0 (25.9%) | 7.0 (38.9%) | ||||
| Unknown/missing | 33 | 14 | 15 | 1 | 3 | ||||
| Charlson comorbidity index | 0.263 1 | 0.354 1 | 0.115 1 | 0.353 1 | |||||
| 1 | 2.0 (0.8%) | 2.0 (1.8%) | 0.0 (0.0%) | 0.0 (0.0%) | 0.0 (0.0%) | ||||
| 2 | 223.0 (88.1%) | 101.0 (88.6%) | 81.0 (90.0%) | 25.0 (89.3%) | 16.0 (76.2%) | ||||
| 3 | 12.0 (4.7%) | 8.0 (7.0%) | 4.0 (4.4%) | 0.0 (0.0%) | 0.0 (0.0%) | ||||
| ≥4 | 16.0 (6.3%) | 3.0 (2.6%) | 5.0 (5.6%) | 3.0 (10.7%) | 5.0 (23.8%) | ||||
| Preoperative mMS | 0.017 1 | 0.003 1 | 0.686 1 | 0.147 1 | |||||
| I | 30.0 (11.9%) | 6.0 (5.3%) | 17.0 (18.9%) | 3.0 (10.7%) | 4.0 (19.0%) | ||||
| II | 151.0 (59.7%) | 79.0 (69.3%) | 41.0 (45.6%) | 20.0 (71.4%) | 11.0 (52.4%) | ||||
| III | 51.0 (20.2%) | 21.0 (18.4%) | 22.0 (24.4%) | 4.0 (14.3%) | 4.0 (19.0%) | ||||
| IV | 12.0 (4.7%) | 4.0 (3.5%) | 7.0 (7.8%) | 0.0 (0.0%) | 1.0 (4.8%) | ||||
| V | 9.0 (3.6%) | 4.0 (3.5%) | 3.0 (3.3%) | 1.0 (3.6%) | 1.0 (4.8%) | ||||
| Duraplasty | 38.0 (15.4%) | 16.0 (14.7%) | 14.0 (15.9%) | 3.0 (10.7%) | 5.0 (23.8%) | 0.795 1 | 0.811 1 | 0.764 3 | 0.760 3 |
| Unknown/missing | 7 | 5 | 2 | 0 | 0 | ||||
| Chemotherapy | 74.0 (29.2%) | 14.0 (12.3%) | 48.0 (53.3%) | 2.0 (7.1%) | 10.0 (47.6%) | <0.001 1 | <0.001 1 | 0.734 3 | <0.001 3 |
| Radiotherapy | 92.0 (36.4%) | 27.0 (23.7%) | 42.0 (46.7%) | 10.0 (35.7%) | 13.0 (61.9%) | 0.003 1 | <0.001 1 | 0.194 1 | 0.308 1 |
| Max radiotherapy dose if used, Gray | 4214.2 (±1257.7) | 4432.5 (±1124.1) | 4653.5 (±892.3) | 2626.7 (±948.7) | 4291.1 (±1456.6) | <0.001 2 | 0.498 4 | <0.001 4 | <0.001 4 |
| Intra-operative complications | 20.0 (8.0%) | 12.0 (10.8%) | 3.0 (3.4%) | 1.0 (3.6%) | 4.0 (19.0%) | 0.100 1 | 0.061 3 | 0.464 3 | 1.000 3 |
| Follow-up length, Months | 45.3 (±48.2) | 48.4 (±49.6) | 42.5 (±48.6) | 44.9 (±44.8) | 40.4 (±45.0) | 0.685 2 | 0.393 4 | 0.727 4 | 0.821 4 |
| Mortality | 48.0 (19.0%) | 12.0 (10.5%) | 27.0 (30.0%) | 5.0 (17.9%) | 4.0 (19.0%) | 0.002 1 | <0.001 1 | 0.284 1 | 0.207 1 |
| Histology or Classification | N (%) |
|---|---|
| Classification | |
| WHO grade 1 | 87.0 (34.4%) |
| WHO grade 2 | 130.0 (51.4%) |
| WHO grade 3 | 25.0 (9.9%) |
| WHO grade 4 | 11.0 (4.3%) |
| Histology | |
| Ependymal tumors * | 114 (45.1%) |
| WHO grade 1 | 11.0 (9.6%) |
| WHO grade 2 | 101.0 (88.6%) |
| WHO grade 3 | 2.0 (1.8%) |
| Astrocytic tumors | 90 (35.6%) |
| WHO grade 1 | 36.0 (40.0%) |
| WHO grade 2 | 27.0 (30.0%) |
| WHO grade 3 | 16.0 (17.8%) |
| WHO grade 4 | 11.0 (12.2%) |
| Hemangioblastoma | 28 (11.1%) |
| Miscellaneous | 21 (8.3%) |
| WHO grade 1 | 12.0 (57.1%) |
| WHO grade 2 | 2.0 (9.5%) |
| WHO grade 3 | 7.0 (33.4%) |
| Total (N = 253) | EPND (N = 114) | ASTR (N = 90) | HMNG (N = 28) | MISC (N = 21) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Without MISC | EPND vs. ASTR | EPND vs. HMNG | ASTR vs. HMNG | ||||||
| Tumor location | 0.069 1 | 0.437 1 | 0.046 1 | 0.024 1 | |||||
| Cervical | 95.0 (37.5%) | 46.0 (40.4%) | 27.0 (30.0%) | 15.0 (53.6%) | 7.0 (33.3%) | ||||
| Cervicothoracic | 84.0 (33.2%) | 39.0 (34.2%) | 33.0 (36.7%) | 3.0 (10.7%) | 9.0 (42.9%) | ||||
| Thoracic | 48.0 (19.0%) | 21.0 (18.4%) | 21.0 (23.3%) | 5.0 (17.9%) | 1.0 (4.8%) | ||||
| Thoracolumbar/conus | 26.0 (10.3%) | 8.0 (7.0%) | 9.0 (10.0%) | 5.0 (17.9%) | 4.0 (19.0%) | ||||
| Type of Resection | <0.001 1 | <0.001 3 | <0.001 1 | <0.001 3 | |||||
| En-bloc | 34.0 (13.4%) | 19.0 (16.7%) | 0.0 (0.0%) | 15.0 (53.6%) | 0.0 (0.0%) | ||||
| Intralesional | 219.0 (86.6%) | 95.0 (83.3%) | 90.0 (100.0%) | 13.0 (46.4%) | 21.0 (100.0%) | ||||
| Extent of resection | <0.001 1 | <0.001 1 | 0.076 3 | <0.001 3 | |||||
| GTR | 185.0 (73.1%) | 94.0 (82.5%) | 50.0 (55.6%) | 27.0 (96.4%) | 14.0 (66.7%) | ||||
| STR | 68.0 (26.9%) | 20.0 (17.5%) | 40.0 (44.4%) | 1.0 (3.6%) | 7.0 (33.3%) | ||||
| Tumor levels span | 3.8 (±2.0) | 3.7 (±2.0) | 4.3 (±2.1) | 2.7 (±1.4) | 3.7 (±1.2) | <0.001 2 | 0.036 4 | 0.016 4 | <0.001 4 |
| Blood transfusion during surgery | 10.0 (6.3%) | 5.0 (7.1%) | 2.0 (3.5%) | 2.0 (13.3%) | 1.0 (6.2%) | 0.353 1 | 0.458 3 | 0.602 3 | 0.189 3 |
| Unknown/missing | 95 | 44 | 33 | 13 | 5 | ||||
| Surgery duration, mins | 270.3 (±103.0) | 287.1 (±121.7) | 249.0 (±94.8) | 300.1 (±75.7) | 234.5 (±46.6) | 0.141 2 | 0.109 4 | 0.689 4 | 0.059 4 |
| Estimated blood loss during surgery, mL | 188.0 (±393.1) | 230.4 (±461.6) | 152.9 (±401.6) | 191.3 (±147.9) | 138.8 (±227.5) | 0.529 2 | 0.291 4 | 0.691 4 | 0.656 4 |
| Length of stay, Days | 8.0 (±8.3) | 8.0 (±7.7) | 7.6 (±8.5) | 9.4 (±10.5) | 7.9 (±7.4) | 0.628 2 | 0.749 4 | 0.431 4 | 0.370 4 |
| Hospital discharges | |||||||||
| Home | 114.0 (45.1%) | 50.0 (43.9%) | 38.0 (42.2%) | 17.0 (60.7%) | 9.0 (42.9%) | 0.210 1 | 0.815 1 | 0.109 1 | 0.087 1 |
| Subacute rehab | 20.0 (7.9%) | 7.0 (6.1%) | 12.0 (13.3%) | 1.0 (3.6%) | 0.0 (0.0%) | 0.115 1 | 0.079 1 | 1.000 3 | 0.297 3 |
| ACIR | 116.0 (45.8%) | 55.0 (48.2%) | 40.0 (44.4%) | 10.0 (35.7%) | 11.0 (52.4%) | 0.481 1 | 0.589 1 | 0.233 1 | 0.414 1 |
| Hospice care | 1.0 (0.4%) | 0.0 (0.0%) | 0.0 (0.0%) | 0.0 (0.0%) | 1.0 (4.8%) | - | - | - | - |
| Death | 2.0 (0.8%) | 2.0 (1.8%) | 0.0 (0.0%) | 0.0 (0.0%) | 0.0 (0.0%) | 0.3521 | 0.5043 | 1.0003 | - |
| Readmission within 30 days | 27.0 (10.7%) | 8.0 (7.0%) | 12.0 (13.3%) | 1.0 (3.6%) | 6.0 (28.6%) | 0.165 1 | 0.132 1 | 0.689 3 | 0.297 3 |
| Reoperation within 30 days | 16.0 (6.3%) | 7.0 (6.1%) | 6.0 (6.7%) | 1.0 (3.6%) | 2.0 (9.5%) | 0.833 1 | 0.879 1 | 1.000 3 | 1.000 3 |
| Total (N = 249) | EPND (N = 111) | ASTR (N = 89) | HMNG (N = 28) | MISC (N = 21) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Without MISC | EPND | ASTR | HMNG | ||||||
| Numbness | <0.001 1 | <0.001 1 | <0.001 1 | 0.021 1 | |||||
| Preoperative | 186.0 (74.7%) | 90.0 (81.1%) | 60.0 (67.4%) | 24.0 (85.7%) | 12.0 (57.1%) | ||||
| Postoperative | 130.0 (52.2%) | 66.0 (59.5%) | 38.0 (42.7%) | 16.0 (57.1%) | 10.0 (47.6%) | ||||
| Bladder incontinence | <0.001 1 | <0.001 1 | 0.210 1 | 1.000 1 | |||||
| Preoperative | 59.0 (23.7%) | 28.0 (25.2%) | 23.0 (25.8%) | 3.0 (10.7%) | 5.0 (23.8%) | ||||
| Postoperative | 29.0 (11.6%) | 6.0 (5.4%) | 16.0 (18.0%) | 2.0 (7.1%) | 5.0 (23.8%) | ||||
| Bowel dysfunctions | 1.000 1 | 0.219 1 | 1.000 1 | 1.000 1 | |||||
| Preoperative | 22.0 (8.8%) | 5.0 (4.5%) | 12.0 (13.5%) | 2.0 (7.1%) | 3.0 (14.3%) | ||||
| Postoperative | 21.0 (8.4%) | 1.0 (0.9%) | 12.0 (13.5%) | 2.0 (7.1%) | 6.0 (28.6%) | ||||
| Back/neck pain | <0.001 1 | <0.001 1 | 0.291 1 | 0.092 1 | |||||
| Preoperative | 105.0 (42.2%) | 48.0 (43.2%) | 38.0 (42.7%) | 11.0 (39.3%) | 8.0 (38.1%) | ||||
| Postoperative | 64.0 (25.7%) | 22.0 (19.8%) | 30.0 (33.7%) | 4.0 (14.3%) | 8.0 (38.1%) | ||||
| Radicular symptoms | <0.001 1 | <0.001 1 | 0.035 1 | 0.219 1 | |||||
| Preoperative | 51.0 (20.5%) | 31.0 (27.9%) | 13.0 (14.6%) | 5.0 (17.9%) | 2.0 (9.5%) | ||||
| Postoperative | 16.0 (6.4%) | 8.0 (7.2%) | 4.0 (4.5%) | 1.0 (3.6%) | 3.0 (14.3%) | ||||
| Muscle weakness | 0.175 1 | 0.007 1 | 0.170 1 | 0.065 1 | |||||
| Preoperative | 144.0 (57.8%) | 66.0 (59.5%) | 50.0 (56.2%) | 19.0 (67.9%) | 9.0 (42.9%) | ||||
| Postoperative | 130.0 (52.2%) | 46.0 (41.4%) | 58.0 (65.2%) | 12.0 (42.9%) | 14.0 (66.7%) | ||||
| Ambulation ability | <0.001 1 | 0.180 1 | 0.013 1 | 1.000 1 | |||||
| Preoperative | 222.0 (89.2%) | 104.0 (93.7%) | 74.0 (83.1%) | 26.0 (92.9%) | 18.0 (85.7%) | ||||
| Postoperative | 202.0 (81.1%) | 99.0 (89.2%) | 63.0 (70.8%) | 25.0 (89.3%) | 15.0 (71.4%) | ||||
| modified McCormick Scale (mMS) | |||||||||
| Preoperative | |||||||||
| I | 28.0 (11.2%) | 5.0 (4.5%) | 16.0 (18.0%) | 3.0 (10.7%) | 4.0 (19.0%) | ||||
| II | 150.0 (60.2%) | 78.0 (70.3%) | 41.0 (46.1%) | 20.0 (71.4%) | 11.0 (52.4%) | ||||
| III | 50.0 (20.1%) | 20.0 (18.0%) | 22.0 (24.7%) | 4.0 (14.3%) | 4.0 (19.0%) | ||||
| IV | 12.0 (4.8%) | 4.0 (3.6%) | 7.0 (7.9%) | 0.0 (0.0%) | 1.0 (4.8%) | ||||
| V | 9.0 (3.6%) | 4.0 (3.6%) | 3.0 (3.4%) | 1.0 (3.6%) | 1.0 (4.8%) | ||||
| Postoperative | <0.001 1 | <0.001 1 | 0.036 1 | 0.300 1 | |||||
| I | 83.0 (33.3%) | 45.0 (40.5%) | 27.0 (30.3%) | 7.0 (25.0%) | 4.0 (19.0%) | ||||
| II | 79.0 (31.7%) | 34.0 (30.6%) | 24.0 (27.0%) | 14.0 (50.0%) | 7.0 (33.3%) | ||||
| III | 53.0 (21.3%) | 26.0 (23.4%) | 17.0 (19.1%) | 6.0 (21.4%) | 4.0 (19.0%) | ||||
| IV | 19.0 (7.6%) | 3.0 (2.7%) | 10.0 (11.2%) | 0.0 (0.0%) | 6.0 (28.6%) | ||||
| V | 15.0 (6.0%) | 3.0 (2.7%) | 11.0 (12.4%) | 1.0 (3.6%) | 0.0 (0.0%) | ||||
| Change | 0.030 2 | - | - | - | |||||
| Worse (grade or more) | 59.0 (23.7%) | 21.0 (18.9%) | 26.0 (29.2%) | 5.0 (17.9%) | 7.0 (33.3%) | ||||
| Same | 107.0 (43.0%) | 40.0 (36.0%) | 39.0 (43.8%) | 16.0 (57.1%) | 12.0 (57.1%) | ||||
| Improved (grade or more) | 83.0 (33.3%) | 50.0 (45.0%) | 24.0 (27.0%) | 7.0 (25.0%) | 2.0 (9.5%) | ||||
| Local recurrence of the tumor | 79.0 (31.7%) | 16.0 (14.4%) | 39.0 (43.8%) | 10.0 (35.7%) | 10.0 (47.6%) | <0.001 2 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mistarehi, A.-H.; Zaitoun, K.J.; Javed, S.; Xia, Y.; Hersh, A.; Ghaith, A.K.; Weber-Levine, C.; Jiang, K.; Khan, M.; Mendelson, B.; et al. Survival and Functional Outcomes Following Surgical Resection of Intramedullary Spinal Cord Tumors: A Series of 253 Patients over 22 Years. Cancers 2025, 17, 2112. https://doi.org/10.3390/cancers17132112
Al-Mistarehi A-H, Zaitoun KJ, Javed S, Xia Y, Hersh A, Ghaith AK, Weber-Levine C, Jiang K, Khan M, Mendelson B, et al. Survival and Functional Outcomes Following Surgical Resection of Intramedullary Spinal Cord Tumors: A Series of 253 Patients over 22 Years. Cancers. 2025; 17(13):2112. https://doi.org/10.3390/cancers17132112
Chicago/Turabian StyleAl-Mistarehi, Abdel-Hameed, Khaled J. Zaitoun, Sania Javed, Yuanxuan Xia, Andrew Hersh, Abdul Karim Ghaith, Carly Weber-Levine, Kelly Jiang, Majid Khan, Benjamin Mendelson, and et al. 2025. "Survival and Functional Outcomes Following Surgical Resection of Intramedullary Spinal Cord Tumors: A Series of 253 Patients over 22 Years" Cancers 17, no. 13: 2112. https://doi.org/10.3390/cancers17132112
APA StyleAl-Mistarehi, A.-H., Zaitoun, K. J., Javed, S., Xia, Y., Hersh, A., Ghaith, A. K., Weber-Levine, C., Jiang, K., Khan, M., Mendelson, B., Ksabi, N., Sciubba, D. M., Gokaslan, Z. L., Jallo, G. I., Wolinsky, J.-P., Theodore, N., & Lubelski, D. (2025). Survival and Functional Outcomes Following Surgical Resection of Intramedullary Spinal Cord Tumors: A Series of 253 Patients over 22 Years. Cancers, 17(13), 2112. https://doi.org/10.3390/cancers17132112







