Impact of HPV Testing Based on the 2020 Update of the German Cervical Cancer Screening Program—Data from a Retrospective Monocentric Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. General Patient Data
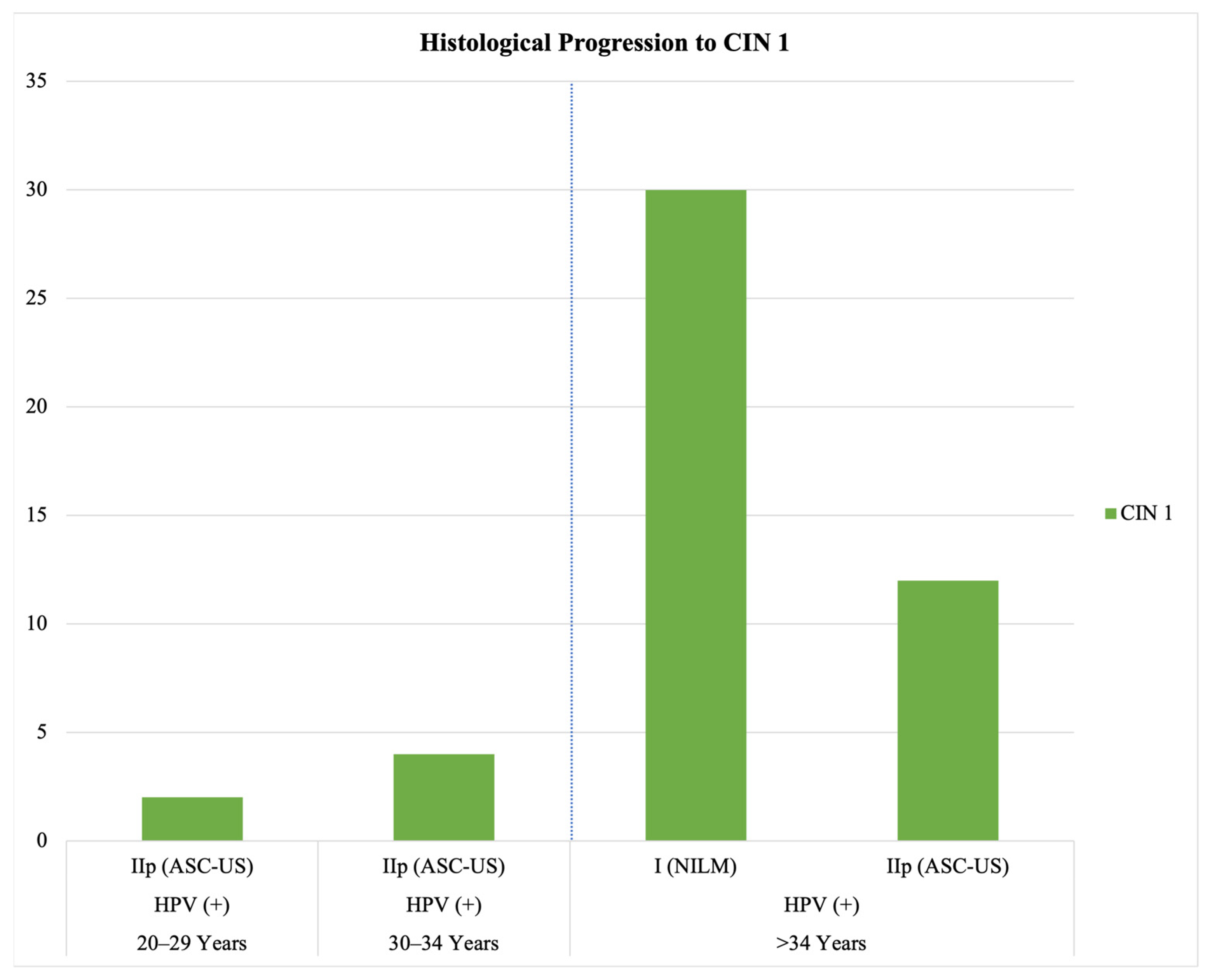
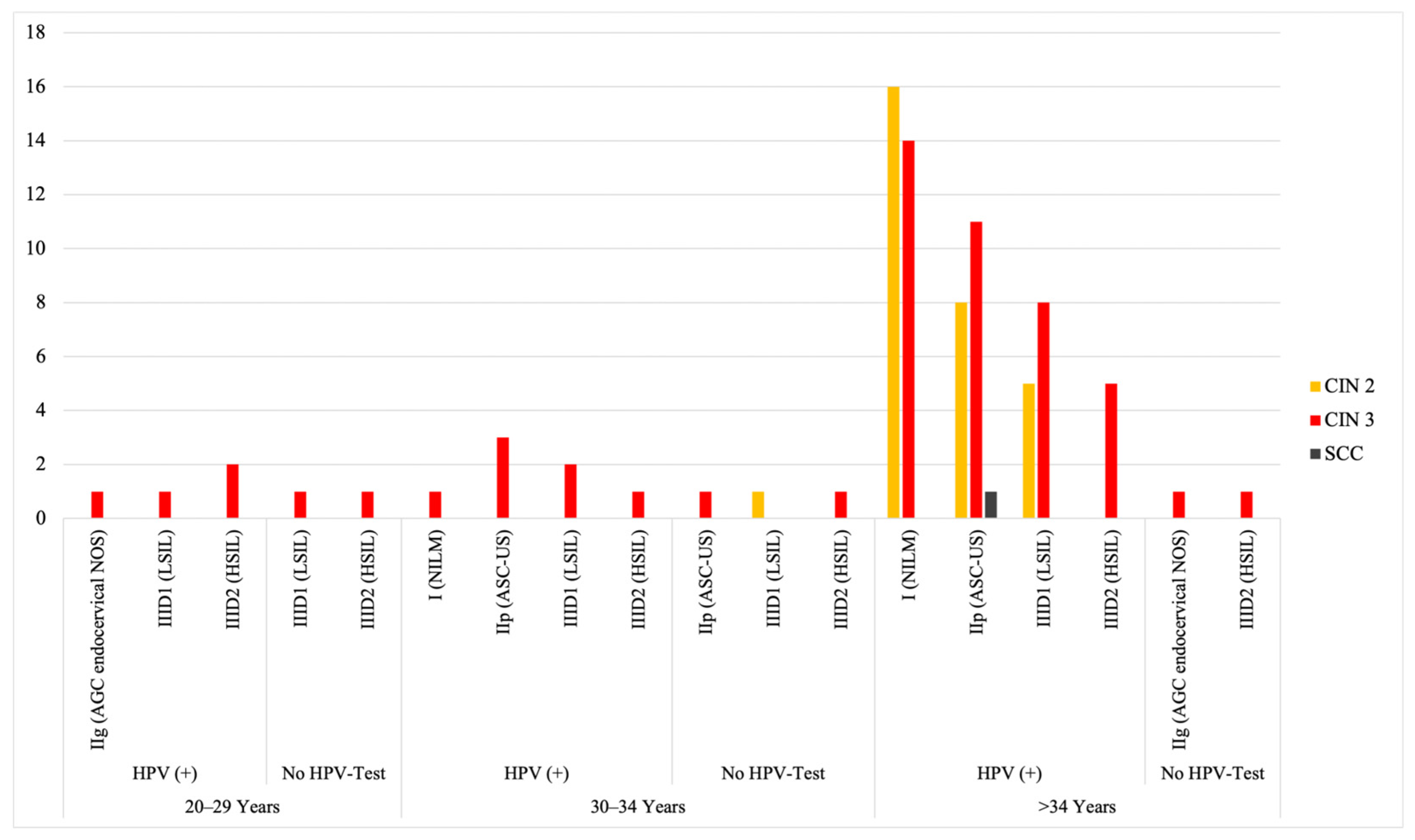
3.2. Clinical Implications of HPV Testing and Cytology: Histological Progression, Repeated Testing, HPV Clearance, and Treatment Following Persistent Positivity
4. Discussion
5. Conclusions
- Since we report a rise in CIN II/III diagnoses in 2020/21 compared to 2018/19, the implementation of HPV co-testing will increase the detection rate of high-grade cervical dysplasia.
- No histological progression was observed in HPV-negative cases with initial abnormal cytology within our study—a negative HPV test correlates with a low risk of progression and potential spontaneous regression.
- As already determined in studies such as Swedescreen, Pobascam, Artistic, and HPV-Focal trial, an initially higher number of colposcopies is observed [4,5,7,10]. In our study, the overall number of colposcopic interventions increased due to the updated screening program. However, it remains to be resolved whether these referral rates can be considered as an earlier detection of dysplastic lesions rather than overdiagnosis per se (as postulated in the abovementioned studies).
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HPV | Human Papillomavirus |
| CIN | Cervical intraepithelial neoplasia |
| IQWiG | Institute for Quality and Efficiency in Health Care |
| QA | Quality Assurance |
| WHO | World Health Organization |
| NILM | Negative for intraepithelial lesion or malignancy |
| ASC-US | Atypical squamous cells of undetermined significance |
| AGC endocervical NOS | Atypical glandular endocervical cells not otherwise specified |
| LSIL | Low-grade squamous intraepithelial lesion |
| HSIL | High-grade squamous intraepithelial lesion |
| AIS | Adenocarcinoma in situ |
References
- Anttila, A.; Ronco, G. Working Group on the Registration and Monitoring of Cervical Cancer Screening Programmes in the European Union; within the European Network for Information on Cancer (EUNICE). Description of the National Situation of Cervical Cancer Screening in the Member States of the European Union. Eur. J. Cancer 2009, 45, 2685–2708. [Google Scholar] [CrossRef]
- Schneider, V. Gynäkologische Krebsvorsorge in Deutschland. Der Pathol. 2012, 33, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Walboomers, J.M.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.F.; Peto, J.; Meijer, C.J.L.M.; Muñoz, N. Human Papillomavirus Is a Necessary Cause of Invasive Cervical Cancer Worldwide. J. Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Bulkmans, N.W.J.; Rozendaal, L.; Snijders, P.J.F.; Voorhorst, F.J.; Boeke, A.J.P.; Zandwijken, G.R.J.; van Kemenade, F.J.; Verheijen, R.H.M.; v Groningen, K.; Boon, M.E.; et al. POBASCAM, a Population-Based Randomized Controlled Trial for Implementation of High-Risk HPV Testing in Cervical Screening: Design, Methods and Baseline Data of 44,102 Women. Int. J. Cancer 2004, 110, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Elfström, K.M.; Smelov, V.; Johansson, A.L.V.; Eklund, C.; Nauclér, P.; Arnheim-Dahlström, L.; Dillner, J. Long Term Duration of Protective Effect for HPV Negative Women: Follow-up of Primary HPV Screening Randomised Controlled Trial. BMJ 2014, 348, g130. [Google Scholar] [CrossRef]
- Bergeron, C.; Giorgi-Rossi, P.; Cas, F.; Schiboni, M.L.; Ghiringhello, B.; Dalla Palma, P.; Minucci, D.; Rosso, S.; Zorzi, M.; Naldoni, C.; et al. Informed Cytology for Triaging HPV-Positive Women: Substudy Nested in the NTCC Randomized Controlled Trial. J. Natl. Cancer Inst. 2015, 107, dju423. [Google Scholar] [CrossRef] [PubMed]
- Gilham, C.; Sargent, A.; Kitchener, H.C.; Peto, J. HPV Testing Compared with Routine Cytology in Cervical Screening: Long-Term Follow-up of ARTISTIC RCT. Health Technol. Assess. 2019, 23, 1–44. [Google Scholar] [CrossRef]
- Ronco, G.; Dillner, J.; Elfström, K.M.; Tunesi, S.; Snijders, P.J.F.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of HPV-Based Screening for Prevention of Invasive Cervical Cancer: Follow-up of Four European Randomised Controlled Trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
- Wright, T.C.; Stoler, M.H.; Behrens, C.M.; Sharma, A.; Zhang, G.; Wright, T.L. Primary Cervical Cancer Screening with Human Papillomavirus: End of Study Results from the ATHENA Study Using HPV as the First-Line Screening Test. Gynecol. Oncol. 2015, 136, 189–197. [Google Scholar] [CrossRef]
- Ogilvie, G.S.; Krajden, M.; van Niekerk, D.; Smith, L.W.; Cook, D.; Ceballos, K.; Lee, M.; Gentile, L.; Gondara, L.; Elwood-Martin, R.; et al. HPV for Cervical Cancer Screening (HPV FOCAL): Complete Round 1 Results of a Randomized Trial Comparing HPV-Based Primary Screening to Liquid-Based Cytology for Cervical Cancer. Int. J. Cancer 2017, 140, 440–448. [Google Scholar] [CrossRef]
- European Commission: Directorate-General for Health and Food Safety; Karsa, L.V.; Dillner, J.; Suonio, E.; Törnberg, S.; Anttila, A.; Ronco, G.; Franceschi, S.; De Vuyst, H.; Dillner, L.; et al. European Guidelines for Quality Assurance in Cervical Cancer Screening—Second Edition—Supplements; Karsa, L.V., Dillner, J., Suonio, E., Törnberg, S., Anttila, A., Ronco, G., Franceschi, S., De Vuyst, H., Dillner, L., Patnick, J., et al., Eds.; Publications Office: Luxembourg, 2015. [Google Scholar]
- Arbyn, M.; Ronco, G.; Anttila, A.; Meijer, C.J.; Poljak, M.; Ogilvie, G.; Koliopoulos, G.; Naucler, P.; Sankaranarayanan, R.; Peto, J. Evidence regarding human papillomavirus testing in secondary prevention of cervical cancer. Vaccine 2012, 30 (Suppl. 5), F88–F99. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, M.; Nieminen, P.; Kotaniemi-Talonen, L.; Malila, N.; Tarkkanen, J.; Laurila, P.; Anttila, A. Age-specific evaluation of primary human papillomavirus screening vs conventional cytology in a randomized setting. J. Natl. Cancer Inst. 2009, 101, 1612–1623. [Google Scholar] [CrossRef]
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Nutzenbewertung Eines HPV-Tests im Primärscreening des Zervixkarzinoms; Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG): Köln, Germany, 2011; pp. 1–193. Available online: https://www.iqwig.de/download/s13-03_rapid-report_hpv-test-im-primaerscreening-des-zervixkarzinoms.pdf (accessed on 12 April 2025).
- Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Nutzenbewertung Eines HPV-Tests im Primärscreening des Zervixkarzinoms—Aktualisierung; Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG): Köln, Germany, 2014; pp. 1–59. [Google Scholar]
- von Karsa, L.; Arbyn, M.; De Vuyst, H.; Dillner, J.; Dillner, L.; Franceschi, S.; Patnick, J.; Ronco, G.; Segnan, N.; Suonio, E.; et al. European Guidelines for Quality Assurance in Cervical Cancer Screening. Summary of the Supplements on HPV Screening and Vaccination. Papillomavirus Res. 2015, 1, 22–31. [Google Scholar] [CrossRef]
- Gemeinsamer Bundesausschuss. Richtlinie Für Organisierte Krebsfrüherkennungsprogramme und Krebsfrüherkennungs-Richtlinie: Programm Zur Früherkennung von Zervixkarzinomen; Gemeinsamer Bundesausschuss: Berlin, Germany, 2018; Available online: https://www.g-ba.de/beschluesse/3597/ (accessed on 21 January 2024).
- Xhaja, A.; Ahr, A.; Zeiser, I.; Ikenberg, H. Two Years of Cytology and HPV Co-Testing in Germany: Initial Experience. Geburtshilfe Frauenheilkd. 2022, 82, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Kim, T.-J.; Hwang, C.-S.; Cho, C.H.; Jeong, D.H.; Seong, S.J.; Lee, J.-K.; Hur, S.; Kee, M.-K.; Seong, J.; et al. Risk of Cervical Dysplasia among Human Papillomavirus-Infected Women in Korea: A Multicenter Prospective Study. J. Gynecol. Oncol. 2019, 30, e50. [Google Scholar] [CrossRef]
- Travassos, A.G.; Netto, E.; Xavier-Souza, E.; Nóbrega, I.; Adami, K.; Timbó, M.; Abbehusen, K.; Fernandes, S.; Duran, C.; Haguihara, T.; et al. Predictors of HPV Incidence and Clearance in a Cohort of Brazilian HIV-Infected Women. PLoS ONE 2017, 12, e0185423. [Google Scholar] [CrossRef] [PubMed]
- Bosch, F.X.; Lorincz, A.; Muñoz, N.; Meijer, C.J.L.M.; Shah, K.V. The Causal Relation between Human Papillomavirus and Cervical Cancer. J. Clin. Pathol. 2002, 55, 244–265. [Google Scholar] [CrossRef]
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications-detail-redirect/9789240014107 (accessed on 21 January 2024).
- Leitlinienprogramm Onkologie. Deutsche Krebsgesellschaft, Deutsche Krebshilfe, AWMF. In S3-Leitlinie Prävention des Zervixkarzinoms; AWMF: Frankfurt am Main, Germany, 2017; Leitlinienreport 1.0. [Google Scholar]
- Sroczynski, G. Uwe Siebert Decision Analysis for the Evaluation of Benefits, Harms and Cost-Effectiveness of Different Cervical Cancer Screening Strategies to Inform the S3 Clinical Guideline “Prevention of Cervical Cancer” in the Context of the German Health Care System. 2016, pp. 1–77. Available online: https://www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Zervixkarzinom_Praevention/S3LL_EvidenceReport_Modeling_SroczynskiAndSiebert.pdf (accessed on 14 April 2025).
- Zhang, W.; Gao, K.; Fowkes, F.J.I.; Adeloye, D.; Rudan, I.; Song, P.; Jin, M.; Chen, K. Associated Factors and Global Adherence of Cervical Cancer Screening in 2019: A Systematic Analysis and Modelling Study. Glob. Health 2022, 18, 101. [Google Scholar] [CrossRef]
- Bundeszentrale für politische Bildung. Bevölkerung Nach Altersgruppen und Geschlecht; Bundeszentrale für politische Bildung: Bonn, Germany, 2020; Available online: https://www.bpb.de/kurz-knapp/zahlen-und-fakten/soziale-situation-in-deutschland/61538/bevoelkerung-nach-altersgruppen-und-geschlecht/ (accessed on 1 May 2024).
- Cuzick, J.; Clavel, C.; Petry, K.-U.; Meijer, C.J.L.M.; Hoyer, H.; Ratnam, S.; Szarewski, A.; Birembaut, P.; Kulasingam, S.; Sasieni, P.; et al. Overview of the European and North American Studies on HPV Testing in Primary Cervical Cancer Screening. Int. J. Cancer 2006, 119, 1095–1101. [Google Scholar] [CrossRef]
- O’Connor, M.; O’Brien, K.; Waller, J.; Gallagher, P.; D’Arcy, T.; Flannelly, G.; Martin, C.; McRae, J.; Prendiville, W.; Ruttle, C.; et al. Physical After-Effects of Colposcopy and Related Procedures, and Their Inter-Relationship with Psychological Distress: A Longitudinal Survey. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1402–1410. [Google Scholar] [CrossRef]
- Cook, D.A.; Smith, L.W.; Law, J.; Mei, W.; van Niekerk, D.J.; Ceballos, K.; Gondara, L.; Franco, E.L.; Coldman, A.J.; Ogilvie, G.S.; et al. Aptima HPV Assay versus Hybrid Capture® 2 HPV Test for Primary Cervical Cancer Screening in the HPV FOCAL Trial. J. Clin. Virol. 2017, 87, 23–29. [Google Scholar] [CrossRef]
- Gottschlich, A.; Gondara, L.; Smith, L.W.; Anderson, J.J.; Cook, D.; Krajden, M.; Lee, M.; Martin, R.E.; Melnikow, J.; Peacock, S.; et al. Colposcopy Referral Rates Post-Introduction of Primary Screening with Human Papillomavirus Testing: Evidence from a Large British Columbia Cohort Study. Lancet Reg. Health—Am. 2023, 26, 100598. [Google Scholar] [CrossRef] [PubMed]
- Franco, E.L.; Cuzick, J.; Hildesheim, A.; de Sanjosé, S. Chapter 20: Issues in Planning Cervical Cancer Screening in the Era of HPV Vaccination. Vaccine 2006, 24, S171–S177. [Google Scholar] [CrossRef] [PubMed]
- Wentzensen, N.; Arbyn, M. HPV-Based Cervical Cancer Screening- Facts, Fiction, and Misperceptions. Prev. Med. 2017, 98, 33–35. [Google Scholar] [CrossRef]
- Isidean, S.D.; Mayrand, M.-H.; Ramanakumar, A.V.; Gilbert, L.; Reid, S.L.; Rodrigues, I.; Ferenczy, A.; Ratnam, S.; Coutlée, F.; Franco, E.L.; et al. Human Papillomavirus Testing versus Cytology in Primary Cervical Cancer Screening: End-of-Study and Extended Follow-up Results from the Canadian Cervical Cancer Screening Trial. Int. J. Cancer 2016, 139, 2456–2466. [Google Scholar] [CrossRef] [PubMed]
- Chrysostomou, A.C.; Stylianou, D.C.; Constantinidou, A.; Kostrikis, L.G. Cervical Cancer Screening Programs in Europe: The Transition Towards HPV Vaccination and Population-Based HPV Testing. Viruses 2018, 10, 729. [Google Scholar] [CrossRef]
- Gibb, R.K.; Martens, M.G. The Impact of Liquid-Based Cytology in Decreasing the Incidence of Cervical Cancer. Rev. Obs. Gynecol. 2011, 4, S2–S11. [Google Scholar]
- Siebers, A.G.; Klinkhamer, P.J.J.M.; Grefte, J.M.M.; Massuger, L.F.A.G.; Vedder, J.E.M.; Beijers-Broos, A.; Bulten, J.; Arbyn, M. Comparison of Liquid-Based Cytology with Conventional Cytology for Detection of Cervical Cancer Precursors: A Randomized Controlled Trial. JAMA 2009, 302, 1757–1764. [Google Scholar] [CrossRef]
- Wentzensen, N.; Schiffman, M.; Palmer, T.; Arbyn, M. Triage of HPV Positive Women in Cervical Cancer Screening. J. Clin. Virol. 2016, 76, S49–S55. [Google Scholar] [CrossRef]
- Basu, P.; Meheus, F.; Chami, Y.; Hariprasad, R.; Zhao, F.; Sankaranarayanan, R. Management Algorithms for Cervical Cancer Screening and Precancer Treatment for Resource-Limited Settings. Int. J. Gynecol. Obstet. 2017, 138, 26–32. [Google Scholar] [CrossRef]
- Basu, P.; Mittal, S.; Bhadra Vale, D.; Chami Kharaji, Y. Secondary Prevention of Cervical Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 47, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.W.; Lipold, L.; Foucher, J.; Sikon, A.; Brainard, J.; Belinson, J.; Schramm, S.; Nottingham, K.; Hu, B.; Rothberg, M.B. Cost-Effectiveness of Primary HPV Testing, Cytology and Co-Testing as Cervical Cancer Screening for Women Above Age 30 Years. J. Gen. Intern. Med. 2016, 31, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, L.; Klamminger, G.G.; Nigdelis, M.P.; Eltze, E. Impact of HPV Testing Based on the 2020 Update of the German Cervical Cancer Screening Program—Data from a Retrospective Monocentric Study. Cancers 2025, 17, 2024. https://doi.org/10.3390/cancers17122024
Jung L, Klamminger GG, Nigdelis MP, Eltze E. Impact of HPV Testing Based on the 2020 Update of the German Cervical Cancer Screening Program—Data from a Retrospective Monocentric Study. Cancers. 2025; 17(12):2024. https://doi.org/10.3390/cancers17122024
Chicago/Turabian StyleJung, Leonard, Gilbert Georg Klamminger, Meletios P. Nigdelis, and Elke Eltze. 2025. "Impact of HPV Testing Based on the 2020 Update of the German Cervical Cancer Screening Program—Data from a Retrospective Monocentric Study" Cancers 17, no. 12: 2024. https://doi.org/10.3390/cancers17122024
APA StyleJung, L., Klamminger, G. G., Nigdelis, M. P., & Eltze, E. (2025). Impact of HPV Testing Based on the 2020 Update of the German Cervical Cancer Screening Program—Data from a Retrospective Monocentric Study. Cancers, 17(12), 2024. https://doi.org/10.3390/cancers17122024






