Comparison of Stereotactic Body Radiotherapy and Surgery for Stage I Lung Cancer: A Multidisciplinary Cohort Study Utilizing Propensity Score Overlap Weighting and AI-Based CT Imaging Analysis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
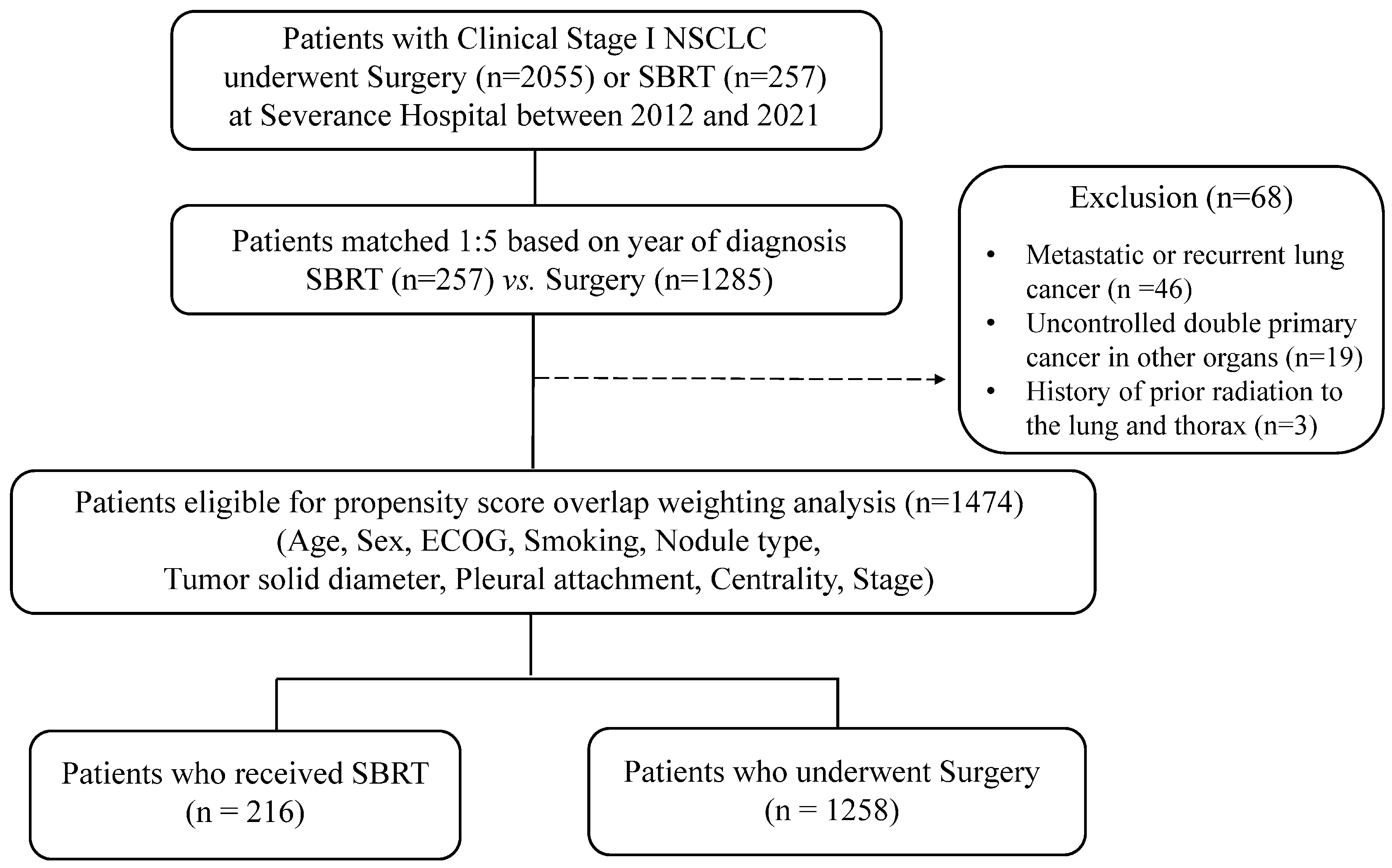
2.1. Patients and Clinical Data
2.2. Treatment
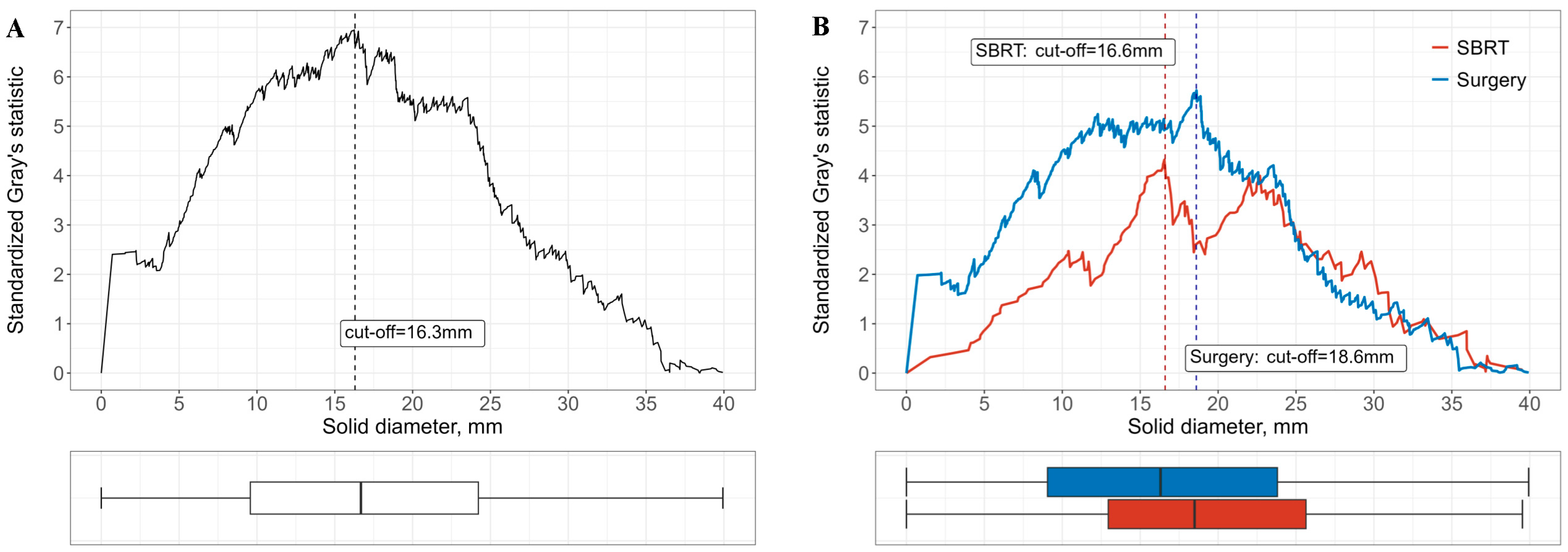
2.3. CT Image Acquisition and AI-Assisted Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Study Cohort Characteristics
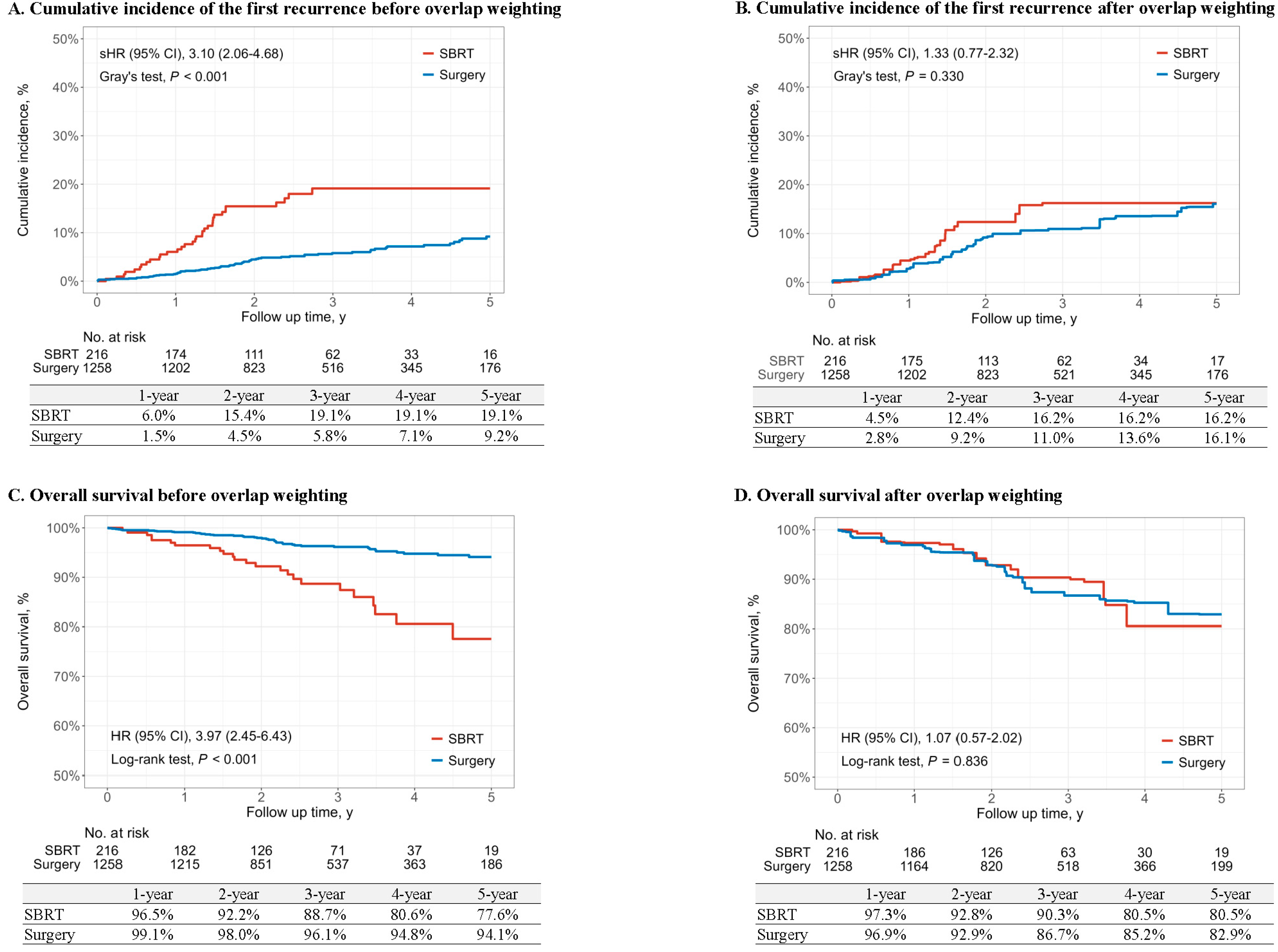
3.2. Survival Outcomes
3.3. Subgroup Analyses Stratified by Risk Factors
3.4. Mortality and Cause of Death Within 90 Days After Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Potter, A.L.; Rosenstein, A.L.; Kiang, M.V.; Shah, S.A.; Gaissert, H.A.; Chang, D.C.; Fintelmann, F.J.; Yang, C.J. Association of computed tomography screening with lung cancer stage shift and survival in the united states: Quasi-experimental study. BMJ 2022, 376, e069008. [Google Scholar] [CrossRef] [PubMed]
- Health Insurance Review and Assessment Service. 5th Lung Cancer Adequacy Assessment Report. Available online: https://www.hira.or.kr/cms/open/04/04/12/2020_14.pdf (accessed on 1 March 2025).
- Health Insurance Review and Assessment Service. Lung Cancer Adequacy Assessment Report (Based on 2013 Treatment Data). Available online: https://www.hira.or.kr/cms/open/04/04/12/2014_16.pdf (accessed on 1 March 2025).
- Kidane, B.; Bott, M.; Spicer, J.; Backhus, L.; Chaft, J.; Chudgar, N.; Colson, Y.; D’Amico, T.A.; David, E.; Lee, J.; et al. The american association for thoracic surgery (aats) 2023 expert consensus document: Staging and multidisciplinary management of patients with early-stage non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2023, 166, 637–654. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Non-Small Cell Lung Cancer. Version 4.2025. 23 May 2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf (accessed on 6 June 2025).
- Stokes, W.A.; Bronsert, M.R.; Meguid, R.A.; Blum, M.G.; Jones, B.L.; Koshy, M.; Sher, D.J.; Louie, A.V.; Palma, D.A.; Senan, S.; et al. Post-treatment mortality after surgery and stereotactic body radiotherapy for early-stage non-small-cell lung cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V. The iaslc lung cancer staging project: Proposals for revision of the tnm stage groupings in the forthcoming (eighth) edition of the tnm classification for lung cancer. J. Thorac. Oncol. 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised recist guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Benedict, S.H.; Yenice, K.M.; Followill, D.; Galvin, J.M.; Hinson, W.; Kavanagh, B.; Keall, P.; Lovelock, M.; Meeks, S.; Papiez, L.; et al. Stereotactic body radiation therapy: The report of aapm task group 101. Med. Phys. 2010, 37, 4078–4101. [Google Scholar] [CrossRef]
- Suh, Y.J.; Han, K.; Kwon, Y.; Kim, H.; Lee, S.; Hwang, S.H.; Kim, M.H.; Shin, H.J.; Lee, C.Y.; Shim, H.S. Computed tomography radiomics for preoperative prediction of spread through air spaces in the early stage of surgically resected lung adenocarcinomas. Yonsei Med. J. 2024, 65, 163–173. [Google Scholar] [CrossRef]
- Casal, R.F.; Sepesi, B.; Sagar, A.S.; Tschirren, J.; Chen, M.; Li, L.; Sunny, J.; Williams, J.; Grosu, H.B.; Eapen, G.A.; et al. Centrally located lung cancer and risk of occult nodal disease: An objective evaluation of multiple definitions of tumour centrality with dedicated imaging software. Eur. Respir. J. 2019, 53, 1802220. [Google Scholar] [CrossRef]
- Kim, H.; Goo, J.M.; Kim, Y.T.; Park, C.M. Ct-defined visceral pleural invasion in t1 lung adenocarcinoma: Lack of relationship to disease-free survival. Radiology 2019, 292, 741–749. [Google Scholar] [CrossRef]
- Li, F.; Thomas, L.E.; Li, F. Addressing extreme propensity scores via the overlap weights. Am. J. Epidemiol. 2019, 188, 250–257. [Google Scholar] [CrossRef]
- Thomas, L.E.; Li, F.; Pencina, M.J. Overlap weighting: A propensity score method that mimics attributes of a randomized clinical trial. JAMA 2020, 323, 2417–2418. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef]
- Austin, P.C. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Woo, S.-y.; Kim, S.; Kim, J. Determining cutoff values of prognostic factors in survival data with competing risks. Comput. Stat. 2016, 31, 369–386. [Google Scholar] [CrossRef]
- Chang, J.Y.; Senan, S.; Paul, M.A.; Mehran, R.J.; Louie, A.V.; Balter, P.; Groen, H.J.; McRae, S.E.; Widder, J.; Feng, L.; et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage i non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol. 2015, 16, 630–637. [Google Scholar] [CrossRef]
- Chang, J.Y.; Mehran, R.J.; Feng, L.; Verma, V.; Liao, Z.; Welsh, J.W.; Lin, S.H.; O’Reilly, M.S.; Jeter, M.D.; Balter, P.A.; et al. Stereotactic ablative radiotherapy for operable stage i non-small-cell lung cancer (revised stars): Long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol. 2021, 22, 1448–1457. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, S.M.; Jiang, J.; Chang, J.Y.; Welsh, J.; Likhacheva, A.; Buchholz, T.A.; Swisher, S.G.; Smith, B.D. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg. 2014, 149, 1244–1253. [Google Scholar] [CrossRef]
- Chi, A.; Fang, W.; Sun, Y.; Wen, S. Comparison of long-term survival of patients with early-stage non-small cell lung cancer after surgery vs stereotactic body radiotherapy. JAMA Netw. Open 2019, 2, e1915724. [Google Scholar] [CrossRef] [PubMed]
- Yerokun, B.A.; Yang, C.J.; Gulack, B.C.; Li, X.; Mulvihill, M.S.; Gu, L.; Wang, X.; Harpole, D.H.; D’Amico, T.A.; Berry, M.F.; et al. A national analysis of wedge resection versus stereotactic body radiation therapy for stage ia non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2017, 154, 675–686.e674. [Google Scholar] [CrossRef]
- Hamaji, M.; Chen, F.; Matsuo, Y.; Kawaguchi, A.; Morita, S.; Ueki, N.; Sonobe, M.; Nagata, Y.; Hiraoka, M.; Date, H. Video-assisted thoracoscopic lobectomy versus stereotactic radiotherapy for stage i lung cancer. Ann. Thorac. Surg. 2015, 99, 1122–1129. [Google Scholar] [CrossRef]
- Schneider, B.J.; Daly, M.E.; Kennedy, E.B.; Antonoff, M.B.; Broderick, S.; Feldman, J.; Jolly, S.; Meyers, B.; Rocco, G.; Rusthoven, C. Stereotactic body radiotherapy for early-stage non–small-cell lung cancer: American society of clinical oncology endorsement of the american society for radiation oncology evidence-based guideline. J. Clin. Oncol. 2018, 36, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Verstegen, N.E.; Oosterhuis, J.W.; Palma, D.A.; Rodrigues, G.; Lagerwaard, F.J.; van der Elst, A.; Mollema, R.; van Tets, W.F.; Warner, A.; Joosten, J.J.; et al. Stage i-ii non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (sabr) or lobectomy by video-assisted thoracoscopic surgery (vats): Outcomes of a propensity score-matched analysis. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Palma, D.; Visser, O.; Lagerwaard, F.J.; Belderbos, J.; Slotman, B.; Senan, S. Treatment of stage i nsclc in elderly patients: A population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2011, 101, 240–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, B.; Zhu, F.; Ma, X.; Tian, Y.; Cao, D.; Luo, S.; Xuan, Y.; Liu, L.; Wei, Y. Matched-pair comparisons of stereotactic body radiotherapy (sbrt) versus surgery for the treatment of early stage non-small cell lung cancer: A systematic review and meta-analysis. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2014, 112, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Borghetti, P.; Costantino, G.; Santoro, V.; Mataj, E.; Singh, N.; Vitali, P.; Greco, D.; Volpi, G.; Sepulcri, M.; Guida, C.; et al. Artificial intelligence-suggested predictive model of survival in patients treated with stereotactic radiotherapy for early lung cancer. In Vivo 2024, 38, 1359–1366. [Google Scholar] [CrossRef]
- Park, S.; Park, J.W.; Lee, E.H.; Suh, Y.J.; Lee, C.Y.; Park, B.J.; Lee, C.G.; Yoon, H.I.; Lee, S.H.; Cui, R.; et al. Stereotactic body radiotherapy for early-stage non-small cell lung cancer: Comprehensive analysis of outcomes and recurrence from a single-center experience. Oncol. Lett. 2025, 29, 314. [Google Scholar] [CrossRef]
- Rochon, P.A.; Gurwitz, J.H.; Sykora, K.; Mamdani, M.; Streiner, D.L.; Garfinkel, S.; Normand, S.L.; Anderson, G.M. Reader’s guide to critical appraisal of cohort studies: 1. Role and design. BMJ 2005, 330, 895–897. [Google Scholar] [CrossRef]

| Characteristic | Before Overlap Weighting | After Overlap Weighting | ||||
|---|---|---|---|---|---|---|
| SBRT (n = 216) | Surgery (n = 1258) | ASD | SBRT (n = 216) | Surgery (n = 1258) | ASD | |
| Age | 79.0 (74.0, 83.0) | 65.0 (58.0, 71.0) | 1.630 | 75.0 (69.0, 79.0) | 75.0 (71.0, 78.0) | <0.001 |
| Sex | 0.552 | <0.001 | ||||
| Male | 155 (71.8%) | 573 (45.5%) | 145 (67.2%) | 846 (67.2%) | ||
| Female | 61 (28.2%) | 685 (54.5%) | 71 (32.8%) | 412 (32.8%) | ||
| Smoking | 0.505 | <0.001 | ||||
| Non-smoker | 84 (38.9%) | 797 (63.4%) | 92 (42.6%) | 536 (42.6%) | ||
| Ever-smoker | 132 (61.1%) | 461 (36.6%) | 124 (57.4%) | 722 (57.4%) | ||
| ECOG | 0.452 | <0.001 | ||||
| 0~1 | 196 (90.7%) | 1258 (100.0%) | 216 (100.0%) | 1258 (100.0%) | ||
| 2~3 | 20 (9.3%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Solid diameter | 18.5 (12.7, 25.4) | 13.6 (6.2, 21.3) | 0.483 | 17.5 (11.5, 23.8) | 17.8 (11.2, 24.7) | <0.001 |
| Nodule type | 0.541 | <0.001 | ||||
| Solid | 119 (55.1%) | 418 (33.2%) | 111 (51.6%) | 649 (51.6%) | ||
| Part-Solid | 92 (42.6%) | 686 (54.5%) | 99 (45.8%) | 576 (45.8%) | ||
| Non-Solid | 5 (2.3%) | 154 (12.2%) | 6 (2.6%) | 33 (2.6%) | ||
| Pleural attachment | 0.337 | <0.001 | ||||
| No pleural invasion | 69 (31.9%) | 366 (29.1%) | 69 (32.1%) | 404 (32.1%) | ||
| >1/4 tumor pleural contact | 33 (15.3%) | 156 (12.4%) | 30 (14.0%) | 176 (14.0%) | ||
| Pleural/fissural retraction | 63 (29.2%) | 341 (27.1%) | 63 (29.0%) | 364 (29.0%) | ||
| Pleural tags with thickening | 30 (13.9%) | 126 (10.0%) | 28 (12.9%) | 163 (12.9%) | ||
| <1/4 contact or non-thickened tags | 21 (9.7%) | 269 (21.4%) | 26 (12.1%) | 152 (12.1%) | ||
| Centrality | 0.117 | <0.001 | ||||
| Peripheral | 195 (90.3%) | 1176 (93.5%) | 197 (91.3%) | 1149 (91.3%) | ||
| Central | 21 (9.7%) | 82 (6.5%) | 19 (8.7%) | 109 (8.7%) | ||
| Stage | 0.108 | <0.001 | ||||
| IA | 175 (81.0%) | 1070 (85.1%) | 176 (81.4%) | 1024 (81.4%) | ||
| IB | 41 (19.0%) | 188 (14.9%) | 40 (18.6%) | 234 (18.6%) | ||
| Variable | Before Overlap Weighting | After Overlap Weighting | ||||
|---|---|---|---|---|---|---|
| SBRT | Surgery | p-Value | SBRT | Surgery | p-Value | |
| First recurrence of lung cancer | ||||||
| No. of events/No. of patients | 33/216 | 74/1258 | - | 27/216 | 138/1258 | - |
| Time to event, median (IQR), y | 2.03 (1.34, 3.21) | 2.50 (1.54, 4.10) | - | 2.04 (1.45, 3.10) | 2.42 (1.49, 4.04) | - |
| IR, per 100 person-years | 6.42 | 1.96 | - | 5.36 | 3.83 | - |
| RD (95% CI) | 4.46 (2.22, 6.70) | 0 [Reference] | <0.001 | 1.54 (−0.58, 3.65) | 0 [Reference] | 0.156 |
| sHR (95% CI) | 3.10 (2.06, 4.68) | 1 [Reference] | <0.001 | 1.33 (0.77, 2.32) | 1 [Reference] | 0.310 |
| Overall survival | ||||||
| No. of events/No. of patients | 26/216 | 47/1258 | - | 23/216 | 144/1258 | - |
| Time to event, median (IQR), y | 2.35 (1.49, 3.33) | 2.54 (1.56, 4.26) | - | 2.30 (1.65, 3.21) | 2.49 (1.52, 4.29) | - |
| IR, per 100 person-years | 4.70 | 1.21 | - | 4.18 | 3.78 | - |
| RD (95% CI) | 3.48 (1.65, 5.32) | 0 [Reference] | <0.001 | 0.40 (−1.43, 2.23) | 0 [Reference] | 0.668 |
| HR (95% CI) | 3.97 (2.45, 6.43) | 1 [Reference] | <0.001 | 1.07 (0.57, 2.02) | 1 [Reference] | 0.835 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.H.; Suh, Y.J.; Park, J.W.; Moon, J.; Park, S.; Lee, C.G.; Yoon, H.I.; Park, B.J.; Lee, J.G.; Kim, D.J.; et al. Comparison of Stereotactic Body Radiotherapy and Surgery for Stage I Lung Cancer: A Multidisciplinary Cohort Study Utilizing Propensity Score Overlap Weighting and AI-Based CT Imaging Analysis. Cancers 2025, 17, 2015. https://doi.org/10.3390/cancers17122015
Lee EH, Suh YJ, Park JW, Moon J, Park S, Lee CG, Yoon HI, Park BJ, Lee JG, Kim DJ, et al. Comparison of Stereotactic Body Radiotherapy and Surgery for Stage I Lung Cancer: A Multidisciplinary Cohort Study Utilizing Propensity Score Overlap Weighting and AI-Based CT Imaging Analysis. Cancers. 2025; 17(12):2015. https://doi.org/10.3390/cancers17122015
Chicago/Turabian StyleLee, Eun Hye, Young Joo Suh, Jong Won Park, Jisu Moon, Sangjoon Park, Chang Geol Lee, Hong In Yoon, Byung Jo Park, Jin Gu Lee, Dae Joon Kim, and et al. 2025. "Comparison of Stereotactic Body Radiotherapy and Surgery for Stage I Lung Cancer: A Multidisciplinary Cohort Study Utilizing Propensity Score Overlap Weighting and AI-Based CT Imaging Analysis" Cancers 17, no. 12: 2015. https://doi.org/10.3390/cancers17122015
APA StyleLee, E. H., Suh, Y. J., Park, J. W., Moon, J., Park, S., Lee, C. G., Yoon, H. I., Park, B. J., Lee, J. G., Kim, D. J., Yong, S. H., Lee, S. H., Lee, C. Y., Cho, J., & Kim, E. Y. (2025). Comparison of Stereotactic Body Radiotherapy and Surgery for Stage I Lung Cancer: A Multidisciplinary Cohort Study Utilizing Propensity Score Overlap Weighting and AI-Based CT Imaging Analysis. Cancers, 17(12), 2015. https://doi.org/10.3390/cancers17122015






