Repeated COVID-19 Vaccination as a Poor Prognostic Factor in Pancreatic Cancer: A Retrospective, Single-Center Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
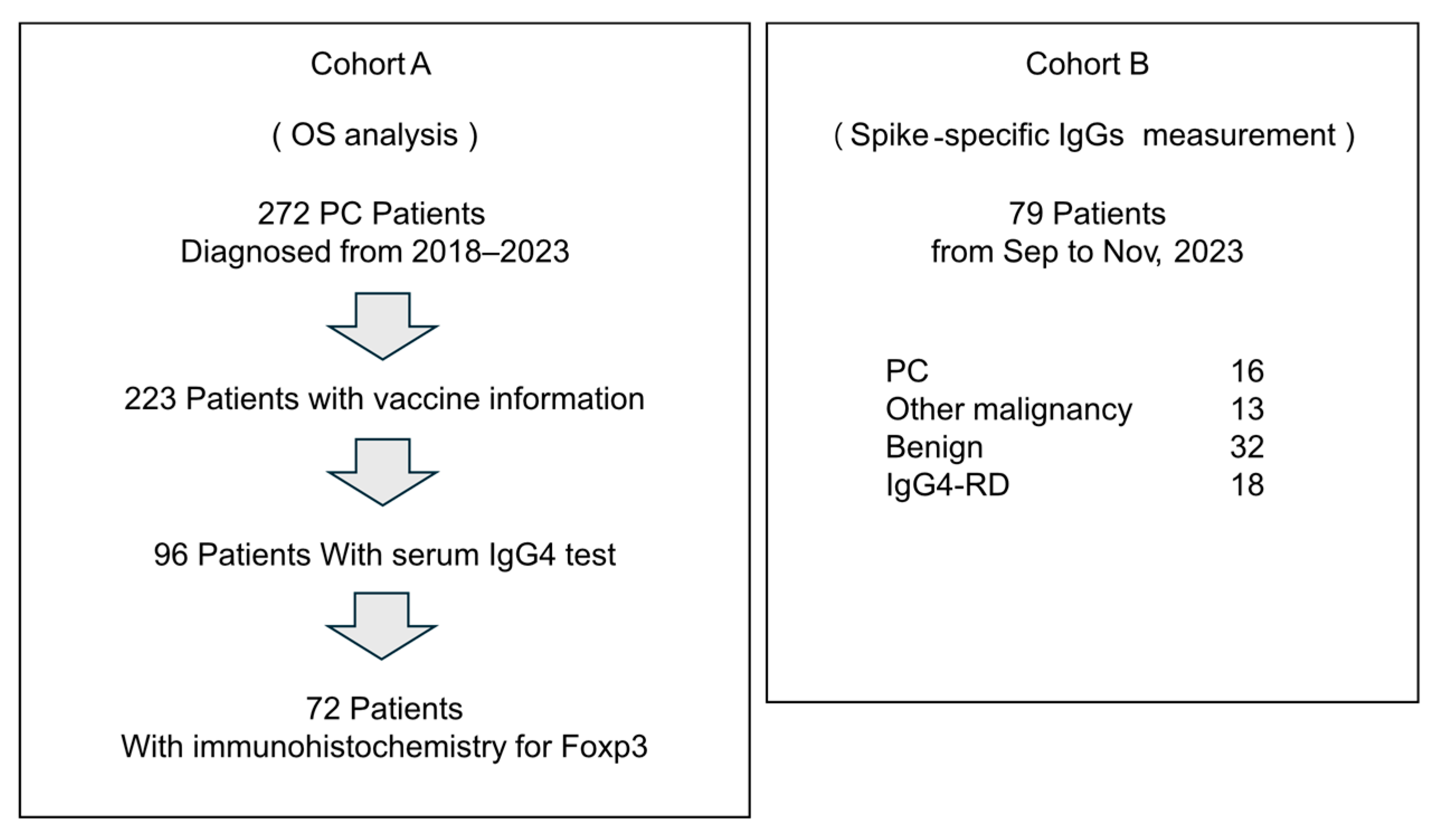
2.1. Patients
2.2. Immunohistochemistry
2.3. Enzyme-Linked Immunosorbent Assay
2.4. Statistics Analysis
3. Results
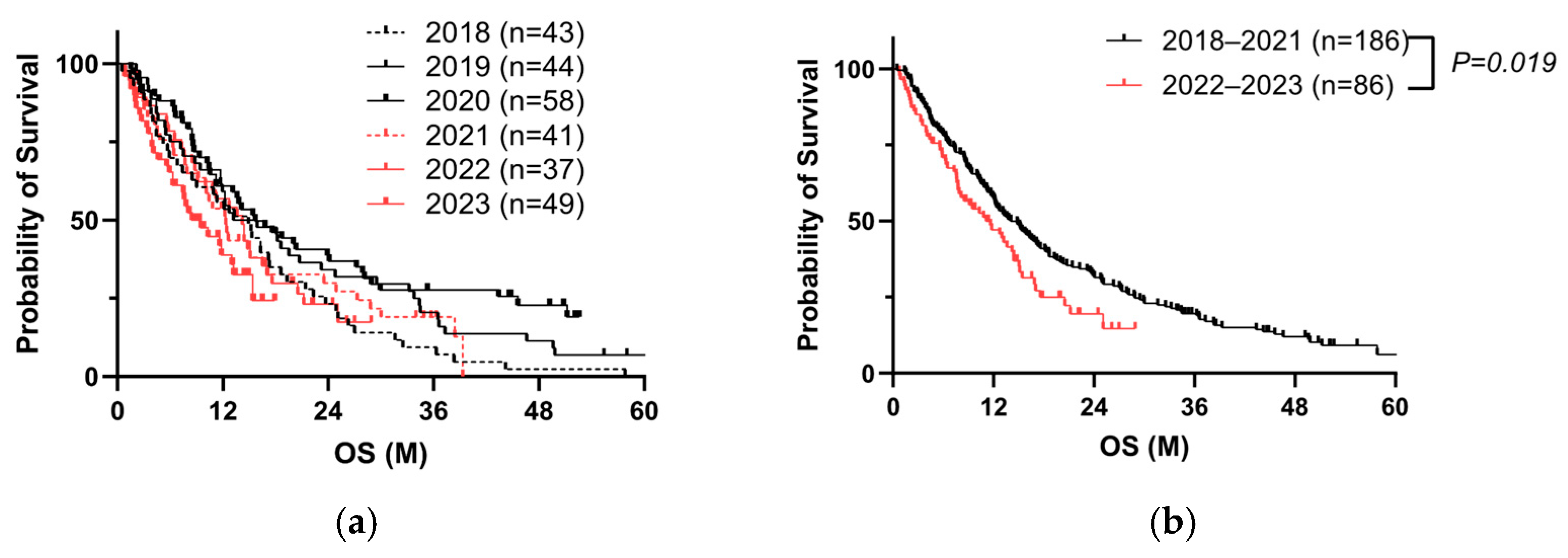
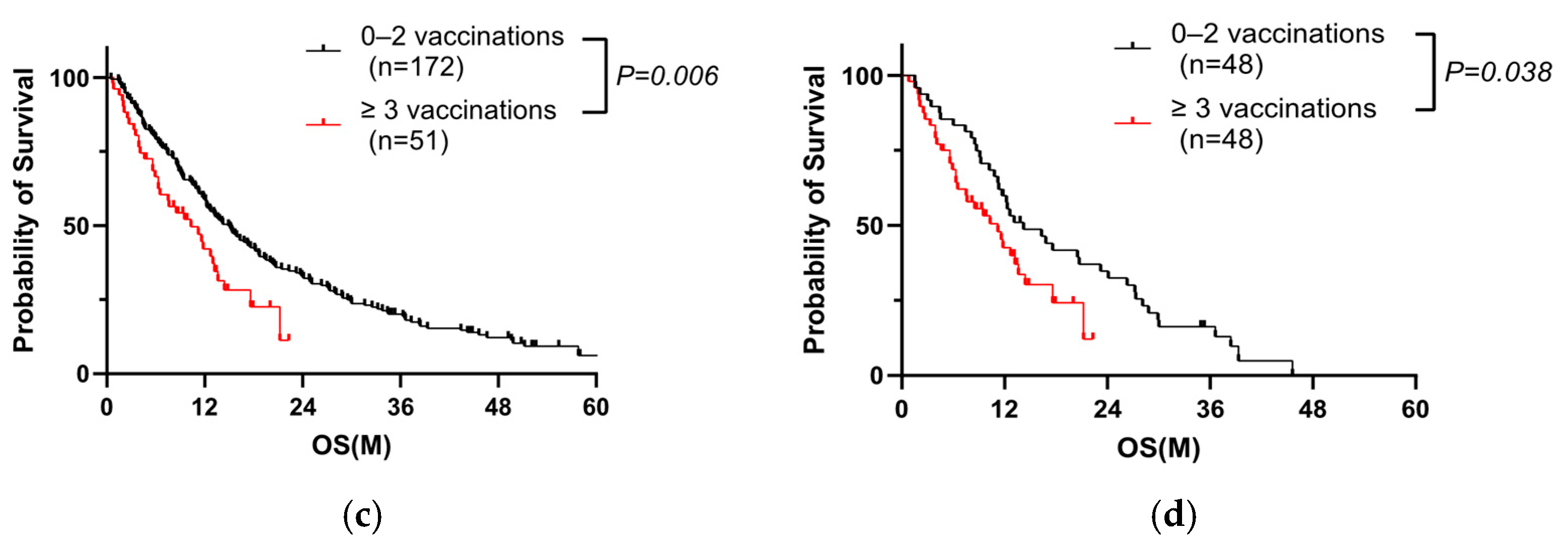
3.1. Repeated COVID-19 Vaccination Worsens the Prognosis of PC Patients
3.2. High Total IgG4 Level Correlates with a Poor PC Prognosis
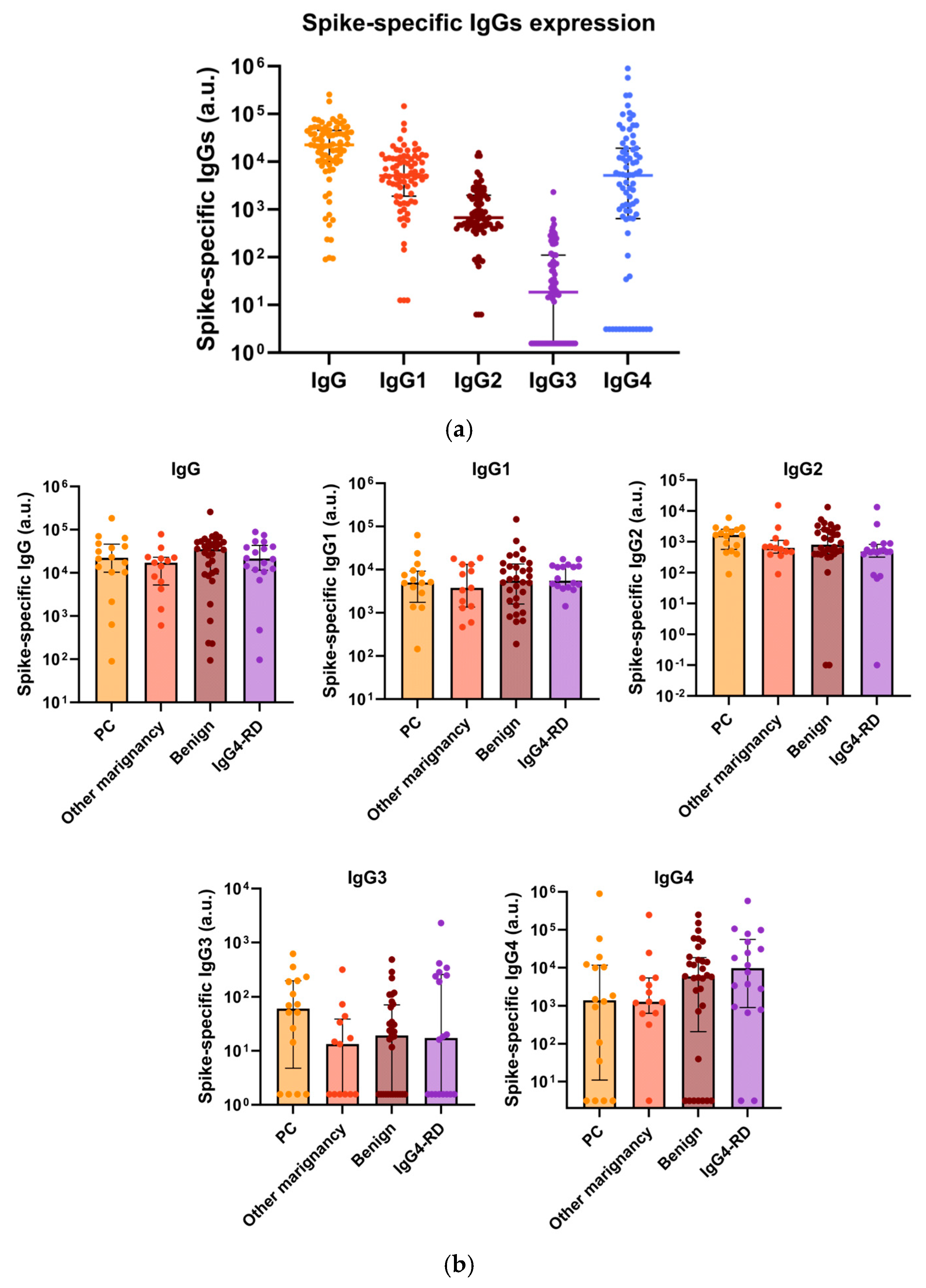
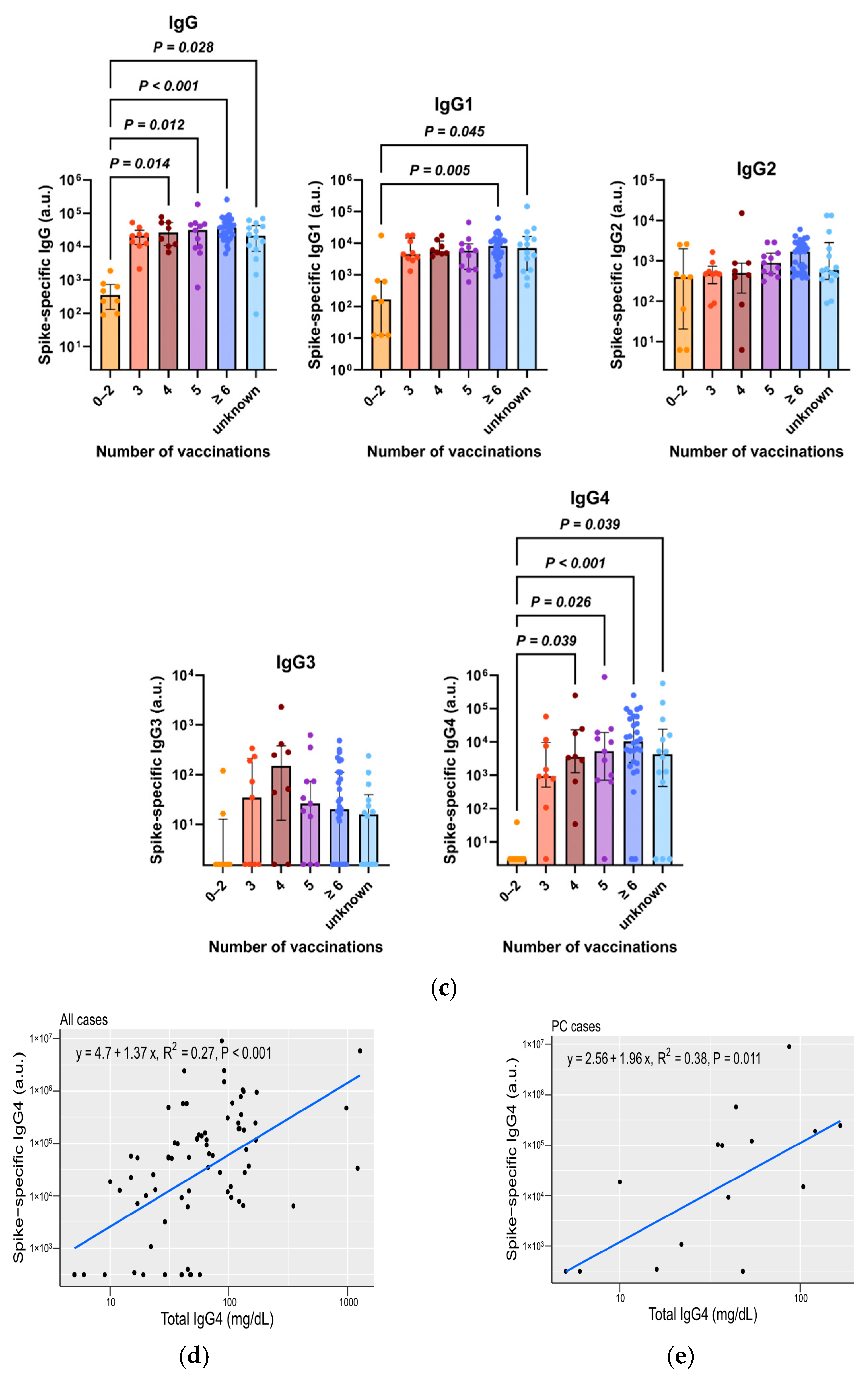
3.3. Total IgG4, Along with Spike-Specific IgG4, Is Increased in Patients with Repeated COVID-19 Vaccination
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| PC | Pancreatic cancer |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | Coronavirus disease 2019 |
| IgG | Immunoglobulin G |
| PS | Performance status |
| CEA | Carcinoembryonic antigen |
| CA19-9 | Carbohydrate Antigen 19-9 |
| IgG4-RD | IgG4-related disease |
| ELISA | Enzyme-linked immunosorbent assay |
| NLR | Neutrophil-to-lymphocyte ratio |
| mGPS | modified Glasgow prognostic score |
| PNI | Prognostic Nutritional Index |
| CRP | C-Reactive Protein |
| AUC | Area Under the Curve. |
| ROC | Receiver Operating Characteristic |
| OS | Overall Survival |
References
- American Cancer Society Cancer Facts & Figures. 2023. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/2023-cancer-facts-figures.html (accessed on 12 January 2024).
- National Cancer Center Japan Projected Cancer Statistics. 2022. Available online: https://ganjoho.jp/reg_stat/statistics/stat/short_pred_en.html (accessed on 11 January 2024).
- Abe, K.; Kitago, M.; Kitagawa, Y.; Hirasawa, A. Hereditary Pancreatic Cancer. Int. J. Clin. Oncol. 2021, 26, 1784–1792. [Google Scholar] [CrossRef]
- Matsubayashi, H.; Takaori, K.; Morizane, C.; Maguchi, H.; Mizuma, M.; Takahashi, H.; Wada, K.; Hosoi, H.; Yachida, S.; Suzuki, M.; et al. Familial Pancreatic Cancer: Concept, Management and Issues. World J. Gastroenterol. 2017, 23, 935–948. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic Cancer Epidemiology: Understanding the Role of Lifestyle and Inherited Risk Factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef]
- Hassan, M.M.; Bondy, M.L.; Wolff, R.A.; Abbruzzese, J.L.; Vauthey, J.-N.; Pisters, P.W.; Evans, D.B.; Khan, R.; Chou, T.-H.; Lenzi, R.; et al. Risk Factors for Pancreatic Cancer: Case-Control Study. Am. J. Gastroenterol. 2007, 102, 2696–2707. [Google Scholar] [CrossRef]
- Maitra, A.; Sharma, A.; Brand, R.E.; Van Den Eeden, S.K.; Fisher, W.E.; Hart, P.A.; Hughes, S.J.; Mather, K.J.; Pandol, S.J.; Park, W.G.; et al. A Prospective Study to Establish a New-Onset Diabetes Cohort: From the Consortium for the Study of Chronic Pancreatitis, Diabetes, and Pancreatic Cancer. Pancreas 2018, 47, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernández-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of International Consensus Fukuoka Guidelines for the Management of IPMN of the Pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P.; Clinical Guidelines Committee; American Gastroenterology Association. American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Asymptomatic Neoplastic Pancreatic Cysts. Gastroenterology 2015, 148, 819–822; quize12–13. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas. European Evidence-Based Guidelines on Pancreatic Cystic Neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef]
- Kirkegård, J.; Mortensen, F.V.; Cronin-Fenton, D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2017, 112, 1366–1372. [Google Scholar] [CrossRef]
- Gandhi, S.; de la Fuente, J.; Murad, M.H.; Majumder, S. Chronic Pancreatitis Is a Risk Factor for Pancreatic Cancer, and Incidence Increases with Duration of Disease: A Systematic Review and Meta-Analysis. Clin. Transl. Gastroenterol. 2022, 13, e00463. [Google Scholar] [CrossRef]
- Michaud, D.S.; Giovannucci, E.; Willett, W.C.; Colditz, G.A.; Stampfer, M.J.; Fuchs, C.S. Physical Activity, Obesity, Height, and the Risk of Pancreatic Cancer. JAMA 2001, 286, 921–929. [Google Scholar] [CrossRef]
- Kanno, A.; Masamune, A.; Hanada, K.; Maguchi, H.; Shimizu, Y.; Ueki, T.; Hasebe, O.; Ohtsuka, T.; Nakamura, M.; Takenaka, M.; et al. Multicenter Study of Early Pancreatic Cancer in Japan. Pancreatology 2018, 18, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Singhi, A.D.; Koay, E.J.; Chari, S.T.; Maitra, A. Early Detection of Pancreatic Cancer: Opportunities and Challenges. Gastroenterology 2019, 156, 2024–2040. [Google Scholar] [CrossRef]
- Pereira, S.P.; Oldfield, L.; Ney, A.; Hart, P.A.; Keane, M.G.; Pandol, S.J.; Li, D.; Greenhalf, W.; Jeon, C.Y.; Koay, E.J.; et al. Early Detection of Pancreatic Cancer. Lancet Gastroenterol. Hepatol. 2020, 5, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402.e1. [Google Scholar] [CrossRef]
- Goggins, M.; Overbeek, K.A.; Brand, R.; Syngal, S.; Del Chiaro, M.; Bartsch, D.K.; Bassi, C.; Carrato, A.; Farrell, J.; Fishman, E.K.; et al. Management of Patients with Increased Risk for Familial Pancreatic Cancer: Updated Recommendations from the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut 2020, 69, 7–17. [Google Scholar] [CrossRef]
- Partyka, O.; Pajewska, M.; Kwaśniewska, D.; Czerw, A.; Deptała, A.; Budzik, M.; Cipora, E.; Gąska, I.; Gazdowicz, L.; Mielnik, A.; et al. Overview of Pancreatic Cancer Epidemiology in Europe and Recommendations for Screening in High-Risk Populations. Cancers 2023, 15, 3634. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; De La Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with Nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Li, C.-P.; Bodoky, G.; Dean, A.; Shan, Y.-S.; Jameson, G.; Macarulla, T.; Lee, K.-H.; Cunningham, D.; Blanc, J.F.; et al. Nanoliposomal Irinotecan with Fluorouracil and Folinic Acid in Metastatic Pancreatic Cancer after Previous Gemcitabine-Based Therapy (NAPOLI-1): A Global, Randomised, Open-Label, Phase 3 Trial. Lancet 2016, 387, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Unno, M.; Motoi, F.; Matsuyama, Y.; Satoi, S.; Matsumoto, I.; Aosasa, S.; Shirakawa, H.; Wada, K.; Fujii, T.; Yoshitomi, H.; et al. Randomized Phase II/III Trial of Neoadjuvant Chemotherapy with Gemcitabine and S-1 versus Upfront Surgery for Resectable Pancreatic Cancer (Prep-02/JSAP-05). Jpn. J. Clin. Oncol. 2019, 37, 189. [Google Scholar] [CrossRef]
- Oettle, H.; Neuhaus, P.; Hochhaus, A.; Hartmann, J.T.; Gellert, K.; Ridwelski, K.; Niedergethmann, M.; Zülke, C.; Fahlke, J.; Arning, M.B.; et al. Adjuvant Chemotherapy with Gemcitabine and Long-Term Outcomes among Patients with Resected Pancreatic Cancer: The CONKO-001 Randomized Trial. JAMA 2013, 310, 1473–1481. [Google Scholar] [CrossRef]
- Uesaka, K.; Boku, N.; Fukutomi, A.; Okamura, Y.; Konishi, M.; Matsumoto, I.; Kaneoka, Y.; Shimizu, Y.; Nakamori, S.; Sakamoto, H.; et al. Adjuvant Chemotherapy of S-1 versus Gemcitabine for Resected Pancreatic Cancer: A Phase 3, Open-Label, Randomised, Non-Inferiority Trial (JASPAC 01). Lancet 2016, 388, 248–257. [Google Scholar] [CrossRef]
- Conroy, T.; Hammel, P.; Hebbar, M.; Ben Abdelghani, M.; Wei, A.C.; Raoul, J.-L.; Choné, L.; Francois, E.; Artru, P.; Biagi, J.J.; et al. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N. Engl. J. Med. 2018, 379, 2395–2406. [Google Scholar] [CrossRef]
- Lu, H.; Stratton, C.W.; Tang, Y. Outbreak of Pneumonia of Unknown Etiology in Wuhan, China: The Mystery and the Miracle. J. Med. Virol. 2020, 92, 401–402. [Google Scholar] [CrossRef]
- Altmann, D.M.; Boyton, R.J. COVID-19 Vaccination: The Road Ahead. Science 2022, 375, 1127–1132. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Baden, L.R.; El Sahly, H.M.; Essink, B.; Kotloff, K.; Frey, S.; Novak, R.; Diemert, D.; Spector, S.A.; Rouphael, N.; Creech, C.B.; et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021, 384, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Prime Minister’s Office of Japan Ongoing Topics, COVID-19 Vaccines. Available online: https://japan.kantei.go.jp/ongoingtopics/index.html (accessed on 12 January 2024).
- Verbeke, R.; Lentacker, I.; De Smedt, S.C.; Dewitte, H. The Dawn of mRNA Vaccines: The COVID-19 Case. J. Control. Release 2021, 333, 511–520. [Google Scholar] [CrossRef]
- Teo, S.P. Review of COVID-19 mRNA Vaccines: BNT162b2 and mRNA-1273. J. Pharm. Pract. 2022, 35, 947–951. [Google Scholar] [CrossRef]
- Evans, J.P.; Zeng, C.; Carlin, C.; Lozanski, G.; Saif, L.J.; Oltz, E.M.; Gumina, R.J.; Liu, S.-L. Neutralizing Antibody Responses Elicited by SARS-CoV-2 mRNA Vaccination Wane over Time and Are Boosted by Breakthrough Infection. Sci. Transl. Med. 2022, 14, eabn8057. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, L.; Li, J.; Bai, S.; Wang, Y.; Zhang, B.; Zheng, Q.; Chen, M.; Zhao, W.; Wu, J. The Kinetics of IgG Subclasses and Contributions to Neutralizing Activity against SARS-CoV-2 Wild-Type Strain and Variants in Healthy Adults Immunized with Inactivated Vaccine. Immunology 2022, 167, 221–232. [Google Scholar] [CrossRef]
- Vidarsson, G.; Dekkers, G.; Rispens, T. IgG Subclasses and Allotypes: From Structure to Effector Functions. Front. Immunol. 2014, 5, 520. [Google Scholar] [CrossRef]
- Kiszel, P.; Sík, P.; Miklós, J.; Kajdácsi, E.; Sinkovits, G.; Cervenak, L.; Prohászka, Z. Class Switch towards Spike Protein-Specific IgG4 Antibodies after SARS-CoV-2 mRNA Vaccination Depends on Prior Infection History. Sci. Rep. 2023, 13, 13166. [Google Scholar] [CrossRef]
- Irrgang, P.; Gerling, J.; Kocher, K.; Lapuente, D.; Steininger, P.; Habenicht, K.; Wytopil, M.; Beileke, S.; Schäfer, S.; Zhong, J.; et al. Class Switch toward Noninflammatory, Spike-Specific IgG4 Antibodies after Repeated SARS-CoV-2 mRNA Vaccination. Sci. Immunol. 2023, 8, eade2798. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, Q.; Zhao, C.; Zhu, Z.; Zhu, X.; Zhou, J.; Zhang, S.; Yang, T.; Zhang, B.; Li, J.; et al. An Immune Evasion Mechanism with IgG4 Playing an Essential Role in Cancer and Implication for Immunotherapy. J. Immunother. Cancer 2020, 8, e000661. [Google Scholar] [CrossRef]
- Ghazale, A.; Chari, S.T.; Smyrk, T.C.; Levy, M.J.; Topazian, M.D.; Takahashi, N.; Clain, J.E.; Pearson, R.K.; Pelaez-Luna, M.; Petersen, B.T.; et al. Value of Serum IgG4 in the Diagnosis of Autoimmune Pancreatitis and in Distinguishing It from Pancreatic Cancer. Am. J. Gastroenterol. 2007, 102, 1646–1653. [Google Scholar] [CrossRef]
- Liu, Q.; Niu, Z.; Li, Y.; Wang, M.; Pan, B.; Lu, Z.; Liao, Q.; Zhao, Y. Immunoglobulin G4 (IgG4)-Positive Plasma Cell Infiltration Is Associated with the Clinicopathologic Traits and Prognosis of Pancreatic Cancer after Curative Resection. Cancer Immunol. Immunother. 2016, 65, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Uehara, T.; Iwaya, M.; Asaka, S.; Nakajima, T.; Kinugawa, Y.; Shimizu, A.; Kubota, K.; Notake, T.; Masuo, H.; et al. IgG4 Expression and IgG4/IgG Ratio in the Tumour Invasion Front Predict Long-Term Outcomes for Patients with Intrahepatic Cholangiocarcinoma. Pathology 2023, 55, 508–513. [Google Scholar] [CrossRef]
- Harada, K.; Shimoda, S.; Kimura, Y.; Sato, Y.; Ikeda, H.; Igarashi, S.; Ren, X.-S.; Sato, H.; Nakanuma, Y. Significance of Immunoglobulin G4 (IgG4)-Positive Cells in Extrahepatic Cholangiocarcinoma: Molecular Mechanism of IgG4 Reaction in Cancer Tissue. Hepatology 2012, 56, 157–164. [Google Scholar] [CrossRef]
- Miyatani, K.; Saito, H.; Murakami, Y.; Watanabe, J.; Kuroda, H.; Matsunaga, T.; Fukumoto, Y.; Osaki, T.; Nakayama, Y.; Umekita, Y.; et al. A High Number of IgG4-Positive Cells in Gastric Cancer Tissue Is Associated with Tumor Progression and Poor Prognosis. Virchows Arch. 2016, 468, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, N.; Onozato, K.; Kosuge, T.; Hirohashi, S. Prevalence of FOXP3+ Regulatory T Cells Increases during the Progression of Pancreatic Ductal Adenocarcinoma and Its Premalignant Lesions. Clin. Cancer Res. 2006, 12, 5423–5434. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Nakanuma, Y. Cholangiocarcinoma with Respect to IgG4 Reaction. Int. J. Hepatol. 2014, 2014, 803876. [Google Scholar] [CrossRef] [PubMed]
- Sinkovits, G.; Szilágyi, Á.; Farkas, P.; Inotai, D.; Szilvási, A.; Tordai, A.; Rázsó, K.; Réti, M.; Prohászka, Z. Concentration and Subclass Distribution of Anti-ADAMTS13 IgG Autoantibodies in Different Stages of Acquired Idiopathic Thrombotic Thrombocytopenic Purpura. Front. Immunol. 2018, 9, 1646. [Google Scholar] [CrossRef]
- Cheng, H.; Long, F.; Jaiswar, M.; Yang, L.; Wang, C.; Zhou, Z. Prognostic Role of the Neutrophil-to-Lymphocyte Ratio in Pancreatic Cancer: A Meta-Analysis. Sci. Rep. 2015, 5, 11026. [Google Scholar] [CrossRef]
- Imaoka, H.; Mizuno, N.; Hara, K.; Hijioka, S.; Tajika, M.; Tanaka, T.; Ishihara, M.; Yogi, T.; Tsutsumi, H.; Fujiyoshi, T.; et al. Evaluation of Modified Glasgow Prognostic Score for Pancreatic Cancer: A Retrospective Cohort Study. Pancreas 2016, 45, 211–217. [Google Scholar] [CrossRef]
- Geng, Y.; Qi, Q.; Sun, M.; Chen, H.; Wang, P.; Chen, Z. Prognostic Nutritional Index Predicts Survival and Correlates with Systemic Inflammatory Response in Advanced Pancreatic Cancer. Eur. J. Surg. Oncol. 2015, 41, 1508–1514. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef]
- Ahmad, J.; Grimes, N.; Farid, S.; Morris-Stiff, G. Inflammatory Response Related Scoring Systems in Assessing the Prognosis of Patients with Pancreatic Ductal Adenocarcinoma: A Systematic Review. Hepatobiliary Pancreat. Dis. Int. 2014, 13, 474–481. [Google Scholar] [CrossRef]
- Rafeeq, M.; Jabir, M.S.; Al-Kuraishy, H.M.; Jeddoa, Z.M.A.; Jawad, S.F.; Najm, M.a.A.; Almulla, A.F.; Elekhnawy, E.; Tayyeb, J.Z.; Turkistani, A.; et al. Evaluation of the Hematological and Immunological Markers after the First and Second Doses of BNT162b2 mRNA Vaccine. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 2605–2614. [Google Scholar] [CrossRef]
- Seneff, S.; Nigh, G.; Kyriakopoulos, A.M.; McCullough, P.A. Innate Immune Suppression by SARS-CoV-2 mRNA Vaccinations: The Role of G-Quadruplexes, Exosomes, and MicroRNAs. Food Chem. Toxicol. 2022, 164, 113008. [Google Scholar] [CrossRef] [PubMed]
- Arbel, R.; Peretz, A.; Sergienko, R.; Friger, M.; Beckenstein, T.; Duskin-Bitan, H.; Yaron, S.; Hammerman, A.; Bilenko, N.; Netzer, D. Effectiveness of a Bivalent mRNA Vaccine Booster Dose to Prevent Severe COVID-19 Outcomes: A Retrospective Cohort Study. Lancet Infect. Dis. 2023, 23, 914–921. [Google Scholar] [CrossRef]
- La Gualana, F.; Maiorca, F.; Marrapodi, R.; Villani, F.; Miglionico, M.; Santini, S.A.; Pulcinelli, F.; Gragnani, L.; Piconese, S.; Fiorilli, M. Opposite Effects of mRNA-Based and Adenovirus-Vectored SARS-CoV-2 Vaccines on Regulatory T Cells: A Pilot Study. Biomedicines 2023, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Yang, K.; Zhu, L.; Yin, D.; Zhou, Y. Regulatory T Cells as Crucial Trigger and Potential Target for Hyperprogressive Disease Subsequent to PD-1/PD-L1 Blockade for Cancer Treatment. Int. Immunopharmacol. 2024, 132, 111934. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) Cells in Cancer: Can Treg Cells Be a New Therapeutic Target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Gao, F.-X.; Wu, R.-X.; Shen, M.-Y.; Huang, J.-J.; Li, T.-T.; Hu, C.; Luo, F.-Y.; Song, S.-Y.; Mu, S.; Hao, Y.-N.; et al. Extended SARS-CoV-2 RBD Booster Vaccination Induces Humoral and Cellular Immune Tolerance in Mice. iScience 2022, 25, 105479. [Google Scholar] [CrossRef]
- Chevaisrakul, P.; Lumjiaktase, P.; Kietdumrongwong, P.; Chuatrisorn, I.; Chatsangjaroen, P.; Phanuphak, N. Hybrid and Herd Immunity 6 Months after SARS-CoV-2 Exposure among Individuals from a Community Treatment Program. Sci. Rep. 2023, 13, 763. [Google Scholar] [CrossRef]
- Lawton, M.L.; Inge, M.M.; Blum, B.C.; Smith-Mahoney, E.L.; Bolzan, D.; Lin, W.; McConney, C.; Porter, J.; Moore, J.; Youssef, A.; et al. Multiomic Profiling of Chronically Activated CD4+ T Cells Identifies Drivers of Exhaustion and Metabolic Reprogramming. PLoS Biol. 2024, 22, e3002943. [Google Scholar] [CrossRef]
- Nair, R.; Somasundaram, V.; Kuriakose, A.; Krishn, S.R.; Raben, D.; Salazar, R.; Nair, P. Deciphering T-Cell Exhaustion in the Tumor Microenvironment: Paving the Way for Innovative Solid Tumor Therapies. Front. Immunol. 2025, 16, 1548234. [Google Scholar] [CrossRef]
- Singh, N.; Bharara Singh, A. S2 Subunit of SARS-nCoV-2 Interacts with Tumor Suppressor Protein P53 and BRCA: An In Silico Study. Transl. Oncol. 2020, 13, 100814. [Google Scholar] [CrossRef]
- Zhang, S.; El-Deiry, W.S. Transfected SARS-CoV-2 Spike DNA for Mammalian Cell Expression Inhibits P53 Activation of P21(WAF1), TRAIL Death Receptor DR5 and MDM2 Proteins in Cancer Cells and Increases Cancer Cell Viability after Chemotherapy Exposure. Oncotarget 2024, 15, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Kawa, S.; Kamisawa, T.; Notohara, K.; Fujinaga, Y.; Inoue, D.; Koyama, T.; Okazaki, K. Japanese Clinical Diagnostic Criteria for Autoimmune Pancreatitis, 2018. Pancreas 2020, 49, e13–e14. [Google Scholar] [CrossRef] [PubMed]
- Wallace, Z.S.; Naden, R.P.; Chari, S.; Choi, H.; Della-Torre, E.; Dicaire, J.-F.; Hart, P.A.; Inoue, D.; Kawano, M.; Khosroshahi, A.; et al. The 2019 American College of Rheumatology/European League Against Rheumatism Classification Criteria for IgG4-Related Disease. Arthritis Rheumatol. 2020, 72, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Gelderloos, A.T.; Verheul, M.K.; Middelhof, I.; de Zeeuw-Brouwer, M.-L.; van Binnendijk, R.S.; Buisman, A.-M.; van Kasteren, P.B. Repeated COVID-19 mRNA Vaccination Results in IgG4 Class Switching and Decreased NK Cell Activation by S1-Specific Antibodies in Older Adults. Immun. Ageing 2024, 21, 63. [Google Scholar] [CrossRef]
- Aurelia, L.C.; Purcell, R.A.; Theisen, R.M.; Kelly, A.; Esterbauer, R.; Ramanathan, P.; Lee, W.S.; Wines, B.D.; Hogarth, P.M.; Juno, J.A.; et al. Increased SARS-CoV-2 IgG4 Has Variable Consequences Dependent upon Fc Function, Fc Receptor Polymorphism, and Viral Variant. Sci. Adv. 2025, 11, eads1482. [Google Scholar] [CrossRef]

| All Case (n = 272) | 2018–2021 (n = 186) | 2022–2023 (n = 86) | p-Value | |
|---|---|---|---|---|
| Age (mean ± SD) | 70.9 ± 9.6 | 70.9 ± 9.6 | 70.5 ± 9.9 | n.s. a |
| Age ≥ 75, no. (%) | 103 (37.9) | 71 (38.2) | 32 (37.2) | n.s. b |
| Female, no. (%) | 133 (48.9) | 92 (49.5) | 41 (47.7) | n.s. b |
| PS ≥ 2, no. (%) | 42 (15.4) | 27 (14.5) | 15 (17.4) | n.s. b |
| Jaundice, no. (%) | 92 (33.8) | 63 (33.9) | 29 (33.7) | n.s. b |
| Diabetes Mellites, no. (%) | 144 (52.9) | 97 (52.2) | 47 (54.7) | n.s. b |
| Location (head), no. (%) | 126 (46.3) | 90 (48.4) | 36 (41.9) | n.s. b |
| UICC TNM classification | ||||
| T (3–4), no. (%) | 148 (54.4) | 95 (51.1) | 53 (61.6) | n.s. b |
| N, no. (%) | 144 (52.9) | 97 (52.2) | 47 (54.7) | n.s. b |
| M, no. (%) | 146 (53.7) | 97 (52.2) | 49 (57.0) | n.s. b |
| Surgery, no. (%) | 61 (22.4) | 51 (27.4) | 10 (11.6) | 0.005 b |
| Chemotherapy, no. (%) | 201 (73.9) | 140 (75.3) | 61 (70.9) | n.s. b |
| COVID-19 mRNA ≥3 vaccinations (Yes/No/unknown) | 51/190/31 | 0/186/0 | 51/4/31 | <0.001 b |
| Number of vaccinations (3/4/5/6/7) | 12/8/19/9/3 | - | 12/8/19/9/3 | |
| CEA median (min–max) ng/mL | 4.8 (0.6–2660.6) | 4.6 (0.6–2205.1) | 5.1 (0.6–2660.6) | n.s. a |
| CEA ≥ 10, no. (%) | 77 (28.3) | 55 (29.6) | 22 (25.6) | n.s. b |
| CA19-9 median (min–max) U/mL | 442.0 (0–150,000) | 449.0 (0–150,000) | 450.2 (0–150,000) | n.s. a |
| CA19-9 ≥ 500, no. (%) | 132 (48.5) | 90 (48.4) | 42 (48.8) | n.s. b |
| HR | 95%CI | p-Value (Univariate) | |
|---|---|---|---|
| Age ≥ 75 | 0.94 | (0.71–1.23) | n.s. |
| Sex (Female/Male) | 1.15 | (0.88–1.50) | n.s. |
| PS ≥ 2 | 2.66 | (1.85–3.74) | <0.001 |
| Jaundice (Yes/No) | 1.49 | (1.12–1.97) | 0.005 |
| Diabetes Mellites (Yes/No) | 0.77 | (0.59–1.00) | n.s. |
| Location (head/body-tail) | 0.95 | (0.73–1.24) | n.s. |
| UICC TNM classification | |||
| T (3–4/1–2) | 2.31 | (1.76–3.05) | <0.001 |
| N (Yes/No) | 1.57 | (1.22–2.00) | <0.001 |
| M (Yes/No) | 3.88 | (2.92–5.19) | <0.001 |
| Surgery (Yes/No) | 0.19 | (0.13–0.28) | <0.001 |
| Chemotherapy (Yes/No) | 0.58 | (0.43–0.79) | <0.001 |
| COVID-19 mRNA ≥3 vaccinations (Yes/No) | 1.72 | (1.15–2.51) | 0.006 |
| CEA > 10 (ng/mL) | 2.06 | (1.54–2.73) | <0.001 |
| CA19-9 > 500 (U/mL) | 2.04 | (1.56–2.69) | <0.001 |
| Before Matching | After Matching | |||||
|---|---|---|---|---|---|---|
| 0–2 Vaccinations (n = 172) | ≥3 Vaccinations (n = 51) | p-Value | 0–2 Vaccinations (n = 48) | ≥3 Vaccinations (n = 48) | p-Value | |
| Age (mean ± SD) | 70.6 ± 9.7 | 71.4 ± 9.3 | n.s. a | 69.8 ± 9.5 | 71.4 ± 9.2 | n.s. a |
| Age ≥ 75, no. (%) | 65 (37.8) | 23 (45.1) | n.s. b | 16 (33.3) | 21 (43.8) | n.s. b |
| Female, no. (%) | 84 (48.8) | 25 (49.0) | n.s. b | 24 (50.0) | 23 (47.9) | n.s. b |
| PS ≥ 2, no. (%) | 20 (11.6) | 11 (21.6) | n.s. b | 8 (16.7) | 11 (22.9) | n.s. b |
| Jaundice, no. (%) | 60 (34.9) | 21 (41.2) | n.s. b | 15 (31.3) | 18 (37.5) | n.s. b |
| Diabetes Mellites, no. (%) | 84 (48.8) | 29 (56.9) | n.s. b | 28 (58.3) | 28 (58.3) | n.s. b |
| Location (head), no. (%) | 87 (50.6) | 24 (47.1) | n.s. b | 22 (45.8) | 21 (43.8) | n.s. b |
| UICC TNM classification | ||||||
| T (3–4), no. (%) | 85 (49.4) | 33 (64.7) | n.s. b | 30 (62.5) | 30 (62.5) | n.s. b |
| N, no. (%) | 90 (52.3) | 30 (58.8) | n.s. b | 29 (60.4) | 29 (60.4) | n.s. b |
| M, no. (%) | 89 (51.7) | 30 (58.8) | n.s. b | 28 (58.3) | 28 (58.3) | n.s. b |
| Surgery, no. (%) | 48 (27.9) | 7 (13.7) | 0.043 b | 7 (14.6) | 7 (14.6) | n.s. b |
| Chemotherapy, no. (%) | 128 (74.4) | 33 (64.7) | n.s. b | 33 (68.8) | 33 (68.8) | n.s. b |
| CEA ≥ 10, no. (%) | 50 (29.1) | 10 (19.6) | n.s. b | 16 (33.3) | 9 (18.8) | n.s. b |
| CA19-9 ≥ 500, no. (%) | 82 (47.7) | 25 (49.0) | n.s. b | 26 (54.2) | 22 (45.8) | n.s. b |
| IgG4 measurement, no. (%) | 47 (27.3) | 49 (96.1) | <0.001 b | 11 (22.9) | 46 (93.9) | <0.001 b |
| IgG4 (mean ± SD) | 38.6 ± 31.7 | 54.7 ± 37.0 | 0.025 a | 44.2 ± 16.5 | 53.6 ± 36.8 | n.s. a |
| Patients (n = 96) | 0–2 Vaccinations (n = 47) | ≥3 Vaccination (n = 49) | p-Value | |
|---|---|---|---|---|
| Age (mean ± SD) | 71.4 ± 8.2 | 70.9 ± 9.6 | 70.9 ± 9.6 | n.s. a |
| Age ≥ 75, no. (%) | 37 (38.5) | 15 (31.9) | 22 (44.9) | n.s. b |
| Female, no. (%) | 50 (52.1) | 26 (55.3) | 24 (49.0) | n.s. b |
| PS ≥ 2, no. (%) | 14 (14.6) | 4 (8.5) | 10 (20.4) | n.s. b |
| Jaundice, no. (%) | 40 (41.7) | 19 (40.4) | 21 (42.9) | n.s. b |
| Diabetes Mellites, no. (%) | 53 (55.2) | 25 (53.2) | 28 (57.1) | n.s. b |
| Location (head), no. (%) | 55 (57.3) | 31 (66.0) | 24 (49.0) | n.s. b |
| UICC TNM classification | ||||
| T (3–4), no. (%) | 44 (45.8) | 13 (27.7) | 31 (63.3) | <0.001 b |
| N, no. (%) | 47 (49.0) | 18 (38.3) | 29 (59.2) | 0.045 b |
| M, no. (%) | 41 (42.7) | 12 (25.5) | 29 (59.2) | 0.001 b |
| Surgery, no. (%) | 30 (31.3) | 23 (48.9) | 7 (14.3) | <0.001 b |
| Chemotherapy, no. (%) | 67 (69.8) | 36 (76.6) | 31 (63.3) | n.s. b |
| CEA ≥ 10, no. (%) | 15 (15.6) | 6 (12.8) | 9 (18.4) | n.s. b |
| CA19-9 ≥ 500, no. (%) | 40 (41.7) | 15 (31.9) | 25 (51.0) | n.s. b |
| Other factors | ||||
| NLR (mean ± SD) | 3.8 ± 2.5 | 3.0 ± 1.2 | 4.5 ± 3.2 | 0.008 a |
| NLR ≥ 2.8, no. (%) | 57 (59.4) | 21 (44.7) | 36 (73.5) | 0.007 b |
| mGPS (0/1/2) | 68/19/9 | 36/8/3 | 32/11/6 | n.s. b |
| mGPS ≥ 1, no. (%) | 28 (29.2) | 11 (23.4) | 17 (34.7) | n.s. b |
| PNI (mean ± SD) | 45.7 ± 5.4 | 47.3 ± 5.2 | 44.2 ± 5.3 | 0.002 a |
| PNI ≥ 46.8, no. (%) | 41 (42.7) | 28 (59.6) | 13 (26.5) | 0.002 b |
| IgG4 (mean ± SD) | 46.8 ± 35.3 | 38.6 ± 31.7 | 54.7 ± 37.0 | 0.025 a |
| IgG4 ≥ 47.5 mg/dL, no. (%) | 35 (36.5) | 12 (25.5) | 23 (46.9) | 0.035 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abue, M.; Mochizuki, M.; Shibuya-Takahashi, R.; Ota, K.; Wakui, Y.; Iwai, W.; Kusaka, J.; Saito, M.; Suzuki, S.; Sato, I.; et al. Repeated COVID-19 Vaccination as a Poor Prognostic Factor in Pancreatic Cancer: A Retrospective, Single-Center Cohort Study. Cancers 2025, 17, 2006. https://doi.org/10.3390/cancers17122006
Abue M, Mochizuki M, Shibuya-Takahashi R, Ota K, Wakui Y, Iwai W, Kusaka J, Saito M, Suzuki S, Sato I, et al. Repeated COVID-19 Vaccination as a Poor Prognostic Factor in Pancreatic Cancer: A Retrospective, Single-Center Cohort Study. Cancers. 2025; 17(12):2006. https://doi.org/10.3390/cancers17122006
Chicago/Turabian StyleAbue, Makoto, Mai Mochizuki, Rie Shibuya-Takahashi, Kensuke Ota, Yuta Wakui, Wataru Iwai, Jun Kusaka, Masashi Saito, Shinichi Suzuki, Ikuro Sato, and et al. 2025. "Repeated COVID-19 Vaccination as a Poor Prognostic Factor in Pancreatic Cancer: A Retrospective, Single-Center Cohort Study" Cancers 17, no. 12: 2006. https://doi.org/10.3390/cancers17122006
APA StyleAbue, M., Mochizuki, M., Shibuya-Takahashi, R., Ota, K., Wakui, Y., Iwai, W., Kusaka, J., Saito, M., Suzuki, S., Sato, I., & Tamai, K. (2025). Repeated COVID-19 Vaccination as a Poor Prognostic Factor in Pancreatic Cancer: A Retrospective, Single-Center Cohort Study. Cancers, 17(12), 2006. https://doi.org/10.3390/cancers17122006





