Blood-Based Biomarkers as Predictive and Prognostic Factors in Immunotherapy-Treated Patients with Solid Tumors—Currents and Perspectives
Simple Summary
Abstract
1. Introduction
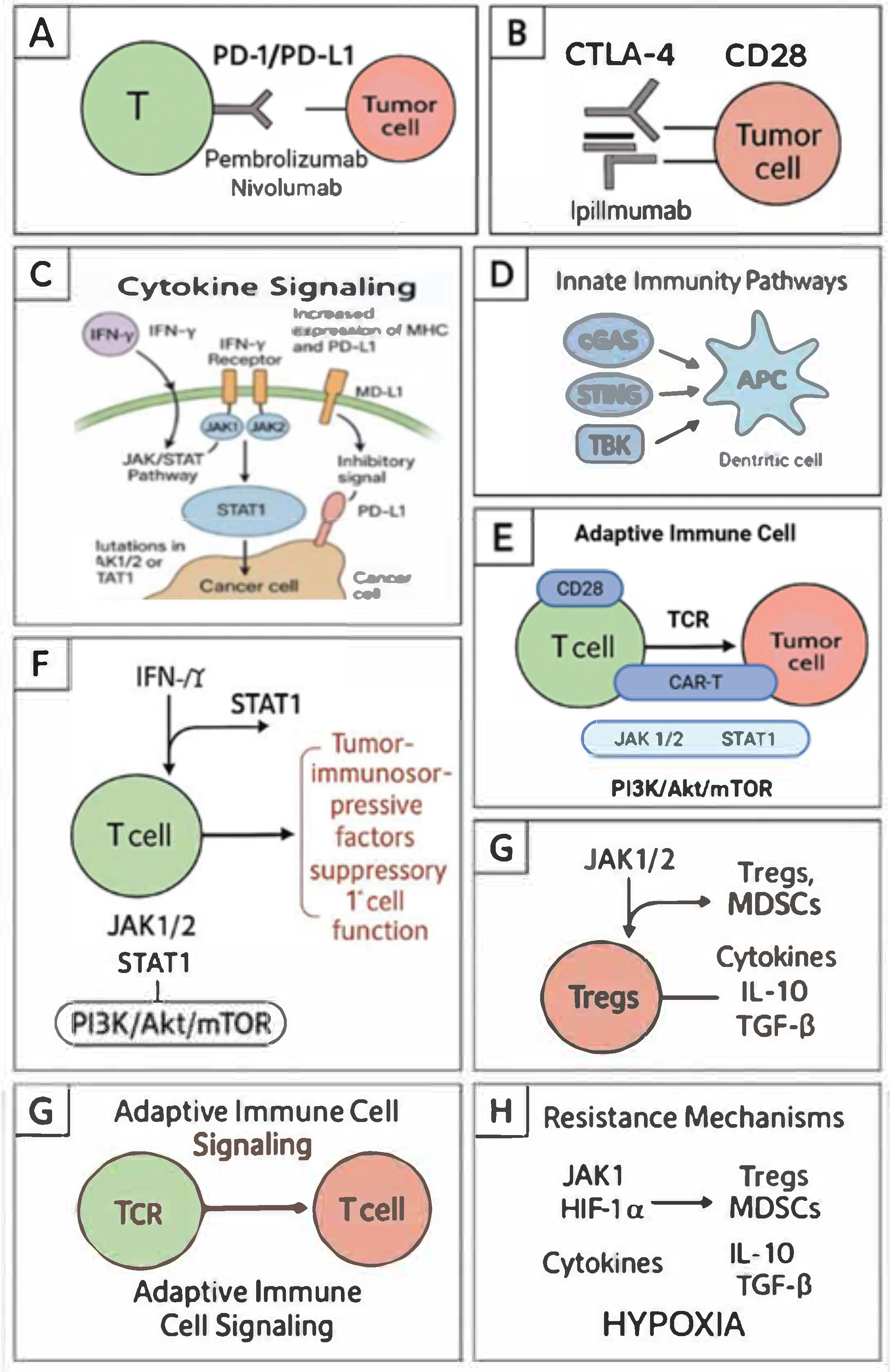
2. Key Signaling Pathways in Cancer Immunotherapy
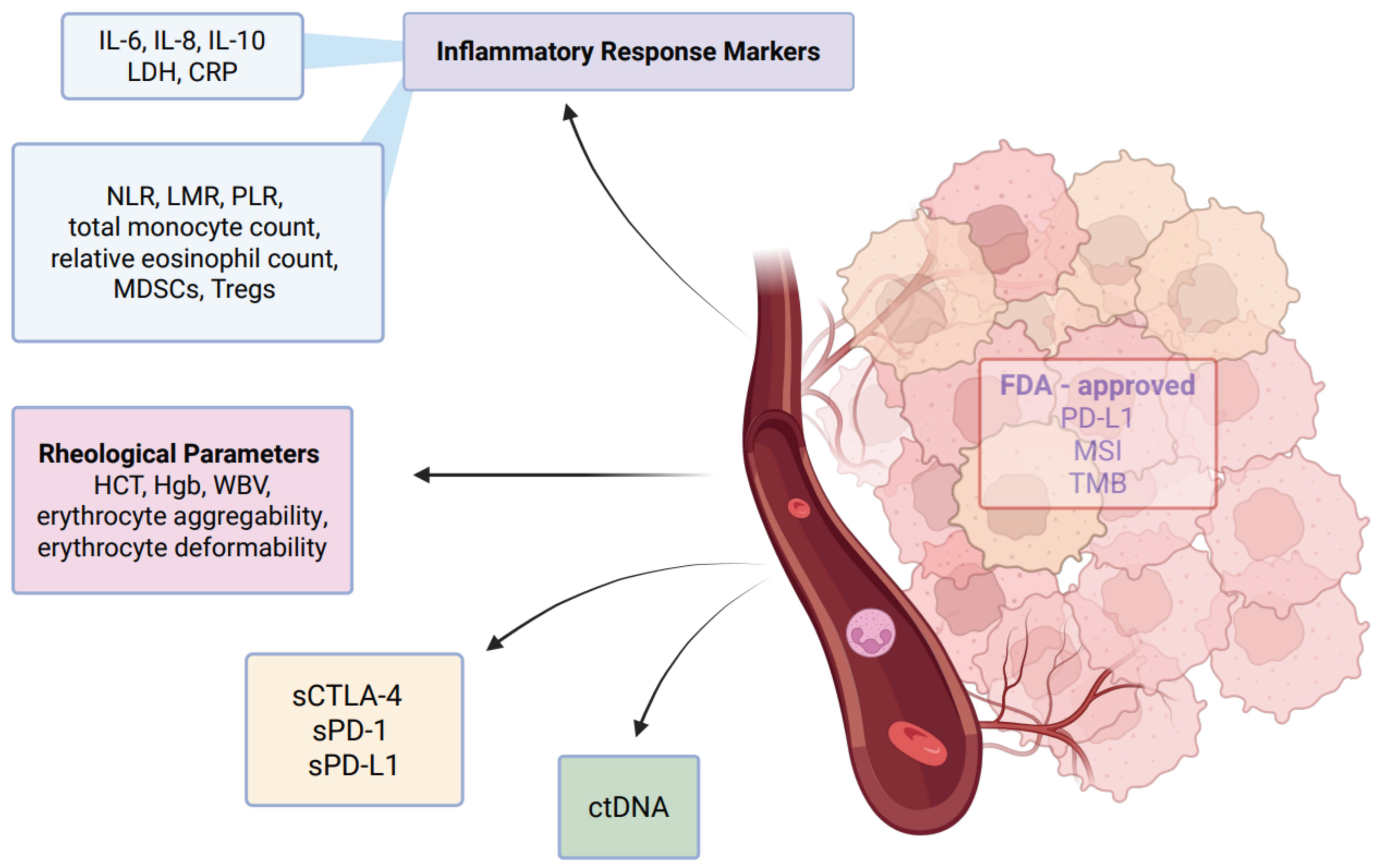
3. Blood Based Biomarkers
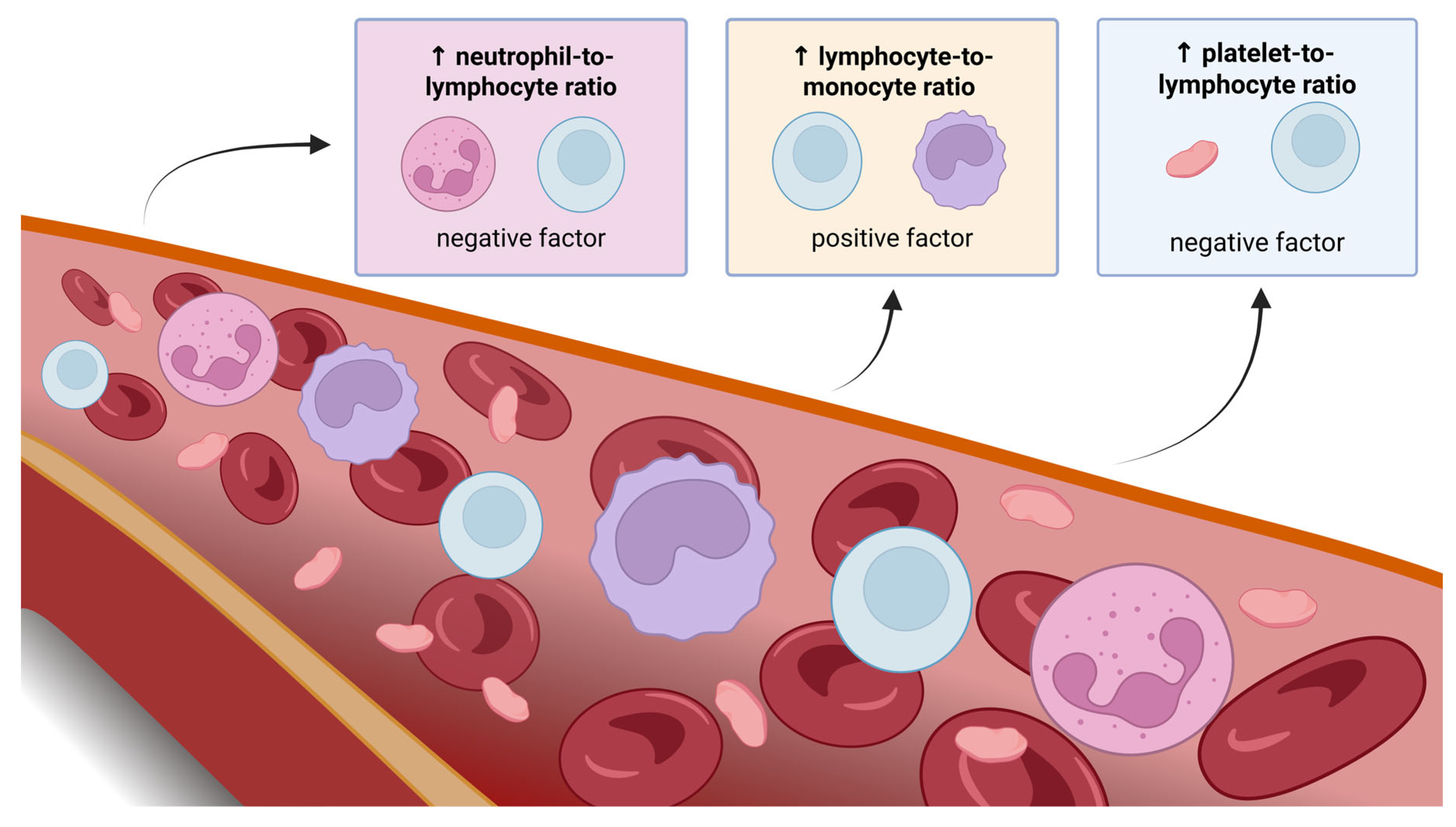
3.1. Inflammatory Response Markers—NLR, LMR, and PLR
3.1.1. NLR
3.1.2. LMR
3.1.3. PLR
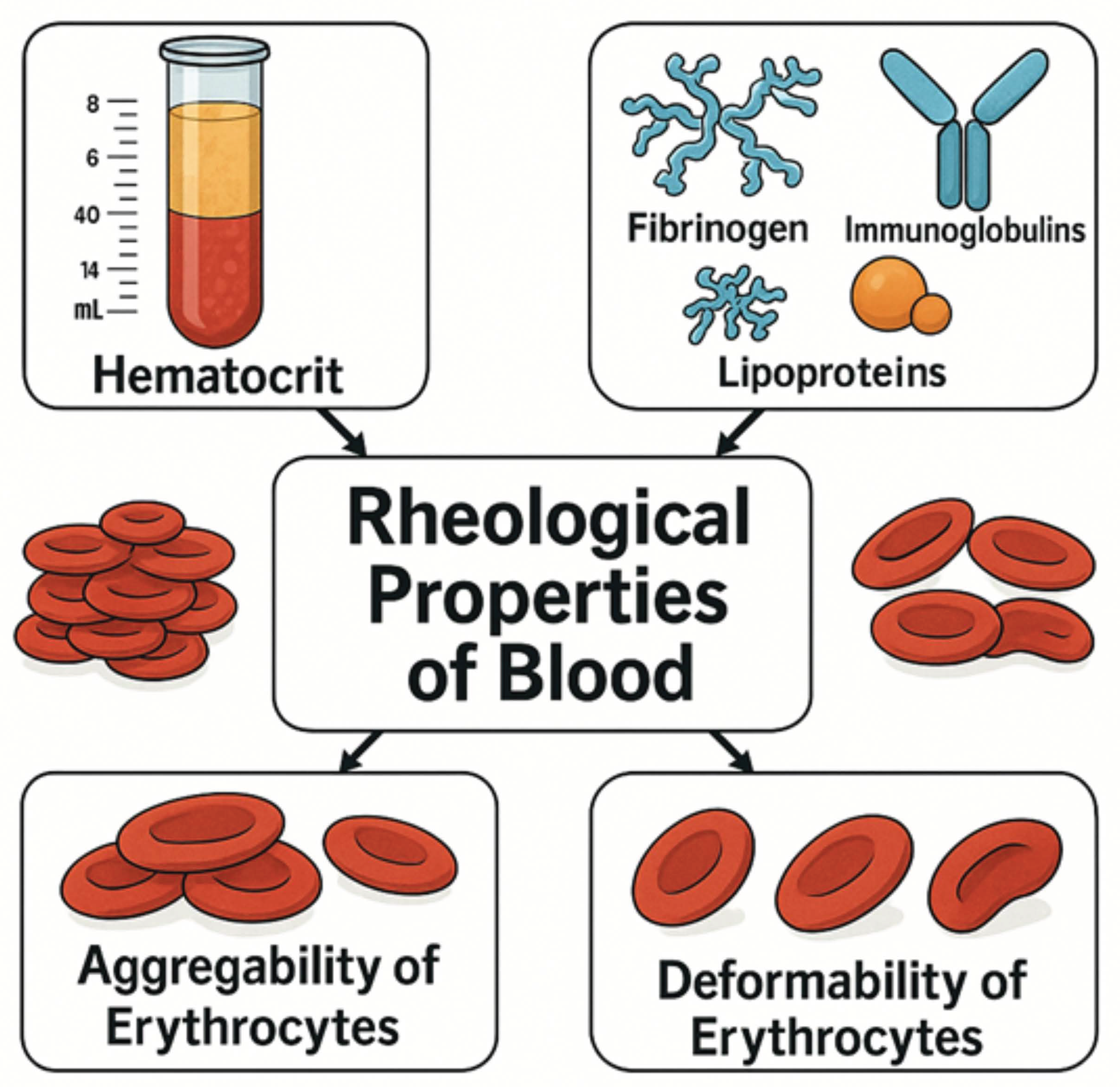
3.2. Rheological Parameters
3.3. Integrated Significance of Inflammatory Markers and Blood Rheological Parameters in Assessing the Response to Immunotherapy
3.4. Blood-Based Biomarkers in Preclinical and Clinical Studies
3.5. New Players in Immunotherapy: Molecular Basis of Emerging Peripheral Blood Biomarkers
3.6. ctDNA
3.7. LDH and CRP
3.8. Cytokine Signaling
3.9. Eosinophiles
3.10. Tregs
3.11. MDSCs
3.12. Monocytes
3.13. Mechanistic Insights into Blood Rheology Alterations and Their Impact on Tumor Biology and Immunotherapy Response
3.14. Inflammatory Signaling
3.15. Tumor-Associated Inflammation and Blood Rheology
3.16. Impact of Blood Rheology on the Tumor Microenvironment
3.17. Strategies to Enhance Immunotherapy Responses
3.18. Prognostic and Predictive Values of Four Blood- and Tumor-Based Biomarkers
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MDSCs | Myeloid-Derived Suppressor Cells |
| Tregs | Regulatory T Cells |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| LDH | Lactate Dehydrogenase |
| CRP | C-Reactive Protein |
| CTLA-4 | Cytotoxic T-Lymphocyte-Associated Protein 4 |
| PD-1 | Programmed Cell Death Protein 1 |
| PD-L1 | Programmed Death-Ligand 1 |
| ctDNA | Circulating Tumor DNA |
| NLR | Neutrophil-to-Lymphocyte Ratio |
| LMR | Lymphocyte-to-Monocyte Ratio |
| PLR | Platelet-to-Lymphocyte Ratio |
| CAR | Chimeric Antigen Receptor |
| TCR | T-Cell Receptor |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription |
| PI3K/AKT/mTOR | Phosphoinositide 3-Kinase/Protein Kinase B/Mammalian Target of Rapamycin |
| MAPK/ERK | Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase |
| ICIs | Immune Checkpoint Inhibitors |
| TMB | Tumor Mutational Burden |
| PDL1 | Programmed Death-Ligand 1 |
| MSI | Microsatellite Instability |
| IFN-γ | Interferon-Gamma |
| cGAS | Cyclic GMP-AMP Synthase |
| STING | Stimulator of Interferon Genes |
| TBK1 | TANK-Binding Kinase 1 |
| IRF3 | Interferon Regulatory Factor 3 |
| TLRs | Toll-Like Receptors |
| NF-κB | Nuclear Factor Kappa B |
| IRF3/7 | Interferon Regulatory Factor 3/7 |
| TANs | Tumor-Associated Neutrophils |
| OS | Overall Survival |
| PFS | Progression-Free Survival |
| ORR | Objective Response Rate |
| DCR | Disease Control Rate |
| irAE | Immune-Related Adverse Event |
| dNLR | Derived Neutrophil–Lymphocyte Ratio |
| ANC | Absolute Neutrophil Count |
| WBC | White Blood Cell |
| LIPI | Lung Immune Prognostic Index |
| LIPS-3 | Lung Immuno-oncology Prognostic Score |
| ECOG | Eastern Cooperative Oncology Group |
| PLT | Platelet |
| CRC | Colorectal Cancer |
| ROS | Reactive Oxygen Species |
| WBV | Whole Blood Viscosity |
| PV | Plasma Viscosity |
| RBC | Red Blood Cell |
| TME | Tumor Microenvironment |
| HCC | Hepatocellular Carcinoma |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| NSCLC | Non-Small-Cell Lung Cancer |
| RCC | Renal Cell Carcinoma |
| MCV | Mean Corpuscular Volume |
| RDW | Red Cell Distribution Width |
| TKI | Tyrosine Kinase Inhibitor |
| ICB | Immune Checkpoint Blockade |
| bTMB | Blood Tumor Mutational Burden |
| LYM% | Lymphocyte Percentage |
| MSAF | Maximum Somatic Allele Frequency |
| ITH | Intratumor Heterogeneity |
| LAF-bTMB | Low Allele Frequency Blood Tumor Mutational Burden |
| CTCs | Circulating Tumor Cells |
| VEGF | Vascular Endothelial Growth Factor |
| IL-1 | Interleukin-1 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| CircPACRGL | Circular RNA PACRGL |
| TGF-β | Transforming Growth Factor Beta |
| PRRs | Pattern Recognition Receptors |
| IL-1β | Interleukin-1 Beta |
| IL-18 | Interleukin-18 |
| VEGFC | Vascular Endothelial Growth Factor C |
| VEGFD | Vascular Endothelial Growth Factor D |
| LAR | LDH-to-Albumin Ratio |
| TNM | Tumor, Node, Metastasis |
| MMPs | Matrix Metalloproteinases |
| CTLs | Cytotoxic T Lymphocytes |
| EPO | Eosinophil Peroxidase |
| MBP | Major Basic Protein |
| ECP | Eosinophil Cationic Protein |
| EDN | Eosinophil-Derived Neurotoxin |
| IL-5 | Interleukin-5 |
| IL-13 | Interleukin-13 |
| TNF-α | Tumor Necrosis Factor Alpha |
| Foxp3 | Forkhead Box P3 |
| APCs | Antigen-Presenting Cells |
| ARG1 | Arginase 1 |
| iNOS/NOS2 | Inducible Nitric Oxide Synthase |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
| AMPK | AMP-Activated Protein Kinase |
| LPS | Lipopolysaccharide |
| IL-12 | Interleukin-12 |
| IL-4 | Interleukin-4 |
| TAMs | Tumor-Associated Macrophages |
| sPD-L1 | Soluble Programmed Death-Ligand 1 |
| sCTLA-4 | Soluble Cytotoxic T-Lymphocyte-Associated Protein 4 |
| IL-2R | Interleukin-2 Receptor |
References
- Fontsa, M.L.; Padonou, F.; Willard-Gallo, K. Biomarkers and immunotherapy: Where are we? Curr. Opin. Oncol. 2022, 34, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.P.; Ming, L.C.; Dhaliwal, J.S.; Gupta, M.; Ardianto, C.; Goh, K.W.; Hussain, Z.; Shafqat, N. Role of Immunotherapy in the Treatment of Cancer: A Systematic Review. Cancers 2022, 14, 5205. [Google Scholar] [CrossRef] [PubMed]
- Holder, A.M.; Dedeilia, A.; Sierra-Davidson, K.; Cohen, S.; Liu, D.; Parikh, A.; Boland, G.M. Defining clinically useful biomarkers of immune checkpoint inhibitors in solid tumours. Nat. Rev. Cancer 2024, 24, 498–512. [Google Scholar] [CrossRef] [PubMed]
- Stadler, J.-C.; Belloum, Y.; Deitert, B.; Sementsov, M.; Heidrich, I.; Gebhardt, C.; Keller, L.; Pantel, K. Current and Future Clinical Applications of ctDNA in Immuno-Oncology. Cancer Res. 2022, 82, 349–358. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Saenger, Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist 2008, 13, 2–9. [Google Scholar] [CrossRef]
- Han, J.; Wu, M.; Liu, Z. Dysregulation in IFN-γ signaling and response: The barricade to tumor immunotherapy. Front. Immunol. 2023, 14, 1190333. [Google Scholar] [CrossRef]
- Jeon, D.; Hill, E.; McNeel, D.G. Toll-like receptor agonists as cancer vaccine adjuvants. Hum. Vaccin Immunother. 2024, 20, 2297453. [Google Scholar] [CrossRef]
- Milone, M.C.; Xu, J.; Chen, S.J.; Collins, M.A.; Zhou, J.; Powell Jr, D.J.; Melenhorst, J.J. Engineering enhanced CAR T-cells for improved cancer therapy. Nat. Cancer. 2021, 2, 780–793. [Google Scholar] [CrossRef]
- Sahraei, M.; Bose, M.; Sanders, J.A.; De, C.; DasRoy, L.; Nath, S.; Brouwer, C.R.; Mukherjee, P. Repression of MUC1 Promotes Expansion and Suppressive Function of Myeloid-Derived Suppressor Cells in Pancreatic and Breast Cancer Murine Models. Int. J. Mol. Sci. 2021, 22, 5587. [Google Scholar] [CrossRef]
- Buonacera, A.; Stancanelli, B.; Colaci, M.; Malatino, L. Neutrophil to Lymphocyte Ratio: An Emerging Marker of the Relationships between the Immune System and Diseases. Int. J. Mol. Sci. 2022, 23, 3636. [Google Scholar] [CrossRef]
- Larsen, M.K.; Skov, V.; Kjær, L.; Eickhardt-Dalbøge, C.S.; Knudsen, T.A.; Kristiansen, M.H.; Sørensen, A.L.; Wienecke, T.; Andersen, M.; Ottesen, J.T.; et al. Neutrophil-to-lymphocyte ratio and all-cause mortality with and without myeloproliferative neoplasms-a Danish longitudinal study. Blood Cancer J. 2024, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Drăgoescu, A.N.; Pădureanu, V.; Stănculescu, A.D.; Chiuțu, L.C.; Tomescu, P.; Geormăneanu, C.; Pădureanu, R.; Iovănescu, V.F.; Ungureanu, B.S.; Pănuș, A.; et al. Neutrophil to Lymphocyte Ratio (NLR)-A Useful Tool for the Prognosis of Sepsis in the ICU. Biomedicines 2021, 10, 75. [Google Scholar] [CrossRef] [PubMed]
- Mosca, M.; Nigro, M.C.; Pagani, R.; De Giglio, A.; Di Federico, A. Neutrophil-to-Lymphocyte Ratio (NLR) in NSCLC, Gastrointestinal, and Other Solid Tumors: Immunotherapy and Beyond. Biomolecules 2023, 13, 1803. [Google Scholar] [CrossRef]
- Cupp, M.A.; Cariolou, M.; Tzoulaki, I.; Aune, D.; Evangelou, E.; Berlanga-Taylor, A.J. Neutrophil to lymphocyte ratio and cancer prognosis: An umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020, 18, 360. [Google Scholar] [CrossRef] [PubMed]
- Anpalakhan, S.; Signori, A.; Cortellini, A.; Verzoni, E.; Giusti, R.; Aprile, G.; Ermacora, P.; Catino, A.; Pipitone, S.; Di Napoli, M.; et al. Using peripheral immune-inflammatory blood markers in tumors treated with immune checkpoint inhibitors: An INVIDIa-2 study sub-analysis. iScience 2023, 26, 107970. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Ma, F.; Sun, B.; Wang, Y.; Luo, J.; Liu, M.; Luo, Z. Peripheral Blood Markers Associated with Immune-Related Adverse Effects in Patients Who Had Advanced Non-Small Cell Lung Cancer Treated with PD-1 Inhibitors. Cancer Manag. Res. 2021, 13, 765–771. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Lin, H.; Wang, J.; Cao, B. Predictive biomarkers for immune-related adverse events in cancer patients treated with immune-checkpoint inhibitors. BMC Immunol. 2024, 25, 8. [Google Scholar] [CrossRef]
- Tan, S.; Zheng, Q.; Zhang, W.; Zhou, M.; Xia, C.; Feng, W. Prognostic value of inflammatory markers NLR, PLR, and LMR in gastric cancer patients treated with immune checkpoint inhibitors: A meta-analysis and systematic review. Front. Immunol. 2024, 15, 1408700. [Google Scholar] [CrossRef]
- Ou, Y.; Liang, S.; Gao, Q.; Shang, Y.; Liang, J.; Zhang, W.; Liu, S. Prognostic value of inflammatory markers NLR, PLR, LMR, dNLR, ANC in melanoma patients treated with immune checkpoint inhibitors: A meta-analysis and systematic review. Front. Immunol. 2024, 15, 1482746. [Google Scholar] [CrossRef]
- Takenaka, Y.; Oya, R.; Takemoto, N.; Inohara, H. Neutrophil-to-lymphocyte ratio as a prognostic marker for head and neck squamous cell carcinoma treated with immune checkpoint inhibitors: Meta-analysis. Head Neck. 2022, 44, 1237–1245. [Google Scholar] [CrossRef]
- Guo, Y.; Xiang, D.; Wan, J.; Yang, L.; Zheng, C. Focus on the Dynamics of Neutrophil-to-Lymphocyte Ratio in Cancer Patients Treated with Immune Checkpoint Inhibitors: A Meta-Analysis and Systematic Review. Cancers 2022, 14, 5297. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Meng, F.; Jiang, R. Neutrophil-to-Lymphocyte Ratio as a Prognostic Biomarker for Patients With Metastatic Renal Cell Carcinoma Treated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 746976. [Google Scholar] [CrossRef] [PubMed]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Banna, G.L.; Cortellini, A.; Cortinovis, D.L.; Tiseo, M.; Aerts, J.G.J.V.; Barbieri, F.; Giusti, R.; Bria, E.; Grossi, F.; Pizzutilo, P.; et al. The lung immuno-oncology prognostic score (LIPS-3): A prognostic classification of patients receiving first-line pembrolizumab for PD-L1 ≥ 50% advanced non-small-cell lung cancer. ESMO Open. 2021, 6, 100078. [Google Scholar] [CrossRef]
- Criscitiello, C.; Marra, A.; Morganti, S.; Zagami, P.; Viale, G.; Esposito, A.; Curigliano, G. Pretreatment Blood Parameters Predict Efficacy from Immunotherapy Agents in Early Phase Clinical Trials. Oncologist 2020, 25, e1732–e1742. [Google Scholar] [CrossRef]
- Tan, D.; Fu, Y.; Tong, W.; Li, F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int. J. Surg. 2018, 55, 128–138. [Google Scholar] [CrossRef]
- Issa, M.; Klamer, B.G.; Mladkova, N.; Laliotis, G.I.; Karivedu, V.; Bhateja, P.; Byington, C.; Dibs, K.; Pan, X.; Chakravarti, A.; et al. Update of a prognostic survival model in head and neck squamous cell carcinoma patients treated with immune checkpoint inhibitors using an expansion cohort. BMC Cancer 2022, 22, 767. [Google Scholar] [CrossRef]
- Wan, L.; Wu, C.; Luo, S.; Xie, X.; Falzone, L. Prognostic Value of Lymphocyte-to-Monocyte Ratio (LMR) in Cancer Patients Undergoing Immune Checkpoint Inhibitors. Dis. Markers 2022, 2022, 3610038. [Google Scholar] [CrossRef]
- Balta, S.; Ozturk, C. The platelet-lymphocyte ratio: A simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets 2015, 26, 680–681. [Google Scholar] [CrossRef]
- Misiewicz, A.; Dymicka-Piekarska, V. Fashionable, but What is Their Real Clinical Usefulness? NLR, LMR, and PLR as a Promising Indicator in Colorectal Cancer Prognosis: A Systematic Review. J. Inflamm. Res. 2023, 16, 69–81. [Google Scholar] [CrossRef]
- Li, B.; Zhou, P.; Liu, Y.; Wei, H.; Yang, X.; Chen, T.; Xiao, J. Platelet-to-lymphocyte ratio in advanced Cancer: Review and meta-analysis. Clin. Chim. Acta 2018, 483, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Lai, Q.; Santa, E.C.; Juri, J.M.R.; Pinheiro, R.S.; Lerut, J. Neutrophil and platelet-to-lymphocyte ratio as new predictors of dropout and recurrence after liver transplantation for hepatocellular cancer. Transpl. Int. 2013, 27, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, Y.; Wang, X.; Jiang, Z.; Zhu, L.; Fang, S. Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and Monocyte-to-Lymphocyte Ratio in Depression: An Updated Systematic Review and Meta-Analysis. Front. Psychiatry 2022, 13, 893097. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.; Dormandy, J.A.; Ernst, E.; Matrai, A. Clinical Hemorheology: Applications in Cardiovascular and Hematological Disease, Diabetes, Surgery and Gynecology; Springer: Amsterdam, The Netherlands, 2012; Available online: https://books.google.pl/books?id=jYx9CAAAQBAJ (accessed on 9 April 2025).
- Oka, S. Cardiovascular Hemorheology, 1st ed; Cambridge University Press: Cambridge, UK, 1981. [Google Scholar]
- Baskurt, O.K.; Yalcin, O.; Meiselman, H.J. Hemorheology and vascular control mechanisms. Clin. Hemorheol. Microcirc. 2004, 30, 169–178. [Google Scholar]
- Kwaan, H.C. Role of plasma proteins in whole blood viscosity: A brief clinical review. Clin. Hemorheol. Microcirc. 2010, 44, 167–176. [Google Scholar] [CrossRef]
- Reinhart, W.H. Molecular biology and self-regulatory mechanisms of blood viscosity: A review. Biorheology 2001, 38, 203–212. [Google Scholar] [CrossRef]
- Késmárky, G.; Kenyeres, P.; Rábai, M.; Tóth, K. Plasma viscosity: A forgotten variable. Clin. Hemorheol. Microcirc. 2008, 39, 243–246. [Google Scholar] [CrossRef]
- Gertz, M.A. Acute hyperviscosity: Syndromes and management. Blood 2018, 132, 1379–1385. [Google Scholar] [CrossRef]
- Gurkan, U.A. Biophysical and rheological biomarkers of red blood cell physiology and pathophysiology. Curr. Opin. Hematol. 2021, 28, 138–149. [Google Scholar] [CrossRef]
- Pryzwan, T.; Dolibog, P.; Kierszniok, K.; Pietrzyk, B. Blood rheological properties and methods of their measurement. Ann. Acad. Medicae Silesiensis 2024, 78, 1–10. [Google Scholar] [CrossRef]
- Beris, A.N.; Horner, J.S.; Jariwala, S.; Armstrong, M.J.; Wagner, N.J. Recent advances in blood rheology: A review. Soft Matter. 2021, 17, 10591–10613. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Determinants of tumor blood flow: A review. Cancer Res. 1988, 48, 2641–2658. [Google Scholar] [PubMed]
- de Arruda, M.V.; Silva, A.C.; Galduróz, J.C.F.; Galduróz, R.F. Standardization for obtaining blood viscosity: A systematic review. Eur. J. Haematol. 2021, 106, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Ptaszek, B.; Podsiadło, S.; Jandziś, Z.; Teległów, A.; Piotrowska, A.; Jurczyszyn, A.; Czerwińska-Ledwig, O. Rheological properties of blood in multiple myeloma patients. Sci. Rep. 2024, 14, 4260. [Google Scholar] [CrossRef]
- Bhuria, V.; Baldauf, C.K.; Schraven, B.; Fischer, T. Thromboinflammation in Myeloproliferative Neoplasms (MPN)—A Puzzle Still to Be Solved. Int. J. Mol. Sci. 2022, 23, 3206. [Google Scholar] [CrossRef]
- O’Reilly, M.A.; Malhi, A.; Cheok, K.P.; Ings, S.; Balsa, C.; Keane, H.; Jalowiec, K.; Neill, L.; Peggs, K.S.; Roddie, C. A novel predictive algorithm to personalize autologous T-cell harvest for chimeric antigen receptor T-cell manufacture. Cytotherapy 2022, 25, 323–329. [Google Scholar] [CrossRef]
- Zheng, X.; Fang, Z.; Liu, X.; Deng, S.; Zhou, P.; Wang, X.; Zhang, C.; Yin, R.; Hu, H.; Chen, X.; et al. Increased vessel perfusion predicts the efficacy of immune checkpoint blockade. J. Clin. Investig. 2018, 128, 2104–2115. [Google Scholar] [CrossRef]
- Marcinkowska-Gapińska, A. Obraz hemoreologiczny w chorobach nowotworowych. Lett. Oncol. Sci. 2019, 4, 36–42. [Google Scholar] [CrossRef]
- von Tempelhoff, G.-F.; Heilmann, L.; Hommel, G.; Pollow, K. Impact of Rheological Variables in Cancer. Semin. Thromb. Hemost. 2003, 29, 499–514. [Google Scholar] [CrossRef]
- Han, J.W.; Sung, P.S.; Jang, J.W.; Choi, J.Y.; Yoon, S.K.; Kanda, T. Whole blood viscosity is associated with extrahepatic metastases and survival in patients with hepatocellular carcinoma. PLoS ONE 2021, 16, e0260311. [Google Scholar] [CrossRef]
- Ucgul, E.; Guven, D.C.; Ucgul, A.N.; Ozbay, Y.; Onur, M.R.; Akin, S. Factors Influencing Immunotherapy Outcomes in Cancer: Sarcopenia and Systemic Inflammation. Cancer Control 2024, 31, 10732748241302248. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, F.; Yuan, F.; Li, Y.; Ma, J.; Ou, Q.; Liu, Z.; Yang, B.; Wang, L.; Tao, H.; et al. Pretreatment hemoglobin level as a predictor to evaluate the efficacy of immune checkpoint inhibitors in patients with advanced non-small cell lung cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920970049. [Google Scholar] [CrossRef] [PubMed]
- Krizova, L.; Benesova, I.; Zemanova, P.; Spacek, J.; Strizova, Z.; Humlova, Z.; Mikulova, V.; Petruzelka, L.; Vocka, M. Immunophenotyping of peripheral blood in NSCLC patients discriminates responders to immune checkpoint inhibitors. J. Cancer Res. Clin. Oncol. 2024, 150, 99. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ren, T.; Ji, C.; Zhao, L.; Wang, X. The baseline hemoglobin level is a positive biomarker for immunotherapy response and can improve the predictability of tumor mutation burden for immunotherapy response in cancer. Front. Pharmacol. 2024, 15, 1456833. [Google Scholar] [CrossRef] [PubMed]
- Mazzaschi, G.; Lazzarin, A.; Santoni, M.; Trentini, F.; De Giorgi, U.; Brighi, N.; Tommasi, C.; Puglisi, S.; Caffo, O.; Kinspergher, S.; et al. Integrating Red Blood Cell Features and Hemoglobin Levels in Metastatic Renal Cell Carcinoma Patients Treated with Pazopanib or Cabozantinib: An Easily Exploitable Prognostic Score. Front. Biosci. 2023, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Maffezzoli, M.; Santoni, M.; Mazzaschi, G.; Rodella, S.; Lai, E.; Maruzzo, M.; Basso, U.; Bimbatti, D.; Iacovelli, R.; Anghelone, A.; et al. External validation of a red cell-based blood prognostic score in patients with metastatic renal cell carcinoma treated with first-line immunotherapy combinations. Clin. Exp. Metastasis 2024, 41, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Anpalakhan, S.; Banna, G.L.; Rebuzzi, S.E.; Fornarini, G.; Maruzzo, M.; Zucali, P.A.; Catalano, F.; Antonj, L.; Tudini, M.; Fratino, L.; et al. A red blood cell-based score in the prognostication of patients with metastatic RCC of the Meet-URO 15 study. Immunotherapy 2024, 16, 963–973. [Google Scholar] [CrossRef]
- Cogels, M.M.; Rouas, R.; Ghanem, G.E.; Martinive, P.; Awada, A.; Van Gestel, D.; Krayem, M. Humanized Mice as a Valuable Pre-Clinical Model for Cancer Immunotherapy Research. Front. Oncol. 2021, 11, 784947. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Huang, G.; Cheng, L.; Li, Z.; Xiao, Y.; Deng, Q.; Jiang, Y.; Li, B.; Lin, S.; Wang, S.; et al. Establishment of peripheral blood mononuclear cell-derived humanized lung cancer mouse models for studying efficacy of PD-L1/PD-1 targeted immunotherapy. mAbs 2018, 10, 1301–1311. [Google Scholar] [CrossRef] [PubMed]
- An, H.J.; Chon, H.J.; Kim, C. Peripheral Blood-Based Biomarkers for Immune Checkpoint Inhibitors. Int. J. Mol. Sci. 2021, 22, 9414. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Pang, G.; Wang, P. Emerging Blood-Based Biomarkers for Predicting Response to Checkpoint Immunotherapy in Non-Small-Cell Lung Cancer. Front. Immunol. 2020, 11, 603157. [Google Scholar] [CrossRef] [PubMed]
- Oitabén, A.; Fonseca, P.; Villanueva, M.J.; García-Benito, C.; López-López, A.; Garrido-Fernández, A.; González-Ojea, C.; Juaneda-Magdalena, L.; Lázaro, M.E.; Martínez-Fernández, M. Emerging Blood-Based Biomarkers for Predicting Immunotherapy Response in NSCLC. Cancers 2022, 14, 2626. [Google Scholar] [CrossRef] [PubMed]
- Gungabeesoon, J.; Gort-Freitas, N.A.; Kiss, M.; Bolli, E.; Messemaker, M.; Siwicki, M.; Hicham, M.; Bill, R.; Koch, P.; Cianciaruso, C.; et al. A neutrophil response linked to tumor control in immunotherapy. Cell 2023, 186, 1448–1464.e20. [Google Scholar] [CrossRef] [PubMed]
- van Elsas, M.; Kleinovink, J.W.; Moerland, M.; Feiss, G.; Beyrend, G.; Arens, R.; Mei, H.; Nibbering, P.H.; Jirka, S.M.; van Hall, T.; et al. Host genetics and tumor environment determine the functional impact of neutrophils in mouse tumor models. J. Immunother. Cancer 2020, 8, e000877. [Google Scholar] [CrossRef]
- Linde, I.L.; Prestwood, T.R.; Qiu, J.; Pilarowski, G.; Linde, M.H.; Zhang, X.; Shen, L.; Reticker-Flynn, N.E.; Chiu, D.K.-C.; Sheu, L.Y.; et al. Neutrophil-activating therapy for the treatment of cancer. Cancer Cell 2023, 41, 356–372.e10. [Google Scholar] [CrossRef]
- Zheng, Y.; Sefik, E.; Astle, J.; Karatepe, K.; Öz, H.H.; Solis, A.G.; Jackson, R.; Luo, H.R.; Bruscia, E.M.; Halene, S.; et al. Human neutrophil development and functionality are enabled in a humanized mouse model. Proc. Natl. Acad. Sci. USA 2022, 119, e2121077119. [Google Scholar] [CrossRef]
- Ye, C.; Yang, H.; Cheng, M.; Shultz, L.D.; Greiner, D.L.; Brehm, M.A.; Keck, J.G. A rapid, sensitive, and reproducible in vivo PBMC humanized murine model for determining therapeutic-related cytokine release syndrome. FASEB J. 2020, 34, 12963–12975. [Google Scholar] [CrossRef]
- Marín-Jiménez, J.A.; Capasso, A.; Lewis, M.S.; Bagby, S.M.; Hartman, S.J.; Shulman, J.; Navarro, N.M.; Yu, H.; Rivard, C.J.; Wang, X.; et al. Testing Cancer Immunotherapy in a Human Immune System Mouse Model: Correlating Treatment Responses to Human Chimerism, Therapeutic Variables and Immune Cell Phenotypes. Front. Immunol. 2021, 12, 607282. [Google Scholar] [CrossRef]
- Wang, X.; Chen, D.; Ma, Y.; Mo, D.; Yan, F. Variation of peripheral blood-based biomarkers for response of anti-PD-1 immunotherapy in non-small-cell lung cancer. Clin. Transl. Oncol. 2024, 26, 1934–1943. [Google Scholar] [CrossRef]
- Peters, S.; Dziadziuszko, R.; Morabito, A.; Felip, E.; Gadgeel, S.M.; Cheema, P.; Cobo, M.; Andric, Z.; Barrios, C.H.; Yamaguchi, M.; et al. Atezolizumab versus chemotherapy in advanced or metastatic NSCLC with high blood-based tumor mutational burden: Primary analysis of BFAST cohort C randomized phase 3 trial. Nat. Med. 2022, 28, 1831–1839. [Google Scholar] [CrossRef]
- Gandara, D.R.; Paul, S.M.; Kowanetz, M.; Schleifman, E.; Zou, W.; Li, Y.; Rittmeyer, A.; Fehrenbacher, L.; Otto, G.; Malboeuf, C.; et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat. Med. 2018, 24, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.; Wang, Z.-J.; Zhang, K.; Li, B.; Cai, Y.-R.; Wen, F.-C.; Zhang, D.; Bai, Y.-Z.; Zhang, X.-Y.; Wang, S.-Y.; et al. ctDNA-adjusted bTMB as a predictive biomarker for patients with NSCLC treated with PD-(L)1 inhibitors. BMC Med. 2022, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zhu, Y.; Zhuo, M.; Chen, X.; Xie, Y.; Duan, J.; Bai, H.; Hao, S.; Yu, Z.; Yi, Y.; et al. Maximum Somatic Allele Frequency-Adjusted Blood-Based Tumor Mutational Burden Predicts the Efficacy of Immune Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer. Cancers 2022, 14, 5649. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Duan, J.; Wang, G.; Zhao, J.; Xu, J.; Han, J.; Zhao, Z.; Zhao, J.; Zhu, B.; Zhuo, M.; et al. Allele Frequency–Adjusted Blood-Based Tumor Mutational Burden as a Predictor of Overall Survival for Patients With NSCLC Treated With PD-(L)1 Inhibitors. J. Thorac. Oncol. 2020, 15, 556–567. [Google Scholar] [CrossRef]
- Tamminga, M.; de Wit, S.; Hiltermann, T.J.N.; Timens, W.; Schuuring, E.; Terstappen, L.W.M.M.; Groen, H.J.M. Circulating tumor cells in advanced non-small cell lung cancer patients are associated with worse tumor response to checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 173. [Google Scholar] [CrossRef]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- Tucci, M.; Passarelli, A.; Mannavola, F.; Stucci, L.S.; Ascierto, P.A.; Capone, M.; Madonna, G.; Lopalco, P.; Silvestris, F. Serum exosomes as predictors of clinical response to ipilimumab in metastatic melanoma. OncoImmunology 2017, 7, e1387706. [Google Scholar] [CrossRef]
- Huang, X.; Nepovimova, E.; Adam, V.; Sivak, L.; Heger, Z.; Valko, M.; Wu, Q.; Kuca, K. Neutrophils in Cancer immunotherapy: Friends or foes? Mol. Cancer 2024, 23, 107. [Google Scholar] [CrossRef]
- Zhou, X.; Du, Y.; Huang, Z.; Xu, J.; Qiu, T.; Wang, J.; Wang, T.; Zhu, W.; Liu, P.; Scheurer, M. Prognostic value of PLR in various cancers: A meta-analysis. PLoS ONE 2014, 9, e101119. [Google Scholar] [CrossRef]
- Krysko, D.V.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef]
- Dao, J.; Conway, P.J.; Subramani, B.; Meyyappan, D.; Russell, S.; Mahadevan, D. Using cfDNA and ctDNA as Oncologic Markers: A Path to Clinical Validation. Int. J. Mol. Sci. 2023, 24, 13219. [Google Scholar] [CrossRef] [PubMed]
- Moon, G.Y.; Dalkiran, B.; Park, H.S.; Shin, D.; Son, C.; Choi, J.H.; Bang, S.; Lee, H.; Doh, I.; Kim, D.H.; et al. Dual Biomarker Strategies for Liquid Biopsy: Integrating Circulating Tumor Cells and Circulating Tumor DNA for Enhanced Tumor Monitoring. Biosensors 2025, 15, 74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Monick, S.; Rosenthal, A. Circulating Tumor DNA as a Complementary Prognostic Biomarker during CAR-T Therapy in B-Cell Non-Hodgkin Lymphomas. Cancers 2024, 16, 1881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nakamura, Y.; Shitara, K. Development of circulating tumour DNA analysis for gastrointestinal cancers. ESMO Open 2020, 5, e000600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, J.; Wang, H.; Zang, W.; Li, B.; Rao, G.; Li, L.; Yu, Y.; Li, Z.; Dong, B.; Lu, Z.; et al. Circulating tumor DNA functions as an alternative for tissue to overcome tumor heterogeneity in advanced gastric cancer. Cancer Sci. 2017, 108, 1881–1887. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balkwill, F.R.; Mantovani, A. Cancer-related inflammation: Common themes and therapeutic opportunities. Semin. Cancer Biol. 2012, 22, 33–40. [Google Scholar] [CrossRef]
- Xie, Z.; Zhou, H.; Wang, L.; Wu, Y. The Significance of the preoperative lactate dehydrogenase/albumin Ratio in the Prognosis of Colon Cancer: A retrospective study. PeerJ 2022, 10, e13091. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Aukrust, P.; Gullestad, L.; Ueland, T.; Damås, J.K.; Yndestad, A. Inflammatory and anti-inflammatory cytokines in chronic heart failure: Potential therapeutic implications. Ann. Med. 2005, 37, 74–85. [Google Scholar] [CrossRef]
- Deichmann, M.; Kahle, B.; Moser, K.; Wacker, J.; Wüst, K. Diagnosing melanoma patients entering American Joint Committee on Cancer stage IV, C-reactive protein in serum is superior to lactate dehydrogenase. Br. J. Cancer 2004, 91, 699–702. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, Y.; Xu, J.; Huang, X.; Xie, S.; Lin, P.; Wang, C.; Guo, Y.; Zou, S.; Zhao, Z.; Wen, W.; et al. C-reactive protein and lactate dehydrogenase serum levels potentially predict the response to checkpoint inhibitors in patients with advanced non-small cell lung cancer. J. Thorac. Dis. 2023, 15, 1892–1900. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, X.; Xu, J.; Zhang, Q.; Yang, L.; Duan, Y. Quantification analysis of lactate dehydrogenase and C-reactive protein in evaluation of the severity and prognosis of the acute pancreatitis. Cell. Mol. Biol. 2020, 66, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Kaur, J.; Kaur, R.; Jaswal, S.; Aggarwal, D.; Saini, V.; Bhatia, C.; Sodhi, M.K.; Aggarwal, P. Evaluation of a Panel of Biomarkers in the Diagnosis of Lung Cancer: An Observational Study. Indian J. Respir. Care 2023, 12, 244–247. [Google Scholar] [CrossRef]
- Safaei, S.; Yari, A.; Pourbagherian, O.; Maleki, L.A. The role of cytokines in shaping the future of Cancer immunotherapy. Cytokine 2025, 189, 156888. [Google Scholar] [CrossRef]
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef]
- Waugh, D.J.; Wilson, C. The Interleukin-8 Pathway in Cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef]
- Fisher, D.T.; Appenheimer, M.M.; Evans, S.S. The two faces of IL-6 in the tumor microenvironment. Semin. Immunol. 2014, 26, 38–47. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tyan, K.; Baginska, J.; Brainard, M.; Giobbie-Hurder, A.; Severgnini, M.; Manos, M.; Haq, R.; Buchbinder, E.I.; Ott, P.A.; Hodi, F.S.; et al. Cytokine changes during immune-related adverse events and corticosteroid treatment in melanoma patients receiving immune checkpoint inhibitors. Cancer Immunol. Immunother. 2021, 70, 2209–2221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, J.; Mao, Y.; Mao, C.; Wang, D.; Dong, L.; Zhu, W.; Siddiqui, A. An On-Treatment Decreased Trend of Serum IL-6 and IL-8 as Predictive Markers Quickly Reflects Short-Term Efficacy of PD-1 Blockade Immunochemotherapy in Patients with Advanced Gastric Cancer. J. Immunol. Res. 2024, 2024, 3604935. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guo, A.-J.; Deng, Q.-Y.; Dong, P.; Zhou, L.; Shi, L. Biomarkers associated with immune-related adverse events induced by immune checkpoint inhibitors. World J. Clin. Oncol. 2024, 15, 1002–1020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grisaru-Tal, S.; Itan, M.; Klion, A.D.; Munitz, A. A new dawn for eosinophils in the tumour microenvironment. Nat. Rev. Cancer 2020, 20, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Huang, T.; Dai, M.; Kong, X.; Liu, H.; Zheng, Z.; Sun, G.; Sun, G.; Rong, D.; Jin, Z.; et al. Tumor Microenvironment and its Implications for Antitumor Immunity in Cholangiocarcinoma: Future Perspectives for Novel Therapies. Int. J. Biol. Sci. 2022, 18, 5369–5390. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Sakaguchi, S. Regulatory T cells in tumor immunity. Int. J. Cancer 2010, 127, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef]
- Condamine, T.; Mastio, J.; Gabrilovich, D.I. Transcriptional regulation of myeloid-derived suppressor cells. J. Leukoc. Biol. 2015, 98, 913–922. [Google Scholar] [CrossRef]
- Mandruzzato, S.; Brandau, S.; Britten, C.M.; Bronte, V.; Damuzzo, V.; Gouttefangeas, C.; Maurer, D.; Ottensmeier, C.; van der Burg, S.H.; Welters, M.J.P.; et al. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: Results from an interim study. Cancer Immunol. Immunother. 2016, 65, 161–169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flores-Campos, R.; García-Domínguez, D.J.; Hontecillas-Prieto, L.; Jiménez-Cortegana, C.; de la Cruz-Merino, L.; Sánchez-Margalet, V. Flow cytometry analysis of myeloid derived suppressor cells using 6 color labeling. Methods Cell Biol. 2024, 190, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ostrand-Rosenberg, S.; Fenselau, C. Myeloid-Derived Suppressor Cells: Immune-Suppressive Cells That Impair Antitumor Immunity and Are Sculpted by Their Environment. J. Immunol. 2018, 200, 422–431. [Google Scholar] [CrossRef]
- Qian, B.-Z.; Pollard, J.W. Macrophage diversity enhances tumor progression and metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Rieth, J.; Subramanian, S. Mechanisms of Intrinsic Tumor Resistance to Immunotherapy. Int. J. Mol. Sci. 2018, 19, 1340. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, M.; Linstra, R.; van Vugt, M.A. Genomic instability, inflammatory signaling and response to cancer immunotherapy. Biochim. Biophys Acta Rev. Cancer. 2022, 1877, 188661. [Google Scholar] [CrossRef] [PubMed]
- Currenti, J.; Mishra, A.; Wallace, M.; George, J.; Sharma, A. Immunosuppressive mechanisms of oncofetal reprogramming in the tumor microenvironment: Implications in immunotherapy response. Biochem. Soc. Trans. 2023, 51, 597–612. [Google Scholar] [PubMed]
- Tikhomirova, I.; Petrochenko, E.; Muravyov, A.; Malysheva, Y.; Petrochenko, A.; Yakusevich, V.; Oslyakova, A. Microcirculation and blood rheology abnormalities in chronic heart failure. Clin. Hemorheol. Microcirc. 2016, 65, 383–391. [Google Scholar] [CrossRef]
- Sevick, E.M.; Jain, R.K. Geometric resistance to blood flow in solid tumors perfused ex vivo: Effects of tumor size and perfusion pressure. Cancer Res. 1989, 49, 3506–3512. [Google Scholar] [PubMed]
- Noman, M.Z.; Parpal, S.; Van Moer, K.; Xiao, M.; Yu, Y.; Viklund, J.; De Milito, A.; Hasmim, M.; Andersson, M.; Amaravadi, R.K.; et al. Inhibition of Vps34 reprograms cold into hot inflamed tumors and improves anti–PD-1/PD-L1 immunotherapy. Sci. Adv. 2020, 6, eaax7881, Erratum in Sci. Adv. 2021, 7, eabf5801. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mpekris, F.; Voutouri, C.; Baish, J.W.; Duda, D.G.; Munn, L.L.; Stylianopoulos, T.; Jain, R.K. Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2020, 117, 3728–3737. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kim, R.; An, M.; Lee, H.; Mehta, A.; Heo, Y.J.; Kim, K.-M.; Lee, S.-Y.; Moon, J.; Kim, S.T.; Min, B.-H.; et al. Early Tumor–Immune Microenvironmental Remodeling and Response to First-Line Fluoropyrimidine and Platinum Chemotherapy in Advanced Gastric Cancer. Cancer Discov. 2022, 12, 984–1001. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, C.; Zhang, H.; Li, S.; Zhang, D.; Li, J.; Dionigi, G.; Liang, N.; Sun, H. Prognostic Impact of Inflammatory Markers PLR, LMR, PDW, MPV in Medullary Thyroid Carcinoma. Front. Endocrinol. 2022, 13, 861869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Von Tempelhoff, G.F.; Nieman, F.; Heilmann, L.; Hommel, G. Association between blood rheology, thrombosis and cancer survival in patients with gynecologic malignancy. Clin Hemorheol. Microcirc. 2000, 22, 107–130. [Google Scholar] [PubMed]
- Zhou, J.; Wei, S.; Guo, X.; Huang, Y.; Zhang, Y.; Hong, Y.; Chen, X.; Lu, M.; Zheng, F.; Zheng, C. Correlation between preoperative peripheral blood NLR, PLR, LMR and prognosis of patients with head and neck squamous cell carcinoma. BMC Cancer 2023, 23, 1247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, U.; Park, H.S.; Im, S.Y.; Yoo, C.Y.; Jung, J.H.; Suh, Y.J.; Choi, H.J.; Lafrenie, R.M. Prognostic value of systemic inflammatory markers and development of a nomogram in breast cancer. PLoS ONE 2018, 13, e0200936. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Maloney, S.; Pavlakis, N.; Itchins, M.; Arena, J.; Mittal, A.; Hudson, A.; Colvin, E.; Sahni, S.; Diakos, C.; Chan, D.; et al. The Prognostic and Predictive Role of the Neutrophil-to-Lymphocyte Ratio (NLR), Platelet-to-Lymphocyte Ratio (PLR), and Lymphocyte-to-Monocyte Ratio (LMR) as Biomarkers in Resected Pancreatic Cancer. J. Clin. Med. 2023, 12, 1989. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pongbala, F.H.; Bahrun, U.; Arif, M. Prognostic Analysis of NLR, PLR, and, LMR in Osteosarcoma at Dr. Wahidin Sudirohusodo Hospital. Indones. J. Clin. Pathol. Med. Lab. 2024, 30, 172–176. [Google Scholar] [CrossRef]
- Lin, J.; Dai, Y.; Sang, C.; Song, G.; Xiang, B.; Zhang, M.; Dong, L.; Xia, X.; Ma, J.; Shen, X.; et al. Multimodule characterization of immune subgroups in intrahepatic cholangiocarcinoma reveals distinct therapeutic vulnerabilities. J. Immunother. Cancer 2022, 10, e004892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Skrip, L.-M.; Moosburner, S.; Tang, P.; Guo, J.; Görner, S.; Tzschätzsch, H.; Brüggemann, K.; Walter, K.A.; Hosse, C.; Fehrenbach, U.; et al. Viscoelastic properties of colorectal liver metastases reflect tumour cell viability. J. Transl. Med. 2024, 22, 774. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Miller, B.; Heilmann, L. Hemorheological parameters during chemotherapy. Clin. Hemorheol. Microcirc. 1987, 7, 287–293. [Google Scholar] [CrossRef]
- Dessard, M.; Manneville, J.-B.; Berret, J.-F. Cytoplasmic viscosity is a potential biomarker for metastatic breast cancer cells. Nanoscale Adv. 2024, 6, 1727–1738. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Deptuła, P.; Łysik, D.; Pogoda, K.; Cieśluk, M.; Namiot, A.; Mystkowska, J.; Król, G.; Głuszek, S.; Janmey, P.A.; Bucki, R. Tissue Rheology as a Possible Complementary Procedure to Advance Histological Diagnosis of Colon Cancer. ACS Biomater. Sci. Eng. 2020, 6, 5620–5631. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, Y.; Wang, C.; Wang, Y.; Dai, L. Soluble PD-L1 as a predictive biomarker in lung cancer: A systematic review and meta-analysis. Futur. Oncol. 2022, 18, 261–273. [Google Scholar] [CrossRef]
- Pistillo, M.P.; Fontana, V.; Morabito, A.; Dozin, B.; Laurent, S.; Carosio, R.; Banelli, B.; Ferrero, F.; Spano, L.; Italian Melanoma Intergroup; et al. Soluble CTLA-4 as a favorable predictive biomarker in metastatic melanoma patients treated with ipilimumab: An Italian melanoma intergroup study. Cancer Immunol. Immunother. 2019, 68, 97–107. [Google Scholar] [CrossRef]
- Hou, W.; Yi, C.; Zhu, H. Predictive biomarkers of colon cancer immunotherapy: Present and future. Front. Immunol. 2022, 13, 1032314. [Google Scholar] [CrossRef]
- Büttner, R.; Gosney, J.R.; Skov, B.G.; Adam, J.; Motoi, N.; Bloom, K.J.; Dietel, M.; Longshore, J.W.; López-Ríos, F.; Penault-Llorca, F.; et al. Programmed Death-Ligand 1 Immunohistochemistry Testing: A Review of Analytical Assays and Clinical Implementation in Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2017, 35, 3867–3876. [Google Scholar] [CrossRef]
- Adam, J.; Le Stang, N.; Rouquette, I.; Cazes, A.; Badoual, C.; Pinot-Roussel, H.; Tixier, L.; Danel, C.; Damiola, F.; Damotte, D.; et al. Multicenter harmonization study for PD-L1 IHC testing in non-small-cell lung cancer. Ann. Oncol. 2018, 29, 953–958. [Google Scholar] [CrossRef]
- Kim, M.-S.; Park, C.-J.; Namgoong, S.; Kim, S.-I.; Cho, Y.-U.; Jang, S. Effective and Practical Complete Blood Count Delta Check Method and Criteria for the Quality Control of Automated Hematology Analyzers. Ann. Lab. Med. 2023, 43, 418–424. [Google Scholar] [CrossRef]
- Åstrand, A.; Wingren, C.; Walton, C.; Mattsson, J.; Agrawal, K.; Lindqvist, M.; Odqvist, L.; Burmeister, B.; Eck, S.; Hughes, G.; et al. A comparative study of blood cell count in four automated hematology analyzers: An evaluation of the impact of preanalytical factors. PLoS ONE 2024, 19, e0301845. [Google Scholar] [CrossRef]
- Oliveira, L.R.; Simionatto, M.; Cruz, B.R.; Bittencourt, J.I.M.; Krum, E.A.; Moss, M.F.; Borato, D.C.K. Stability of complete blood count in different storage conditions using the ABX PENTRA 60 analyzer. Int. J. Lab. Hematol. 2018, 40, 359–365. [Google Scholar] [CrossRef]
- Rosencranz, R.; Bogen, S.A. Clinical laboratory measurement of serum, plasma, and blood viscosity. Am. J. Clin. Pathol. 2006, 125, S78–S86. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Z.; Zhang, W.; Zhang, W.; Buzdin, A.; Mu, X.; Yan, Q.; Zhao, X.; Chang, H.-H.; Duhon, M.; et al. FDA-Approved and Emerging Next Generation Predictive Biomarkers for Immune Checkpoint Inhibitors in Cancer Patients. Front. Oncol. 2021, 11, 683419. [Google Scholar] [CrossRef]
- Diaz-Montero, C.M.; Salem, M.L.; Nishimura, M.I.; Garrett-Mayer, E.; Cole, D.J.; Montero, A.J. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 2009, 58, 49–59. [Google Scholar] [CrossRef]
- Gabitass, R.F.; Annels, N.E.; Stocken, D.D.; Pandha, H.A.; Middleton, G.W. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol. Immunother. 2011, 60, 1419–1430. [Google Scholar] [CrossRef]
- Shou, D.; Wen, L.; Song, Z.; Yin, J.; Sun, Q.; Gong, W. Suppressive role of myeloid-derived suppressor cells (MDSCs) in the microenvironment of breast cancer and targeted immunotherapies. Oncotarget 2016, 7, 64505–64511. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Z.; Wu, L.; Zhang, M.; Li, W.; Ding, J.; Zhu, J.; Wei, H.; Zhao, K.; Unutmaz, D. Circulating and tumor-infiltrating myeloid-derived suppressor cells in patients with colorectal carcinoma. PLoS ONE 2013, 8, e57114. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Y.-H.; Li, B.-G.; Yang, Q.; Zhang, P.-Y.; Wang, H.-T. Prognostic value of pretreatment serum lactate dehydrogenase level in patients with solid tumors: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 9800. [Google Scholar] [CrossRef]
- Petrelli, F.; Cabiddu, M.; Coinu, A.; Borgonovo, K.; Ghilardi, M.; Lonati, V.; Barni, S. Prognostic role of lactate dehydrogenase in solid tumors: A systematic review and meta-analysis of 76 studies. Acta Oncol. 2015, 54, 961–970. [Google Scholar] [CrossRef]
- Leek, R.; Harris, A.; Lewis, C.E. Cytokine networks in solid human tumors: Regulation of angiogenesis. J. Leukoc. Biol. 1994, 56, 423–435. [Google Scholar] [CrossRef]
- Rovelli, F.; Lissoni, P.; Crispino, S.; Barni, S.; Fumagalli, G.; Paolorossi, F.; Tancini, G. Increased level of soluble interleukin-2 receptor in advanced solid tumors: A preliminary study. Tumori J. 1988, 74, 633–637. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Zhu, M.; He, W.; Hua, H.; Ye, F.; Zhou, X.; Chen, N.; Li, Y.; Zhong, W.; et al. Soluble PD-L1: A potential dynamic predictive biomarker for immunotherapy in patients with proficient mismatch repair colorectal cancer. J. Transl. Med. 2023, 21, 25. [Google Scholar] [CrossRef]
- Park, J.J.; Thi, E.P.; Carpio, V.H.; Bi, Y.; Cole, A.G.; Dorsey, B.D.; Fan, K.; Harasym, T.; Iott, C.L.; Kadhim, S.; et al. Checkpoint inhibition through small molecule-induced internalization of programmed death-ligand 1. Nat. Commun. 2021, 12, 1222. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goto, M.; Chamoto, K.; Higuchi, K.; Yamashita, S.; Noda, K.; Iino, T.; Miura, M.; Yamasaki, T.; Ogawa, O.; Sonobe, M.; et al. Analytical performance of a new automated chemiluminescent magnetic immunoassays for soluble PD-1, PD-L1, and CTLA-4 in human plasma. Sci. Rep. 2019, 9, 10144. [Google Scholar] [CrossRef]
- Kang, Y.; Zhu, X.; Lin, Z.; Zeng, M.; Shi, P.; Cao, Y.; Chen, F. Compare the Diagnostic and Prognostic Value of MLR, NLR and PLR in CRC Patients. Clin. Lab. 2021, 9, 2003. [Google Scholar] [CrossRef]
- Fang, T.; Wang, Y.; Yin, X.; Zhai, Z.; Zhang, Y.; Yang, Y.; You, Q.; Li, Z.; Ma, Y.; Li, C.; et al. Diagnostic sensitivity of NLR and PLR in early diagnosis of gastric cancer. J. Immunol. Res. 2020, 2020, 9146042. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, W.; Wang, J.; Ao, X.; Xue, J. Non-coding RNAs in lung cancer: Molecular mechanisms and clinical applications. Front. Oncol. 2023, 13, 1256537. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lu, H.; Zhang, Z.; Yuan, X.; Song, H.; Li, P. The role of circular RNA hsa_circ_0001789 as a diagnostic biomarker in gastric carcinoma. Scand. J. Gastroenterol. 2023, 58, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ye, J.; Xu, S.; Wang, J. Circulating noncoding RNAs: Promising biomarkers in liquid biopsy for the diagnosis, prognosis, and therapy of NSCLC. Discov. Oncol. 2023, 14, 142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamphorst, A.O.; Pillai, R.N.; Yang, S.; Nasti, T.H.; Akondy, R.S.; Wieland, A.; Sica, G.L.; Yu, K.; Koenig, L.; Patel, N.T.; et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1–targeted therapy in lung cancer patients. Proc. Natl. Acad. Sci. USA 2017, 114, 4993–4998. [Google Scholar] [CrossRef]
- Wei, S.C.; Levine, J.H.; Cogdill, A.P.; Zhao, Y.; Anang, N.-A.A.S.; Andrews, M.C.; Sharma, P.; Wang, J.; Wargo, J.A.; Pe’Er, D.; et al. Distinct Cellular Mechanisms Underlie Anti-CTLA-4 and Anti-PD-1 Checkpoint Blockade. Cell 2017, 170, 1120–1133.e17. [Google Scholar] [CrossRef]
- Wei, S.C.; Anang, N.-A.A.S.; Sharma, R.; Andrews, M.C.; Reuben, A.; Levine, J.H.; Cogdill, A.P.; Mancuso, J.J.; Wargo, J.A.; Pe’er, D.; et al. Combination anti–CTLA-4 plus anti–PD-1 checkpoint blockade utilizes cellular mechanisms partially distinct from monotherapies. Proc. Natl. Acad. Sci. USA 2019, 116, 22699–22709. [Google Scholar] [CrossRef]
- Masucci, G.V.; Cesano, A.; Hawtin, R.; Janetzki, S.; Zhang, J.; Kirsch, I.; Dobbin, K.K.; Alvarez, J.; Robbins, P.B.; Selvan, S.R.; et al. Validation of biomarkers to predict response to immunotherapy in cancer: Volume I—Pre-analytical and analytical validation. J. Immunother. Cancer 2016, 4, 76. [Google Scholar] [CrossRef]
| Rheological Parameter | Cancer Type/Treatment | Association with Treatment Outcomes | Statistical Data | Reference |
|---|---|---|---|---|
| Whole blood viscosity (WBV) | HCC and treated with nivolumab | A higher WBV (>16.0 cP) was associated with worse OS and PFS. | OS: p = 0.069; PFS: p = 0.067; ORR: 25% (low WBV) vs. 0% (high WBV), p = 0.409 | [52] |
| Hemoglobin (Hb) | Non-small-cell lung cancer (NSCLC) and immunotherapy | Hb ≥ 110 g/L associated with better OS and PFS | OS: 17.6 vs. 10.5 months, HR 0.56, p < 0.001; PFS: 10.0 vs. 4.0 months, HR 0.63, p = 0.001 | [54] |
| Hemoglobin (Hb) | NSCLC and ICI | Higher baseline Hb associated with better OS and PFS | Detailed stats not disclosed; trend observed in 224 patients | [55] |
| Red cell-based rheologic score (Hb ≥ 12 g/dL, MCV > 87 fL, and RDW ≤ 16%) | Metastatic renal cell carcinoma (mRCC), treated with TKIs and/or ICIs | Higher score correlated with better OS and PFS | OS: 42.0 vs. 17.3 months, HR 0.60, p < 0.001; PFS: 17.4 vs. 8.2 months, HR 0.66, p < 0.001 | [58] |
| Animal Model | Tumor Subsite/Biomarkers | Immunotherapy | Biomarkers | Key Findings | Reference |
|---|---|---|---|---|---|
| mouse (KP lung adenocarcinoma and MC38) | lung adenocarcinoma and colon carcinoma | not specified | neutrophil gene signatures and, likely, blood neutrophil counts | Therapy-elicited neutrophils with interferon gene signatures are essential for successful immunotherapy. | [65] |
| mouse (various strains: BL/6 and BALB/c) | TC-1, CT26, B16F10, MC38, and 4T1 | antimicrobial peptides and vaccination | blood neutrophil percentages | Neutrophil abundance and phenotype vary with host genetics and tumor type, affecting tumor growth control. | [66] |
| mouse | not specified | TNF, CD40 agonist, and tumor-binding antibody | neutrophil activation and infiltration | Neutrophils induced tumor eradication through oxidative damage. | [67] |
| humanized mouse (MISTRGGR) | not specified | not specified | human neutrophil counts | improved reconstitution of human neutrophils, enabling potential NLR calculation | [68] |
| PBMC humanized (NSG, etc.) | - | TGN1412 analog, a CD28 superagonist | cytokine levels (e.g., IL-6) | robust cytokine release in response to CD28 superagonist; useful for CRS assessment | [69] |
| HIS-BRGS mice | breast, colorectal, pancreatic, lung, adrenocortical, melanoma, and hematological malignancies | block CTLA-4 and/or PD-1/PD-L1 | human chimerism and T cell subsets | correlated blood immune profiles with tumor infiltration; variable chimerism noted | [70] |
| Clinical Study Type—Phase | Tumor Subsite | Biomarkers | Immunotherapy | Key Findings | Reference |
|---|---|---|---|---|---|
| retrospective study | NSCLC | CD4/CD8, LYM%, PD-1+ T-cells, NLR, and MLR | anti-PD-(L)1 | Elevated expression of CD4/CD8 and LYM% are positively associated with effective immunotherapy, while PD-1+ on T cells, NLR, and MLR have a negative impact. | [71] |
| phase 3 trial | NSCLC | bTMB | atezolizumab | Additional exploration of bTMB to identify optimal cutoffs, confounding factors, assay improvements, or cooperative biomarkers is warranted. | [72] |
| phase 2 and phase 3 trials | NSCLC | bTMB | atezolizumab | bTMB identifies patients who derive clinically significant improvements in PFS from atezolizumab. | [73] |
| multiple cohorts | NSCLC | CD4/CD8, LYM%, PD-1+ T-cells, NLR, and MLR | anti-PD-(L)1 | ctDNA-adjusted bTMB might predict OS benefit in NSCLC patients receiving ICIs. | [74] |
| multiple cohorts | NSCLC | bTMB | anti-PD-(L)1 | Ma-bTMB could reduce the confounding effect of MSAF and ITH on bTMB calculation and effectively identify beneficiaries of ICIs. | [75] |
| multiple cohorts | NSCLC | bTMB | anti-PD-(L)1 | LAF-bTMB is a feasible predictor of OS, PFS, and ORR. | [76] |
| prospective cohort study | NSCLC | ctDNA-adjusted bTMB | anti-PD-(L)1 | Presence of CTCs is a predictive factor for a worse durable response rate to ICIs. | [77] |
| prospective cohort study | melanoma | MSAF-adjusted bTMB | pembrolizumab | Early on-treatment increase in circulating exosomal PD-L1 stratifies clinical responders from nonresponders. | [78] |
| prospective cohort study | melanoma | Allele frequency-adjusted bTMB | ipilimumab | Increased exosomal PD-1 and CD28 on T-cells were correlated with longer PFS and OS. | [79] |
| Cancer Type | NLR Prognostic Value | PLR Prognostic Value | LMR Prognostic Value | Reference |
|---|---|---|---|---|
| Gastric Cancer (ICI) | Elevated NLR associated with poorer OS (HR = 2.01) and PFS (HR = 1.59) | Elevated PLR associated with poorer OS (HR = 1.57) and PFS (HR = 1.52) | Elevated LMR associated with improved OS (HR = 0.62) and PFS (HR = 0.69) | [18] |
| Melanoma (ICI) | Elevated NLR associated with poorer OS and PFS | Elevated PLR associated with poorer OS and PFS | Elevated LMR associated with improved OS and PFS | [19] |
| Head and Neck SCC | Elevated NLR identified as an independent negative prognostic factor for OS | PLR not specified as a significant prognostic factor | LMR not specified as a significant prognostic factor | [123] |
| Breast Cancer | Elevated NLR correlated with poorer DSS and DFS | PLR identified as an independent prognostic marker with superior predictive value for DSS and DFS compared to NLR and LMR | Lower LMR associated with poorer DSS and DFS | [124] |
| Pancreatic Cancer | Elevated NLR associated with worse OS | Elevated PLR correlated with greater tumor viability post-neoadjuvant chemotherapy | LMR not significant as a prognostic marker | [125] |
| Osteosarcoma | Elevated NLR significantly correlated with advanced disease stage and poorer prognosis | Elevated PLR significantly correlated with advanced disease stage and poorer prognosis | Lower LMR significantly correlated with advanced disease stage and poorer prognosis | [126] |
| Laryngeal Carcinoma | Elevated NLR associated with increased mortality | Elevated PLR associated with increased mortality | Lower LMR associated with better survival outcomes | [121] |
| Hilar Cholangiocarcinoma | NLR negatively correlated with CD3+ and CD8+ TILs; associated with poorer OS | PLR showed no correlation with TILs | Elevated LMR positively correlated with CD3+ TILs; identified as an independent prognostic factor for OS | [127] Początek formularza |
| Dół formularza |
| Cancer Type | Rheological Parameter(s) | Prognostic/Predictive Value | Reference |
| Gynecologic Cancers | Plasma viscosity and RBC aggregation | Elevated plasma viscosity is an independent prognostic marker for overall survival in breast and ovarian cancers; higher plasma viscosity correlates with increased risk of thrombosis and poorer survival outcomes. | [122] |
| Hepatocellular Carcinoma | Whole blood viscosity | Increased whole blood viscosity is associated with extrahepatic metastases and reduced survival, indicating its potential as a prognostic marker. | [52] |
| Colorectal Liver Metastases | Tissue stiffness (shear wave speed) and viscoelastic parameters (α and µ) | Higher tissue stiffness correlates with better histopathological response to chemotherapy; viscoelastic parameters can predict treatment response with high diagnostic accuracy (AUC > 0.8). | [128] |
| Multiple Myeloma | RBC aggregation index and deformability | Patients exhibit higher RBC aggregation and reduced deformability compared to healthy controls, which may contribute to disease progression and could serve as prognostic indicators. | [46] |
| Various Cancers (Pre/Post-Chemotherapy) | Hematocrit, ESR, plasma viscosity, and whole blood viscosity | Chemotherapy induces significant changes in rheological parameters; post-chemotherapy reductions in whole blood viscosity and hematocrit may reflect treatment response and impact prognosis. | [129] |
| Breast Cancer (Cellular Level) | Cytoplasmic viscosity | Lower cytoplasmic viscosity in highly metastatic breast cancer cells suggests its potential as a biomarker for metastatic potential and aggressiveness. | [130] |
| Colon Cancer | Tissue rheology (compressional stiffening and shear weakening) | Cancerous colon tissues exhibit distinct rheological properties compared to healthy tissues; these mechanical characteristics may serve as complementary diagnostic markers alongside histopathology. | [131] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczmarek, F.; Marcinkowska-Gapińska, A.; Bartkowiak-Wieczorek, J.; Nowak, M.; Kmiecik, M.; Brzezińska, K.; Dotka, M.; Brosz, P.; Firlej, W.; Wojtyła-Buciora, P. Blood-Based Biomarkers as Predictive and Prognostic Factors in Immunotherapy-Treated Patients with Solid Tumors—Currents and Perspectives. Cancers 2025, 17, 2001. https://doi.org/10.3390/cancers17122001
Kaczmarek F, Marcinkowska-Gapińska A, Bartkowiak-Wieczorek J, Nowak M, Kmiecik M, Brzezińska K, Dotka M, Brosz P, Firlej W, Wojtyła-Buciora P. Blood-Based Biomarkers as Predictive and Prognostic Factors in Immunotherapy-Treated Patients with Solid Tumors—Currents and Perspectives. Cancers. 2025; 17(12):2001. https://doi.org/10.3390/cancers17122001
Chicago/Turabian StyleKaczmarek, Franciszek, Anna Marcinkowska-Gapińska, Joanna Bartkowiak-Wieczorek, Michał Nowak, Michał Kmiecik, Kinga Brzezińska, Mariusz Dotka, Paweł Brosz, Wojciech Firlej, and Paulina Wojtyła-Buciora. 2025. "Blood-Based Biomarkers as Predictive and Prognostic Factors in Immunotherapy-Treated Patients with Solid Tumors—Currents and Perspectives" Cancers 17, no. 12: 2001. https://doi.org/10.3390/cancers17122001
APA StyleKaczmarek, F., Marcinkowska-Gapińska, A., Bartkowiak-Wieczorek, J., Nowak, M., Kmiecik, M., Brzezińska, K., Dotka, M., Brosz, P., Firlej, W., & Wojtyła-Buciora, P. (2025). Blood-Based Biomarkers as Predictive and Prognostic Factors in Immunotherapy-Treated Patients with Solid Tumors—Currents and Perspectives. Cancers, 17(12), 2001. https://doi.org/10.3390/cancers17122001








