As Radical as Technically Feasible—Surgical Treatment for Mobile Spine Chordoma
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
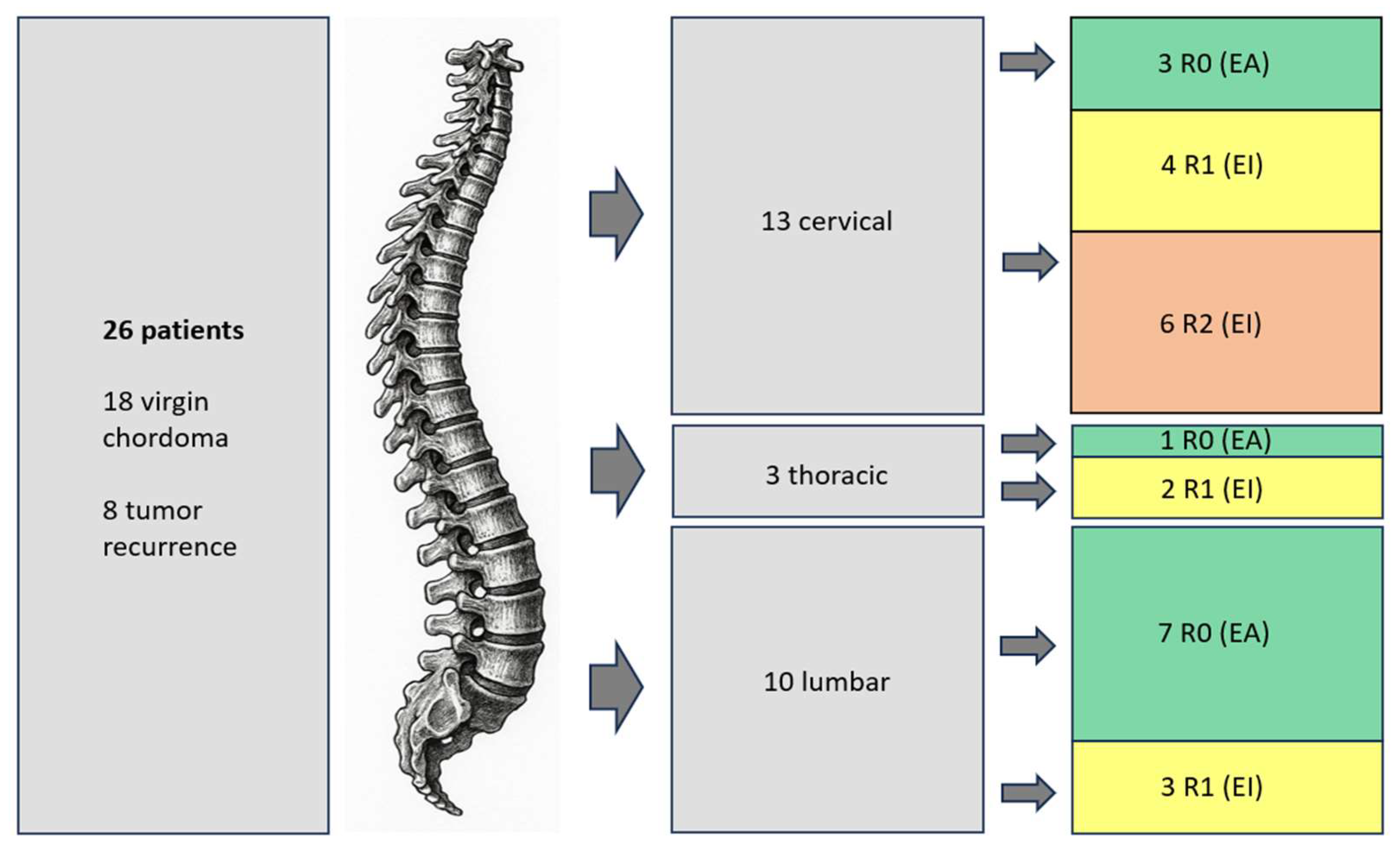
3.1. Clinical Characteristics
3.2. Treatment
- A young patient with an incidental finding of the tumor during routine imaging follow-up for familial cavernomatosis. Given the patient’s pre-existing condition and the minimal residual tumor burden on imaging, the multidisciplinary tumor board recommended carbon ion therapy in lieu of further surgery.
- A patient with a complicated postoperative course, including fulminant ventriculitis, following prior surgery abroad. Due to the clinical condition, re-resection was deemed unfeasible.
- Two patients had undergone prior surgery abroad and presented with distant metastases at the time of evaluation, precluding further aggressive surgical intervention.
- Postoperative imaging revealed residual bony tumor fragments, which were completely resected during a third surgical procedure.
- A preoperative balloon occlusion test yielded negative results, indicating that occlusion of the vertebral artery was not tolerated, thus rendering an en bloc resection technically impossible.
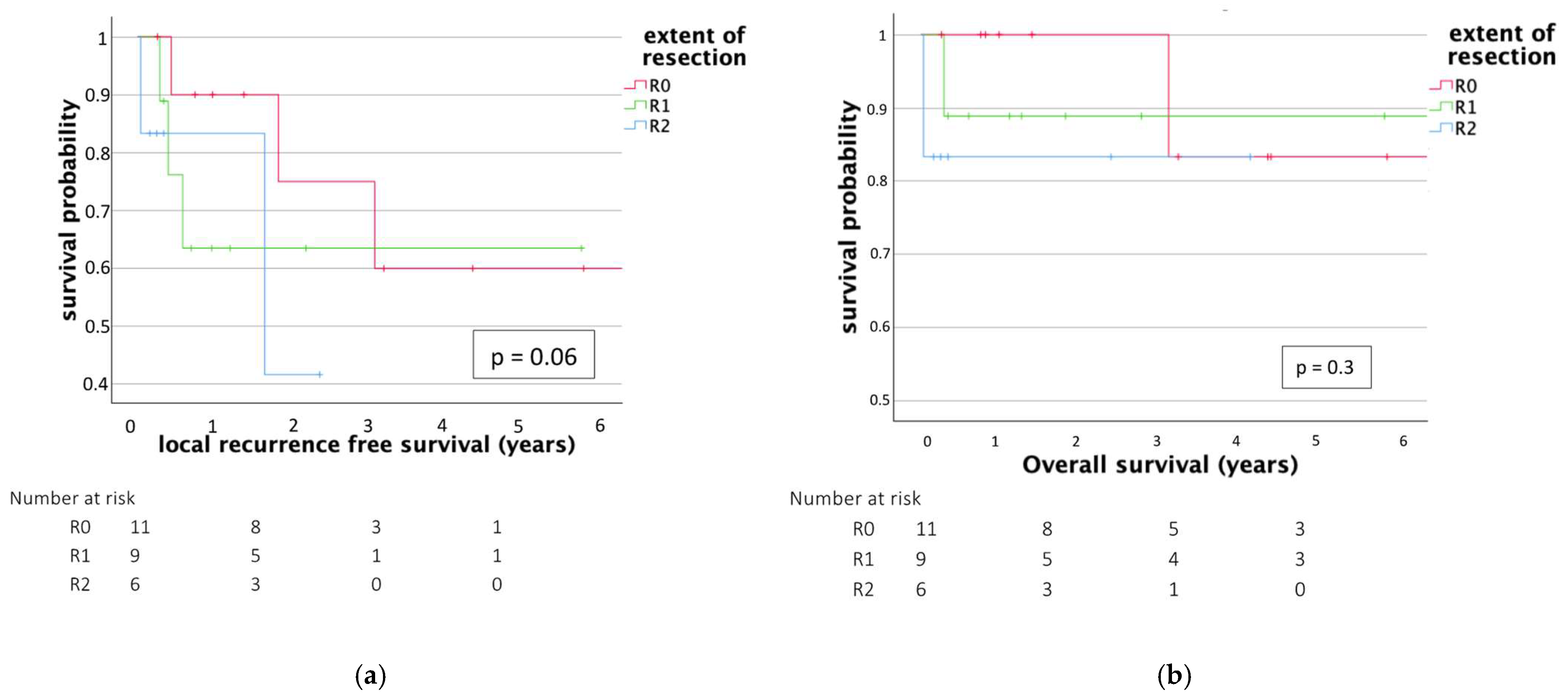
3.3. Outcome
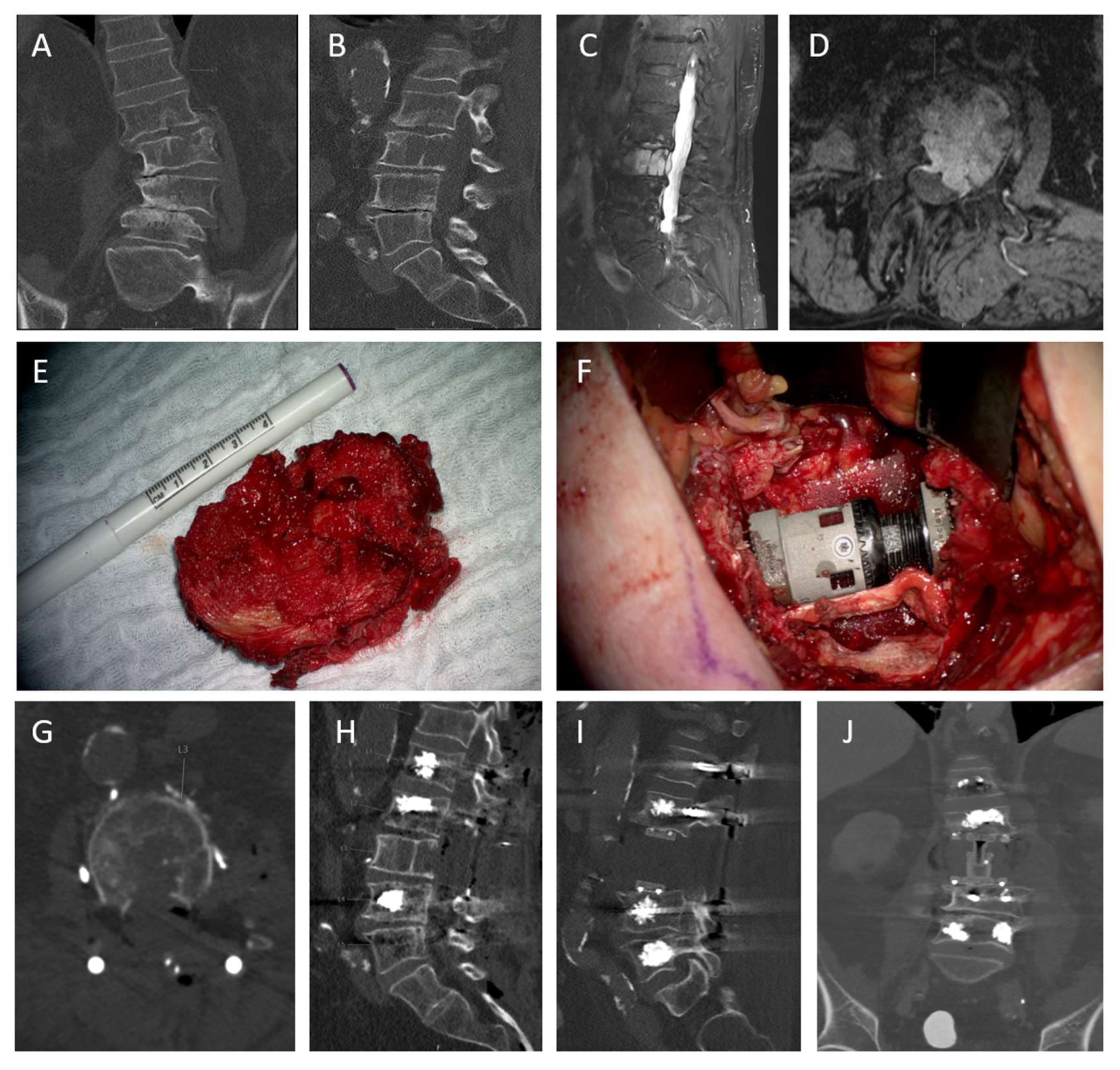
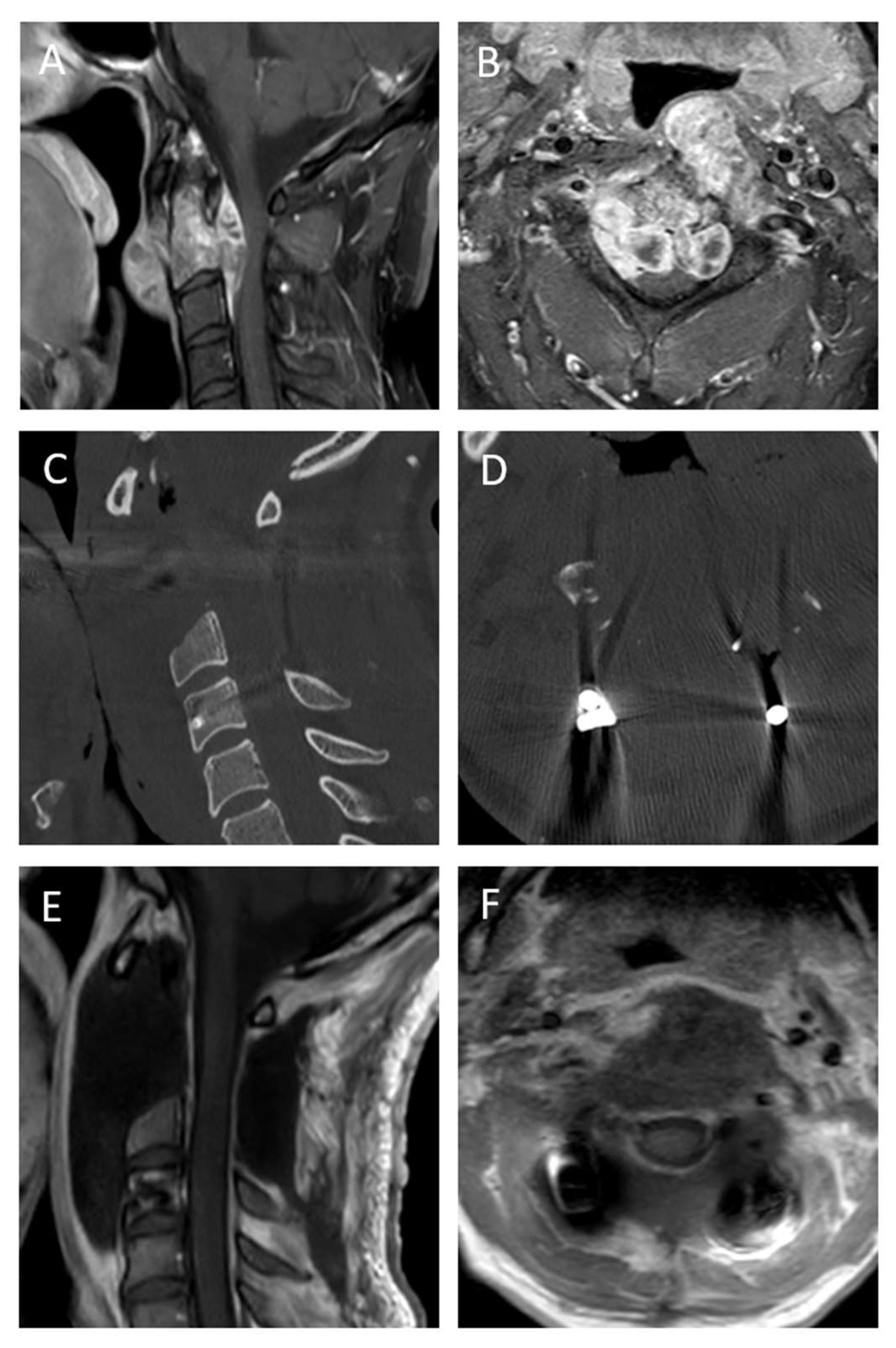
3.4. Case Descriptions
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barber, S.M.; Sadrameli, S.S.; Lee, J.J.; Fridley, J.S.; Teh, B.S.; Oyelese, A.A.; Telfeian, A.E.; Gokaslan, Z.L. Chordoma-Current Understanding and Modern Treatment Paradigms. J. Clin. Med. 2021, 10, 1054. [Google Scholar] [CrossRef] [PubMed]
- Enneking, W.F.; Spanier, S.S.; Goodman, M.A. A system for the surgical staging of musculoskeletal sarcoma. Clin. Orthop. 1980, 153, 106–120. [Google Scholar] [CrossRef]
- Meng, T.; Yin, H.; Li, B.; Li, Z.; Xu, W.; Zhou, W.; Cheng, M.; Wang, J.; Zhou, L.; Yang, X.; et al. Clinical features and prognostic factors of patients with chordoma in the spine: A retrospective analysis of 153 patients in a single center. Neuro-Oncology 2015, 17, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, S.; Sommer, J.; Chordoma Global Consensus Group. Building a global consensus approach to chordoma: A position paper from the medical and patient community. Lancet Oncol. 2015, 16, e71–e83. [Google Scholar] [CrossRef]
- De Robertis, M.; Ghermandi, R.; Pipola, V.; Griffoni, C.; Cianchetti, M.; Rotondi, M.; Asunis, E.; Tosini, G.; Cini, C.; Morenghi, E.; et al. Therapeutic strategies for mobile spine chordoma: En bloc Versus intralesional surgery with adjuvant charged-particle therapy. J. Neurooncol. 2025, 171, 229–240. [Google Scholar] [CrossRef]
- Lange, N.; Jörger, A.-K.; Ryang, Y.-M.; Liesche-Starnecker, F.; Gempt, J.; Meyer, B. Primary Bone Tumors of the Spine—Proposal for Treatment Based on a Single Centre Experience. Diagnostics 2022, 12, 2264. [Google Scholar] [CrossRef]
- Denaro, L.; Berton, A.; Ciuffreda, M.; Loppini, M.; Candela, V.; Brandi, M.L.; Longo, U.G. Surgical management of chordoma: A systematic review. J. Spinal Cord Med. 2020, 43, 797–812. [Google Scholar] [CrossRef]
- Lange, N.; Quiring, L.; Backhaus, P.; Eisenkolb, V.; Butenschoen, V.M.; Meyer, B. Two-staged en-bloc vertebrectomy for primary spinal bone tumors: A surgical strategy for optimal resection with low complication rates. Spine J. Off. J. N. Am. Spine Soc. 2025, submitted.
- Fourney, D.; DiPaola, C.; Fisher, C. A novel classification system for spinal instability in neoplastic disease: An evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine J. 2010, 35, E1221–E1229. [Google Scholar] [CrossRef]
- Boriani, S.; Weinstein, J.N.; Biagini, R. Primary bone tumors of the spine. Terminology and surgical staging. Spine 1997, 22, 1036–1044. [Google Scholar] [CrossRef]
- Jawad, M.U.; Scully, S.P. In brief: Classifications in brief: Enneking classification: Benign and malignant tumors of the musculoskeletal system. Clin. Orthop. 2010, 468, 2000–2002. [Google Scholar] [CrossRef]
- Choi, D.; Crockard, A.; Bunger, C.; Harms, J.; Kawahara, N.; Mazel, C.; Melcher, R.; Tomita, K. Review of metastatic spine tumour classification and indications for surgery: The consensus statement of the Global Spine Tumour Study Group. Eur. Spine J. 2010, 19, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.B.; Greene, F.L. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Krasovec, K.; Altawelbeh, G.; Quiring, A.L.; Meyer, B.; Lange, N. Solitary space-occupying lesion in L3 vertebra with en bloc resection, posterior instrumentation and vertebral body replacement. Cureus 2025. in revision. [Google Scholar]
- Fisher, C.G.; Saravanja, D.D.; Dvorak, M.F.; Rampersaud, Y.R.; Clarkson, P.W.; Hurlbert, J.; Fox, R.; Zhang, H.; Lewis, S.; Riaz, S. Surgical management of primary bone tumors of the spine: Validation of an approach to enhance cure and reduce local recurrence. Spine 2011, 36, 830–836. [Google Scholar] [CrossRef]
- Xiao, Y.; Yu, W.; Zheng, J.; Cheng, L.; Ding, X.; Qiao, L.; Wu, X.; Ma, J. Bibliometric Insights in Advances of Chordoma: Global Trends and Research Development in the Last Decade. Orthop. Surg. 2023, 15, 2505–2514. [Google Scholar] [CrossRef]
- Boriani, S.; Bandiera, S.; Biagini, R.; Bacchini, P.; Boriani, L.; Cappuccio, M.; Chevalley, F.; Gasbarrini, A.; Picci, P.; Weinstein, J.N.D. Chordoma of the mobile spine: Fifty years of experience. Spine 2006, 31, 493–503. [Google Scholar] [CrossRef]
- Kolz, J.M.; Wellings, E.P.; Houdek, M.T.; Clarke, M.J.; Yaszemski, M.J.; Rose, P.S. Surgical treatment of primary mobile spine chordoma. J. Surg. Oncol. 2021, 123, 1284–1291. [Google Scholar] [CrossRef]
- Gokaslan, Z.L.; Zadnik, P.L.; Sciubba, D.M.; Germscheid, N.; Goodwin, C.R.; Wolinsky, J.-P.; Bettegowda, C.; Groves, M.L.; Luzzati, A.; Rhines, L.D.; et al. Mobile spine chordoma: Results of 166 patients from the AOSpine Knowledge Forum Tumor database. J. Neurosurg. Spine 2016, 24, 644–651. [Google Scholar] [CrossRef]
- Akinduro, O.O.; Garcia, D.P.; Domingo, R.A.; Vivas-Buitrago, T.; Sousa-Pinto, B.; Bydon, M.; Clarke, M.J.; Gokaslan, Z.L.; Kalani, M.A.; Abode-Iyamah, K.; et al. Cervical chordomas: Multicenter case series and meta-analysis. J. Neurooncol. 2021, 153, 65–77. [Google Scholar] [CrossRef]
- Stacchiotti, S.; Gronchi, A.; Fossati, P.; Akiyama, T.; Alapetite, C.; Baumann, M.; Blay, J.Y.; Bolle, S.; Boriani, S.; Bruzzi, P.; et al. Best practices for the management of local-regional recurrent chordoma: A position paper by the Chordoma Global Consensus Group. Ann. Oncol. 2017, 28, 1230–1242. [Google Scholar] [CrossRef]
- Dong, M.; Liu, R.; Zhang, Q.; Wang, D.; Luo, H.; Wang, Y.; Chen, J.; Ou, Y.; Wang, X. Efficacy and safety of carbon ion radiotherapy for chordomas: A systematic review and meta-analysis. Radiat. Oncol. 2023, 18, 152. [Google Scholar] [CrossRef] [PubMed]
- Kolz, J.M.; Wellings, E.P.; Houdek, M.T.; Clarke, M.J.; Yaszemski, M.J.; Rose, P.S. Outcomes of Recurrent Mobile Spine Chordomas. J. Am. Acad. Orthop. Surg. 2023, 31, e278–e286. [Google Scholar] [CrossRef] [PubMed]
- Redmond, K.J.; Schaub, S.K.; Lo, S.-f.L.; Khan, M.; Lubelski, D.; Bilsky, M.; Yamada, Y.; Fehlings, M.; Gogineni, E.; Vajkoczy, P.; et al. Radiotherapy for Mobile Spine and Sacral Chordoma: A Critical Review and Practical Guide from the Spine Tumor Academy. Cancers 2023, 15, 2359. [Google Scholar] [CrossRef] [PubMed]
- Pennington, Z.; Ehresman, J.; Elsamadicy, A.A.; Shin, J.H.; Goodwin, C.R.; Schwab, J.H.; Sciubba, D.M. Systematic review of charged-particle therapy for chordomas and sarcomas of the mobile spine and sacrum. Neurosurg. Focus 2021, 50, E17. [Google Scholar] [CrossRef]
- de Araujo, A.O.; Narazaki, D.K.; Teixeira, W.G.J.; Ghilardi, C.S.; Araujo, P.H.X.N.; Zerati, A.E.; Marcon, R.M.; Cristante, A.F.; Barros Filho, T.E.P. En bloc vertebrectomy for the treatment of spinal lesions. Five years of experience in a single institution: A case series. Clinics 2018, 73, e95. [Google Scholar] [CrossRef]
- Molina, C.A.; Ames, C.P.; Chou, D.; Rhines, L.D.; Hsieh, P.C.; Zadnik, P.L.; Wolinsky, J.-P.; Gokaslan, Z.L.; Sciubba, D.M. Outcomes following attempted en bloc resection of cervical chordomas in the C-1 and C-2 region versus the subaxial region: A multiinstitutional experience. J. Neurosurg. Spine 2014, 21, 348–356. [Google Scholar] [CrossRef]
- Xia, Y.; Papali, P.; Al-Mistarehi, A.-H.; Hansen, L.J.; Azad, T.D.; Ahmed, A.K.; Meyer, C.; Gross, J.; Khan, M.; Bettegowda, C.; et al. Outcomes After Definitive Surgery for Spinal and Sacral Chordoma in 101 Patients Over 20 Years. Neurosurgery 2025, 96, 494–504. [Google Scholar] [CrossRef]
| Characteristic | Enneking-Appropriate Resection EA | Enneking-Inappropriate Resection EI | p-Value |
|---|---|---|---|
| Number of patients | 11 | 15 | |
| Sex (male) | 8 (72.7%) | 11 (73.3%) | 0.87 |
| Age (mean, range) | 64 (41–82) | 51 (16–88) | 0.81 |
| CCI (mean, range) | 4.63 (2–7) | 5.07 (2–19) | 0.71 |
| BMI (mean, range) | 28.41 (22–45.2) | 26.97 (22.2–37) | 0.93 |
| Tumor size (mean, range) | 43.4 mm (20–94) | 38.9 mm (18–83) | 0.53 |
| Previous RTX | 4 (36.2%) | 4 (26.6%) | 0.61 |
| LOH (days) (mean, range) | 20 (8–57) | 21 (5–60) | 0.9 |
| Blood loss (mean) | 4690 mL | 1775 mL | 0.027 |
| Surgery duration (mean) | 467 min | 312 min | 0.078 |
| Postoperative radiotherapy | 8 (72.7%) | 9 (60%) | 0.62 |
| Time to radiotherapy (days, mean) | 28 | 40 | 0.49 |
| LRFS (days) (mean, range) | 1071 (96–3670) | 422 (13–2119) | 0.06 |
| OS (days) (mean, range) | 1238 (96–4460) | 910 (13–5869) | 0.56 |
| Patient Characteristics | Surgery | Follow Up | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pat. ID | Age | Sex | Localisation | CCI | BMI | KPS | SINS Score | Enneking | Previous Therapies | Date of Surgery | Resection Margins | Surgery Time min | Blood Loss mL | Number of Surgeries | LOH Days | Short-Term Complications | Length Off Follow Up Days | Long Term Complications | Adjuvant Radio- Therapy | KPS At Last Follow Up | Death | Recurrent Tumor |
| 1 | 26 | male | cervical | 2 | 24.1 | 90 | 8 | 1B | surgery, radiotherapy | 6 June 2008 | R1 | 172 | 900 | 1 | 5 | - | 220 | - | 0 | 100 | - | 1 |
| 2 | 45 | male | cervical | 2 | 22.2 | 90 | 7 | 1B | - | 13 May 2009 | R1 | 73 | 650 | 2 | 7 | - | 5869 | - | 1 | 100 | - | - |
| 3 | 25 | male | cervical | 2 | 27.8 | 60 | 8 | 1B | - | 28 November 2011 | R2 | 175 | 700 | 1 | 24 | - | 92 | - | 0 | 70 | - | - |
| 4 | 62 | male | lumbar | 4 | 25.5 | 90 | 11 | 1B | - | 22 March 2013 | R0 | 148 | 500 | 2 | 8 | - | 4460 | - | 1 | 100 | - | - |
| 5 | 64 | male | thoracic | 4 | 37.9 | 50 | 13 | 2B | - | 1 February 2014 | R0 | 608 | 3200 | 2 | 57 | - | 1133 | hardware failure | 1 | 0 | + | - |
| 6 | 44 | female | cervical | 5 | 34.4 | 90 | 8 | 1A | surgery | 17 March 2014 | R2 | 102 | 600 | 2 | 21 | - | 126 | - | 1 | 100 | - | - |
| 7 | 55 | female | lumbar | 3 | 22.7 | 80 | 7 | 2B | radiotherapy | 30 November 2015 | R0 | 224 | 4000 | 4 | 17 | - | 1584 | - | 1 | 90 | - | 1 |
| 8 | 65 | male | cervical | 4 | 26.4 | 80 | 11 | 3 | surgery, radiotherapy | 5 December 2016 | R1 | 114 | 200 | 2 | 23 | - | 462 | - | 0 | 80 | - | 1 |
| 9 | 73 | male | lumbar | 6 | 23.7 | 80 | 11 | 2A | - | 24 May 2018 | R0 | 446 | 10,000 | 2 | 30 | - | 295 | - | 0 | 70 | - | 1 |
| 10 | 64 | male | thoracic | 4 | 26.1 | 80 | 14 | 2B | surgery | 26 March 2019 | R1 | 524 | 3000 | 2 | 17 | - | 2119 | - | 1 | 40 | - | - |
| 11 | 41 | male | lumbar | 2 | 27.2 | 80 | 10 | 2B | neoadjuvant radiotherapy | 16 July 2019 | R0 | 549 | 1000 | 2 | 9 | - | 1598 | hardware failure | 0 | 80 | - | - |
| 12 | 71 | female | cervical | 5 | 27.7 | 80 | 6 | 2B | - | 7 August 2019 | R0 | 350 | 5000 | 3 | 10 | - | 2131 | - | 1 | 90 | - | - |
| 13 | 42 | male | cervical | 4 | 24.5 | 80 | 11 | 2B | - | 30 October 2020 | R2 | 339 | 5000 | 2 | 13 | - | 1504 | - | 1 | 90 | - | 1 |
| 14 | 81 | female | thoracic | 15 | 24.2 | 60 | 6 | 2B | - | 29 July 2021 | R1 | 277 | 800 | 3 | 24 | wound | 108 | - | 1 | 0 | + | - |
| 15 | 67 | female | cervical | 4 | 45.2 | 90 | 8 | 2B | - | 17 March 2022 | R0 | 86 | 200 | 2 | 9 | - | 1178 | hardware failure | 1 | 100 | - | - |
| 16 | 73 | male | lumbar | 6 | 30.9 | 80 | 8 | 3 | surgery, radiotherapy | 25 May 2022 | R1 | 538 | 1900 | 2 | 11 | - | 124 | hardware failure | 0 | 80 | - | - |
| 17 | 43 | male | cervical | 4 | 31.1 | 40 | 9 | 3 | surgery | 17 June 2022 | R2 | 243 | 3300 | 2 | 60 | - | 60 | - | 0 | 10 | - | - |
| 18 | 31 | male | cervical | 2 | 23.8 | 100 | 7 | 2A | neoadjuvant radiotherapy | 1 September 2022 | R1 | 450 | 1100 | 3 | 10 | - | 1010 | hardware failure | 0 | 100 | - | - |
| 19 | 56 | female | cervical | 4 | 25.7 | 90 | 7 | 2B | - | 4 October 2022 | R2 | 490 | 1900 | 2 | 22 | pulm. Embolism | 869 | - | 1 | 70 | - | - |
| 20 | 16 | male | cervical | 6 | 22.5 | 100 | 9 | 3 | surgery, radiotherapy | 4 July 2023 | R2 | 250 | 180 | 2 | 13 | - | 13 | - | 0 | 0 | + | - |
| 21 | 66 | male | lumbar | 9 | 37.0 | 80 | 8 | 2B | surgery | 14 August 2023 | R1 | 377 | 3500 | 2 | 7 | cage malposition | 663 | 1 | 80 | - | - | |
| 22 | 53 | male | lumbar | 3 | 26.8 | 90 | 11 | 2B | radiotherapy | 15 January 2024 | R0 | 585 | 3700 | 2 | 9 | - | 509 | hardware failure | 1 | 90 | - | - |
| 23 | 88 | female | lumbar | 7 | 23.9 | 100 | 9 | 3 | - | 26 April 2024 | R1 | 557 | 2900 | 2 | 58 | wound | 407 | - | 1 | 100 | - | - |
| 24 | 62 | male | cervical | 7 | 22.0 | 80 | 10 | 2B | surgery, radiotherapy | 14 June 2024 | R0 | 826 | 15,000 | 2 | 36 | meningitis | 358 | - | 1 | 60 | - | - |
| 25 | 75 | male | lumbar | 6 | 28.4 | 80 | 8 | 2B | - | 5 September 2024 | R0 | 411 | 2000 | 2 | 26 | - | 275 | - | 1 | 90 | - | - |
| 26 | 82 | male | lumbar | 7 | 25.4 | 80 | 11 | 2B | - | 3 March 2025 | R0 | 912 | 7000 | 2 | 11 | - | 96 | - | 1 | 80 | - | - |
| Risk Factors | OR (95% CI) | p | HR (95% CI) | p |
|---|---|---|---|---|
| Sex (Male) | 0.37 (0.01–23.66) | 0.64 | 0.46 (0.03–22.78) | 0.59 |
| Age | 1.01 (0.92–1.09) | 0.87 | 1.02 (0.89–1.13) | 0.76 |
| SINS score | 0.87 (0.43–1.74) | 1.00 | 0.84 (0.56–1.17) | 0.82 |
| Tumor size | 0.95 (0.85–1.05) | 0.28 | 0.97 (0.88–1.07) | 0.34 |
| Postoperative radiotherapy | 1.5 (0.03–67.42) | 0.83 | 1.3 (0.08–52.34) | 0.56 |
| En bloc resection | 2.32 (0.36–15.06) | 0.38 | 1.9 (0.55–14.07) | 0.34 |
| Carbon fiber-only hardware | 4.26 (0.31–59.11) | 0.28 | 3.89 (0.47–49.22) | 0.54 |
| Surgery duration | 1.01 (0.99–1.03) | 0.09 | 1.24 (0.97–1.39) | 0.37 |
| Blood loss | 0.99 (0.98–1.00) | 0.18 | 0.97 (0.94–1.01) | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiring, A.L.; Sarkis, H.; Backhaus, P.; von Oldershausen, M.; Meyer, B.; Lange, N. As Radical as Technically Feasible—Surgical Treatment for Mobile Spine Chordoma. Cancers 2025, 17, 1989. https://doi.org/10.3390/cancers17121989
Quiring AL, Sarkis H, Backhaus P, von Oldershausen M, Meyer B, Lange N. As Radical as Technically Feasible—Surgical Treatment for Mobile Spine Chordoma. Cancers. 2025; 17(12):1989. https://doi.org/10.3390/cancers17121989
Chicago/Turabian StyleQuiring, Alexander Lars, Hraq Sarkis, Paul Backhaus, Maximilian von Oldershausen, Bernhard Meyer, and Nicole Lange. 2025. "As Radical as Technically Feasible—Surgical Treatment for Mobile Spine Chordoma" Cancers 17, no. 12: 1989. https://doi.org/10.3390/cancers17121989
APA StyleQuiring, A. L., Sarkis, H., Backhaus, P., von Oldershausen, M., Meyer, B., & Lange, N. (2025). As Radical as Technically Feasible—Surgical Treatment for Mobile Spine Chordoma. Cancers, 17(12), 1989. https://doi.org/10.3390/cancers17121989





