Application of the Deep Inspiration Breath-Hold Technique in Proton Therapy for Mediastinal Lymphomas: Initial Experience
Simple Summary
Abstract
1. Introduction
1.1. Proton Therapy for Mediastinal Tumors
1.2. Epidemiological Background
2. Materials and Methods
2.1. Patients Data
2.2. Equipment for Treating Moving Targets/Mediastinal Tumors at CCB
2.3. Patient and Treatment Plan Preparation
2.3.1. Immobilization
2.3.2. Breath-Hold Training and CT with Surface Imaging
2.3.3. Contouring
2.3.4. Treatment Planning
2.3.5. Proton Irradiation with Breath Holding
2.4. Study I Design—DIBH vs. FB Dose Comparison
Statistical Analysis
2.5. Study II Design—Breath-Hold Duration and Patient Position Variability
Statistical Analysis
3. Results
3.1. Study I: FB vs. DIBH Treatment Plan Comparison
3.2. Study II: Verification of Inter-Fraction Breath-Hold Variability
3.2.1. Breath-Hold Duration and Frequency per Radiation Field
3.2.2. Patient Position Variability
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABVD | adriamycin + bleomycin + vinblastine + dacarbazine |
| BEACOPP | bleomycin + etoposide + doxorubicin + cyclophosphamide + vincristine + procarbazine + prednisolone |
| BH | breath -hold |
| BX | biopsy |
| CAR-T | chimeric antigen receptor T-cell therapy |
| CCB | Cyclotron Centre Bronowice |
| CT | computed tomography |
| CTDIvol | computed tomography dose index volume |
| CTV | clinical target volume |
| DIBH | deep inspiration breath hold |
| DRR | digitally reconstructed radiograph |
| DVH | dose-volume histogram |
| ESHAP | etoposide + methylprednisolone + cytarabine + cisplatin |
| FB | free breathing |
| GTV | gross tumor volume |
| HL | Hodgkin lymphoma |
| IBA | ion beam application |
| ITV | internal tumor volume |
| kV | kilovoltage |
| NS | nodular sclerosis |
| OAR | organ at risk |
| PBS | pencil beam scanning |
| PMBCL | primary mediastinal B-Cell lymphoma |
| PT | proton therapy |
| PTV | planning target volume |
| RS | range shifter |
| SGRT | surface-guided radiotherapy |
| TPS | treatment planning system |
| UBTI | universal beam triggering interface |
| UTU | universal trigger unit |
Appendix A
| Parameter | Value |
|---|---|
| Scanning technique | Axial |
| Scanning direction | Caudo-cranial |
| Tube voltage (kV) | 120 (full-dose), 70 (low-dose) |
| Tube current-time product (mAs) | CareDose4D (qual.ref. 190 mAs) |
| Slice (increment) | 2 mm(2 mm) acq. 64 × 0.6 mm |
| Feed | 17 mm |
| Scan time | 0.36 s (quick) |
| Cycle time | 1.25 s |
| Delay | 2 s |
| Field of view (FOV) | 500 mm |
| Reconstruction kernel | B30s medium smooth |
Appendix B

| DVH Parameter | Goal | #1 | #2 | #3 | #4 | #5 | #6 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DIBH | FB | delta | DIBH | FB | delta | DIBH | FB | delta | DIBH | FB | delta | DIBH | FB | delta | DIBH | FB | delta | ||
| D98% ITV/CTV [%] | >95% | 97.28 | 97.28 | 0.00 | 97.02 | 97.05 | 0.03 | 97.60 | 97.60 | 0.00 | 97.75 | 97.79 | 0.04 | 98.10 | 98.10 | 0.00 | 96.40 | 96.40 | 0.00 |
| D98% PTV [%] | >95% | 100.00 | 99.94 | −0.06 | 96.75 | 95.94 | −0.81 | 97.66 | 97.50 | −0.16 | 97.78 | 97.51 | −0.27 | 97.88 | 96.91 | −0.97 | 96.53 | 95.89 | −0.64 |
| D2% PTV [%] | <105% | 104.21 | 101.42 | −2.79 | 105.94 | 104.75 | −1.19 | 104.24 | 102.88 | −1.36 | 105.52 | 103.81 | −1.71 | 103.60 | 103.97 | 0.37 | 105.63 | 101.96 | −3.67 |
| D̅Heart [Gy(RBE)] | <5 Gy * | 3.74 | 6.15 | 2.41 | 3.33 | 4.90 | 1.57 | 0.34 | 1.59 | 1.25 | 11.29 | 18.31 | 7.02 | 4.86 | 5.06 | 0.20 | 5.70 | 8.10 | 2.40 |
| D̅Lungs [Gy(RBE)] | <10 Gy ** | 5.23 | 5.97 | 0.74 | 4.86 | 5.69 | 0.83 | 4.34 | 5.11 | 0.77 | 13.68 | 13.54 | −0.14 | 8.06 | 7.90 | −0.16 | 5.97 | 6.61 | 0.64 |
| V5GyLungs [%] | <55% | 27.21 | 28.70 | 1.49 | 25.98 | 27.80 | 1.82 | 15.66 | 18.69 | 3.03 | 50.78 | 61.82 | 11.04 | 37.88 | 34.89 | −2.99 | 29.40 | 33.50 | 4.10 |
| D̅Breast(L) [Gy(RBE)] | <3 Gy | - | - | - | - | - | - | - | - | - | 4.08 | 4.65 | 0.57 | 1.76 | 1.79 | 0.03 | 1.55 | 2.16 | 0.60 |
| D̅Breast(R) [Gy(RBE)] | <3 Gy | - | - | - | - | - | - | - | - | - | 2.55 | 2.20 | −0.35 | 1.79 | 1.19 | −0.61 | 1.71 | 1.71 | 0 |
Appendix C
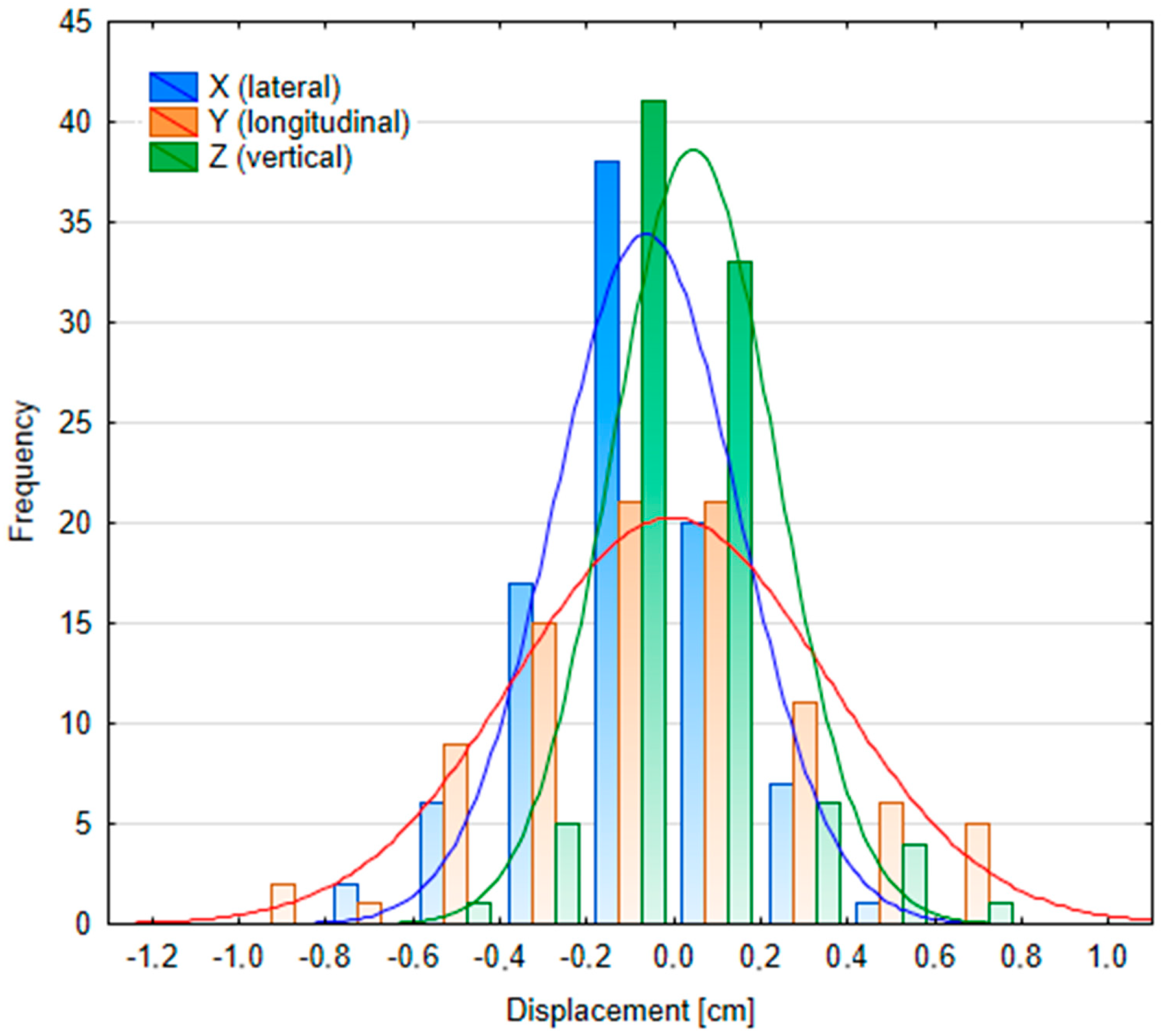
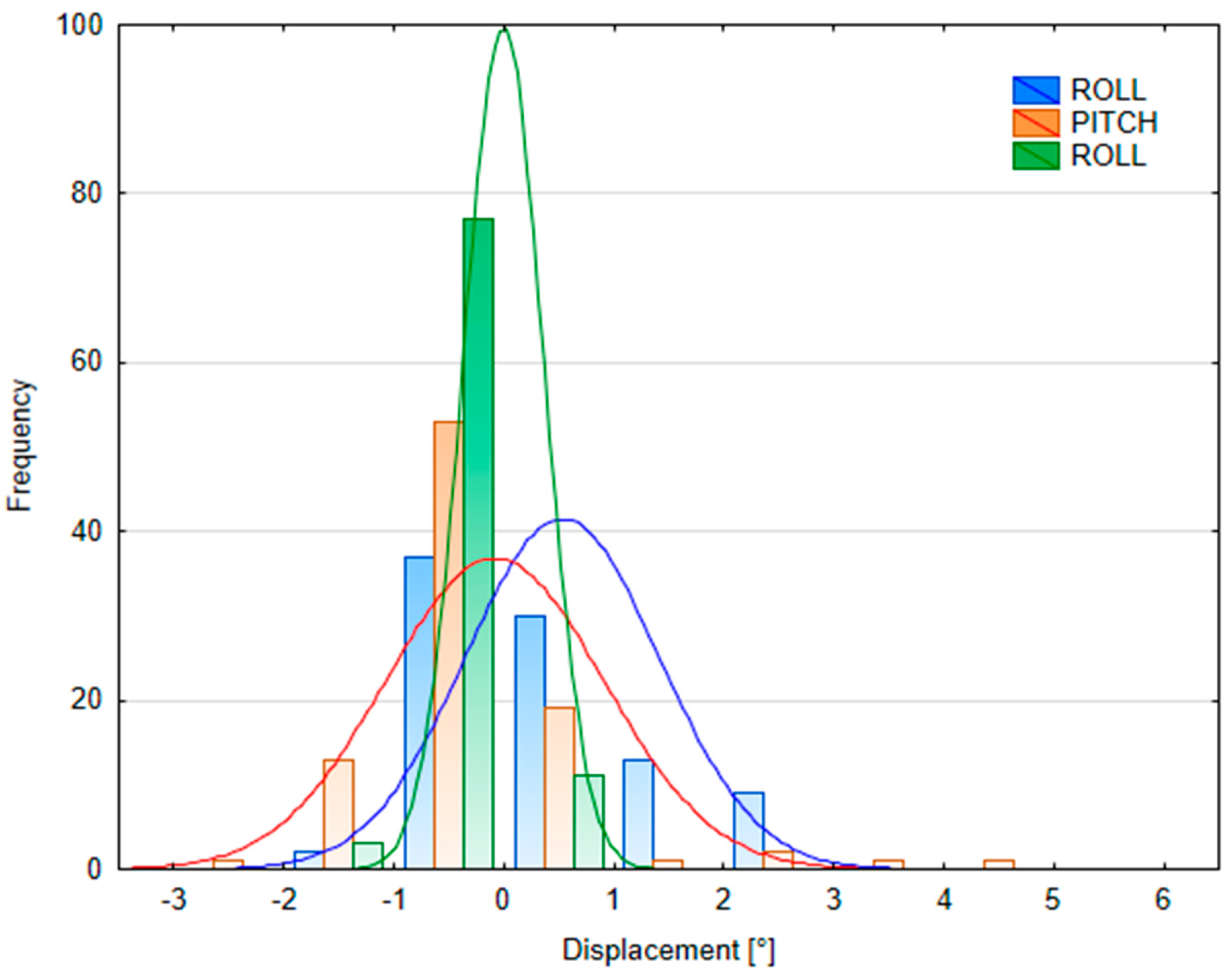
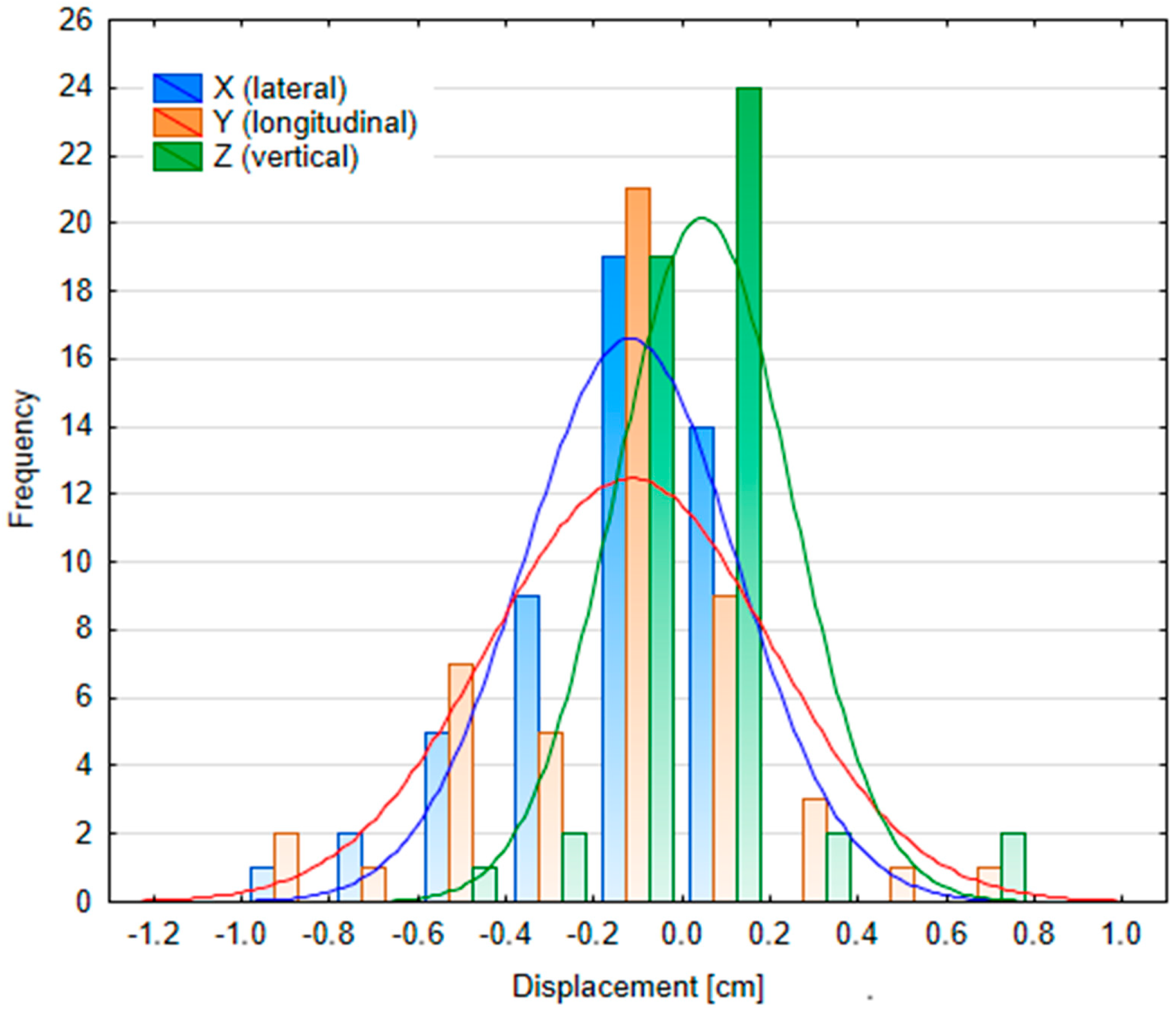
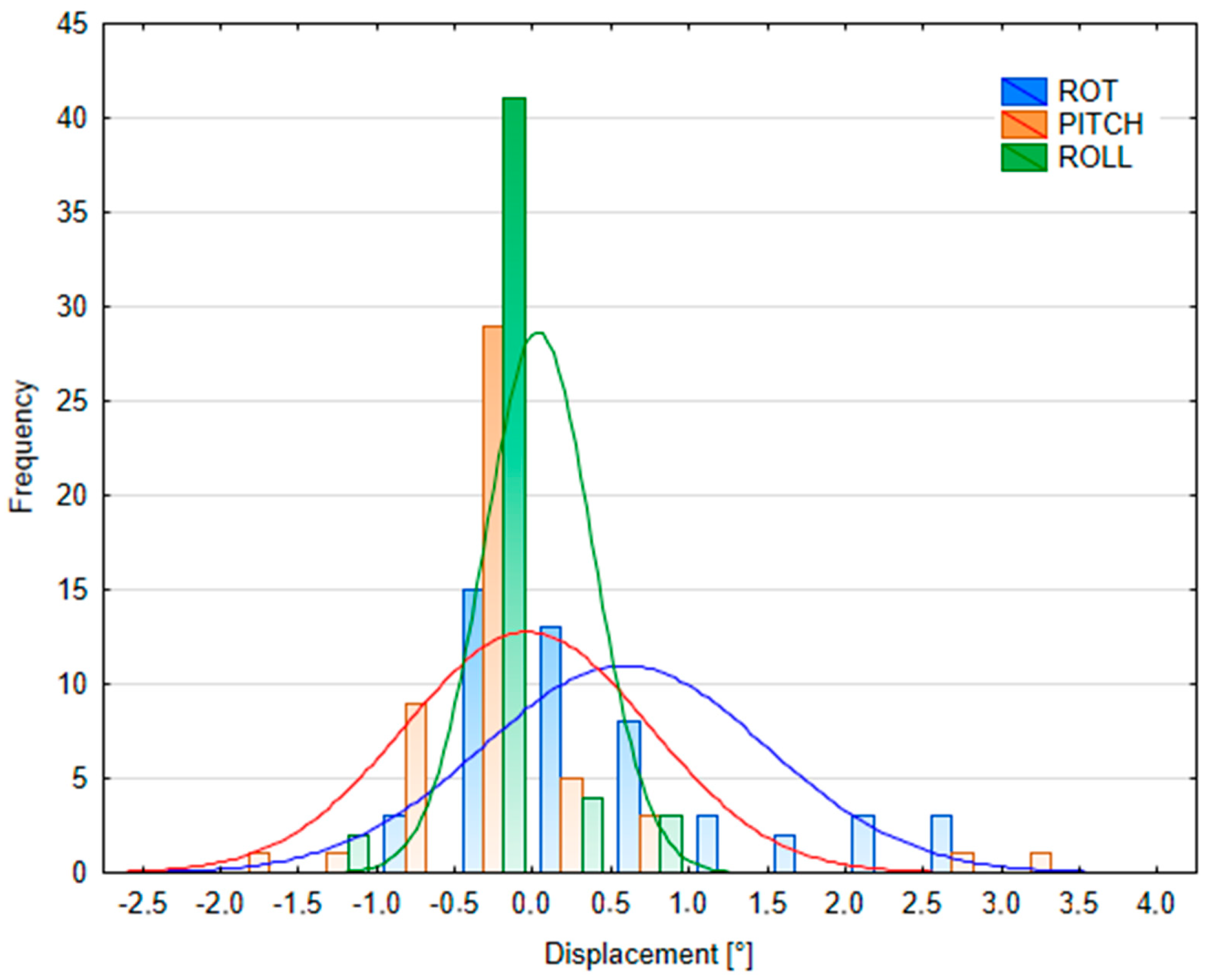
Appendix D
| Characteristic | Median: Male (n = 3) | Median: Female (n = 3) | Z | p-Value |
|---|---|---|---|---|
| Age [years] | 40 [38; 40] | 26 [25; 35] | Z = 13.72 | p < 0.05 |
| Height [m] | 1.69 [1.69; 1.73] | 1.65 [1.62; 1.70] | Z = 10.60 | p < 0.05 |
| Weight [kg] | 65 [65; 71.5] | 63 [59; 93.9] | Z = 7.24 | p < 0.05 |
| BMI | 22.76 [22.76; 23.36] | 23.14 [22.48; 32.47] | Z = −0.43 | p = 0.66 |
References
- Chen, Z.; Dominello, M.M.; Joiner, M.C.; Burmeister, J.W. Proton versus photon radiation therapy: A clinical review. Front. Oncol. 2023, 13, 1133909. [Google Scholar] [CrossRef] [PubMed]
- Loap, P.; Goudjil, F.; Dendale, R.; Kirova, Y. Clinical and technical considerations for mediastinal Hodgkin lymphoma protontherapy based on a single-center early experience. Cancer Radiother. 2021, 25, 779–785. [Google Scholar] [CrossRef]
- Ricardi, U.; Maraldo, M.V.; Levis, M.; Parikh, R.R. Proton Therapy for Lymphomas: Current State of the Art. OncoTargets Ther. 2019, 12, 8033–8046. [Google Scholar] [CrossRef]
- Lideståhl, A.; Mondlane, G.; Gubanski, M.; Lind, P.A.; Siegbahn, A. An in silico planning study comparing doses and estimated risk of toxicity in 3D-CRT, IMRT and proton beam therapy of patients with thymic tumours. Phys. Medica 2019, 60, 120–126. [Google Scholar] [CrossRef]
- Houlihan, O.A.; Rangaswamy, G.; Dunne, M.; Rohan, C.; O’Neill, L.; Chalke, S.; Daly, P.; Gillham, C.; McArdle, O. Deep inspiration breath hold versus free breathing technique in mediastinal radiotherapy for lymphoma. BJR Open 2021, 3, 20200067. [Google Scholar] [CrossRef] [PubMed]
- Korreman, S.S.; Pedersen, A.N.; Nøttrup, T.J.; Specht, L.; Nyström, H. Breathing adapted radiotherapy for breast cancer: Comparison of free breathing gating with the breath-hold technique. Radiother. Oncol. 2005, 76, 311–318. [Google Scholar] [CrossRef]
- Righetto, R.; Fracchiolla, F.; Widesott, L.; Lorentini, S.; Dionisi, F.; Rombi, B.; Scartoni, D.; Vennarini, S.; Schwarz, M.; Farace, P. Technical challenges in the treatment of mediastinal lymphomas by proton pencil beam scanning and deep inspiration breath-hold. Radiother. Oncol. 2022, 169, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Wojciechowska, U.; Barańska, K.; Michałek, I.; Olasek, P.; Miklewska, M.; Didkowska, J.A. Nowotwory Złośliwe w Polsce w 2020 Roku; Report No.: 0867-8251; Krajowy Rejestr Nowotworów: Warsaw, Poland, 2022. [Google Scholar]
- Zaucha Jan Maciej Ewa Paszkiewicz-Kozik Monika Prochorec-Sobieszek “Chłoniak Hodgkina” w “Hematoonkologia Tom 2 Nowotwory układu chłonnego” Wydanie, I. ISBN 978-83-67263-49-8. Via Medica, Gdańsk. 2022.
- Robak, T.; Walewski, J.; Romejko-Jarosińska, J.; Medica, V. Hematoonkologia: T.1; Narodowy Instytut Onkologii: Warsaw, Poland, 2022. [Google Scholar]
- National Comprehensive Cancer Network® (NCCN®). NCCN Guidelines for Patients® Hodgkin Lymphoma; National Comprehensive Cancer Network® (NCCN®): Plymouth Meeting, PA, USA, 2019. [Google Scholar]
- Aznar, M.C.; Corradini, S.; Mast, M.; McNair, H.; Meattini, I.; Persson, G.; van Haaren, P. ESTRO-ACROP guideline: Recommendations on implementation of breath-hold techniques in radiotherapy. Radiother. Oncol. 2023, 185, 109734. [Google Scholar] [CrossRef]
- Wiant, D.; Liu, H.; Hayes, T.L.; Shang, Q.; Mutic, S.; Sintay, B. Direct comparison between surface imaging and orthogonal radiographic imaging for SRS localization in phantom. J. Appl. Clin. Med. Phys. 2019, 20, 137–144. [Google Scholar] [CrossRef]
- Fiandra, C.; Filippi, A.R.; Catuzzo, P.; Botticella, A.; Ciammella, P.; Franco, P.; Borca, V.C.; Ragona, R.; Tofani, S.; Ricardi, U. Different IMRT solutions vs. 3D-conformal radiotherapy in early stage Hodgkin’s Lymphoma: Dosimetric comparison and clinical considerations. Radiat. Oncol. 2012, 7, 186. [Google Scholar] [CrossRef]
- Hoppe, B.S.; Mendenhall, N.P.; Louis, D.; Li, Z.; Flampouri, S. Comparing Breath Hold and Free Breathing During Intensity-Modulated Radiation Therapy and Proton Therapy in Patients with Mediastinal Hodgkin Lymphoma. Int. J. Part. Ther. 2017, 3, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Baues, C.; Marnitz, S.; Engert, A.; Baus, W.; Jablonska, K.; Fogliata, A.; Vásquez-Torres, A.; Scorsetti, M.; Cozzi, L. Proton versus photon deep inspiration breath hold technique in patients with hodgkin lymphoma and mediastinal radiation: A Planning Comparison of Deep Inspiration Breath Hold Intensity Modulation Radiotherapy and Intensity Modulated Proton Therapy. Radiat. Oncol. 2018, 13, 122. [Google Scholar] [CrossRef] [PubMed]
- Rechner, L.A.; Maraldo, M.V.; Vogelius, I.R.; Zhu, X.R.; Dabaja, B.S.; Brodin, N.P.; Petersen, P.M.; Specht, L.; Aznar, M.C. Life years lost attributable to late effects after radiotherapy for early stage Hodgkin lymphoma: The impact of proton therapy and/or deep inspiration breath hold. Radiother. Oncol. 2017, 125, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.Y.; Zhang, X.; Knopf, A.; Li, H.; Mori, S.; Dong, L.; Lu, H.M.; Liu, W.; Badiyan, S.N.; Both, S.; et al. Consensus Guidelines for Implementing Pencil-Beam Scanning Proton Therapy for Thoracic Malignancies on Behalf of the PTCOG Thoracic and Lymphoma Subcommittee. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 41–50. [Google Scholar] [CrossRef]
- Gelover, E.; Deisher, A.J.; Herman, M.G.; Johnson, J.E.; Kruse, J.J.; Tryggestad, E.J. Clinical implementation of respiratory-gated spot-scanning proton therapy: An efficiency analysis of active motion management. J. Appl. Clin. Med. Phys. 2019, 20, 99–108. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Box, J.F. Gosset, Fisher, and the t Distribution. Am. Stat. 1981, 35, 61. [Google Scholar] [CrossRef]
- Wilcoxon, F. Individual Comparisons by Ranking Methods. Biom. Bull. 1945, 1, 80–83. [Google Scholar] [CrossRef]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat. Methods 2020, 17, 261–272. [Google Scholar] [CrossRef]
- Andersson, K.M.; Edvardsson, A.; Hall, A.; Enmark, M.; Kristensen, I. Pencil beam scanning proton therapy of Hodgkin’s lymphoma in deep inspiration breath-hold: A case series report. Tech. Innov. Patient Support Radiat. Oncol. 2020, 13, 6–10. [Google Scholar] [CrossRef][Green Version]
- Mann, H.B.; Whitney, D.R. On a Test of Whether one of Two Random Variables is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Patel, C.G.; Peterson, J.; Aznar, M.; Tseng, Y.D.; Lester, S.; Pafundi, D.; Flampouri, S.; Mohindra, P.; Parikh, R.R.; Vega, R.M.; et al. Systematic review for deep inspiration breath hold in proton therapy for mediastinal lymphoma: A PTCOG Lymphoma Subcommittee report and recommendations. Radiother. Oncol. 2022, 177, 21–32. [Google Scholar] [CrossRef]
- Tseng, Y.D.; Cutter, D.J.; Plastaras, J.P.; Parikh, R.R.; Cahlon, O.; Chuong, M.D.; Dedeckova, K.; Khan, M.K.; Lin, S.Y.; McGee, L.A.; et al. Evidence-Based Review on the Use of Proton Therapy in Lymphoma from the Particle Therapy Cooperative Group (PTCOG) Lymphoma Subcommittee. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 825–842. [Google Scholar] [CrossRef] [PubMed]
- Edvardsson, A.; Kügele, M.; Alkner, S.; Enmark, M.; Nilsson, J.; Kristensen, I.; Kjellén, E.; Engelholm, S.; Ceberg, S. Comparative treatment planning study for mediastinal Hodgkin’s lymphoma: Impact on normal tissue dose using deep inspiration breath hold proton and photon therapy. Acta Oncol. 2019, 58, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.D.; Maes, S.M.; Kicska, G.; Sponsellor, P.; Traneus, E.; Wong, T.; Stewart, R.D.; Saini, J. Comparative photon and proton dosimetry for patients with mediastinal lymphoma in the era of Monte Carlo treatment planning and variable relative biological effectiveness. Radiat. Oncol. 2019, 14, 243. [Google Scholar] [CrossRef]
- Nguyen, D.; Reinoso, R.; Farah, J.; Yossi, S.; Lorchel, F.; Passerat, V.; Louet, E.; Pouchard, I.; Khodri, M.; Barbet, N. Reproducibility of surface-based deep inspiration breath-hold technique for lung stereotactic body radiotherapy on a closed-bore gantry linac. Phys. Imaging Radiat. Oncol. 2023, 26, 100448. [Google Scholar] [CrossRef]
- Johansen, J.; Bertelsen, A.; Hansen, C.R.; Westberg, J.; Hansen, O.; Brink, C. Set-up errors in patients undergoing image guided radiation treatment. Relationship to body mass index and weight loss. Acta Oncol. 2008, 47, 1454–1458. [Google Scholar] [CrossRef]
- Hörberger, F.; Andersson, K.M.; Enmark, M.; Kristensen, I.; Flejmer, A.; Edvardsson, A. Pencil beam scanning proton therapy for mediastinal lymphomas in deep inspiration breath-hold: A retrospective assessment of plan robustness. Acta Oncol. 2024, 63, 62–69. [Google Scholar] [CrossRef]
- Paganetti, H.; Blakely, E.; Carabe-Fernandez, A.; Carlson, D.J.; Das, I.J.; Dong, L.; Grosshans, D.; Held, K.D.; Mohan, R.; Moiseenko, V.; et al. Report of the AAPM TG-256 on the relative biological effectiveness of proton beams in radiation therapy. Med. Phys. 2019, 46, e53–e78. [Google Scholar] [CrossRef]
- Garbacz, M.; Gajewski, J.; Durante, M.; Kisielewicz, K.; Krah, N.; Kopeć, R.; Olko, P.; Patera, V.; Rinaldi, I.; Rydygier, M.; et al. Quantification of biological range uncertainties in patients treated at the Krakow proton therapy centre. Radiat. Oncol. 2022, 17, 50. [Google Scholar] [CrossRef]
- Rechner, L.A.; Maraldo, M.V.; Smith, E.A.; Lundgaard, A.Y.; Hjalgrim, L.L.; MacKay, R.I.; Aitkenhead, A.H.; Aznar, M.C. Proton linear energy transfer and variable relative biological effectiveness for adolescent patients with Hodgkin lymphoma. BJR Open 2023, 5, 20230012. [Google Scholar] [CrossRef] [PubMed]


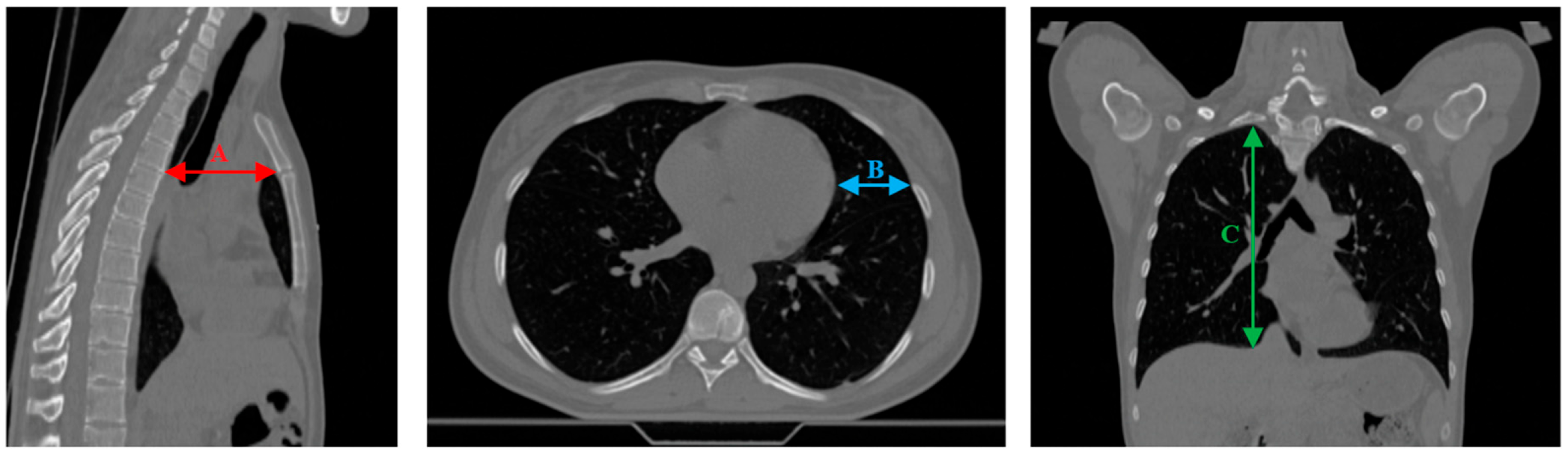
| Patient ID | #1 | #2 | #3 | #4 | #5 | #6 |
|---|---|---|---|---|---|---|
| Age | 27 | 38 | 40 | 26 | 25 | 35 |
| Gender | male | male | male | female | female | female |
| Diagnosis/ Tumor subtype | HL, NS | HL, NS | PMBCL | HL, NS | HL, NS | HL, NS |
| Stage | 1 A | 2 A | 2 BX | 2 BX | 2 A | 4 |
| Chemotherapy | 4 × ××ABVD | × x ABVD | × x R-CHOP-14 × x ESHAP CAR-T | × x ABVD | × x ABVD | × x ABVD |
| PTV volume DIBH/FB [cm3] | 545.65/601.93 | 516.04/680.54 | 216.67/306.0 | 1034.38/1301.35 | 772.56/871.39 | 377.03/513.92 |
| Fraction dose/no of fractions | 2.0 Gy(RBE)/15 | 2.0 Gy(RBE)/15 | 2.0 Gy(RBE)/ 25 | 2.0 Gy(RBE)/15 | 2.0 Gy(RBE)/15 | 2.0 Gy(RBE)/15 |
| Beam arrangement | 0° RS/0°/330° RS/30° RS | 0° RS/0° RS/330°/30° | 0° RS/0° RS/20° RS/340° RS | 0° RS/0° RS/340° RS/340° RS/13° RS | 0° RS/0° RS/20° RS/340° RS | 0° RS/0° RS/343° RS/17° RS |
| Distance | #1 | #2 | #3 | #4 | #5 | #6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Max | Mean | Max | Mean | Max | Mean | Max | Mean | Max | Mean | Max | |
| ∆A [cm] | 0.27 | 0.56 | 0.29 | 0.45 | 0.41 | 0.68 | 0.07 | 0.28 | 0.01 | 0.02 | 0.06 | 0.34 |
| ∆B [cm] | 0.70 | 0.90 | 0.37 | 0.77 | 0.45 | 1.54 | 0.88 | 1.58 | 0.23 | 0.57 | 0.32 | 1.51 |
| ∆C [cm] | 0.29 | 1.31 | 0.38 | 0.55 | 0.03 | 0.56 | 0.09 | 0.24 | 0.10 | 0.43 | 0.78 | 1.87 |
| Table Shift Coordinates | Median SGRT FB Setup Error | Median SGRT DIBH Setup Error | Median SGRT DIBH Reproducibility Error | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Male (n = 58) | Female (n = 46) | p-Value | Male (n = 49) | Female (n = 40) | p-Value | Male (n = 62) | Female (n = 69) | p-Value | |
| X (lateral) [cm] | 0.02 (−0.1; 0.04) | 0 (−0.06; 0.11) | p = 0.06 | −0.08 (−0.14; 0) | 0.08 (−0.03; 0.23) | p < 0.05 | 0 (−0.05; 0.03) | 0.03 (−0.01; 0.08) | p < 0.05 |
| Y (longitudinal) [cm] | 0.03 (−0,07; 0,13) | 0.08 (−0.07; 0.16) | p = 0.27 | −0.24 (−0.54; −0.02) | −0.25 (−0.43; −0.15) | p = 0.72 | 0.02 (−0.12; 0.13) | 0.11 (0.01; 0.19) | p < 0.05 |
| Z (vertical) [cm] | 0.08 (0.01; 0.13) | 0.12 (−0.06; 0.21) | p < 0.05 | −0.1 (−0.2; 0.06) | −0.16 (−0.27; −0.09) | p < 0.05 | 0.07 (−0.02; 0.13) | 0.02 (−0.01; 0.07) | p < 0.05 |
| MAG (magnitude) [cm] | 0.17 (0.14; 0.25) | 0.24 (0.15; 0.29) | p = 0.54 | 0.41 (0.22; 0.68) | 0.40 (0.23; 0.54) | p = 0.38 | 0.19 (0.13; 0.27) | 0.17 (0.12; 0.23) | p = 0.26 |
| ROT [°] | 0 (−0.44; 0.37) | 0.1 (−0.27; 0.53) | p = 0.24 | 0.23 (−0.02; 0.38) | −0.95 (−1.44; −0.12) | p < 0.05 | 0.22 (0.07; 0.35) | 0.03 (−0.19; 0.29) | p < 0.05 |
| PITCH [°] | 0.05 (−0.75; 1.17) | −0.97 (−1.72; −0.23) | p < 0.05 | 0.47 (−0.08; 1.21) | 0.06 (−1.14; 1.23) | p = 0.07 | −0.07 (−0.35; 0.3) | −0.28 (−0.61; 0.02) | p < 0.05 |
| ROLL [°] | 0.18 (−0.26; 0.69) | −0.13 (−0.70; 0.52) | p < 0.05 | −0.04 (−0.17; 0.17) | −0.21 (−0.77; 0.05) | p < 0.05 | 0.05 (−0.06; 0.16) | 0.19 (0.05; 0.28) | p < 0.05 |
| Table Shift Coordinates | Median kV Portal Imaging Setup Error | Median kV Portal Imaging DIBH Reproducibility | ||||
|---|---|---|---|---|---|---|
| Male (n = 45) | Female (n = 38) | p-Value | Male (n = 17) | Female (n = 33) | p-Value | |
| X (lateral) [cm] | 0 (−0.06; 0.10) | −0.14 (−0.30; 0) | p < 0.05 | 0 (−0.06; 0.03) | −0.09 (−0.34; 0) | p < 0.05 |
| Y (longitudinal) [cm] | 0.02 (−0.14; 0.24) | −0.03 (−0.34; 0.12) | p = 0.16 | 0 (−0.15; 0.06) | −0.07 (−0.45; 0) | p = 0.13 |
| Z (vertical) [cm] | 0 (−0.06; 0.11) | 0.01 (−0.06; 0.12) | p = 0.74 | 0.05 (−0.1; 0.09) | 0.02 (0; 0.12) | p = 0.39 |
| MAG (magnitude) [cm] | 0.34 (0.16; 0.43) | 0.33 (0.17; 0.68) | p = 0.18 | 0 (0; 0.08) | 0 (0; 0.26) | p = 0.06 |
| ROT [°] | 0 (−0.2; 0.4) | 0.65 (0; 1.5) | p < 0.05 | 0 (−0.2; 0.3) | 0.5 (0; 1.5) | p < 0.05 |
| PITCH [°] | −0.31 (−0.93; 0) | −0.05 (−0.3; 0.5) | p < 0.05 | −0.27 (−0.5; 0) | 0 (−0.21; 0.07) | p = 0.11 |
| ROLL [°] | 0 (0; 0) | 0 (0; 0) | p = 0.09 | 0 (0; 0) | 0 (0; 0) | p = 0.43 |
| Tolerances Within | kV Portal Imaging Setup Error (Interfractional, n = 91) | kV Portal Imaging DIBH Reproducibility (Intrafractional, n = 48) | ||||
|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | |
| 1 mm | 45.1% | 34.1% | 50.5% | 45.8% | 41.7% | 56.3% |
| 2 mm | 63.7% | 46.2% | 80.2% | 64.6% | 56.3% | 85.4% |
| 3 mm | 82.4% | 58.2% | 87.9% | 75.0% | 66.7% | 91.7% |
| 4 mm | 90.1% | 74.7% | 93.4% | 83.3% | 75.0% | 93.8% |
| 5 mm | 96.7% | 82.4% | 97.8% | 93.8% | 85.4% | 93.8% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garbacz, M.; Skóra, T.; Cepiga, A.; Foltyńska, G.; Gajewski, J.; Góra, E.; Kędzierska-Pardel, D.; Komenda, W.; Krzempek, D.; Krzywonos, E.; et al. Application of the Deep Inspiration Breath-Hold Technique in Proton Therapy for Mediastinal Lymphomas: Initial Experience. Cancers 2025, 17, 1985. https://doi.org/10.3390/cancers17121985
Garbacz M, Skóra T, Cepiga A, Foltyńska G, Gajewski J, Góra E, Kędzierska-Pardel D, Komenda W, Krzempek D, Krzywonos E, et al. Application of the Deep Inspiration Breath-Hold Technique in Proton Therapy for Mediastinal Lymphomas: Initial Experience. Cancers. 2025; 17(12):1985. https://doi.org/10.3390/cancers17121985
Chicago/Turabian StyleGarbacz, Magdalena, Tomasz Skóra, Anna Cepiga, Gabriela Foltyńska, Jan Gajewski, Eleonora Góra, Dominika Kędzierska-Pardel, Wiktor Komenda, Dawid Krzempek, Emilia Krzywonos, and et al. 2025. "Application of the Deep Inspiration Breath-Hold Technique in Proton Therapy for Mediastinal Lymphomas: Initial Experience" Cancers 17, no. 12: 1985. https://doi.org/10.3390/cancers17121985
APA StyleGarbacz, M., Skóra, T., Cepiga, A., Foltyńska, G., Gajewski, J., Góra, E., Kędzierska-Pardel, D., Komenda, W., Krzempek, D., Krzywonos, E., Mikołajski, T., Ruciński, A., Sobkowicz, K., Sowa, U., Wochnik, A., Kisielewicz, K., & Kopeć, R. (2025). Application of the Deep Inspiration Breath-Hold Technique in Proton Therapy for Mediastinal Lymphomas: Initial Experience. Cancers, 17(12), 1985. https://doi.org/10.3390/cancers17121985






