What Is the Survivorship of Megaprosthetic Reconstruction Following the Resection of Renal Cell Carcinoma Long Bone Metastases and What Are the Potential Risk Factors for a Prosthetic Complication? †
Simple Summary
Abstract
1. Introduction
- What is the overall survival of patients with RCC bone metastases treated with megaprosthetic reconstruction, and which factors influence survival?
- What is the revision-free implant survivorship of modular megaprostheses used in the reconstruction of bone defects following the resection of a RCC metastasis in a competing risk framework, and what are types and timing of implant complications?
- Which factors are associated with a risk of implant complications?
2. Patients and Methods
2.1. Study Design and Setting
2.2. Patients and Treatment Approach
2.3. Definitions
2.4. Demographic Details
2.5. Ethical Approval
2.6. Statistical Analysis
3. Results
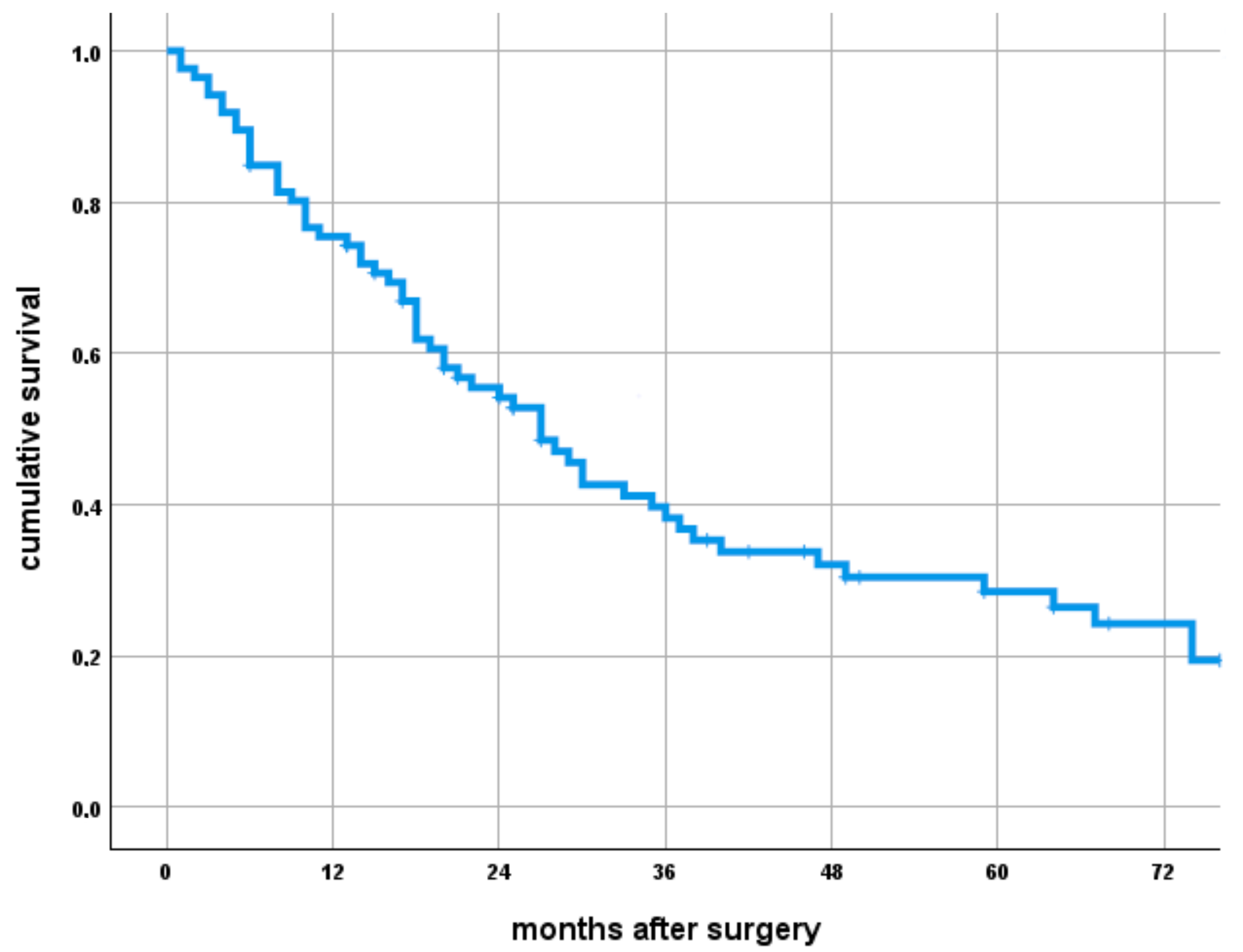
3.1. What Is the Overall Survival of Patients with RCC Bone Metastases Treated with Megaprosthetic Reconstruction and Which Factors Influence Survival?
3.2. What Is the Revision-Free Implant Survivorship of Modular Megaprostheses Used in the Reconstruction of Bone Defects Following the Resection of a RCC Metastasis in a Competing Risk Framework, and What Are Types and Timing of Implant Complications?
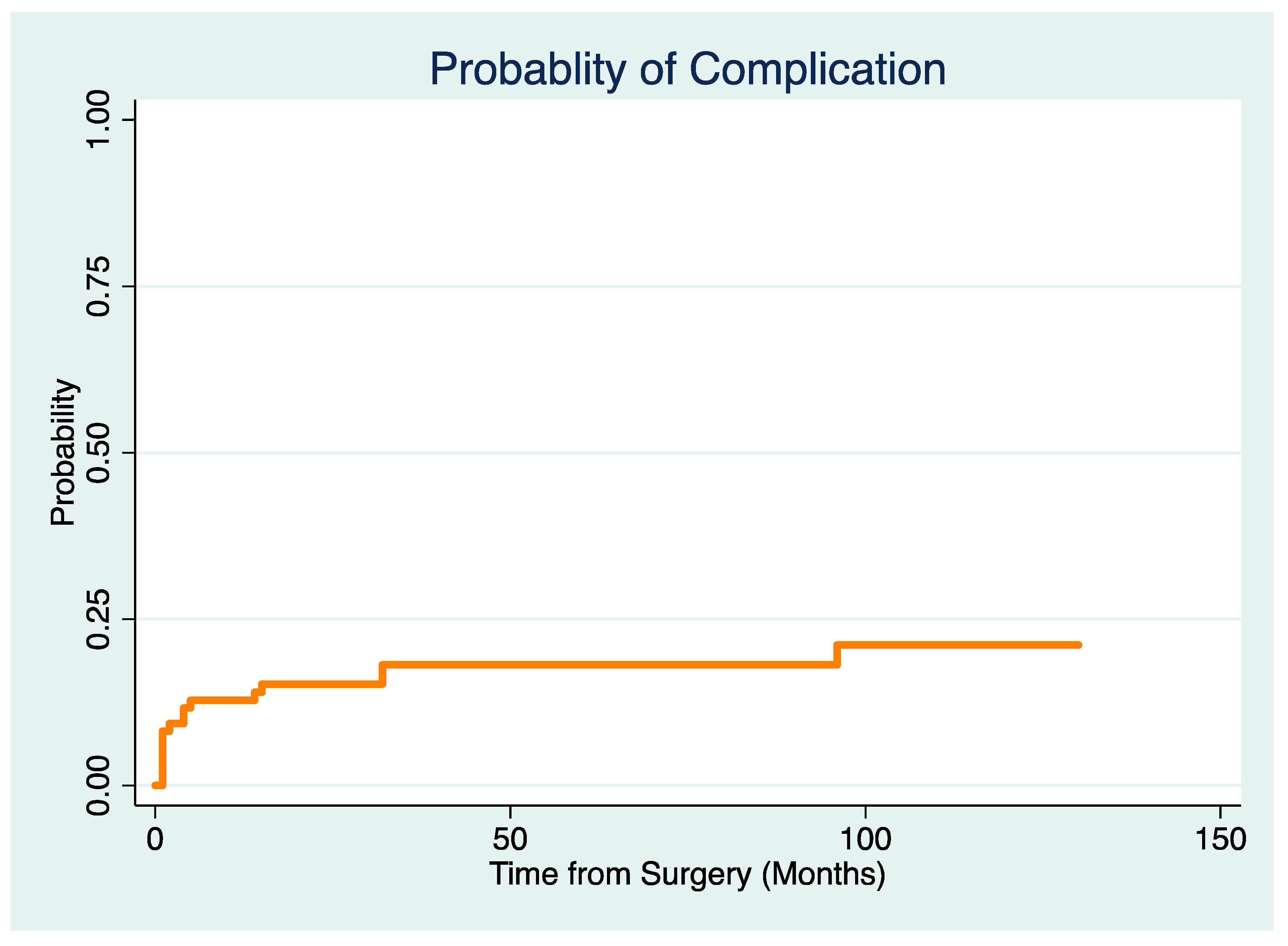
3.3. Which Factors Are Associated with the Risk for Implant Complications in a Competing Risk Model?
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flanigan, R.C.; Campbell, S.C.; Clark, J.I.; Picken, M.M. Metastatic renal cell carcinoma. Curr. Treat. Options Oncol. 2003, 4, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, M.; Sun, M.; Jeldres, C.; Shariat, S.F.; Trinh, Q.D.; Briganti, A.; Tian, Z.; Schmitges, J.; Graefen, M.; Perrotte, P.; et al. Distribution of metastatic sites in renal cell carcinoma: A population-based analysis. Ann. Oncol. 2012, 23, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Procopio, G.; Porta, C.; Ibrahim, T.; Barni, S.; Mazzara, C.; Fontana, A.; Berruti, A.; Berardi, R.; Vincenzi, B.; et al. Natural history of malignant bone disease in renal cancer: Final results of an Italian bone metastasis survey. PLoS ONE 2013, 8, e83026. [Google Scholar] [CrossRef] [PubMed]
- Ratasvuori, M.; Wedin, R.; Hansen, B.H.; Keller, J.; Trovik, C.; Zaikova, O.; Bergh, P.; Kalen, A.; Laitinen, M. Prognostic role of en-bloc resection and late onset of bone metastasis in patients with bone-seeking carcinomas of the kidney, breast, lung, and prostate: SSG study on 672 operated skeletal metastases. J. Surg. Oncol. 2014, 110, 360–365. [Google Scholar] [CrossRef]
- Higuchi, T.; Yamamoto, N.; Hayashi, K.; Takeuchi, A.; Abe, K.; Taniguchi, Y.; Kato, S.; Murakami, H.; Tsuchiya, H. Long-term patient survival after the surgical treatment of bone and soft-tissue metastases from renal cell carcinoma. Bone Jt. J. 2018, 100-B, 1241–1248. [Google Scholar] [CrossRef]
- Grunwald, V.; Eberhardt, B.; Bex, A.; Florcken, A.; Gauler, T.; Derlin, T.; Panzica, M.; Durr, H.R.; Grotz, K.A.; Giles, R.H.; et al. An interdisciplinary consensus on the management of bone metastases from renal cell carcinoma. Nat. Rev. Urol. 2018, 15, 511–521. [Google Scholar] [CrossRef]
- Willeumier, J.J.; Kaynak, M.; van der Zwaal, P.; Meylaerts, S.A.G.; Mathijssen, N.M.C.; Jutte, P.C.; Tsagozis, P.; Wedin, R.; van de Sande, M.A.J.; Fiocco, M.; et al. What Factors Are Associated With Implant Breakage and Revision After Intramedullary Nailing for Femoral Metastases? Clin. Orthop. Relat. Res. 2018, 476, 1823–1833. [Google Scholar] [CrossRef]
- Chafey, D.H.; Lewis, V.O.; Satcher, R.L.; Moon, B.S.; Lin, P.P. Is a Cephalomedullary Nail Durable Treatment for Patients With Metastatic Peritrochanteric Disease? Clin. Orthop. Relat. Res. 2018, 476, 2392–2401. [Google Scholar] [CrossRef]
- Steensma, M.; Boland, P.J.; Morris, C.D.; Athanasian, E.; Healey, J.H. Endoprosthetic treatment is more durable for pathologic proximal femur fractures. Clin. Orthop. Relat. Res. 2012, 470, 920–926. [Google Scholar] [CrossRef]
- Fuchs, B.; Trousdale, R.T.; Rock, M.G. Solitary bony metastasis from renal cell carcinoma: Significance of surgical treatment. Clin. Orthop. Relat. Res. 2005, 431, 187–192. [Google Scholar] [CrossRef]
- Ratasvuori, M.; Wedin, R.; Keller, J.; Nottrott, M.; Zaikova, O.; Bergh, P.; Kalen, A.; Nilsson, J.; Jonsson, H.; Laitinen, M. Insight opinion to surgically treated metastatic bone disease: Scandinavian Sarcoma Group Skeletal Metastasis Registry report of 1195 operated skeletal metastasis. Surg. Oncol. 2013, 22, 132–138. [Google Scholar] [CrossRef]
- Szendroi, A.; Dinya, E.; Kardos, M.; Szasz, A.M.; Nemeth, Z.; Ats, K.; Kiss, J.; Antal, I.; Romics, I.; Szendroi, M. Prognostic factors and survival of renal clear cell carcinoma patients with bone metastases. Pathol. Oncol. Res. 2010, 16, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Hutson, T.E.; Tomczak, P.; Michaelson, M.D.; Bukowski, R.M.; Oudard, S.; Negrier, S.; Szczylik, C.; Pili, R.; Bjarnason, G.A.; et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2009, 27, 3584–3590. [Google Scholar] [CrossRef] [PubMed]
- Laitinen, M.; Parry, M.; Ratasvuori, M.; Wedin, R.; Albergo, J.I.; Jeys, L.; Abudu, A.; Carter, S.; Gaston, L.; Tillman, R.; et al. Survival and complications of skeletal reconstructions after surgical treatment of bony metastatic renal cell carcinoma. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2015, 41, 886–892. [Google Scholar] [CrossRef]
- Fottner, A.; Szalantzy, M.; Wirthmann, L.; Stahler, M.; Baur-Melnyk, A.; Jansson, V.; Durr, H.R. Bone metastases from renal cell carcinoma: Patient survival after surgical treatment. BMC Musculoskelet. Disord. 2010, 11, 145. [Google Scholar] [CrossRef]
- Henrichs, M.P.; Krebs, J.; Gosheger, G.; Streitbuerger, A.; Nottrott, M.; Sauer, T.; Hoell, S.; Singh, G.; Hardes, J. Modular tumor endoprostheses in surgical palliation of long-bone metastases: A reduction in tumor burden and a durable reconstruction. World J. Surg. Oncol. 2014, 12, 330. [Google Scholar] [CrossRef]
- Angelini, A.; Trovarelli, G.; Berizzi, A.; Pala, E.; Breda, A.; Maraldi, M.; Ruggieri, P. Treatment of pathologic fractures of the proximal femur. Injury 2018, 49 (Suppl. 3), S77–S83. [Google Scholar] [CrossRef] [PubMed]
- Bischel, O.E.; Suda, A.J.; Bohm, P.M.; Lehner, B.; Bitsch, R.G.; Seeger, J.B. En-Bloc Resection of Metastases of the Proximal Femur and Reconstruction by Modular Arthroplasty is Not Only Justified in Patients with a Curative Treatment Option-An Observational Study of a Consecutive Series of 45 Patients. J. Clin. Med. 2020, 9, 758. [Google Scholar] [CrossRef]
- Theil, C.; Roder, J.; Gosheger, G.; Deventer, N.; Dieckmann, R.; Schorn, D.; Hardes, J.; Andreou, D. What is the Likelihood That Tumor Endoprostheses Will Experience a Second Complication After First Revision in Patients With Primary Malignant Bone Tumors And What Are Potential Risk Factors? Clin. Orthop. Relat. Res. 2019, 477, 2705–2714. [Google Scholar] [CrossRef]
- Hwang, N.; Nandra, R.; Grimer, R.J.; Carter, S.R.; Tillman, R.M.; Abudu, A.; Jeys, L.M. Massive endoprosthetic replacement for bone metastases resulting from renal cell carcinoma: Factors influencing patient survival. Eur. J. Surg. Oncol. 2014, 40, 429–434. [Google Scholar] [CrossRef]
- Evenski, A.; Ramasunder, S.; Fox, W.; Mounasamy, V.; Temple, H.T. Treatment and survival of osseous renal cell carcinoma metastases. J. Surg. Oncol. 2012, 106, 850–855. [Google Scholar] [CrossRef]
- Langerhuizen, D.W.; Janssen, S.J.; van der Vliet, Q.M.; Raskin, K.A.; Ferrone, M.L.; Hornicek, F.J.; Schwab, J.H.; Lozano-Calderon, S.A. Metastasectomy, intralesional resection, or stabilization only in the treatment of bone metastases from renal cell carcinoma. J. Surg. Oncol. 2016, 114, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Lacny, S.; Wilson, T.; Clement, F.; Roberts, D.J.; Faris, P.D.; Ghali, W.A.; Marshall, D.A. Kaplan-Meier Survival Analysis Overestimates the Risk of Revision Arthroplasty: A Meta-analysis. Clin. Orthop. Relat. Res. 2015, 473, 3431–3442. [Google Scholar] [CrossRef] [PubMed]
- Henderson, E.R.; Groundland, J.S.; Pala, E.; Dennis, J.A.; Wooten, R.; Cheong, D.; Windhager, R.; Kotz, R.I.; Mercuri, M.; Funovics, P.T.; et al. Failure mode classification for tumor endoprostheses: Retrospective review of five institutions and a literature review. J. Bone Jt. Surg. Am. 2011, 93, 418–429. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Mantel, N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother. Rep. Part 1 1966, 50, 163–170. [Google Scholar]
- Prentice, R.L.; Kalbfleisch, J.D.; Peterson, A.V., Jr.; Flournoy, N.; Farewell, V.T.; Breslow, N.E. The analysis of failure times in the presence of competing risks. Biometrics 1978, 34, 541–554. [Google Scholar] [CrossRef]
- Gutowski, C.J.; Zmistowski, B.; Fabbri, N.; Boland, P.J.; Healey, J.H. Should the Use of Biologic Agents in Patients with Renal and Lung Cancer Affect Our Surgical Management of Femoral Metastases? Clin. Orthop. Relat. Res. 2019, 477, 707–714. [Google Scholar] [CrossRef]
- Park, D.H.; Jaiswal, P.K.; Al-Hakim, W.; Aston, W.J.; Pollock, R.C.; Skinner, J.A.; Cannon, S.R.; Briggs, T.W. The use of massive endoprostheses for the treatment of bone metastases. Sarcoma 2007, 2007, 62151. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Takahashi, A.; Takei, F.; Hotta, H.; Miyao, N.; Shindo, T.; Igarashi, M.; Tachiki, H.; Kunishima, Y.; Muranaka, T.; et al. Molecular-targeted Therapy and Surgery May Prolong Survival of Renal Cell Carcinoma Patients with Bone Metastasis: A Multi-institutional Retrospective Study in Japan. Anticancer. Res. 2016, 36, 5531–5536. [Google Scholar] [CrossRef]
- Alt, A.L.; Boorjian, S.A.; Lohse, C.M.; Costello, B.A.; Leibovich, B.C.; Blute, M.L. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 2011, 117, 2873–2882. [Google Scholar] [CrossRef]
- Janssen, S.J.; Kortlever, J.T.; Ready, J.E.; Raskin, K.A.; Ferrone, M.L.; Hornicek, F.J.; Lozano-Calderon, S.A.; Schwab, J.H. Complications After Surgical Management of Proximal Femoral Metastasis: A Retrospective Study of 417 Patients. J. Am. Acad. Orthop. Surg. 2016, 24, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Janssen, S.J.; Teunis, T.; Hornicek, F.J.; van Dijk, C.N.; Bramer, J.A.; Schwab, J.H. Outcome after fixation of metastatic proximal femoral fractures: A systematic review of 40 studies. J. Surg. Oncol. 2016, 114, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Wedin, R.; Hansen, B.H.; Laitinen, M.; Trovik, C.; Zaikova, O.; Bergh, P.; Kalen, A.; Schwarz-Lausten, G.; Vult von Steyern, F.; Walloe, A.; et al. Complications and survival after surgical treatment of 214 metastatic lesions of the humerus. J. Shoulder Elb. Surg. 2012, 21, 1049–1055. [Google Scholar] [CrossRef]
- Brinkmann, O.A.; Bruns, F.; Gosheger, G.; Micke, O.; Hertle, L. Treatment of bone metastases and local recurrence from renal cell carcinoma with immunochemotherapy and radiation. World J. Urol. 2005, 23, 185–190. [Google Scholar] [CrossRef]
- Jeys, L.M.; Luscombe, J.S.; Grimer, R.J.; Abudu, A.; Tillman, R.M.; Carter, S.R. The risks and benefits of radiotherapy with massive endoprosthetic replacement. J. Bone Jt. Surg. Br. 2007, 89, 1352–1355. [Google Scholar] [CrossRef]
- Sevelda, F.; Waldstein, W.; Panotopoulos, J.; Kaider, A.; Funovics, P.T.; Windhager, R. Is Total Femur Replacement a Reliable Treatment Option for Patients With Metastatic Carcinoma of the Femur? Clin. Orthop. Relat. Res. 2018, 476, 977–983. [Google Scholar] [CrossRef]
- Kotwal, S.; Moon, B.; Lin, P.; Satcher, R., Jr.; Lewis, V. Total Humeral Endoprosthetic Replacement following Excision of Malignant Bone Tumors. Sarcoma 2016, 2016, 6318060. [Google Scholar] [CrossRef]
| Variable | % (n) |
|---|---|
| Male | 71 (61/86) |
| Smokers | 14 (12/86) |
| Diabetes | 12 (10/86) |
| Pathologic fracture | 54 (46/86) |
| Previous non-megaprosthetic reconstruction | 29 (25/86) |
| Intramedullary nail | 9 (8/25) |
| Plate or screw fixation | 14 (12/25) |
| Arthroplasty | 6 (5/25) |
| Resection margin | |
| Tumor-free margins | 78 (67/86) |
| Intralesional margin | 22 (19/86) |
| Postoperative local radiation | 41 (35/86) |
| Preoperative local radiation | 24 (21/86) |
| Systemic therapy for primary tumor | 37 (32/86) |
| Surgery for renal tumor | 88 (76/86) |
| Visceral metastasis at surgery | 21 (18/86) |
| Pulmonary metastasis at surgery | 41 (36/86) |
| Solitary bone metastasis | 31 (27/86) |
| Time of metastasis | |
| Synchronous | 28 (24/86) |
| Metachronous early (<2 years from the initial diagnosis) | 17 (15/86) |
| Metachronous late (>2 years) | 55 (47/86) |
| Variable | Mean (±SD) |
|---|---|
| Body mass index (BMI in kg/m2) | 29 (7.1) |
| Age at initial surgery in years | 69 (6.8) |
| Reconstruction length in mm | 233 (64) |
| Follow-up in months | 53 (29.5) |
| Time from initial diagnosis to implantation of a megaprosthesis | 84 (39.8) |
| Variable | % (n) |
|---|---|
| Prosthetic location | |
| Proximal femur (PFR) | 38 (33/86) |
| Distal femur (DFR) | 23 (20/86) |
| Proximal humerus (PHR) | 23 (20/86) |
| Intercalary (IP) | 9 (8/86) |
| Total humerus (THR) | 2 (2/86) |
| Proximal tibial replacement (PTR) | 1 (1/86) |
| Total femoral (TFR) | 1 (1/86) |
| Distal humerus (DHR) | 1 (1/86) |
| Variable | Hazard Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|
| Diabetes | 1.01 | 0.237–4.309 | 0.99 |
| Smoking | 0.40 | 0.055–2.971 | 0.37 |
| Age at surgery | 0.99 | 0.960–1.031 | 0.77 |
| Pathological fracture | 1.17 | 0.450–3.045 | 0.75 |
| Previous non-megaprosthetic surgery | 1.55 | 0.575–4.186 | 0.39 |
| Cemented stem | 0.49 | 0.151–1.577 | 0.23 |
| Blood transfusion | 2.06 | 0.789–5.352 | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bockholt, S.; Schneider, K.N.; Gosheger, G.; Smolle, M.A.; Deventer, N.; Andreou, D.; Theil, C. What Is the Survivorship of Megaprosthetic Reconstruction Following the Resection of Renal Cell Carcinoma Long Bone Metastases and What Are the Potential Risk Factors for a Prosthetic Complication? Cancers 2025, 17, 1982. https://doi.org/10.3390/cancers17121982
Bockholt S, Schneider KN, Gosheger G, Smolle MA, Deventer N, Andreou D, Theil C. What Is the Survivorship of Megaprosthetic Reconstruction Following the Resection of Renal Cell Carcinoma Long Bone Metastases and What Are the Potential Risk Factors for a Prosthetic Complication? Cancers. 2025; 17(12):1982. https://doi.org/10.3390/cancers17121982
Chicago/Turabian StyleBockholt, Sebastian, Kristian Nikolaus Schneider, Georg Gosheger, Maria Anna Smolle, Niklas Deventer, Dimosthenis Andreou, and Christoph Theil. 2025. "What Is the Survivorship of Megaprosthetic Reconstruction Following the Resection of Renal Cell Carcinoma Long Bone Metastases and What Are the Potential Risk Factors for a Prosthetic Complication?" Cancers 17, no. 12: 1982. https://doi.org/10.3390/cancers17121982
APA StyleBockholt, S., Schneider, K. N., Gosheger, G., Smolle, M. A., Deventer, N., Andreou, D., & Theil, C. (2025). What Is the Survivorship of Megaprosthetic Reconstruction Following the Resection of Renal Cell Carcinoma Long Bone Metastases and What Are the Potential Risk Factors for a Prosthetic Complication? Cancers, 17(12), 1982. https://doi.org/10.3390/cancers17121982







