Neoantigen-Based Immunotherapy in Lung Cancer: Advances, Challenges and Prospects
Simple Summary
Abstract
1. Introduction
2. The Production and Immune Mechanism of Neoantigens in Lung Cancer
3. The Prediction and Identification of Neoantigens in Lung Cancer
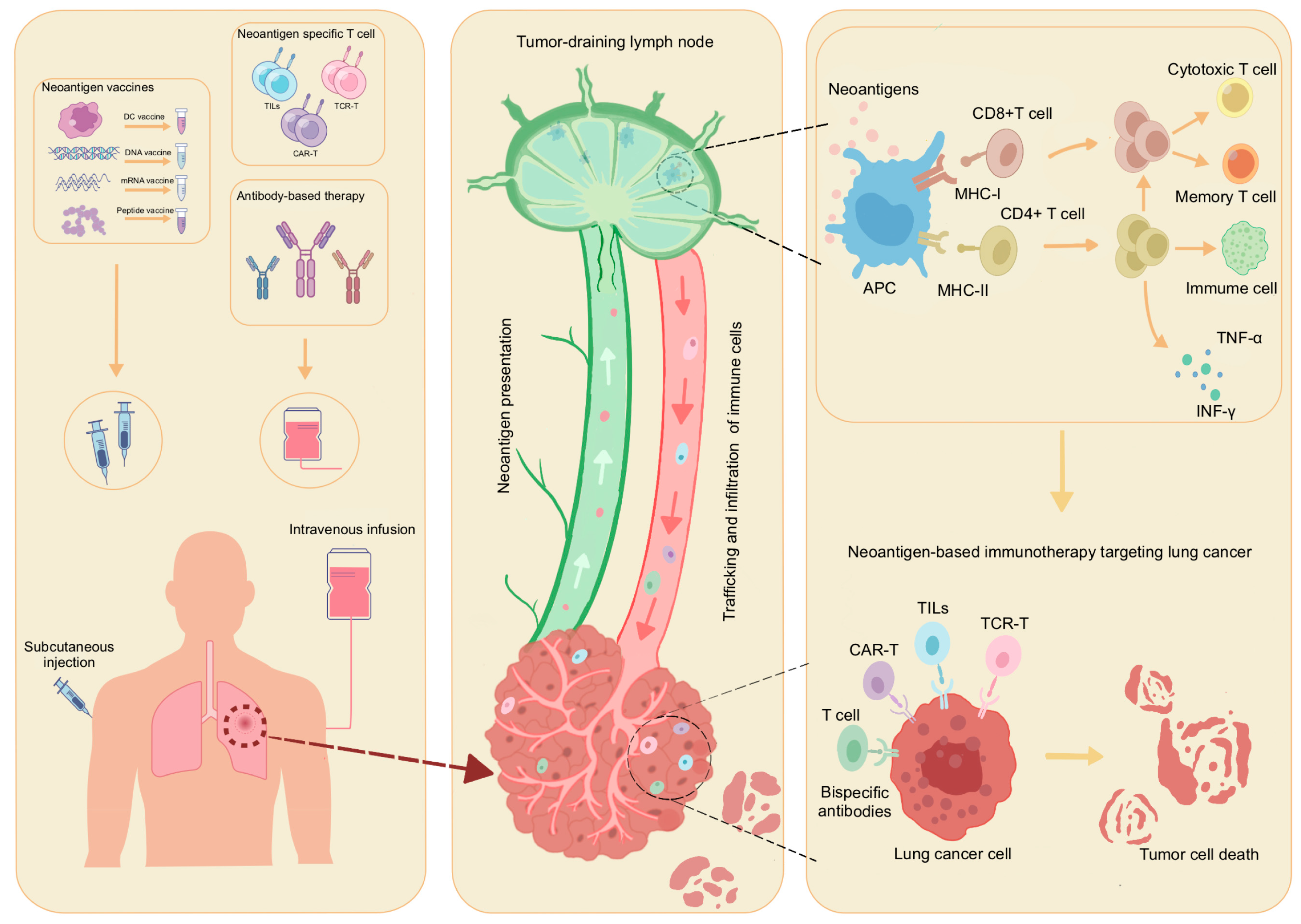
4. Application of Neoantigen-Based Therapy in Lung Cancer
4.1. The Use of Neoantigen-Based Therapy in the Lung Cancer
4.1.1. Personalized Neoantigen Vaccine
4.1.2. Adoptive Cell Therapy Based on Neoantigens
4.1.3. Neoantigen-Based Antibody Therapy
4.1.4. Clinical Trials of Neoantigen-Based Therapies
4.2. Combination Therapy
4.2.1. Neoantigen Vaccine Combined with Adoptive Cell Therapy
4.2.2. Neoantigen Immunotherapy Combined with ICIs
4.2.3. Neoantigen Immunotherapy Combined with Conventional Therapy
5. Challenges of Neoantigen Immunotherapy in Lung Cancer
5.1. Tumor Heterogeneity and Patient HLA Heterogeneity
5.2. Dilemmas to Predict and Identify Neoantigens
5.3. Immune Escape and Immune Interference
5.4. Tumor Associated Antigens and Public Neoantigens
5.5. Production Cycle and Cost of Neoantigen Vaccine
5.6. Neoantigen Prognostic Monitoring
5.7. Drug Resistance
6. Future Prospect and Research Direction of Neoantigens
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| IARC | International Agency for Research on Cancer |
| SCLC | small cell lung cancer |
| NSCLC | non-small cell lung cancer |
| ICI | immune checkpoint inhibitor |
| TSA | tumor-specific antigen |
| TAA | tumor associated antigen |
| SNV | single nucleotide variant |
| INDELS | insertions/deletions |
| MHC | major histocompatibility complex |
| PTM | post-translational modification |
| APC | antigen-presenting cell |
| DC | dendritic cell |
| TDLN | tumor draining lymph node |
| TCR | T cell receptor |
| NGS | next-generation sequencing |
| WGS | whole genome sequencing |
| WES | whole exome sequencing |
| RNA-seq | RNA sequencing |
| HLA | human leukocyte antigen |
| SHERPA | systematic hla epitope ranking pan algorithm |
| TAP | tumor abnormal protein |
| CNN | convolutional neural network |
| SABR | signaling and antigen-presenting bifunctional receptor |
| MCR | MHC-TCR chimeric receptor |
| ACT | adoptive cell therapy |
| TCR-T | T cell receptor modified T cell |
| TIL | tumor-infiltrating lymphocyte |
| PFS | progression-free survival |
| OS | overall survival |
| WTL | whole tumor lysate |
| CEA | carcinoembryonic antigen |
| IFN-γ | interferon-γ |
| IL-2 | interleukin-2 |
| PEV | personalized vaccine |
| CAR-T | chimeric antigen receptor modified T cells |
| PFS | progression-free survival |
| TCRm | TCR mimics |
| MANA | mutation-associated neoantigen |
| ADCs | antibody-drug conjugates |
| BsAbs | bispecific antibodies |
| scFvs | single-stranded variable fragments |
| scDbs | single-stranded disomes |
| TME | tumor microenvironment |
| SD | stable disease |
| PR | partial response |
| ctDNA | circulating tumor DNA |
| Treg | T regulatory |
References
- Nasim, F.; Sabath, B.F.; Eapen, G.A. Lung Cancer. Med. Clin. N. Am. 2019, 103, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The global burden of lung cancer: Current status and future trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Li, Q.; Zhang, R.; Xie, L.; Shu, Y.; Gao, S.; Wang, P.; Su, X.; Qin, Y.; Wang, Y.; et al. Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal Transduct. Target. Ther. 2021, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.S.; Pyo, K.H.; Chung, J.M.; Cho, B.C. Artificial intelligence-based non-small cell lung cancer transcriptome RNA-sequence analysis technology selection guide. Front. Bioeng. Biotechnol. 2023, 11, 1081950. [Google Scholar] [CrossRef]
- Pirlog, R.; Chiroi, P.; Rusu, I.; Jurj, A.M.; Budisan, L.; Pop-Bica, C.; Braicu, C.; Crisan, D.; Sabourin, J.C.; Berindan-Neagoe, I. Cellular and Molecular Profiling of Tumor Microenvironment and Early-Stage Lung Cancer. Int. J. Mol. Sci. 2022, 23, 5346. [Google Scholar] [CrossRef]
- Ye, L.; Creaney, J.; Redwood, A.; Robinson, B. The Current Lung Cancer Neoantigen Landscape and Implications for Therapy. J. Thorac. Oncol. 2021, 16, 922–932. [Google Scholar] [CrossRef]
- Abdel-Rahman, O. Global trends in mortality from malignant mesothelioma: Analysis of WHO mortality database (1994–2013). Clin. Respir. J. 2018, 12, 2090–2100. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef]
- Zhu, Y.J.; Li, X.; Chen, T.T.; Wang, J.X.; Zhou, Y.X.; Mu, X.L.; Du, Y.; Wang, J.L.; Tang, J.; Liu, J.Y. Personalised neoantigen-based therapy in colorectal cancer. Clin. Transl. Med. 2023, 13, e1461. [Google Scholar] [CrossRef]
- Liu, Q.; Li, J.; Zheng, H.; Yang, S.; Hua, Y.; Huang, N.; Kleeff, J.; Liao, Q.; Wu, W. Adoptive cellular immunotherapy for solid neoplasms beyond CAR-T. Mol. Cancer 2023, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Biswas, N.; Chakrabarti, S.; Padul, V.; Jones, L.D.; Ashili, S. Designing neoantigen cancer vaccines, trials, and outcomes. Front. Immunol. 2023, 14, 1105420. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Liu, B. Critical factors in chimeric antigen receptor-modified T-cell (CAR-T) therapy for solid tumors. Onco Targets Ther. 2019, 12, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Shi, T.; Zhang, H.; Hu, J.; Song, Y.; Wei, J.; Ren, S.; Zhou, C. Tumor neoantigens: From basic research to clinical applications. J. Hematol. Oncol. 2019, 12, 93. [Google Scholar] [CrossRef]
- Su, S.; Chen, F.; Xu, M.; Liu, B.; Wang, L. Recent advances in neoantigen vaccines for treating non-small cell lung cancer. Thorac. Cancer 2023, 14, 3361–3368. [Google Scholar] [CrossRef]
- Kretzschmar, K. Cancer research using organoid technology. J. Mol. Med. 2021, 99, 501–515. [Google Scholar] [CrossRef]
- Li, S.N.; Wang, X.; Bai, H.; Wang, J. Predictive value of tumor mutational burden on the efficacy of immunotherapy for non-small cell lung cancer. Chin. J. Front. Med. Sci. 2019, 11, 18–23. [Google Scholar]
- Thundimadathil, J. Cancer treatment using peptides: Current therapies and future prospects. J. Amino Acids 2012, 2012, 967347. [Google Scholar] [CrossRef]
- Yao, M.; Liu, X.; Qian, Z.; Fan, D.; Sun, X.; Zhong, L.; Wu, P. Research progress of nanovaccine in anti-tumor immunotherapy. Front. Oncol. 2023, 13, 1211262. [Google Scholar] [CrossRef]
- Rossi, J.F.; Céballos, P.; Lu, Z.Y. Immune precision medicine for cancer: A novel insight based on the efficiency of immune effector cells. Cancer Commun. 2019, 39, 34. [Google Scholar] [CrossRef]
- Coulie, P.G.; Van den Eynde, B.J.; van der Bruggen, P.; Boon, T. Tumour antigens recognized by T lymphocytes: At the core of cancer immunotherapy. Nat. Rev. Cancer 2014, 14, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Dietlein, F.; Wang, A.B.; Fagre, C.; Tang, A.; Besselink, N.J.M.; Cuppen, E.; Li, C.; Sunyaev, S.R.; Neal, J.T.; Van Allen, E.M. Genome-wide analysis of somatic noncoding mutation patterns in cancer. Science 2022, 376, eabg5601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, J.; Bian, J.; Lu, X. The efficacy and safety of chimeric antigen receptor T cells in digestive system cancers: A systematic review and meta-analysis. Ann. Transl. Med. 2022, 10, 508. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lee, K.W.; Srivastava, R.M.; Kuo, F.; Krishna, C.; Chowell, D.; Makarov, V.; Hoen, D.; Dalin, M.G.; Wexler, L.; et al. Immunogenic neoantigens derived from gene fusions stimulate T cell responses. Nat. Med. 2019, 25, 767–775. [Google Scholar] [CrossRef]
- Turajlic, S.; Litchfield, K.; Xu, H.; Rosenthal, R.; McGranahan, N.; Reading, J.L.; Wong, Y.N.S.; Rowan, A.; Kanu, N.; Al Bakir, M.; et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: A pan-cancer analysis. Lancet Oncol. 2017, 18, 1009–1021. [Google Scholar] [CrossRef]
- Capietto, A.H.; Hoshyar, R.; Delamarre, L. Sources of Cancer Neoantigens beyond Single-Nucleotide Variants. Int. J. Mol. Sci. 2022, 23, 10131. [Google Scholar] [CrossRef]
- Fotakis, G.; Trajanoski, Z.; Rieder, D. Computational cancer neoantigen prediction: Current status and recent advances. Immunooncol. Technol. 2021, 12, 100052. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, A.; Yang, F.; Du, W. Immunotherapeutic Strategies for Head and Neck Squamous Cell Carcinoma (HNSCC): Current Perspectives and Future Prospects. Vaccines 2022, 10, 1272. [Google Scholar] [CrossRef]
- Xie, N.; Shen, G.; Gao, W.; Huang, Z.; Huang, C.; Fu, L. Neoantigens: Promising targets for cancer therapy. Signal Transduct. Target. Ther. 2023, 8, 9. [Google Scholar] [CrossRef]
- Blass, E.; Ott, P.A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 2021, 18, 215–229. [Google Scholar] [CrossRef]
- Sobhani, N.; Scaggiante, B.; Morris, R.; Chai, D.; Catalano, M.; Tardiel-Cyril, D.R.; Neeli, P.; Roviello, G.; Mondani, G.; Li, Y. Therapeutic cancer vaccines: From biological mechanisms and engineering to ongoing clinical trials. Cancer Treat. Rev. 2022, 109, 102429. [Google Scholar] [CrossRef] [PubMed]
- Vansteenkiste, J.F.; Cho, B.C.; Vanakesa, T.; De Pas, T.; Zielinski, M.; Kim, M.S.; Jassem, J.; Yoshimura, M.; Dahabreh, J.; Nakayama, H.; et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2016, 17, 822–835. [Google Scholar] [CrossRef] [PubMed]
- Quoix, E.; Ramlau, R.; Westeel, V.; Papai, Z.; Madroszyk, A.; Riviere, A.; Koralewski, P.; Breton, J.L.; Stoelben, E.; Braun, D.; et al. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: A controlled phase 2B trial. Lancet Oncol. 2011, 12, 1125–1133. [Google Scholar] [CrossRef]
- Lv, L.; Chen, W.; Chen, N.; Cui, E. Advances of cell therapy in lung cancer: A narrative review. J. Thorac. Dis. 2023, 15, 7050–7062. [Google Scholar] [CrossRef]
- Pei, B.; Hsu, Y.H. IConMHC: A deep learning convolutional neural network model to predict peptide and MHC-I binding affinity. Immunogenetics 2020, 72, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Stolk, D.A.; Horrevorts, S.K.; Schetters, S.T.T.; Kruijssen, L.J.W.; Duinkerken, S.; Keuning, E.; Ambrosini, M.; Kalay, H.; van de Ven, R.; Garcia-Vallejo, J.J.; et al. Palmitoylated antigens for the induction of anti-tumor CD8(+) T cells and enhanced tumor recognition. Mol. Ther. Oncolytics 2021, 21, 315–328. [Google Scholar] [CrossRef]
- Sahin, U.; Türeci, Ö. Personalized vaccines for cancer immunotherapy. Science 2018, 359, 1355–1360. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Wang, L.; Liu, B. Personalized neoantigen vaccination with synthetic long peptides: Recent advances and future perspectives. Theranostics 2020, 10, 6011–6023. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- Zander, R.; Schauder, D.; Xin, G.; Nguyen, C.; Wu, X.; Zajac, A.; Cui, W. CD4(+) T Cell Help Is Required for the Formation of a Cytolytic CD8(+) T Cell Subset that Protects against Chronic Infection and Cancer. Immunity 2019, 51, 1028–1042.e1024. [Google Scholar] [CrossRef]
- Alspach, E.; Lussier, D.M.; Miceli, A.P.; Kizhvatov, I.; DuPage, M.; Luoma, A.M.; Meng, W.; Lichti, C.F.; Esaulova, E.; Vomund, A.N.; et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 2019, 574, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz-Fathi, M.; Rezaei, N. Cancer Immunoprevention: Current Status and Future Directions. Arch. Immunol. Ther. Exp. 2021, 69, 3. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Turcotte, S.; Gros, A.; Robbins, P.F.; Lu, Y.C.; Dudley, M.E.; Wunderlich, J.R.; Somerville, R.P.; Hogan, K.; Hinrichs, C.S.; et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014, 344, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Tran, E.; Robbins, P.F.; Lu, Y.C.; Prickett, T.D.; Gartner, J.J.; Jia, L.; Pasetto, A.; Zheng, Z.; Ray, S.; Groh, E.M.; et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N. Engl. J. Med. 2016, 375, 2255–2262. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Nik-Zainal, S.; Wedge, D.C.; Aparicio, S.A.; Behjati, S.; Biankin, A.V.; Bignell, G.R.; Bolli, N.; Borg, A.; Børresen-Dale, A.L.; et al. Signatures of mutational processes in human cancer. Nature 2013, 500, 415–421. [Google Scholar] [CrossRef]
- Yu, G.J.; Wu, Y.F.; Cao, X.T. Personalized neoantigen vaccines for tumor immunotherapy: A long hard journey to promising land. Chinses J. Cancer Biother. 2022, 29, 1–10. [Google Scholar]
- Song, Y.; Zhang, Y. Research progress of neoantigens in gynecologic cancers. Int. Immunopharmacol. 2022, 112, 109236. [Google Scholar] [CrossRef]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A., Jr.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, W.; Wu, J.; Lu, J.; Ding, Y.; Wu, S.; Wang, H.; Ding, D.; Mo, F.; Zhou, Z.; et al. Neoantigens Derived from Recurrently Mutated Genes as Potential Immunotherapy Targets for Gastric Cancer. Biomed. Res. Int. 2019, 2019, 8103142. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, Q.; Zhang, J.; Zhu, B. Neoantigens in precision cancer immunotherapy: From identification to clinical applications. Chin Med. J. 2022, 135, 1285–1298. [Google Scholar] [CrossRef]
- Hoyos, L.E.; Abdel-Wahab, O. Cancer-Specific Splicing Changes and the Potential for Splicing-Derived Neoantigens. Cancer Cell 2018, 34, 181–183. [Google Scholar] [CrossRef] [PubMed]
- Kye, Y.; Nagineni, L.; Gadad, S.; Ramirez, F.; Riva, H.; Fernandez, L.; Samaniego, M.; Holland, N.; Yeh, R.; Takigawa, K.; et al. The Identification and Clinical Applications of Mutated Antigens in the Era of Immunotherapy. Cancers 2022, 14, 4255. [Google Scholar] [CrossRef] [PubMed]
- Borden, E.S.; Buetow, K.H.; Wilson, M.A.; Hastings, K.T. Cancer Neoantigens: Challenges and Future Directions for Prediction, Prioritization, and Validation. Front. Oncol. 2022, 12, 836821. [Google Scholar] [CrossRef] [PubMed]
- Gopanenko, A.V.; Kosobokova, E.N.; Kosorukov, V.S. Main Strategies for the Identification of Neoantigens. Cancers 2020, 12, 2879. [Google Scholar] [CrossRef]
- Pyke, R.M.; Mellacheruvu, D.; Dea, S.; Abbott, C.; Zhang, S.V.; Phillips, N.A.; Harris, J.; Bartha, G.; Desai, S.; McClory, R.; et al. Precision Neoantigen Discovery Using Large-Scale Immunopeptidomes and Composite Modeling of MHC Peptide Presentation. Mol. Cell Proteom. 2023, 22, 100506. [Google Scholar] [CrossRef]
- Okada, M.; Shimizu, K.; Fujii, S.I. Identification of Neoantigens in Cancer Cells as Targets for Immunotherapy. Int. J. Mol. Sci. 2022, 23, 2594. [Google Scholar] [CrossRef]
- Yadav, M.; Jhunjhunwala, S.; Phung, Q.T.; Lupardus, P.; Tanguay, J.; Bumbaca, S.; Franci, C.; Cheung, T.K.; Fritsche, J.; Weinschenk, T.; et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 2014, 515, 572–576. [Google Scholar] [CrossRef]
- Chen, R.; Fulton, K.M.; Twine, S.M.; Li, J. Identification of Mhc Peptides Using Mass Spectrometry for Neoantigen Discovery and Cancer Vaccine Development. Mass. Spectrom. Rev. 2021, 40, 110–125. [Google Scholar] [CrossRef] [PubMed]
- Bulik-Sullivan, B.; Busby, J.; Palmer, C.D.; Davis, M.J.; Murphy, T.; Clark, A.; Busby, M.; Duke, F.; Yang, A.; Young, L.; et al. Deep learning using tumor HLA peptide mass spectrometry datasets improves neoantigen identification. Nat. Biotechnol. 2018, 37, 55–63. [Google Scholar] [CrossRef]
- Schenck, R.O.; Lakatos, E.; Gatenbee, C.; Graham, T.A.; Anderson, A.R.A. NeoPredPipe: High-throughput neoantigen prediction and recognition potential pipeline. BMC Bioinform. 2019, 20, 264. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Tan, X.; Ouyang, J.; Zhang, M.; Song, X.; Liu, Q.; Leng, Q.; Chen, L.; Xie, L. ProGeo-neo: A customized proteogenomic workflow for neoantigen prediction and selection. BMC Med. Genom. 2020, 13, 52. [Google Scholar] [CrossRef]
- Rieder, D.; Fotakis, G.; Ausserhofer, M.; René, G.; Paster, W.; Trajanoski, Z.; Finotello, F. nextNEOpi: A comprehensive pipeline for computational neoantigen prediction. Bioinformatics 2022, 38, 1131–1132. [Google Scholar] [CrossRef]
- Hundal, J.; Kiwala, S.; Feng, Y.Y.; Liu, C.J.; Govindan, R.; Chapman, W.C.; Uppaluri, R.; Swamidass, S.J.; Griffith, O.L.; Mardis, E.R.; et al. Accounting for proximal variants improves neoantigen prediction. Nat. Genet. 2019, 51, 175–179. [Google Scholar] [CrossRef]
- Richters, M.M.; Xia, H.; Campbell, K.M.; Gillanders, W.E.; Griffith, O.L.; Griffith, M. Best practices for bioinformatic characterization of neoantigens for clinical utility. Genome Med. 2019, 11, 56. [Google Scholar] [CrossRef]
- Chu, Y.; Liu, Q.; Wei, J.; Liu, B. Personalized cancer neoantigen vaccines come of age. Theranostics 2018, 8, 4238–4246. [Google Scholar] [CrossRef]
- Tang, L.; Zhang, R.; Zhang, X.; Yang, L. Personalized Neoantigen-Pulsed DC Vaccines: Advances in Clinical Applications. Front. Oncol. 2021, 11, 701777. [Google Scholar] [CrossRef]
- Peng, M.; Mo, Y.; Wang, Y.; Wu, P.; Zhang, Y.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; Li, X.; et al. Neoantigen vaccine: An emerging tumor immunotherapy. Mol. Cancer 2019, 18, 128. [Google Scholar] [CrossRef]
- Hundal, J.; Kiwala, S.; McMichael, J.; Miller, C.A.; Xia, H.; Wollam, A.T.; Liu, C.J.; Zhao, S.; Feng, Y.Y.; Graubert, A.P.; et al. pVACtools: A Computational Toolkit to Identify and Visualize Cancer Neoantigens. Cancer Immunol. Res. 2020, 8, 409–420. [Google Scholar] [CrossRef]
- Kirchmair, A.; Finotello, F. In Silico Prediction of Tumor Neoantigens with TIminer. Methods Mol. Biol. 2020, 2120, 129–145. [Google Scholar]
- Kisielow, J.; Obermair, F.J.; Kopf, M. Deciphering CD4(+) T cell specificity using novel MHC-TCR chimeric receptors. Nat. Immunol. 2019, 20, 652–662. [Google Scholar] [CrossRef]
- Joglekar, A.V.; Leonard, M.T.; Jeppson, J.D.; Swift, M.; Li, G.; Wong, S.; Peng, S.; Zaretsky, J.M.; Heath, J.R.; Ribas, A.; et al. T cell antigen discovery via signaling and antigen-presenting bifunctional receptors. Nat. Methods 2019, 16, 191–198. [Google Scholar] [CrossRef]
- Zhou, Z.; Lyu, X.; Wu, J.; Yang, X.; Wu, S.; Zhou, J.; Gu, X.; Su, Z.; Chen, S. TSNAD: An integrated software for cancer somatic mutation and tumour-specific neoantigen detection. R. Soc. Open Sci. 2017, 4, 170050. [Google Scholar] [CrossRef]
- Zhou, C.; Wei, Z.; Zhang, Z.; Zhang, B.; Zhu, C.; Chen, K.; Chuai, G.; Qu, S.; Xie, L.; Gao, Y.; et al. pTuneos: Prioritizing tumor neoantigens from next-generation sequencing data. Genome Med. 2019, 11, 67. [Google Scholar] [CrossRef]
- Wang, T.Y.; Wang, L.; Alam, S.K.; Hoeppner, L.H.; Yang, R. ScanNeo: Identifying indel-derived neoantigens using RNA-Seq data. Bioinformatics 2019, 35, 4159–4161. [Google Scholar] [CrossRef]
- Kodysh, J.; Rubinsteyn, A. OpenVax: An Open-Source Computational Pipeline for Cancer Neoantigen Prediction. Methods Mol. Biol. 2020, 2120, 147–160. [Google Scholar]
- Bjerregaard, A.M.; Nielsen, M.; Hadrup, S.R.; Szallasi, Z.; Eklund, A.C. MuPeXI: Prediction of neo-epitopes from tumor sequencing data. Cancer Immunol. Immunother. 2017, 66, 1123–1130. [Google Scholar] [CrossRef]
- Vitiello, A.; Zanetti, M. Neoantigen prediction and the need for validation. Nat. Biotechnol. 2017, 35, 815–817. [Google Scholar] [CrossRef]
- Zhang, R.; Yuan, F.; Shu, Y.; Tian, Y.; Zhou, B.; Yi, L.; Zhang, X.; Ding, Z.; Xu, H.; Yang, L. Personalized neoantigen-pulsed dendritic cell vaccines show superior immunogenicity to neoantigen-adjuvant vaccines in mouse tumor models. Cancer Immunol. Immunother. 2020, 69, 135–145. [Google Scholar] [CrossRef]
- Johanns, T.M.; Miller, C.A.; Liu, C.J.; Perrin, R.J.; Bender, D.; Kobayashi, D.K.; Campian, J.L.; Chicoine, M.R.; Dacey, R.G.; Huang, J.; et al. Detection of neoantigen-specific T cells following a personalized vaccine in a patient with glioblastoma. Oncoimmunology 2019, 8, e1561106. [Google Scholar] [CrossRef]
- De Mattos-Arruda, L.; Vazquez, M.; Finotello, F.; Lepore, R.; Porta, E.; Hundal, J.; Amengual-Rigo, P.; Ng, C.K.Y.; Valencia, A.; Carrillo, J.; et al. Neoantigen prediction and computational perspectives towards clinical benefit: Recommendations from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 978–990. [Google Scholar] [CrossRef]
- Kast, F.; Klein, C.; Umaña, P.; Gros, A.; Gasser, S. Advances in identification and selection of personalized neoantigen/T-cell pairs for autologous adoptive T cell therapies. Oncoimmunology 2021, 10, 1869389. [Google Scholar] [CrossRef]
- Jou, J.; Harrington, K.J.; Zocca, M.B.; Ehrnrooth, E.; Cohen, E.E.W. The Changing Landscape of Therapeutic Cancer Vaccines-Novel Platforms and Neoantigen Identification. Clin. Cancer Res. 2021, 27, 689–703. [Google Scholar] [CrossRef]
- Sneddon, S.; Rive, C.M.; Ma, S.; Dick, I.M.; Allcock, R.J.N.; Brown, S.D.; Holt, R.A.; Watson, M.; Leary, S.; Lee, Y.C.G.; et al. Identification of a CD8+ T-cell response to a predicted neoantigen in malignant mesothelioma. Oncoimmunology 2020, 9, 1684713. [Google Scholar] [CrossRef]
- Balachandran, V.P.; Łuksza, M.; Zhao, J.N.; Makarov, V.; Moral, J.A.; Remark, R.; Herbst, B.; Askan, G.; Bhanot, U.; Senbabaoglu, Y.; et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 2017, 551, 512–516. [Google Scholar] [CrossRef]
- Hiraoka, K.; Miyamoto, M.; Cho, Y.; Suzuoki, M.; Oshikiri, T.; Nakakubo, Y.; Itoh, T.; Ohbuchi, T.; Kondo, S.; Katoh, H. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br. J. Cancer 2006, 94, 275–280. [Google Scholar] [CrossRef]
- Brambilla, E.; Le Teuff, G.; Marguet, S.; Lantuejoul, S.; Dunant, A.; Graziano, S.; Pirker, R.; Douillard, J.Y.; Le Chevalier, T.; Filipits, M.; et al. Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2016, 34, 1223–1230. [Google Scholar] [CrossRef]
- Maby, P.; Galon, J.; Latouche, J.B. Frameshift mutations, neoantigens and tumor-specific CD8(+) T cells in microsatellite unstable colorectal cancers. Oncoimmunology 2016, 5, e1115943. [Google Scholar] [CrossRef]
- Strønen, E.; Toebes, M.; Kelderman, S.; van Buuren, M.M.; Yang, W.; van Rooij, N.; Donia, M.; Böschen, M.L.; Lund-Johansen, F.; Olweus, J.; et al. Targeting of cancer neoantigens with donor-derived T cell receptor repertoires. Science 2016, 352, 1337–1341. [Google Scholar] [CrossRef]
- Wilson, E.A.; Anderson, K.S. Lost in the crowd: Identifying targetable MHC class I neoepitopes for cancer immunotherapy. Expert. Rev. Proteomics 2018, 15, 1065–1077. [Google Scholar] [CrossRef]
- Robbins, P.F.; Lu, Y.C.; El-Gamil, M.; Li, Y.F.; Gross, C.; Gartner, J.; Lin, J.C.; Teer, J.K.; Cliften, P.; Tycksen, E.; et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 2013, 19, 747–752. [Google Scholar] [CrossRef]
- Restifo, N.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat. Rev. Immunol. 2012, 12, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Rowshanravan, B.; Halliday, N.; Sansom, D.M. CTLA-4: A moving target in immunotherapy. Blood 2018, 131, 58–67. [Google Scholar] [CrossRef]
- Ahn, M.J.; Sun, J.M.; Lee, S.H.; Ahn, J.S.; Park, K. EGFR TKI combination with immunotherapy in non-small cell lung cancer. Expert. Opin. Drug Saf. 2017, 16, 465–469. [Google Scholar] [CrossRef]
- Carreno, B.M.; Magrini, V.; Becker-Hapak, M.; Kaabinejadian, S.; Hundal, J.; Petti, A.A.; Ly, A.; Lie, W.R.; Hildebrand, W.H.; Mardis, E.R.; et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015, 348, 803–808. [Google Scholar] [CrossRef]
- Wu, W.; Chen, Y.; Huang, L.; Li, W.; Tao, C.; Shen, H. Point mutation screening of tumor neoantigens and peptide-induced specific cytotoxic T lymphocytes using The Cancer Genome Atlas database. Oncol. Lett. 2020, 20, 123. [Google Scholar] [CrossRef]
- Lybaert, L.; Lefever, S.; Fant, B.; Smits, E.; De Geest, B.; Breckpot, K.; Dirix, L.; Feldman, S.A.; van Criekinge, W.; Thielemans, K.; et al. Challenges in neoantigen-directed therapeutics. Cancer Cell 2023, 41, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Mascola, J.R.; Fauci, A.S. Novel vaccine technologies for the 21st century. Nat. Rev. Immunol. 2020, 20, 87–88. [Google Scholar] [CrossRef]
- Saxena, M.; van der Burg, S.H.; Melief, C.J.M.; Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 2021, 21, 360–378. [Google Scholar] [CrossRef]
- Niemi, J.V.L.; Sokolov, A.V.; Schiöth, H.B. Neoantigen Vaccines; Clinical Trials, Classes, Indications, Adjuvants and Combinatorial Treatments. Cancers 2022, 14, 5163. [Google Scholar] [CrossRef]
- Rock, K.L.; Reits, E.; Neefjes, J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016, 37, 724–737. [Google Scholar] [CrossRef]
- Bijker, M.S.; van den Eeden, S.J.; Franken, K.L.; Melief, C.J.; Offringa, R.; van der Burg, S.H. CD8+ CTL priming by exact peptide epitopes in incomplete Freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J. Immunol. 2007, 179, 5033–5040. [Google Scholar] [CrossRef] [PubMed]
- Slingluff, C.L., Jr. The present and future of peptide vaccines for cancer: Single or multiple, long or short, alone or in combination? Cancer J. 2011, 17, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Hodge, K.; Supabphol, S.; Kumar, P.; Poomipak, W.; Pisitkun, T. Recent developments in neoantigen-based cancer vaccines. Asian Pac. J. Allergy Immunol. 2020, 38, 91–101. [Google Scholar]
- Akazawa, Y.; Saito, Y.; Yoshikawa, T.; Saito, K.; Nosaka, K.; Shimomura, M.; Mizuno, S.; Nakamoto, Y.; Nakatsura, T. Efficacy of immunotherapy targeting the neoantigen derived from epidermal growth factor receptor T790M/C797S mutation in non-small cell lung cancer. Cancer Sci. 2020, 111, 2736–2746. [Google Scholar] [CrossRef]
- Takayama, K.; Sugawara, S.; Saijo, Y.; Maemondo, M.; Sato, A.; Takamori, S.; Harada, T.; Sasada, T.; Kakuma, T.; Kishimoto, J.; et al. Randomized Phase II Study of Docetaxel plus Personalized Peptide Vaccination versus Docetaxel plus Placebo for Patients with Previously Treated Advanced Wild Type EGFR Non-Small-Cell Lung Cancer. J. Immunol. Res. 2016, 2016, 1745108. [Google Scholar] [CrossRef]
- Vergati, M.; Intrivici, C.; Huen, N.Y.; Schlom, J.; Tsang, K.Y. Strategies for cancer vaccine development. J. Biomed. Biotechnol. 2010, 2010, 596432. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Yoshiyuki, K.; Watanabe, Y.; Sogo, Y.; Ohno, T.; Tsuji, N.M.; Ito, A. Cancer Immunotherapy: Comprehensive Mechanism Analysis of Mesoporous-Silica-Nanoparticle-Induced Cancer Immunotherapy (Adv. Healthcare Mater. 10/2016). Adv. Healthc. Mater. 2016, 5, 1246. [Google Scholar] [CrossRef]
- Yu, J.; Sun, H.; Cao, W.; Song, Y.; Jiang, Z. Research progress on dendritic cell vaccines in cancer immunotherapy. Exp. Hematol. Oncol. 2022, 11, 3. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Yang, J.C.; Restifo, N.P. Cancer immunotherapy: Moving beyond current vaccines. Nat. Med. 2004, 10, 909–915. [Google Scholar] [CrossRef]
- Bol, K.F.; Schreibelt, G.; Gerritsen, W.R.; de Vries, I.J.; Figdor, C.G. Dendritic Cell-Based Immunotherapy: State of the Art and Beyond. Clin. Cancer Res. 2016, 22, 1897–1906. [Google Scholar] [CrossRef]
- Sun, C.; Nagaoka, K.; Kobayashi, Y.; Maejima, K.; Nakagawa, H.; Nakajima, J.; Kakimi, K. Immunotherapies targeting neoantigens are effective in PD-1 blockade-resistant tumors. Int. J. Cancer 2023, 152, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Ingels, J.; De Cock, L.; Stevens, D.; Mayer, R.L.; Théry, F.; Sanchez, G.S.; Vermijlen, D.; Weening, K.; De Smet, S.; Lootens, N.; et al. Neoantigen-targeted dendritic cell vaccination in lung cancer patients induces long-lived T cells exhibiting the full differentiation spectrum. Cell Rep. Med. 2024, 101516. [Google Scholar] [CrossRef]
- Sun, C.; Nagaoka, K.; Kobayashi, Y.; Nakagawa, H.; Kakimi, K.; Nakajima, J. Neoantigen Dendritic Cell Vaccination Combined with Anti-CD38 and CpG Elicits Anti-Tumor Immunity against the Immune Checkpoint Therapy-Resistant Murine Lung Cancer Cell Line LLC1. Cancers 2021, 13, 5508. [Google Scholar] [CrossRef]
- Esprit, A.; de Mey, W.; Bahadur Shahi, R.; Thielemans, K.; Franceschini, L.; Breckpot, K. Neo-Antigen mRNA Vaccines. Vaccines 2020, 8, 776. [Google Scholar] [CrossRef] [PubMed]
- Fritah, H.; Rovelli, R.; Chiang, C.L.; Kandalaft, L.E. The current clinical landscape of personalized cancer vaccines. Cancer Treat. Rev. 2022, 106, 102383. [Google Scholar] [CrossRef]
- Gebre, M.S.; Brito, L.A.; Tostanoski, L.H.; Edwards, D.K.; Carfi, A.; Barouch, D.H. Novel approaches for vaccine development. Cell 2021, 184, 1589–1603. [Google Scholar] [CrossRef]
- Veisi Malekshahi, Z.; Hashemi Goradel, N.; Shakouri Khomartash, M.; Maleksabet, A.; Kadkhodazadeh, M.; Kardar, G.A.; Negahdari, B. CEA Plasmid as Therapeutic DNA Vaccination against Colorectal Cancer. Iran. J. Immunol. 2019, 16, 235–245. [Google Scholar]
- Yarchoan, M.; Gane, E.J.; Marron, T.U.; Perales-Linares, R.; Yan, J.; Cooch, N.; Shu, D.H.; Fertig, E.J.; Kagohara, L.T.; Bartha, G.; et al. Personalized neoantigen vaccine and pembrolizumab in advanced hepatocellular carcinoma: A phase 1/2 trial. Nat. Med. 2024, 30, 1044–1053. [Google Scholar] [CrossRef]
- Cheng, R.; Xu, Z.; Luo, M.; Wang, P.; Cao, H.; Jin, X.; Zhou, W.; Xiao, L.; Jiang, Q. Identification of alternative splicing-derived cancer neoantigens for mRNA vaccine development. Brief. Bioinform. 2022, 23, bbab553. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, X.; Wang, Y.; Zhang, Y. Targeting neoantigens for cancer immunotherapy. Biomark. Res. 2021, 9, 61. [Google Scholar] [CrossRef]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Miao, L.; Sui, J.; Hao, Y.; Huang, G. Nanoparticle cancer vaccines: Design considerations and recent advances. Asian J. Pharm. Sci. 2020, 15, 576–590. [Google Scholar] [CrossRef]
- Oosting, L.T.; Franke, K.; Martin, M.V.; Kloosterman, W.P.; Jamieson, J.A.; Glenn, L.A.; de Jager, M.W.; van Zanten, J.; Allersma, D.P.; Gareb, B. Development of a Personalized Tumor Neoantigen Based Vaccine Formulation (FRAME-001) for Use in a Phase II Trial for the Treatment of Advanced Non-Small Cell Lung Cancer. Pharmaceutics 2022, 14, 1515. [Google Scholar] [CrossRef] [PubMed]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef]
- D’Alise, A.M.; Leoni, G.; Cotugno, G.; Siani, L.; Vitale, R.; Ruzza, V.; Garzia, I.; Antonucci, L.; Micarelli, E.; Venafra, V.; et al. Phase I trial of viral vector based personalized vaccination elicits robust neoantigen specific antitumor T cell responses. Clin. Cancer Res. 2024, 30, 2412–2423. [Google Scholar] [CrossRef]
- Rappaport, A.R.; Kyi, C.; Lane, M.; Hart, M.G.; Johnson, M.L.; Henick, B.S.; Liao, C.Y.; Mahipal, A.; Shergill, A.; Spira, A.I.; et al. A shared neoantigen vaccine combined with immune checkpoint blockade for advanced metastatic solid tumors: Phase 1 trial interim results. Nat. Med. 2024, 30, 1013–1022. [Google Scholar] [CrossRef]
- Yamamoto, T.N.; Kishton, R.J.; Restifo, N.P. Developing neoantigen-targeted T cell-based treatments for solid tumors. Nat. Med. 2019, 25, 1488–1499. [Google Scholar] [CrossRef]
- Komuro, H.; Shinohara, S.; Fukushima, Y.; Demachi-Okamura, A.; Muraoka, D.; Masago, K.; Matsui, T.; Sugita, Y.; Takahashi, Y.; Nishida, R.; et al. Single-cell sequencing on CD8(+) TILs revealed the nature of exhausted T cells recognizing neoantigen and cancer/testis antigen in non-small cell lung cancer. J. Immunother. Cancer 2023, 11, e007180. [Google Scholar] [CrossRef]
- Zheng, Y.W.; Li, R.M.; Wei, F.; Liu, L.; Zhang, X.; Ren, X. A Meta-analysis of adoptive immunotherapy combined with chemo /radio therapy in the treatment of non-small cell lung cancer. Chinses J. Cancer Biother. 2013, 20, 461–467. [Google Scholar]
- Xia, Y.; Tian, X.; Wang, J.; Qiao, D.; Liu, X.; Xiao, L.; Liang, W.; Ban, D.; Chu, J.; Yu, J.; et al. Treatment of metastatic non-small cell lung cancer with NY-ESO-1 specific TCR engineered-T cells in a phase I clinical trial: A case report. Oncol. Lett. 2018, 16, 6998–7007. [Google Scholar] [CrossRef]
- Hsiue, E.H.; Wright, K.M.; Douglass, J.; Hwang, M.S.; Mog, B.J.; Pearlman, A.H.; Paul, S.; DiNapoli, S.R.; Konig, M.F.; Wang, Q.; et al. Targeting a neoantigen derived from a common TP53 mutation. Science 2021, 371, eabc8697. [Google Scholar] [CrossRef]
- Pearlman, A.H.; Hwang, M.S.; Konig, M.F.; Hsiue, E.H.; Douglass, J.; DiNapoli, S.R.; Mog, B.J.; Bettegowda, C.; Pardoll, D.M.; Gabelli, S.B.; et al. Targeting public neoantigens for cancer immunotherapy. Nat. Cancer 2021, 2, 487–497. [Google Scholar] [CrossRef]
- Douglass, J.; Hsiue, E.H.; Mog, B.J.; Hwang, M.S.; DiNapoli, S.R.; Pearlman, A.H.; Miller, M.S.; Wright, K.M.; Azurmendi, P.A.; Wang, Q.; et al. Bispecific antibodies targeting mutant RAS neoantigens. Sci. Immunol. 2021, 6, eabd5515. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu-Lieskovan, S.; Chmielowski, B.; Govindan, R.; Naing, A.; Bhardwaj, N.; Margolin, K.; Awad, M.M.; Hellmann, M.D.; Lin, J.J.; et al. A Phase Ib Trial of Personalized Neoantigen Therapy Plus Anti-PD-1 in Patients with Advanced Melanoma, Non-Small Cell Lung Cancer, or Bladder Cancer. Cell 2020, 183, 347–362.e324. [Google Scholar] [CrossRef]
- Palmer, C.D.; Rappaport, A.R.; Davis, M.J.; Hart, M.G.; Scallan, C.D.; Hong, S.J.; Gitlin, L.; Kraemer, L.D.; Kounlavouth, S.; Yang, A.; et al. Individualized, heterologous chimpanzee adenovirus and self-amplifying mRNA neoantigen vaccine for advanced metastatic solid tumors: Phase 1 trial interim results. Nat. Med. 2022, 28, 1619–1629. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Zhang, L.; Wei, F.; Lian, Y.; Wu, Y.; Gong, Z.; Zhang, S.; Zhou, J.; Cao, K.; et al. Role of tumor microenvironment in tumorigenesis. J. Cancer 2017, 8, 761–773. [Google Scholar] [CrossRef]
- Zhou, W.J.; Li, Y.H. Clinical transformation of personalized neoantigen vaccine: Opportunities and challenge. Chinses J. Cancer Biother. 2019, 26, 16–21. [Google Scholar]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C.X. Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 156. [Google Scholar] [CrossRef]
- Li, J.; Xiao, Z.; Wang, D.; Jia, L.; Nie, S.; Zeng, X.; Hu, W. The screening, identification, design and clinical application of tumor-specific neoantigens for TCR-T cells. Mol. Cancer 2023, 22, 141. [Google Scholar] [CrossRef]
- Taghikhani, A.; Farzaneh, F.; Sharifzad, F.; Mardpour, S.; Ebrahimi, M.; Hassan, Z.M. Engineered Tumor-Derived Extracellular Vesicles: Potentials in Cancer Immunotherapy. Front. Immunol. 2020, 11, 221. [Google Scholar] [CrossRef]
- Pan, R.Y.; Chung, W.H.; Chu, M.T.; Chen, S.J.; Chen, H.C.; Zheng, L.; Hung, S.I. Recent Development and Clinical Application of Cancer Vaccine: Targeting Neoantigens. J. Immunol. Res. 2018, 2018, 4325874. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.W.; Cho, W.C. Drug Repurposing to Circumvent Immune Checkpoint Inhibitor Resistance in Cancer Immunotherapy. Pharmaceutics 2023, 15, 2166. [Google Scholar] [CrossRef] [PubMed]
- Zou, D.; Song, A.; Yong, W. Prognostic role of IL-8 in cancer patients treated with immune checkpoint inhibitors: A system review and meta-analysis. Front. Oncol. 2023, 13, 1176574. [Google Scholar] [CrossRef]
- Makaremi, S.; Asadzadeh, Z.; Hemmat, N.; Baghbanzadeh, A.; Sgambato, A.; Ghorbaninezhad, F.; Safarpour, H.; Argentiero, A.; Brunetti, O.; Bernardini, R.; et al. Immune Checkpoint Inhibitors in Colorectal Cancer: Challenges and Future Prospects. Biomedicines 2021, 9, 1075. [Google Scholar] [CrossRef]
- Thommen, D.S.; Koelzer, V.H.; Herzig, P.; Roller, A.; Trefny, M.; Dimeloe, S.; Kiialainen, A.; Hanhart, J.; Schill, C.; Hess, C.; et al. A transcriptionally and functionally distinct PD-1(+) CD8(+) T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 2018, 24, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Gros, A.; Parkhurst, M.R.; Tran, E.; Pasetto, A.; Robbins, P.F.; Ilyas, S.; Prickett, T.D.; Gartner, J.J.; Crystal, J.S.; Roberts, I.M.; et al. Prospective identification of neoantigen-specific lymphocytes in the peripheral blood of melanoma patients. Nat. Med. 2016, 22, 433–438. [Google Scholar] [CrossRef]
- Lopez de Rodas, M.; Schalper, K.A. Tumour antigen-induced T cell exhaustion—The archenemy of immune-hot malignancies. Nat. Rev. Clin. Oncol. 2021, 18, 749–750. [Google Scholar] [CrossRef]
- Kamphorst, A.O.; Pillai, R.N.; Yang, S.; Nasti, T.H.; Akondy, R.S.; Wieland, A.; Sica, G.L.; Yu, K.; Koenig, L.; Patel, N.T.; et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc. Natl. Acad. Sci. USA 2017, 114, 4993–4998. [Google Scholar] [CrossRef]
- Simoni, Y.; Becht, E.; Fehlings, M.; Loh, C.Y.; Koo, S.L.; Teng, K.W.W.; Yeong, J.P.S.; Nahar, R.; Zhang, T.; Kared, H.; et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018, 557, 575–579. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Wang, S.Z.; Falchook, G.S.; Charlton, J.; Burris, H.A. A phase I, open-label, multicenter dose escalation study to assess the safety, tolerability, and pharmacokinetics of AZD2811 nanoparticle in patients with advanced solid tumors. J. Clin. Oncol. 2018, 36, 2592. [Google Scholar] [CrossRef]
- Awad, M.M.; Govindan, R.; Balogh, K.N.; Spigel, D.R.; Garon, E.B.; Bushway, M.E.; Poran, A.; Sheen, J.H.; Kohler, V.; Esaulova, E.; et al. Personalized neoantigen vaccine NEO-PV-01 with chemotherapy and anti-PD-1 as first-line treatment for non-squamous non-small cell lung cancer. Cancer Cell 2022, 40, 1010–1026.e1011. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, J.; Zhu, Z.; Fang, J.; Zhao, Y.; Liu, Z.; Qin, H.; Wei, Y.; Xu, H.; Dan, X.; et al. Effective personalized neoantigen vaccine plus anti-PD-1 in a PD-1 blockade-resistant lung cancer patient. Immunotherapy 2023, 15, 57–69. [Google Scholar] [CrossRef]
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217. [Google Scholar] [CrossRef]
- Formenti, S.C.; Rudqvist, N.P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef]
- Garnett, C.T.; Palena, C.; Chakraborty, M.; Tsang, K.Y.; Schlom, J.; Hodge, J.W. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004, 64, 7985–7994. [Google Scholar] [CrossRef]
- Lhuillier, C.; Rudqvist, N.P.; Yamazaki, T.; Zhang, T.; Charpentier, M.; Galluzzi, L.; Dephoure, N.; Clement, C.C.; Santambrogio, L.; Zhou, X.K.; et al. Radiotherapy-exposed CD8+ and CD4+ neoantigens enhance tumor control. J. Clin. Invest. 2021, 131, e138740. [Google Scholar] [CrossRef]
- Sharma, A.; Bode, B.; Wenger, R.H.; Lehmann, K.; Sartori, A.A.; Moch, H.; Knuth, A.; Boehmer, L.; Broek, M. γ-Radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens In Vitro and In Vivo. PLoS ONE 2011, 6, e28217. [Google Scholar] [CrossRef]
- Lin, X.; Tang, S.; Guo, Y.; Tang, R.; Li, Z.; Pan, X.; Chen, G.; Qiu, L.; Dong, X.; Zhang, L.; et al. Personalized neoantigen vaccine enhances the therapeutic efficacy of bevacizumab and anti-PD-1 antibody in advanced non-small cell lung cancer. Cancer Immunol. Immunother. 2024, 73, 26. [Google Scholar] [CrossRef]
- Frankiw, L.; Baltimore, D.; Li, G. Alternative mRNA splicing in cancer immunotherapy. Nat. Rev. Immunol. 2019, 19, 675–687. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Furness, A.J.; Rosenthal, R.; Ramskov, S.; Lyngaa, R.; Saini, S.K.; Jamal-Hanjani, M.; Wilson, G.A.; Birkbak, N.J.; Hiley, C.T.; et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016, 351, 1463–1469. [Google Scholar] [CrossRef]
- Heide, T.; Househam, J.; Cresswell, G.D.; Spiteri, I.; Lynn, C.; Mossner, M.; Kimberley, C.; Fernandez-Mateos, J.; Chen, B.; Zapata, L.; et al. The co-evolution of the genome and epigenome in colorectal cancer. Nature 2022, 611, 733–743. [Google Scholar] [CrossRef]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Vitale, I.; Shema, E.; Loi, S.; Galluzzi, L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat. Med. 2021, 27, 212–224. [Google Scholar] [CrossRef]
- Shen, Y.; Yu, L.; Xu, X.; Yu, S.; Yu, Z. Neoantigen vaccine and neoantigen-specific cell adoptive transfer therapy in solid tumors: Challenges and future directions. Cancer Innov. 2022, 1, 168–182. [Google Scholar] [CrossRef]
- Yan, T.; Yu, L.; Zhang, N.; Peng, C.; Su, G.; Jing, Y.; Zhang, L.; Wu, T.; Cheng, J.; Guo, Q.; et al. The advanced development of molecular targeted therapy for hepatocellular carcinoma. Cancer Biol. Med. 2022, 19, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Motamedi, H.; Ari, M.M.; Shahlaei, M.; Moradi, S.; Farhadikia, P.; Alvandi, A.; Abiri, R. Designing multi-epitope vaccine against important colorectal cancer (CRC) associated pathogens based on immunoinformatics approach. BMC Bioinform. 2023, 24, 65. [Google Scholar] [CrossRef]
- Kristensen, V.N. The Antigenicity of the Tumor Cell—Context Matters. N. Engl. J. Med. 2017, 376, 491–493. [Google Scholar] [CrossRef]
- McGranahan, N.; Rosenthal, R.; Hiley, C.T.; Rowan, A.J.; Watkins, T.B.K.; Wilson, G.A.; Birkbak, N.J.; Veeriah, S.; Van Loo, P.; Herrero, J.; et al. Allele-Specific HLA Loss and Immune Escape in Lung Cancer Evolution. Cell 2017, 171, 1259–1271.e1211. [Google Scholar] [CrossRef]
- Hov, J.R.; Lleo, A.; Selmi, C.; Woldseth, B.; Fabris, L.; Strazzabosco, M.; Karlsen, T.H.; Invernizzi, P. Genetic associations in Italian primary sclerosing cholangitis: Heterogeneity across Europe defines a critical role for HLA-C. J. Hepatol. 2010, 52, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhang, Z.; Zhu, J.; Wang, Y.; Jiang, P.; Xiao, X.; Bernatchez, C.; Heymach, J.V.; Gibbons, D.L.; Wang, J.; et al. Deep learning-based prediction of the T cell receptor-antigen binding specificity. Nat. Mach. Intell. 2021, 3, 864–875. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Huber, F.; Arnaud, M.; Kraemer, A.I.; Altimiras, E.R.; Michaux, J.; Taillandier-Coindard, M.; Chiffelle, J.; Murgues, B.; Gehret, T.; et al. Machine learning methods and harmonized datasets improve immunogenic neoantigen prediction. Immunity 2023, 56, 2650–2663.e2656. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, J. The Role of Neoantigens in Cancer Immunotherapy. Front. Oncol. 2021, 11, 682325. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, Y.; Wang, J.; Li, M.; Peng, L.; Wei, G.; Zhang, Y.; Li, J.; Gao, Z. TruNeo: An integrated pipeline improves personalized true tumor neoantigen identification. BMC Bioinform. 2020, 21, 532. [Google Scholar] [CrossRef]
- Wu, J.; Chen, W.; Zhou, Y.; Chi, Y.; Hua, X.; Wu, J.; Gu, X.; Chen, S.; Zhou, Z. TSNAdb v2.0: The Updated Version of Tumor-specific Neoantigen Database. Genom. Proteom. Bioinform. 2023, 21, 259–266. [Google Scholar] [CrossRef]
- Lundegaard, C.; Lund, O.; Nielsen, M. Predictions versus high-throughput experiments in T-cell epitope discovery: Competition or synergy? Expert. Rev. Vaccines 2012, 11, 43–54. [Google Scholar] [CrossRef]
- Comber, J.D.; Philip, R. MHC class I antigen presentation and implications for developing a new generation of therapeutic vaccines. Ther. Adv. Vaccines 2014, 2, 77–89. [Google Scholar] [CrossRef]
- Clara, J.A.; Monge, C.; Yang, Y.; Takebe, N. Targeting signalling pathways and the immune microenvironment of cancer stem cells—A clinical update. Nat. Rev. Clin. Oncol. 2020, 17, 204–232. [Google Scholar] [CrossRef]
- Kaymak, I.; Williams, K.S.; Cantor, J.R.; Jones, R.G. Immunometabolic Interplay in the Tumor Microenvironment. Cancer Cell 2021, 39, 28–37. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.; Chang, C.Y.; Wang, H.Y.; Wang, R.F. The Interplay between T Cells and Cancer: The Basis of Immunotherapy. Genes 2023, 14, 1008. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.T.; Yang, K.P.; Zhou, H.N.; Huang, Y.F.; Li, H.; Zong, Z. Landscapes and mechanisms of CD8(+) T cell exhaustion in gastrointestinal cancer. Front. Immunol. 2023, 14, 1149622. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, S.; Wan, L.; Ling, W.; Chen, H.; Wang, J. New developments in the mechanism and application of immune checkpoint inhibitors in cancer therapy (Review). Int. J. Oncol. 2023, 63, 86. [Google Scholar] [CrossRef]
- Grund-Gröschke, S.; Stockmaier, G.; Aberger, F. Hedgehog/GLI signaling in tumor immunity—New therapeutic opportunities and clinical implications. Cell Commun. Signal. 2019, 17, 172. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Y.; Liu, S. The role of epithelial-mesenchymal transition and autophagy in pancreatic ductal adenocarcinoma invasion. Cell Death Dis. 2023, 14, 506. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, R.; Cadieux, E.L.; Salgado, R.; Bakir, M.A.; Moore, D.A.; Hiley, C.T.; Lund, T.; Tanić, M.; Reading, J.L.; Joshi, K.; et al. Neoantigen-directed immune escape in lung cancer evolution. Nature 2019, 567, 479–485. [Google Scholar] [CrossRef]
- Sabbatino, F.; Villani, V.; Yearley, J.H.; Deshpande, V.; Cai, L.; Konstantinidis, I.T.; Moon, C.; Nota, S.; Wang, Y.; Al-Sukaini, A.; et al. PD-L1 and HLA Class I Antigen Expression and Clinical Course of the Disease in Intrahepatic Cholangiocarcinoma. Clin. Cancer Res. 2016, 22, 470–478. [Google Scholar] [CrossRef]
- Rosenbaum, S.R.; Wilski, N.A.; Aplin, A.E. Fueling the Fire: Inflammatory Forms of Cell Death and Implications for Cancer Immunotherapy. Cancer Discov. 2021, 11, 266–281. [Google Scholar] [CrossRef]
- Li, X.; You, J.; Hong, L.; Liu, W.; Guo, P.; Hao, X. Neoantigen cancer vaccines: A new star on the horizon. Cancer Biol. Med. 2023, 21, 274–311. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Z.; Dai, X.; Zhang, X.; Wang, X. Research progress of neoantigen-based dendritic cell vaccines in pancreatic cancer. Front. Immunol. 2023, 14, 1104860. [Google Scholar] [CrossRef]
- Tran, E.; Robbins, P.F.; Rosenberg, S.A. ‘Final common pathway’ of human cancer immunotherapy: Targeting random somatic mutations. Nat. Immunol. 2017, 18, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Schreiber, R.D. Neoantigens in cancer immunotherapy. Science 2015, 348, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, C.S.; Hsu, J.C.; Hosseini, I.; Shen, B.Q.; Rotte, A.; Twomey, P.; Girish, S.; Wu, B. Personalized Cancer Vaccines: Clinical Landscape, Challenges, and Opportunities. Mol. Ther. 2021, 29, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wu, J.; Chen, S.; Zhou, Z. Shared neoantigens: Ideal targets for off-the-shelf cancer immunotherapy. Pharmacogenomics 2020, 21, 637–645. [Google Scholar] [CrossRef]
- Thol, K.; McGranahan, N. Potential Use of Shared Frameshift Mutations in ‘Off-the-Shelf’ Neoantigen Vaccines. Trends Cancer 2021, 7, 175–177. [Google Scholar] [CrossRef]
- Freudenmann, L.K.; Marcu, A.; Stevanović, S. Mapping the tumour human leukocyte antigen (HLA) ligandome by mass spectrometry. Immunology 2018, 154, 331–345. [Google Scholar] [CrossRef]
- van Rooij, N.; van Buuren, M.M.; Philips, D.; Velds, A.; Toebes, M.; Heemskerk, B.; van Dijk, L.J.; Behjati, S.; Hilkmann, H.; El Atmioui, D.; et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J. Clin. Oncol. 2013, 31, e439–e442. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- Anagnostou, V.; Smith, K.N.; Forde, P.M.; Niknafs, N.; Bhattacharya, R.; White, J.; Zhang, T.; Adleff, V.; Phallen, J.; Wali, N.; et al. Evolution of Neoantigen Landscape during Immune Checkpoint Blockade in Non-Small Cell Lung Cancer. Cancer Discov. 2017, 7, 264–276. [Google Scholar] [CrossRef]
- Hoos, A. Development of immuno-oncology drugs—From CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov. 2016, 15, 235–247. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Hoos, A.; O’Day, S.; Weber, J.S.; Hamid, O.; Lebbé, C.; Maio, M.; Binder, M.; Bohnsack, O.; Nichol, G.; et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin. Cancer Res. 2009, 15, 7412–7420. [Google Scholar] [CrossRef] [PubMed]
- Al Bakir, M.; Reading, J.L.; Gamble, S.; Rosenthal, R.; Uddin, I.; Rowan, A.; Przewrocka, J.; Rogers, A.; Wong, Y.N.S.; Bentzen, A.K.; et al. Clonal driver neoantigen loss under EGFR TKI and immune selection pressures. Nature 2025, 639, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Powles, T.; Braiteh, F.; Siu, L.L.; LoRusso, P.; Friedman, C.F.; Balmanoukian, A.S.; Gordon, M.; Yachnin, J.; Rottey, S.; et al. Autogene cevumeran with or without atezolizumab in advanced solid tumors: A phase 1 trial. Nat. Med. 2025, 31, 152–164. [Google Scholar] [CrossRef]
- Emmers, M.; Welters, M.J.P.; Dietz, M.V.; Santegoets, S.J.; Boekesteijn, S.; Stolk, A.; Loof, N.M.; Dumoulin, D.W.; Geel, A.L.; Steinbusch, L.C.; et al. TEIPP-vaccination in checkpoint-resistant non-small cell lung cancer: A first-in-human phase I/II dose-escalation study. Nat. Commun. 2025, 16, 4958. [Google Scholar] [CrossRef]
- Resistance to TIL therapy in lung cancer mediated by clonal T cell loss and neoantigen escape. Nat. Cancer 2025, 6, 749–750. [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Shah, N.M.; Maeng, J.H.; Liang, Y.; Basri, N.L.; Ge, J.; Qu, X.; Mahlokozera, T.; Tzeng, S.C.; Williams, R.B.; et al. Epigenetic therapy potentiates transposable element transcription to create tumor-enriched antigens in glioblastoma cells. Nat. Genet. 2024, 56, 1903–1913. [Google Scholar] [CrossRef]
- Olsen, H.E.; Lynn, G.M.; Valdes, P.A.; Cerecedo Lopez, C.D.; Ishizuka, A.S.; Arnaout, O.; Bi, W.L.; Peruzzi, P.P.; Chiocca, E.A.; Friedman, G.K.; et al. Therapeutic cancer vaccines for pediatric malignancies: Advances, challenges, and emerging technologies. Neurooncol Adv. 2021, 3, vdab027. [Google Scholar] [CrossRef]
- Hu, Z.; Ott, P.A.; Wu, C.J. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat. Rev. Immunol. 2018, 18, 168–182. [Google Scholar] [CrossRef]

| Vaccine Type | Advantages | Challenges | Differentiations | Common Features |
|---|---|---|---|---|
| Peptide/Protein vaccines | Direct antigen presentation Customizable for MHC-I (short peptides) or MHC-II (long peptides) | Low stability (rapid degradation) Limited immunogenicity | Production complexity: Very High Cost: Very High Target cohort: Individualized | 1. Activate tumor-specific T cell immunity against neoantigens. 2. Rely on genomic sequencing and bioinformatic neoantigen prediction. 3. Most show enhanced efficacy with ICIs or conventional therapies. 4. Ranging from fully individualized (DC/peptide vaccines) to semi-/non-personalized (shared/viral vector vaccines). |
| DC vaccines | High antigen-presenting efficiency Induces long-term immune memory | Complex/costly production Requires strict personalized protocols | Production complexity: Low Cost: Medium Target cohort: Semi-personalized | |
| Nucleic acid vaccines | Rapid manufacturing Multi-epitope encoding mRNA avoids insertional mutagenesis risks | Low DNA transfection efficiency mRNA requires cold-chain storage | Production complexity: Low Cost: Low Target cohort: Broad (mutation-dependent) | |
| Viral vector vaccines | High gene delivery efficiency Intrinsic adjuvant effect | Preexisting immunity reduces efficacy Vector-related toxicity risks | Production complexity: Medium Cost: Medium Target cohort: Broad (if no pre-immunity) | |
| Fusion protein vaccines | Boosts immunogenicity Modulates specific immune pathways | Complex design/engineering Risk of uncontrolled inflammation | Production complexity: High Cost: High Target cohort: Pathway-specific | |
| Shared neoantigen vaccines | No full personalization needed Low-cost/scalable production | Limited eligible patients Suboptimal immunogenicity in some cases | Production complexity: LowCost: LowTarget cohort: Broad (mutation-dependent) |
| Trail Number | Therapy Type | Tumor | Intervention | Adjuvant Therapy | Stage | Patients | Status |
|---|---|---|---|---|---|---|---|
| NCT04397926 | Vaccine | NSCLC | Individualized neoantigen peptides vaccine | / | Phase 1 | 20 | Unknown status |
| NCT04266730 | Vaccine | Lung cancer | PANDA-VAC(Peptide vaccine) | Pembrolizumab | Phase 1 | 6 | Not yet recruiting |
| NCT05269381 | Vaccine | Lung Cancer | Neoantigen Peptide Vaccine | Pembrolizumab | Phase 1 | 36 | Recruiting |
| NCT06095934 | Vaccine | NSCLC | Neoantigen Peptide Vaccine | / | / | 20 | Recruiting |
| NCT06751901 | Vaccine | Advanced NSCLC | Neoantigen-based peptide vaccine | Radiotherapy PD-1 inhibitor | Phase 2 | 10 | Recruiting |
| NCT05098210 | Vaccine | Stage III-IV NSCLC | Neoantigen Peptide Vaccine | Nivolumab Poly ICLC | Phase 1 | 25 | Recruiting |
| NCT04397003 | Vaccine | Extensive-stage SCLC | Neoantigen DNA Vaccine | Durvalumab | Phase 2 | 27 | Recruiting |
| NCT02897765 | Vaccine | Lung cancer | NEO-PV-01(DNA Vaccine) | Nivolumab | Phase 1 | 34 | Completed |
| NCT03380871 | Vaccine | NSCLC | NEO-PV-01(DNA Vaccine) | Pembrolizumab Carboplatin Pemetrexed | Phase 1 | 38 | Completed |
| NCT03908671 | Vaccine | NSCLC | Personalized mRNA Tumor Vaccine | / | / | 24 | Recruiting |
| NCT03639714 | Vaccine | NSCLC | GRT-C901/GRT-C902(mRNA Vaccine) | Nivolumab Ipilimumab | Phase1/2 | 29 | Completed |
| NCT06735508 | Vaccine | NSCLC | mRNA Neoantigen Vaccine | Adebrelimab | Phase1 | 40 | Not yet recruiting |
| NCT06685653 | Vaccine | NSCLC | RGL-270 (mRNA vaccine) | Adebrelimab | Phase 1 | 65 | Not yet recruiting |
| NCT04078269 | Vaccine | NSCLC | MIDRIXNEO-LUNG (DC Vaccine) | / | Phase 1 | 6 | Completed |
| NCT03871205 | Vaccine | Lung cancer | Neoantigen loaded DC Vaccine | / | Phase 1 | 30 | Unknown status |
| NCT02956551 | Vaccine | NSCLC | DC Vaccine | / | Phase 1 | 20 | Unknown status |
| NCT04147078 | Vaccine | NSCLC | DC Vaccine | / | Phase 1 | 80 | Recruiting |
| NCT06751849 | Vaccine | Advanced NSCLC | Neoantigen loaded DC vaccine | Radiotherapy PD-1 inhibitor | Phase 2 | 10 | Recruiting |
| NCT05886439 | Vaccine | Advanced lung carcinoma | LK101(personlized neoantigen pulsed DC Vaccine) | Pembrolizumab; Durvalumab | Phase 1 | 40 | Recruiting |
| NCT06329908 | Vaccine | Neo-DCVac | Lung Cancer | ICIs | Phase 1 | 20 | Recruiting |
| NCT04487093 | Vaccine | NSLCL | neoantigen vaccine | EGFR-TKI Anti-angiogenesis drug | Phase 1 | 20 | Unknown status |
| NCT03807102 | Vaccine | Lung cancer | Neoantigen tumor vaccine | / | Phase1/2 | 30 | Not yet recruiting |
| NCT04998474 | Vaccine | NSCLC | FRAME-001 personalized vaccine | / | Phase 2 | 15 | Unknown status |
| NCT03633110 | Vaccine | NSCLC | GEN-009 Adjuvanted Vaccine | Nivolumab; Pembrolizumab | Phase 1/2 | 24 | Completed |
| NCT03715985 | Vaccine | NSCLC Metastatic | EVAX-01- CAF09b | / | Phase1/2 | 12 | Unknown status |
| NCT03953235 | Vaccine | NSCLC | GRT-C903/GRT-C904 | Nivolumab; Ipilimumab | Phase 1/2 | 39 | Completed |
| NCT03552718 | Vaccine | NSCLC | YE-NEO-001 | / | Phase 1 | 16 | Active, not recruiting |
| NCT03205930 | Vaccine | NSCLC Stage IV | Neo-MASCT | / | Phase 1/2 | 20 | Unknown status |
| NCT03289962 | Vaccine | NSCLC | RO7198457 | Atezolizumab | Phase 1 | 272 | Active, not recruiting |
| NCT05292859 | TCR-T | Squamous cell lung Cancer; Adenocarcinoma of lung; Adenosquamous cell Lung Cancer | Neoantigen specific TCR-T cell drug product | / | / | 8 | Active, not recruiting |
| NCT05194735 | TCR-T | NSCLC Squamous Cell Lung Cancer; Lung Adenocarcinoma; Adenosquamous cell lung cancer | Neoantigen specific TCR-T cell drug product | / | Phase 1/2 | 18 | Active, not recruiting |
| NCT03970382 | TCR-T | NSCLC | NeoTCR-P1 adoptive cell therapy | Nivolumab | Phase 1 | 21 | Suspended |
| NCT03412877 | TCR-T | NSCLC | Individual Patient TCR-Transduced PBL | Cyclophosphamide Fludarabine Aldesleukin Pembrolizumab (KEYTRUDA) | Phase 2 | 270 | Recruiting |
| NCT05798533 | ACT | NSCLC | Neo-T | Toripalimab; Tislelizumab | Phase 1 | 6 | Unknown status |
| NCT05798546 | ACT | NSCLC | Neo-T | Cyclophosphamide; Fludarabine; Interleukin-2 | Phase 1 | 21 | Unknown status |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhu, Y.-J.; Xue, Y.; Chen, T.-T.; Sun, X.-K.; Shi, H.-Y. Neoantigen-Based Immunotherapy in Lung Cancer: Advances, Challenges and Prospects. Cancers 2025, 17, 1953. https://doi.org/10.3390/cancers17121953
Li X, Zhu Y-J, Xue Y, Chen T-T, Sun X-K, Shi H-Y. Neoantigen-Based Immunotherapy in Lung Cancer: Advances, Challenges and Prospects. Cancers. 2025; 17(12):1953. https://doi.org/10.3390/cancers17121953
Chicago/Turabian StyleLi, Xiong, Ya-Juan Zhu, Ying Xue, Ting-Ting Chen, Xiao-Ke Sun, and Hong-Yang Shi. 2025. "Neoantigen-Based Immunotherapy in Lung Cancer: Advances, Challenges and Prospects" Cancers 17, no. 12: 1953. https://doi.org/10.3390/cancers17121953
APA StyleLi, X., Zhu, Y.-J., Xue, Y., Chen, T.-T., Sun, X.-K., & Shi, H.-Y. (2025). Neoantigen-Based Immunotherapy in Lung Cancer: Advances, Challenges and Prospects. Cancers, 17(12), 1953. https://doi.org/10.3390/cancers17121953





