Risk for Recurrence After Liver Resection in Patients with Hepatitis C Virus-Related Hepatocellular Carcinoma Detected After Sustained Virological Response by Direct-Acting Antivirals: A Retrospective Multicenter Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
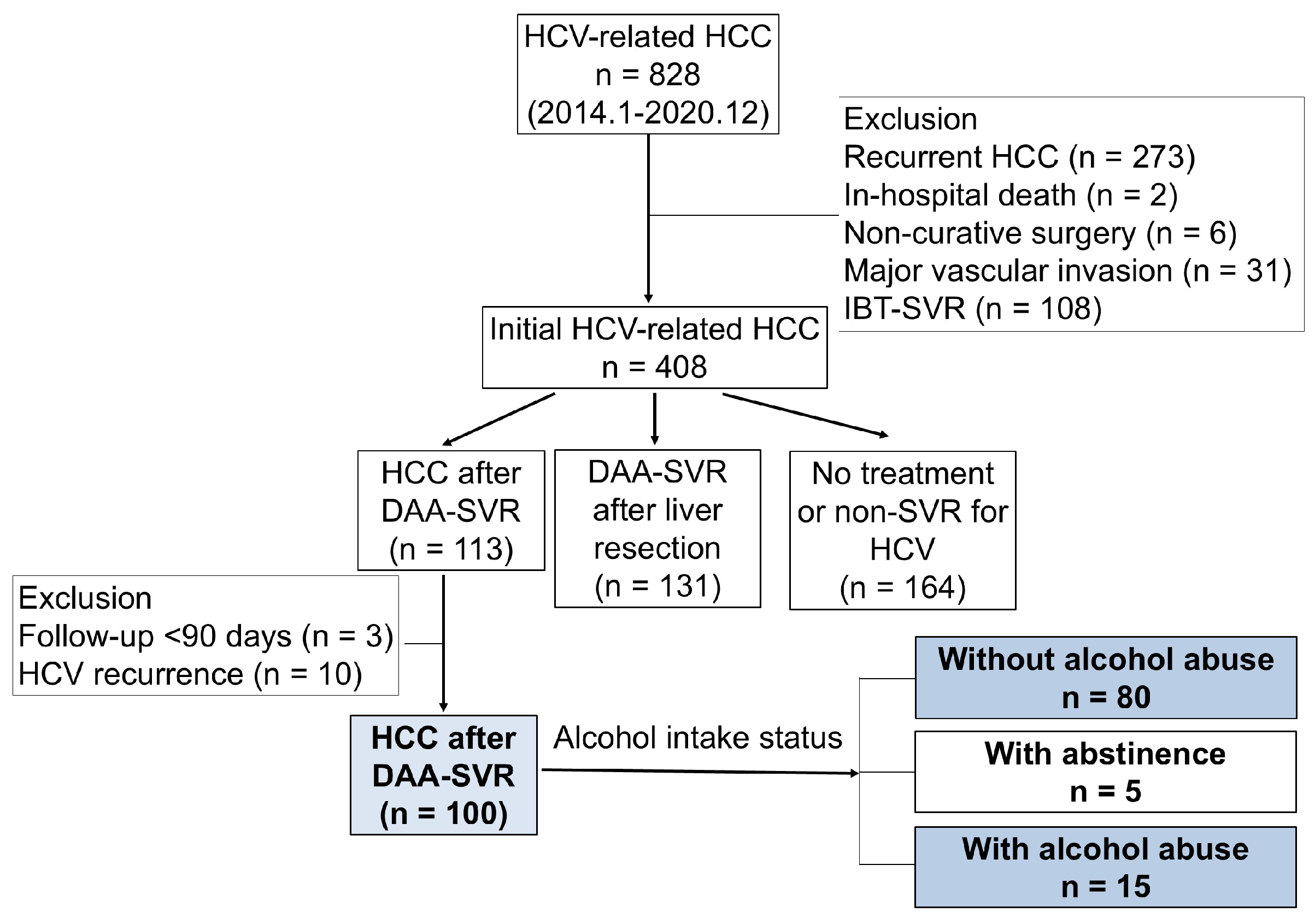
2.1. Patients
2.2. Criteria for Hepatic Resection
2.3. Interferon-Free DAA Therapy for Chronic HCV Treatment
2.4. Definition
2.5. Data Collection
2.6. Pathology
2.7. Follow-Up Evaluations
2.8. Statistical Analyses
3. Results
3.1. Clinicopathological Characteristics of Patients with HCV-Related HCC Detected After DAA-SVR
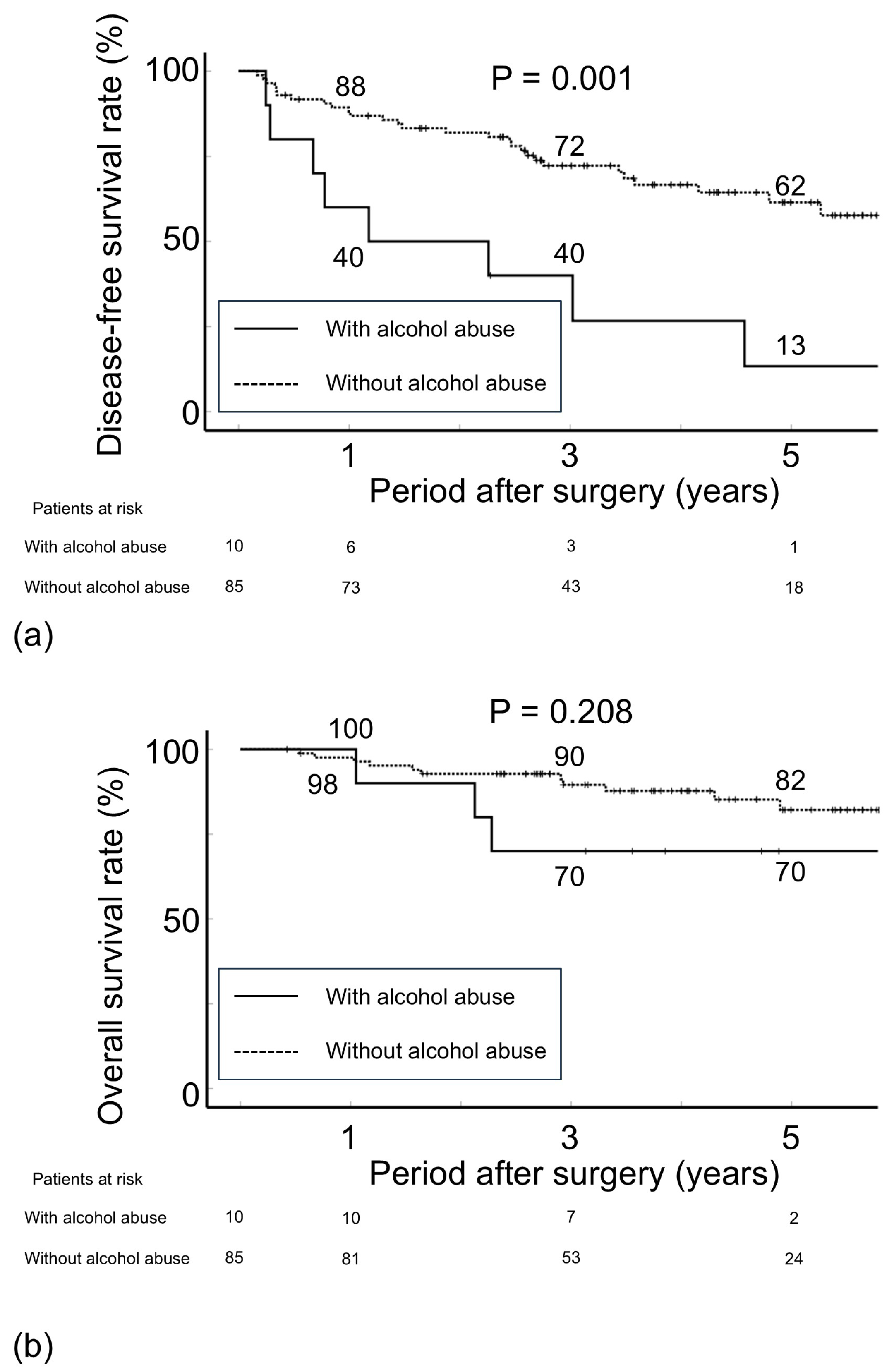
3.2. Postoperative Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DAA | Direct-acting antiviral |
| DFS | Disease-free survival |
| HAI | Histological activity index |
| HCV | Hepatitis C virus |
| HR | Hazard ratio |
| IBT | Interferon-based therapy |
| OS | Overall survival |
| SVR | Sustained virological response |
| UICC | Union for International Cancer Control |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Ueno, Y.; Hiasa, Y.; Nishikawa, H.; Hige, S.; Takikawa, Y.; Taniai, M.; Ishikawa, T.; Yasui, K.; Takaki, A.; et al. The transition in the etiologies of hepatocellular carcinoma-complicated liver cirrhosis in a nationwide survey of Japan. J. Gastroenterol. 2021, 56, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Yatsuhashi, H.; Bekki, S.; Takami, Y.; Tanaka, Y.; Yoshimaru, Y.; Honda, K.; Komorizono, Y.; Harada, M.; Shibata, M.; et al. Trends in hepatocellular carcinoma incident cases in Japan between 1996 and 2019. Sci. Rep. 2022, 12, 1517. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Akuta, N.; Hikita, H.; Suda, G.; Inoue, J.; Tamaki, N.; Ito, K.; Akahane, T.; Kawaoka, T.; Morishita, A.; et al. Etiological changes of liver cirrhosis and hepatocellular carcinoma-complicated liver cirrhosis in Japan: Updated nationwide survey from 2018 to 2021. Hepatol. Res. 2024, 54, 763–772. [Google Scholar] [CrossRef]
- Koda, M.; Tanaka, S.; Takemura, S.; Shinkawa, H.; Kinoshita, M.; Hamano, G.; Ito, T.; Kawada, N.; Shibata, T.; Kubo, S. Long-term prognostic factors after hepatic resection for hepatitis C virus-related hepatocellular carcinoma, with a special reference to viral status. Liver Cancer 2018, 7, 261–276. [Google Scholar] [CrossRef]
- Shindoh, J.; Hasegawa, K.; Inoue, Y.; Ishizawa, T.; Nagata, R.; Aoki, T.; Sakamoto, Y.; Sugawara, Y.; Makuuchi, M.; Kokudo, N. Risk factors of post-operative recurrence and adequate surgical approach to improve long-term outcomes of hepatocellular carcinoma. HPB 2013, 15, 31–39. [Google Scholar] [CrossRef]
- Kubo, S.; Nishiguchi, S.; Hirohashi, K.; Tanaka, H.; Shuto, T.; Yamazaki, O.; Shiomi, S.; Tamori, A.; Oka, H.; Igawa, S.; et al. Effects of long-term postoperative interferon-alpha therapy on intrahepatic recurrence after resection of hepatitis C virus-related hepatocellular carcinoma. A randomized, controlled trial. Ann. Intern. Med. 2001, 134, 963–967. [Google Scholar] [CrossRef]
- Lawitz, E.; Makara, M.; Akarca, U.S.; Thuluvath, P.J.; Preotescu, L.L.; Varunok, P.; Morillas, R.M.; Hall, C.; Mobashery, N.; Redman, R.; et al. Efficacy and safety of ombitasvir, paritaprevir, and ritonavir in an open-label study of patients with genotype 1b chronic hepatitis C virus infection with and without cirrhosis. Gastroenterology 2015, 149, 971–980 e971. [Google Scholar] [CrossRef] [PubMed]
- Poordad, F.; Hezode, C.; Trinh, R.; Kowdley, K.V.; Zeuzem, S.; Agarwal, K.; Shiffman, M.L.; Wedemeyer, H.; Berg, T.; Yoshida, E.M.; et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N. Engl. J. Med. 2014, 370, 1973–1982. [Google Scholar] [CrossRef]
- Reddy, K.R.; Bourliere, M.; Sulkowski, M.; Omata, M.; Zeuzem, S.; Feld, J.J.; Lawitz, E.; Marcellin, P.; Welzel, T.M.; Hyland, R.; et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: An integrated safety and efficacy analysis. Hepatology 2015, 62, 79–86. [Google Scholar] [CrossRef]
- Tanaka, S.; Shinkawa, H.; Tamori, A.; Takemura, S.; Takahashi, S.; Amano, R.; Kimura, K.; Ohira, G.; Kawada, N.; Kubo, S. Surgical outcomes for hepatocellular carcinoma detected after hepatitis C virus eradiation by direct-acting antivirals. J. Surg. Oncol. 2020, 122, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Shinkawa, H.; Tamori, A.; Takemura, S.; Uchida-Kobayashi, S.; Amano, R.; Kimura, K.; Ohira, G.; Nishio, K.; Tauchi, J.; et al. Postoperative direct-acting antiviral treatment after liver resection in patients with hepatitis C virus-related hepatocellular carcinoma. Hepatol. Res. 2021, 51, 1102–1114. [Google Scholar] [CrossRef]
- Makuuchi, M.; Kosuge, T.; Takayama, T.; Yamazaki, S.; Kakazu, T.; Miyagawa, S.; Kawasaki, S. Surgery for small liver cancers. Semin. Surg. Oncol. 1993, 9, 298–304. [Google Scholar] [CrossRef]
- Belghiti, J.; Clavien, P.A.; Gadzijev, E.; Garden, J.O.; Lau, W.Y.; Makuuchi, M.; Strong, R.W. The Brisbane 2000 terminology of liver anatomy and resection. HPB 2000, 2, 333–339. [Google Scholar]
- Hasegawa, K.; Takemura, N.; Yamashita, T.; Watadani, T.; Kaibori, M.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Aikata, H.; et al. Clinical Practice Guidelines for Hepatocellular Carcinoma: The Japan Society of Hepatology 2021 version (5th JSH-HCC Guidelines). Hepatol. Res. 2023, 53, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.C.; Yang, S.S.; Lin, C.C.; Wang, W.L.; Hsu, Y.C.; Chen, Y.S.; Hu, J.T.; Lin, J.Y.; Yu, M.L.; Lin, C.W. Association of Heavy Alcohol Intake and ALDH2 rs671 Polymorphism with Hepatocellular Carcinoma and Mortality in Patients with Hepatitis B Virus-Related Cirrhosis. JAMA Netw. Open 2022, 5, e2223511. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumors, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017. [Google Scholar]
- Facciorusso, A.; Del Prete, V.; Antonino, M.; Crucinio, N.; Neve, V.; Di Leo, A.; Carr, B.I.; Barone, M. Post-recurrence survival in hepatocellular carcinoma after percutaneous radiofrequency ablation. Dig. Liver Dis. 2014, 46, 1014–1019. [Google Scholar] [CrossRef]
- Knodell, R.G.; Ishak, K.G.; Black, W.C.; Chen, T.S.; Craig, R.; Kaplowitz, N.; Kiernan, T.W.; Wollman, J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology 1981, 1, 431–435. [Google Scholar] [CrossRef]
- Desmet, V.J.; Gerber, M.; Hoofnagle, J.H.; Manns, M.; Scheuer, P.J. Classification of chronic hepatitis: Diagnosis, grading and staging. Hepatology 1994, 19, 1513–1520. [Google Scholar] [CrossRef]
- Charlton, M.; Everson, G.T.; Flamm, S.L.; Kumar, P.; Landis, C.; Brown, R.S., Jr.; Fried, M.W.; Terrault, N.A.; O’Leary, J.G.; Vargas, H.E.; et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015, 149, 649–659. [Google Scholar] [CrossRef]
- Poordad, F.; Schiff, E.R.; Vierling, J.M.; Landis, C.; Fontana, R.J.; Yang, R.; McPhee, F.; Hughes, E.A.; Noviello, S.; Swenson, E.S. Daclatasvir with sofosbuvir and ribavirin for hepatitis C virus infection with advanced cirrhosis or post-liver transplantation recurrence. Hepatology 2016, 63, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Ooka, Y.; Miho, K.; Shuntaro, O.; Nakamura, M.; Ogasawara, S.; Suzuki, E.; Yasui, S.; Chiba, T.; Arai, M.; Kanda, T.; et al. Prediction of the very early occurrence of HCC right after DAA therapy for HCV infection. Hepatol. Int. 2018, 12, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Ebel, F.; Deterding, K.; Port, K.; Schlevogt, B.; Manns, M.P.; Maasoumy, B.; Cornberg, M.; Wedemeyer, H. Letter: A 5-year long-term follow-up study after DAA treatment confirms a reduced HCC risk in a central European cohort of HCV patients with liver cirrhosis. Aliment. Pharmacol. Ther. 2020, 51, 194–195. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated with Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1. [Google Scholar] [CrossRef] [PubMed]
- Yoshimasu, Y.; Furuichi, Y.; Kasai, Y.; Takeuchi, H.; Sugimoto, K.; Nakamura, I.; Itoi, T. Predictive factors for hepatocellular carcinoma occurrence or recurrence after direct-acting antiviral agents in patients with chronic hepatitis C. J. Gastrointest. Liver Dis. 2019, 28, 63–71. [Google Scholar] [CrossRef]
- Cheung, M.C.M.; Walker, A.J.; Hudson, B.E.; Verma, S.; McLauchlan, J.; Mutimer, D.J.; Brown, A.; Gelson, W.T.H.; MacDonald, D.C.; Agarwal, K.; et al. Outcomes after successful direct-acting antiviral therapy for patients with chronic hepatitis C and decompensated cirrhosis. J. Hepatol. 2016, 65, 741–747. [Google Scholar] [CrossRef]
- Kobayashi, M.; Suzuki, F.; Fujiyama, S.; Kawamura, Y.; Sezaki, H.; Hosaka, T.; Akuta, N.; Suzuki, Y.; Saitoh, S.; Arase, Y.; et al. Sustained virologic response by direct antiviral agents reduces the incidence of hepatocellular carcinoma in patients with HCV infection. J. Med. Virol. 2017, 89, 476–483. [Google Scholar] [CrossRef]
- Cardoso, H.; Vale, A.M.; Rodrigues, S.; Goncalves, R.; Albuquerque, A.; Pereira, P.; Lopes, S.; Silva, M.; Andrade, P.; Morais, R.; et al. High incidence of hepatocellular carcinoma following successful interferon-free antiviral therapy for hepatitis C associated cirrhosis. J. Hepatol. 2016, 65, 1070–1071. [Google Scholar] [CrossRef]
- Okamura, Y.; Sugiura, T.; Ito, T.; Yamamoto, Y.; Ashida, R.; Ohgi, K.; Uesaka, K. The Achievement of a Sustained Virological Response Either Before or After Hepatectomy Improves the Prognosis of Patients with Primary Hepatitis C Virus-Related Hepatocellular Carcinoma. Ann. Surg. Oncol. 2019, 26, 4566–4575. [Google Scholar] [CrossRef]
- Morgan, T.R.; Mandayam, S.; Jamal, M.M. Alcohol and hepatocellular carcinoma. Gastroenterology 2004, 127, S87–S96. [Google Scholar] [CrossRef]
- Vandenbulcke, H.; Moreno, C.; Colle, I.; Knebel, J.F.; Francque, S.; Serste, T.; George, C.; de Galocsy, C.; Laleman, W.; Delwaide, J.; et al. Alcohol intake increases the risk of HCC in hepatitis C virus-related compensated cirrhosis: A prospective study. J. Hepatol. 2016, 65, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, N.; Ohho, A.; Yamasaki, A.; Kurokawa, M.; Kotoh, K.; Kajiwara, E. Hepatocarcinogenesis in chronic hepatitis C patients achieving a sustained virological response to interferon: Significance of lifelong periodic cancer screening for improving outcomes. J. Gastroenterol. 2014, 49, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Minami, T.; Tateishi, R.; Fujiwara, N.; Nakagomi, R.; Nakatsuka, T.; Sato, M.; Uchino, K.; Enooku, K.; Nakagawa, H.; Fujinaga, H.; et al. Impact of Obesity and Heavy Alcohol Consumption on Hepatocellular Carcinoma Development after HCV Eradication with Antivirals. Liver Cancer 2021, 10, 309–319. [Google Scholar] [CrossRef]
- Franssen, B.; Alshebeeb, K.; Tabrizian, P.; Marti, J.; Pierobon, E.S.; Lubezky, N.; Roayaie, S.; Florman, S.; Schwartz, M.E. Differences in surgical outcomes between hepatitis B- and hepatitis C-related hepatocellular carcinoma: A retrospective analysis of a single North American center. Ann. Surg. 2014, 260, 650–656; discussion 656–658. [Google Scholar] [CrossRef]
- Ikeda, K.; Arase, Y.; Saitoh, S.; Kobayashi, M.; Suzuki, Y.; Suzuki, F.; Tsubota, A.; Chayama, K.; Murashima, N.; Kumada, H. Interferon beta prevents recurrence of hepatocellular carcinoma after complete resection or ablation of the primary tumor-A prospective randomized study of hepatitis C virus-related liver cancer. Hepatology 2000, 32, 228–232. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Jiang, C.; Qiang, Z.Y.; Zhou, Y.F.; Ji, J.; Zeng, Y.; Huang, J.W. Role of microvascular invasion in early recurrence of hepatocellular carcinoma after liver resection: A literature review. Asian J. Surg. 2024, 47, 2138–2143. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.M.; Yang, J.M.; Li, B.; Yin, Z.F.; Xu, F.; Wang, B.; Xu, W.; Kan, T. Risk factors for early recurrence of small hepatocellular carcinoma after curative resection. Hepatobiliary Pancreat. Dis. Int. 2010, 9, 33–37. [Google Scholar]
- Yan, W.T.; Li, C.; Yao, L.Q.; Qiu, H.B.; Wang, M.D.; Xu, X.F.; Zhou, Y.H.; Wang, H.; Chen, T.H.; Gu, W.M.; et al. Predictors and long-term prognosis of early and late recurrence for patients undergoing hepatic resection of hepatocellular carcinoma: A large-scale multicenter study. Hepatobiliary Surg. Nutr. 2023, 12, 155–168. [Google Scholar] [CrossRef]
- Berardi, G.; Morise, Z.; Sposito, C.; Igarashi, K.; Panetta, V.; Simonelli, I.; Kim, S.; Goh, B.K.P.; Kubo, S.; Tanaka, S.; et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J. Hepatol. 2020, 72, 75–84. [Google Scholar] [CrossRef]
| Variables | Data | Variables | Data | ||
|---|---|---|---|---|---|
| Background | Surgery-related variables | ||||
| Age (y.o.) | 72 | (45–88) | Tumor location | ||
| Sex (male/female) | 56/39 | Anterolateral segments | 57 | (60) | |
| History of IBT | 28 | (30) | Posterosuperior segments | 38 | (40) |
| Duration between DAA–SVR and detection of HCC (days) | 788 | (27–2269) | Segmentectomy or more | 31 | (33) |
| BMI (Kg/m2) | 23.3 | (17.0–34.8) | Minimal invasive surgery | 74 | (78) |
| Status of alcohol intake | Operation time (min) | 251 | (86–641) | ||
| Without alcohol abuse | 85 | (89) | Bleeding (cc) | 50 | (0–2340) |
| With alcohol abuse | 10 | (11) | |||
| Diabetes mellitus | 24 | (25) | Tumor-related variables | ||
| AFP (ng/mL) | 6 | (1.0–25,452) | |||
| Preoperative liver function tests | PIVKA-II (U/mL) | 27 | (1.0–15,600) | ||
| Total bilirubin (mg/dL) | 0.7 | (0.2–2.2) | Tumor size (cm) | 2 | (0.7–7.5) |
| Albumin (g/dL) | 4.2 | (3.3–5.0) | Multiplicity | 12 | (13) |
| Prothrombin activity (%) | 91 | (59–125) | Macrovascular invasion | 2 | (2) |
| ICGR 15 min (%) | 13 | (1.0–37.1) | UICC stage 1A/1B/2 | 45/37/13 | |
| Child A/B | 94/1 | ||||
| Platelets (×104/μL) | 12.3 | (4.1–34.9) | Histology | ||
| AST (IU/L) | 26 | (12–56) | Poorly differentiated HCC | 15 | (16) |
| ALT (IU/L) | 18 | (7–66) | Microvascular invasion | 15 | (16) |
| Liver cirrhosis | 35 | (37) | |||
| DFS | Multivariate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | n | 3 Years | 5 Years | p Value | HR | (95% CI) | p Value | |
| Background | ||||||||
| Age | 0.197 | |||||||
| ≥72 y.o. | 49 | 69.1 | 55.7 | |||||
| <71 y.o. | 46 | 68.3 | 44.9 | |||||
| Sex (male/female) | 0.080 | |||||||
| Male | 56 | 66.8 | 45.2 | |||||
| Female | 39 | 71.9 | 71.9 | |||||
| History of IBT | 0.745 | |||||||
| Presence | 28 | 78.0 | 57.0 | |||||
| Absence | 67 | 64.5 | 55.1 | |||||
| Duration between DAA-SVR and detection of HCC <1 year | 0.306 | |||||||
| <1 year | 24 | 64.2 | 49.4 | |||||
| ≥1 year | 71 | 70.0 | 56.7 | |||||
| BMI | 0.722 | |||||||
| ≥23.3 Kg/m2 | 48 | 70.7 | 47.1 | |||||
| <23.3 Kg/m2 | 47 | 66.6 | 60.3 | |||||
| Status of alcohol intake | 0.001 | 3.36 | (1.49–7.61) | 0.004 | ||||
| Without alcohol abuse | 85 | 72.2 | 61.5 | |||||
| Alcohol abuse | 10 | 40.0 | 13.3 | |||||
| Diabetes mellitus | 0.321 | |||||||
| Presence | 24 | 57.6 | 48.0 | |||||
| Absence | 71 | 71.9 | 57.8 | |||||
| Preoperative liver function tests | ||||||||
| Total bilirubin | 0.28 | |||||||
| ≥0.7 mg/dL | 56 | 64.2 | 50.3 | |||||
| <0.7 mg | 39 | 74.6 | 61.7 | |||||
| Albumin | 0.602 | |||||||
| ≤4.2 g/dL | 52 | 68.9 | 58.9 | |||||
| >4.2 g/dL | 43 | 68.5 | 49.7 | |||||
| Prothrombin activity | ||||||||
| ≤91% | 52 | 60.7 | 45.8 | |||||
| >91% | 48 | 75.5 | 65.8 | |||||
| ICGR 15 min | 0.258 | |||||||
| ≥13.0% | 52 | 66.8 | 49.9 | |||||
| <13.0% | 43 | 71.2 | 60.5 | |||||
| Platelets | 0.312 | |||||||
| ≤12.3 × 104/μL | 48 | 65.5 | 49.4 | |||||
| >12.3 × 104/μL | 47 | 69.7 | 59.6 | |||||
| AST | 0.076 | |||||||
| ≥26 IU/L | 48 | 66.1 | 44.5 | |||||
| <26 IU/L | 47 | 71.1 | 71.1 | |||||
| ALT | 0.008 | 2.05 | (0.94–4.48) | 0.072 | ||||
| ≥18 IU/L | 52 | 63.0 | 41.5 | |||||
| <18 IU/L | 43 | 75.8 | 75.8 | |||||
| Surgery-related variables | ||||||||
| Tumor location | 0.982 | |||||||
| Anterolateral segments | 57 | 66.0 | 55.7 | |||||
| Posterosuperior segments | 38 | 72.4 | 55.7 | |||||
| Segmentectomy or more | 0.438 | |||||||
| Performed | 31 | 76.5 | 61.5 | |||||
| Not performed | 64 | 64.8 | 52.5 | |||||
| Approach | 0.617 | |||||||
| Minimal invasive surgery | 74 | 68.4 | 53.9 | |||||
| Open surgery | 21 | 70.0 | 61.3 | |||||
| Operation time | 0.616 | |||||||
| ≥251 min | 48 | 69.1 | 59.4 | |||||
| <251 min | 47 | 68.3 | 51.2 | |||||
| Bleeding | 0.528 | |||||||
| ≥50 cc | 56 | 66.9 | 50.7 | |||||
| <50 cc | 39 | 70.7 | 59.4 | |||||
| Tumor-related variables | ||||||||
| AFP | 0.113 | |||||||
| ≥6 ng/mL | 49 | 63.3 | 46.6 | |||||
| <6 ng/mL | 46 | 74.7 | 66.5 | |||||
| PIVKA-II | 0.354 | |||||||
| ≥27 U/mL | 50 | 61.7 | 48.2 | |||||
| <27 U/mL | 45 | 71.7 | 58.5 | |||||
| Tumor size | 0.006 | 2.53 | (1.25–5.10) | 0.01 | ||||
| ≥2.0 cm | 49 | 54.7 | 47.4 | |||||
| <2.0 cm | 46 | 83.5 | 64.9 | |||||
| Multiplicity | 0.388 | |||||||
| Solitary | 83 | 69.1 | 58.6 | |||||
| Multiple | 12 | 64.8 | 34.6 | |||||
| Macrovascular invasion | 0.666 | |||||||
| Presence | 2 | 50.0 | 50.0 | |||||
| Absence | 93 | 69.1 | 55.2 | |||||
| Histology | ||||||||
| Poorly differentiated HCC | 0.818 | |||||||
| Presence | 15 | 62.2 | 51.8 | |||||
| Absence | 80 | 69.9 | 55.7 | |||||
| Microvascular invasion | 0.077 | |||||||
| Presence | 15 | 53.3 | 53.3 | |||||
| Absence | 80 | 71.6 | 55.7 | |||||
| Liver cirrhosis | 0.961 | |||||||
| Presence | 35 | 72.5 | 47.3 | |||||
| Absence | 60 | 66.4 | 61.3 | |||||
| With Alcohol Abuse | Without Alcohol Abuse | ||||
|---|---|---|---|---|---|
| Variables | (n = 8) | (n = 28) | p Value | ||
| Recurrence sites | 1.00 | ||||
| Liver | 7 | (88) | 25 | (89) | |
| Other organs | 1 | (13) | 3 | (11) | |
| Child–Pugh class (A/B) disease at recurrence | 7/1 | 27/1 | 0.400 | ||
| Treatments | 0.434 | ||||
| Repeat liver resection or ablation therapy | 3 | (38) | 16 | (57) | |
| TACE or other treatments | 5 | (63) | 12 | (43) | |
| Death | 2 | (25) | 9 | (32) | 1.00 |
| Causes of death | |||||
| HCC | 2 | (25) | 8 | (29) | |
| Liver failure | 0 | 1 | (4) | ||
| Post-recurrence survival rate (%) | 0.79 | ||||
| 3 years after surgery | 66.7 | 67.1 | |||
| 5 years after surgery | 66.7 | 67.1 | |||
| With Alcohol Abuse | Without Alcohol Abuse | ||||
|---|---|---|---|---|---|
| Variables | (n = 10) | (n = 85) | p Value | ||
| Background | |||||
| Age (y.o.) | 67 | (48–77) | 73 | (45–88) | 0.012 |
| Sex (male/female) | 10/0 | 46/39 | 0.005 | ||
| History of IBT | 4 | (40.0) | 24 | (28.2) | 0.474 |
| BMI (Kg/m2) | 23.6 | (17.2–33.8) | 23.3 | (17.0–34.8) | 0.396 |
| Diabetes mellitus | 3 | (30.0) | 21 | (24.7) | 0.709 |
| Preoperative liver function tests | |||||
| Total bilirubin (mg/dL) | 0.8 | (0.5–1.5) | 0.7 | (0.2–2.2) | 0.380 |
| Albumin (g/dL) | 4.1 | (3.3–4.7) | 4.2 | (3.3–5.0) | 0.841 |
| Prothrombin activity (%) | 99 | (62–114) | 91 | (59–125) | 0.272 |
| ICGR 15 min (%) | 14.4 | (5.0–26.6) | 13 | (1.0–37.0) | 0.734 |
| Child–Pugh class B | 0 | 1 | (1.2) | 1.00 | |
| Platelets (×104/μL) | 13.8 | (8.1–21.8) | 12.3 | (4.1–34.9) | 0.658 |
| AST (IU/L) | 36 | (19–56) | 25 | (12–52) | 0.016 |
| ALT (IU/L) | 26 | (12–66) | 18 | (7–52) | 0.062 |
| Surgery-related variables | |||||
| Segmentectomy or more | 4 | (40.0) | 27 | (31.8) | 0.724 |
| Minimal invasive surgery | 10 | (100) | 64 | (75.3) | 0.111 |
| Operation time (min) | 334 | (147–641) | 240 | (86–594) | 0.011 |
| Bleeding (cc) | 130 | (15–1800) | 50 | (0–2340) | 0.148 |
| Tumor-related variables | |||||
| AFP (ng/mL) | 10.4 | (1.8–2826) | 6.0 | (1.0–25452) | 0.61 |
| PIVKA-II (U/mL) | 48.5 | (16–188) | 27 | (1.0–15600) | 0.544 |
| Tumor size (cm) | 1.8 | (0.7–3.5) | 2.0 | (0.8–7.5) | 0.322 |
| Multiplicity | 1 | (10.0) | 11 | (12.9) | 1.00 |
| Macrovascular invasion | 0 | 2 | (2.4) | 1.00 | |
| UICC stage 1A/1B/2 | 5/4/1 | 40/33/12 | 0.937 | ||
| Histology | |||||
| Poorly differentiated HCC | 1 | (10.0) | 14 | (16.5) | 1 |
| Microvascular invasion | 2 | (20.0) | 13 | (15.3) | 0.656 |
| Liver cirrhosis | 3 | (30.0) | 32 | (37.6) | 0.741 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, S.; Noda, T.; Komeda, K.; Yasuda, S.; Ueno, M.; Mori, H.; Kosaka, H.; Morimura, R.; Shinkawa, H.; Sekiguchi, N.; et al. Risk for Recurrence After Liver Resection in Patients with Hepatitis C Virus-Related Hepatocellular Carcinoma Detected After Sustained Virological Response by Direct-Acting Antivirals: A Retrospective Multicenter Study. Cancers 2025, 17, 1946. https://doi.org/10.3390/cancers17121946
Tanaka S, Noda T, Komeda K, Yasuda S, Ueno M, Mori H, Kosaka H, Morimura R, Shinkawa H, Sekiguchi N, et al. Risk for Recurrence After Liver Resection in Patients with Hepatitis C Virus-Related Hepatocellular Carcinoma Detected After Sustained Virological Response by Direct-Acting Antivirals: A Retrospective Multicenter Study. Cancers. 2025; 17(12):1946. https://doi.org/10.3390/cancers17121946
Chicago/Turabian StyleTanaka, Shogo, Takehiro Noda, Koji Komeda, Satoshi Yasuda, Masaki Ueno, Haruki Mori, Hisashi Kosaka, Ryo Morimura, Hiroji Shinkawa, Naoko Sekiguchi, and et al. 2025. "Risk for Recurrence After Liver Resection in Patients with Hepatitis C Virus-Related Hepatocellular Carcinoma Detected After Sustained Virological Response by Direct-Acting Antivirals: A Retrospective Multicenter Study" Cancers 17, no. 12: 1946. https://doi.org/10.3390/cancers17121946
APA StyleTanaka, S., Noda, T., Komeda, K., Yasuda, S., Ueno, M., Mori, H., Kosaka, H., Morimura, R., Shinkawa, H., Sekiguchi, N., Ikoma, H., Ishizawa, T., & Kaibori, M. (2025). Risk for Recurrence After Liver Resection in Patients with Hepatitis C Virus-Related Hepatocellular Carcinoma Detected After Sustained Virological Response by Direct-Acting Antivirals: A Retrospective Multicenter Study. Cancers, 17(12), 1946. https://doi.org/10.3390/cancers17121946







