A Meta-Analysis of Patient-Reported Outcomes of Sacituzumab Govitecan Versus Treatment of Physician’s Choice in Previously Treated HR+/HER− mBC Using Two Phase 3 (TROPiCS-02 and EVER-132-002) Trials
Simple Summary
Abstract
1. Background
2. Materials and Methods
2.1. Selection Criteria
2.2. Risk-of-Bias Assessment
2.3. Feasibility Assessment
2.4. PRO Assessments
2.5. Statistical Analysis
3. Results
3.1. Study Characteristics and Risk-of-Bias Assessment
3.2. Least Square Mean Changes from Baseline
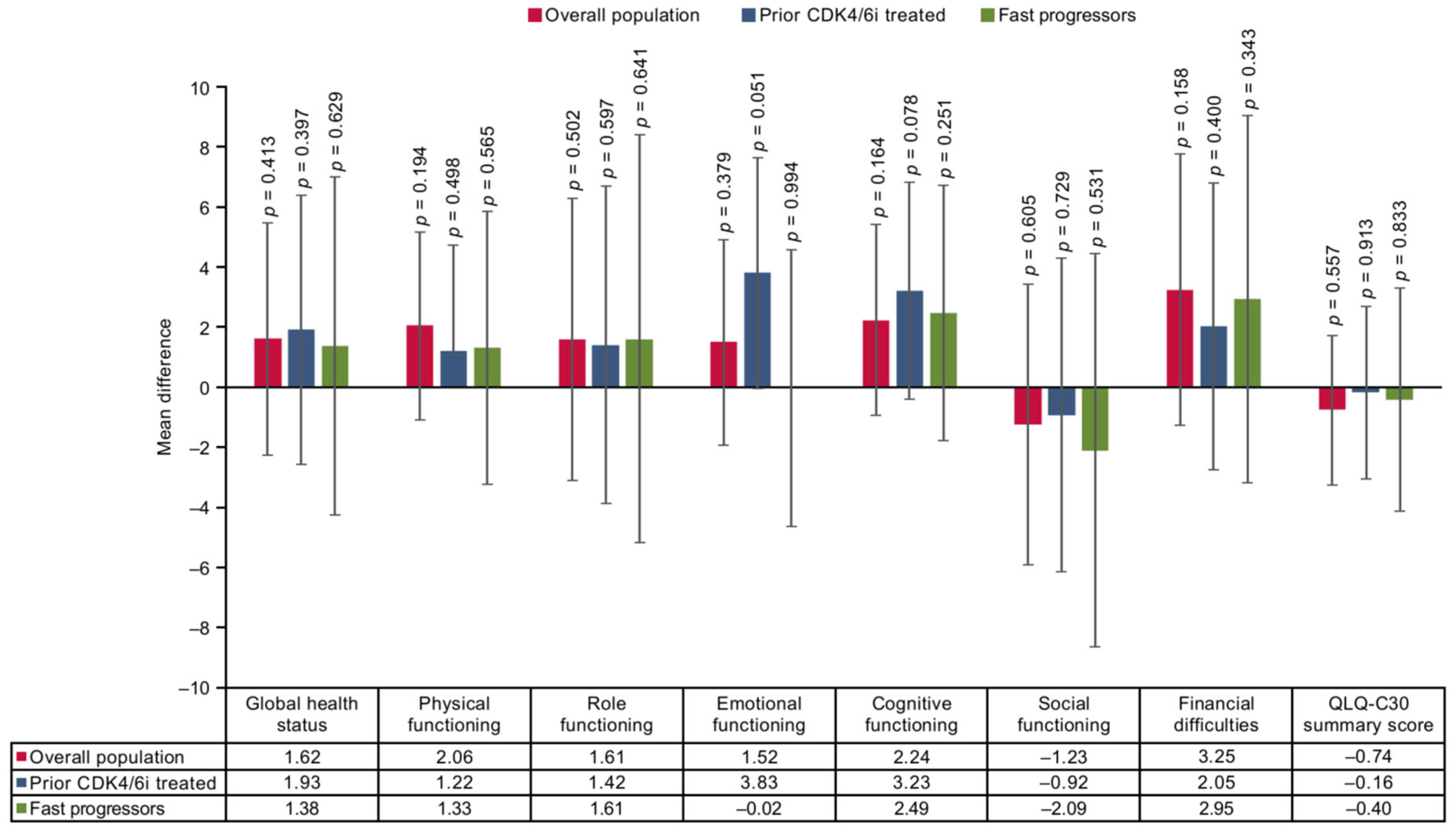
3.3. Mean Difference
3.4. Time to Deterioration
3.5. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phung, M.T.; Tin Tin, S.; Elwood, J.M. Prognostic models for breast cancer: A systematic review. BMC Cancer 2019, 19, 230. [Google Scholar] [CrossRef] [PubMed]
- National Breast Cancer Foundation Inc. Breast Cancer Facts & Stats. Available online: https://www.nationalbreastcancer.org/breast-cancer-facts/ (accessed on 19 November 2024).
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Gómez Pardo, P.; et al. Overall survival with sacituzumab govitecan in hormone receptor-positive and human epidermal growth factor receptor 2-negative metastatic breast cancer (TROPiCS-02): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1423–1433. [Google Scholar] [CrossRef] [PubMed]
- Elfgen, C.; Bjelic-Radisic, V. Targeted therapy in HR+ HER2- metastatic breast cancer: Current clinical trials and their implications for CDK4/6 inhibitor therapy and beyond treatment options. Cancers 2021, 13, 5994. [Google Scholar] [CrossRef]
- Rugo, H.S.; Schmid, P.; Tolaney, S.M.; Dalenc, F.; Marmé, F.; Shi, L.; Verret, W.; Shah, A.; Gharaibeh, M.; Bardia, A.; et al. Health-related quality of life with sacituzumab govitecan in HR+/HER2- metastatic breast cancer in the phase III TROPiCS-02 trial. Oncologist 2024, 29, 768–779. [Google Scholar] [CrossRef]
- Bardia, A.; Messersmith, W.A.; Kio, E.A.; Berlin, J.D.; Vahdat, L.; Masters, G.A.; Moroose, R.; Santin, A.D.; Kalinsky, K.; Picozzi, V.; et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: Final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann. Oncol. 2021, 32, 746–756. [Google Scholar] [CrossRef]
- Xu, B.; Wang, S.; Yan, M.; Sohn, J.; Li, W.; Tang, J.; Wang, X.; Wang, Y.; Im, S.-A.; Jiang, D.; et al. Sacituzumab govitecan in HR+HER2− metastatic breast cancer: The randomized phase 3 EVER-132-002 trial. Nat. Med. 2024, 30, 3709–3716. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab govitecan in metastatic triple-negative breast cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bardia, A.; Tolaney, S.M.; Arteaga, C.; Cortes, J.; Sohn, J.; Marmé, F.; Hong, Q.; Delaney, R.J.; Hafeez, A.; et al. TROPiCS-02: A phase III study investigating sacituzumab govitecan in the treatment of HR+/HER2− metastatic breast cancer. Future Oncol. 2020, 16, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Dai, M.S.; Xu, B.; Ma, F.; Wang, S.; Yan, M.; Li, W.; Tang, J.; Wang, X.; Wang, Y.; Im, S.-A.; et al. Health-related quality of life (HRQOL) in the EVER-132-002 study of sacituzumab govitecan (SG) vs. treatment of physician’s choice (TPC) in Asian patients with HR+/HER2− MBC. In Proceedings of the Global Breast Cancer Conference (GBCC), Seoul, Republic of Korea, 25–27 April 2024; p. 106. [Google Scholar]
- Stewart, L.A.; Clarke, M.; Rovers, M.; Riley, R.D.; Simmonds, M.; Stewart, G.; Tierney, J.F. Preferred reporting items for systematic review and meta-analyses of individual participant data: The PRISMA-IPD statement. JAMA 2015, 313, 1657–1665. [Google Scholar] [CrossRef]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortes, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Sacituzumab govitecan in hormone receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2022, 40, 3365–3376. [Google Scholar] [CrossRef]
- Sacituzumab Govitecan: Primary Clinical Study Report: EVER-132-002. Data on File; Gilead Sciences Inc.: Foster City, CA, USA, 2023.
- Sacituzumab Govitecan: Interim Clinical Study Report TROPiCS-02. Data on File; Gilead Sciences Inc.: Foster City, CA, USA, 2022.
- Sacituzumab Govitecan: Primary Clinical Study Report TROPiCS-02. Data on File; Gilead Sciences Inc.: Foster City, CA, USA, 2022.
- Dossier zur Nutzenbewertung Gemäß § 35a SGB V. Sacituzumab Govitecan (Trodelvy®); Gilead Sciences GmbH: Munich, Germany, 2023.
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane: London, UK, 2023. [Google Scholar]
- Salanti, G.; Del Giovane, C.; Chaimani, A.; Caldwell, D.M.; Higgins, J.P.T. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE 2014, 9, e99682. [Google Scholar] [CrossRef] [PubMed]
- Florez, I.D.; De La Cruz-Mena, J.E.; Veroniki, A.-A. Network meta-analysis: A powerful tool for clinicians, decision-makers, and methodologists. J. Clin. Epidemiol. 2024, 176, 111537. [Google Scholar] [CrossRef] [PubMed]
- Cope, S.; Zhang, J.; Saletan, S.; Smiechowski, B.; Jansen, J.P.; Schmid, P. A process for assessing the feasibility of a network meta-analysis: A case study of everolimus in combination with hormonal therapy versus chemotherapy for advanced breast cancer. BMC Med. 2014, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.J.; Copas, A.J.; Tierney, J.F.; Parmar, M.K.B. A critical review of methods for the assessment of patient-level interactions in individual participant data meta-analysis of randomized trials, and guidance for practitioners. J. Clin. Epidemiol. 2011, 64, 949–967. [Google Scholar] [CrossRef]
- Protocol: IMMU-132-09 Phase 3 Study of Sacituzumab Govitecan (IMMU-132) in Relapsed/Refractory HR+/HER2− MBC. Data on File; Immunomedics: Morris Plains, NJ, USA, 2021.
- Balestroni, G.; Bertolotti, G. EuroQol-5D (EQ-5D): An instrument for measuring quality of life. Monaldi Arch. Chest. Dis. 2012, 78, 155–159. [Google Scholar] [CrossRef]
- Tierney, J.F.; Vale, C.; Riley, R.; Smith, C.T.; Stewart, L.; Clarke, M.; Rovers, M. Individual participant data (IPD) meta-analyses of randomised controlled trials: Guidance on their use. PLoS Med. 2015, 12, e1001855. [Google Scholar] [CrossRef]
- Riley, R.D.; Lambert, P.C.; Abo-Zaid, G. Meta-analysis of individual participant data: Rationale, conduct, and reporting. BMJ 2010, 340, c221. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, A.; Mavridis, D.; Salanti, G. How to interpret meta-analysis models: Fixed effect and random effects meta-analyses. Evid. Based Ment. Health 2014, 17, 64. [Google Scholar] [CrossRef]
- Gluz, O.; Xu, B.; Nanda, R.; Dasgupta, A.; Kaushik, A.; Verret, W.; Baio, G.; Sharma, A.; Singh, B.; Rugo, H.S. 193P Efficacy of sacituzumab govitecan versus treatment of physician’s choice in previously treated hormone receptor-positive/HER2-negative metastatic breast cancer: A meta-analysis of TROPiCS-02 and EVER-132-002 trials. ESMO Open 2024, 9, 103215. [Google Scholar] [CrossRef]
- Loibl, S.; Loirat, D.; Tolaney, S.M.; Punie, K.; Oliveira, M.; Rugo, H.S.; Bardia, A.; Hurvitz, S.A.; Brufsky, A.M.; Kalinsky, K.; et al. Health-related quality of life in the phase III ASCENT trial of sacituzumab govitecan versus standard chemotherapy in metastatic triple-negative breast cancer. Eur. J. Cancer 2023, 178, 23–33. [Google Scholar] [CrossRef]
- Ueno, N.T.; Jacot, W.; Yamashita, T.; Sohn, J.; Tokunaga, E.; Prat, A.; Tsurutani, J.; Park, Y.H.; Rugo, H.S.; Xu, B.; et al. 217O Patient-reported outcomes (PROs) from DESTINY-Breast04, a randomized phase III study of trastuzumab deruxtecan (T-DXd) vs treatment of physician’s choice (TPC) in patients (pts) with HER2-low metastatic breast cancer (MBC). Ann. Oncol. 2022, 33, S632–S633. [Google Scholar] [CrossRef]
- Pernas, S.; Im, S.-A.; Hattori, M.; Xu, B.; Wang, S.; Lu, Y.-S.; Borges, G.; Tsurutani, J.; Jhaveri, K.L.; Zhang, Q.; et al. Datopotamab deruxtecan (Dato-DXd) vs chemotherapy (CT) in previously treated inoperable or metastatic hormone receptor-positive, HER2-negative (HR+/HER2–) breast cancer (BC): Patient-reported outcomes (PROs) from the TROPION-Breast01 study. J. Clin. Oncol. 2024, 42, 1006. [Google Scholar] [CrossRef]
- Sohrabi, C.; Franchi, T.; Mathew, G.; Kerwan, A.; Nicola, M.; Griffin, M.; Agha, M.; Agha, R. PRISMA 2020 statement: What’s new and the importance of reporting guidelines. Int. J. Surg. 2021, 88, 105918. [Google Scholar] [CrossRef] [PubMed]
- Burke, D.L.; Ensor, J.; Riley, R.D. Meta-analysis using individual participant data: One-stage and two-stage approaches, and why they may differ. Stat. Med. 2017, 36, 855–875. [Google Scholar] [CrossRef]
- Javan Biparva, A.; Raoofi, S.; Rafiei, S.; Pashazadeh Kan, F.; Kazerooni, M.; Bagheribayati, F.; Masoumi, M.; Doustmehraban, M.; Sanaei, M.; Zarabi, F.; et al. Global quality of life in breast cancer: Systematic review and meta-analysis. BMJ Support. Palliat. Care 2023, 13, e528–e536. [Google Scholar] [CrossRef] [PubMed]
- Ge, I.; Berner, K.; Mathis, M.; Hensgen, C.; Mayer, S.; Erbes, T.; Juhasz-Böss, I.; Asberger, J. Real-world data analysis of CDK4/6 inhibitor therapy-a patient-centric single center study. Cancers 2024, 16, 1760. [Google Scholar] [CrossRef]
- Ladak, L.A.; Raza, S.F.; Khawaja, S. Cross-cultural considerations in health-related quality of life in cancer. In Handbook of Quality of Life in Cancer; Kassianos, A.P., Ed.; Springer International Publishing: Cham, Switzerland, 2022; pp. 189–207. [Google Scholar]
- Yeom, J.-W.; Yeom, I.-S.; Park, H.-Y.; Lim, S.-H. Cultural factors affecting the self-care of cancer survivors: An integrative review. Eur. J. Oncol. Nurs. 2022, 59, 102165. [Google Scholar] [CrossRef]






| Criteria | TROPiCS-02 | EVER-132-002 |
|---|---|---|
| Population | Patients with HR+/HER2− mBC who have progressed after endocrine therapy, taxane, and at least two systemic therapies in the advanced setting | Patients with HR+/HER2− mBC who have progressed after endocrine therapy, taxane, and at least two systemic therapies in the advanced setting |
| Prior CDK4/6 inhibitor required | Prior CDK4/6 inhibitor not required | |
| Intervention | SG | |
| Comparator | TPC, i.e., gemcitabine, eribulin, capecitabine, vinorelbine | |
| Outcome | Patient-reported outcomes (EORTC QLQ-C30, EQ-5D-5L VAS) measured using: • Change from baseline (analyzed using MMRM; mean difference analyzed at end of treatment) • Time to deterioration (analyzed using stratified Cox proportional hazards regression analysis) | |
| Prior CDK4/6 inhibitor exposure | Mandatory | Not mandatory |
| Study design | Randomized controlled trial | |
| Variable | TROPiCS-02 | EVER-132-002 |
|---|---|---|
| Trial design | Phase 3, randomized controlled trial | |
| Blinding | Open-label | |
| Setting | Multicenter international | |
| Prior CDK4/6i treatment | Mandatory inclusion criterion (100% Prior CDK4/6i) | Not a mandatory criterion (49% Prior CDK4/6i) |
| Geography | Global (US, Belgium, Canada, France, Germany, Great Britain, Italy, the Netherlands, Spain) | Asian (China mainland, Taiwan, and Republic of Korea) |
| Stratification factors | Prior chemotherapy regimens for treatment of metastatic disease (two versus three/four lines) and visceral metastasis (Yes/No) | Prior chemotherapy regimens for treatment of metastatic disease (two versus three/four lines) and visceral metastasis (Yes/No) |
| Endocrine therapy in metastatic setting ≥ 6 months (Yes/No) | Prior CDK4/6i (Yes/No) | |
| ITT population | 543 (SG: 272; TPC: 271) | 331 (SG: 166; TPC: 165) |
| EORTC QLQ-C30-evaluable population | 446 (SG: 236; TPC: 210) | 318 (SG: 161; TPC: 157) |
| EQ-5D-5L-Evaluable Population | 445 (SG: 238; TPC: 207) | 318 (SG: 161; TPC: 157) |
| Data-cut off | 1 December 2022 | 30 April 2023 |
| Treatment | SG 10 mg/kg IV on Days 1 and 8 of 21-day cycles | |
| Comparator | TPC (a single-agent treatment determined by the investigator before randomization from one of the four following choices: eribulin, capecitabine, gemcitabine, or vinorelbine) | |
| Treatment duration, median (SG versus TPC) | 4.1 months vs. 2.3 months | 5.1 months versus 3.25 months |
| Follow-up time | PRO questionnaires evaluated in all patients at baseline (within 3 days of first study treatment), Day 1 of every cycle except Cycle 1 (every 3 weeks for TPC if given weekly), and the final study visit (prior to telling patients that they are being withdrawn from the study); median duration: 12.75 months | PRO assessments evaluated on C1D1, at each tumor assessment visit, and the end of treatment visit. The assessment of PROs was before tumor assessments at the planned visit; median duration: 13.4 months |
| Measures being evaluated | Patient-reported outcomes (EORTC QLQ-C30, EQ-5D-5L VAS) measured using: • Change from baseline • Time to deterioration | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rugo, H.S.; Xu, B.; Dasgupta, A.; Kaushik, A.; Verret, W.; Singh, B. A Meta-Analysis of Patient-Reported Outcomes of Sacituzumab Govitecan Versus Treatment of Physician’s Choice in Previously Treated HR+/HER− mBC Using Two Phase 3 (TROPiCS-02 and EVER-132-002) Trials. Cancers 2025, 17, 1885. https://doi.org/10.3390/cancers17111885
Rugo HS, Xu B, Dasgupta A, Kaushik A, Verret W, Singh B. A Meta-Analysis of Patient-Reported Outcomes of Sacituzumab Govitecan Versus Treatment of Physician’s Choice in Previously Treated HR+/HER− mBC Using Two Phase 3 (TROPiCS-02 and EVER-132-002) Trials. Cancers. 2025; 17(11):1885. https://doi.org/10.3390/cancers17111885
Chicago/Turabian StyleRugo, Hope S., Binghe Xu, Anandaroop Dasgupta, Ankita Kaushik, Wendy Verret, and Barinder Singh. 2025. "A Meta-Analysis of Patient-Reported Outcomes of Sacituzumab Govitecan Versus Treatment of Physician’s Choice in Previously Treated HR+/HER− mBC Using Two Phase 3 (TROPiCS-02 and EVER-132-002) Trials" Cancers 17, no. 11: 1885. https://doi.org/10.3390/cancers17111885
APA StyleRugo, H. S., Xu, B., Dasgupta, A., Kaushik, A., Verret, W., & Singh, B. (2025). A Meta-Analysis of Patient-Reported Outcomes of Sacituzumab Govitecan Versus Treatment of Physician’s Choice in Previously Treated HR+/HER− mBC Using Two Phase 3 (TROPiCS-02 and EVER-132-002) Trials. Cancers, 17(11), 1885. https://doi.org/10.3390/cancers17111885







