Checkpoint Kinase 1 Inhibitor Combined with Low Dose Hydroxyurea Promotes ATM-Activated NF-κB-Dependent Pro-Inflammatory Chemokine Expression in Melanomas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. IncuCyte Assays
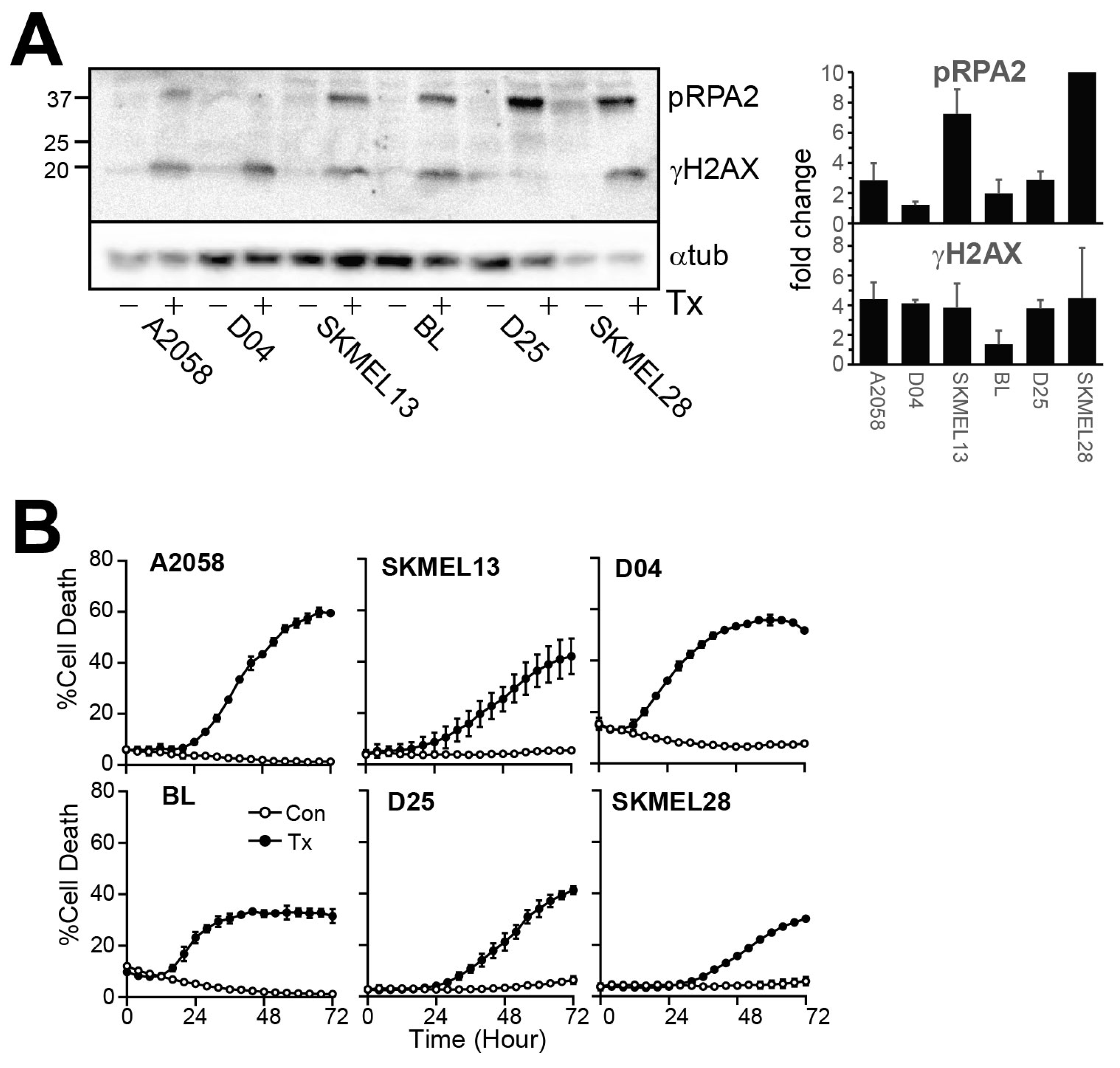
2.3. Immunoblotting
2.4. RT-qPCR
2.5. Cytokine/Chemokine Bead Array
2.6. Immunofluorescence
2.7. Quantitative Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATM | Ataxia telangiectasia mutated |
| CHK1 | Checkpoint kinase 1 |
| CHK1i | CHK1 inhibitor |
| LDHU | Low dose hydroxyurea |
| HU | hydroxyurea |
| IL-6 | Interleukin 6 |
| IRF3 | Interferon regulatory factor 3 |
| NF-κB | Nuclear factor-κB |
| NK | Natural killer |
| TAM | Tumour-associated macrophage |
| TBK1 | TANK binding kinase 1 |
| TIL | Tumour infiltrating leukocytes |
| TME | Tumour microenvironment |
| TNF | Tumour necrosis factor |
| Treg | T regulatory cells |
References
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef]
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The Tumor Microenvironment: A Milieu Hindering and Obstructing Antitumor Immune Responses. Front. Immunol. 2020, 11, 940. [Google Scholar] [CrossRef]
- Verdegaal, E.M.; de Miranda, N.F.; Visser, M.; Harryvan, T.; van Buuren, M.M.; Andersen, R.S.; Hadrup, S.R.; van der Minne, C.E.; Schotte, R.; Spits, H.; et al. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature 2016, 536, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Sánchez, G.S.; Hodge, J.W.; Fabian, K.P. Tipping the scales: Immunotherapeutic strategies that disrupt immunosuppression and promote immune activation. Front. Immunol. 2022, 13, 993624. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Tang, F.; Wei, Y.Q.; Wei, X.W. Immunosuppressive cells in cancer: Mechanisms and potential therapeutic targets. J. Hematol. Oncol. 2022, 15, 61. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Yoshie, O.; Kitahata, K.; Kamei, M.; Hara, Y.; Nakayama, T. Recent Progress in Dendritic Cell-Based Cancer Immunotherapy. Cancers 2021, 13, 2495. [Google Scholar] [CrossRef]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Korneev, K.V.; Atretkhany, K.-S.N.; Drutskaya, M.S.; Grivennikov, S.I.; Kuprash, D.V.; Nedospasov, S.A. TLR-signaling and proinflammatory cytokines as drivers of tumorigenesis. Cytokine 2017, 89, 127–135. [Google Scholar] [CrossRef]
- Nastasi, C.; Mannarino, L.; D’Incalci, M. DNA Damage Response and Immune Defense. Int. J. Mol. Sci. 2020, 21, 7504. [Google Scholar] [CrossRef] [PubMed]
- Pilger, D.; Seymour, L.W.; Jackson, S.P. Interfaces between cellular responses to DNA damage and cancer immunotherapy. Genes Dev 2021, 35, 602–618. [Google Scholar] [CrossRef]
- Barros, E.M.; McIntosh, S.A.; Savage, K.I. The DNA damage induced immune response: Implications for cancer therapy. DNA Repair. 2022, 120, 103409. [Google Scholar] [CrossRef]
- Ng, K.W.; Marshall, E.A.; Bell, J.C.; Lam, W.L. cGAS-STING and Cancer: Dichotomous Roles in Tumor Immunity and Development. Trends Immunol. 2017, 19, 30151–30155. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.; Tschopp, J. Signals from within: The DNA-damage-induced NF-kappaB response. Cell Death Differ. 2006, 13, 773–784. [Google Scholar] [CrossRef]
- Oo, Z.Y.; Proctor, M.; Stevenson, A.J.; Nazareth, D.; Fernando, M.; Daignault, S.M.; Lanagan, C.; Walpole, S.; Bonazzi, V.; Skalamera, D.; et al. Combined use of subclinical hydroxyurea and CHK1 inhibitor effectively controls melanoma and lung cancer progression, with reduced normal tissue toxicity compared to gemcitabine. Mol. Oncol. 2019, 13, 1503–1518. [Google Scholar] [CrossRef] [PubMed]
- Proctor, M.; Gonzalez Cruz, J.L.; Daignault-Mill, S.M.; Veitch, M.; Zeng, B.; Ehmann, A.; Sabdia, M.; Snell, C.; Keane, C.; Dolcetti, R.; et al. Targeting Replication Stress Using CHK1 Inhibitor Promotes Innate and NKT Cell Immune Responses and Tumour Regression. Cancers 2021, 13, 3733. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Pavey, S.; Hayward, N. Confirmation of a BRAF mutation-associated gene expression signature in melanoma. Pigment Cell Res. 2007, 20, 216–221. [Google Scholar] [CrossRef]
- Li, T.; Chen, Z.J. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018, 215, 1287–1299. [Google Scholar] [CrossRef]
- Ghandi, M.; Huang, F.W.; Jane-Valbuena, J.; Kryukov, G.V.; Lo, C.C.; McDonald, E.R., 3rd; Barretina, J.; Gelfand, E.T.; Bielski, C.M.; Li, H.; et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature 2019, 569, 503–508. [Google Scholar] [CrossRef]
- Konno, H.; Yamauchi, S.; Berglund, A.; Putney, R.M.; Mulé, J.J.; Barber, G.N. Suppression of STING signaling through epigenetic silencing and missense mutation impedes DNA damage mediated cytokine production. Oncogene 2018, 37, 2037–2051. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S. Nuclear initiated NF-kappaB signaling: NEMO and ATM take center stage. Cell Res. 2011, 21, 116–130. [Google Scholar] [CrossRef]
- Klose, R.; Krzywinska, E.; Castells, M.; Gotthardt, D.; Putz, E.M.; Kantari-Mimoun, C.; Chikdene, N.; Meinecke, A.-K.; Schrödter, K.; Helfrich, I.; et al. Targeting VEGF-A in myeloid cells enhances natural killer cell responses to chemotherapy and ameliorates cachexia. Nat. Commun. 2016, 7, 12528. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Fowlis, D.J.; Bryson, S.; Duffie, E.; Ireland, H.; Balmain, A.; Akhurst, R.J. TGFβ1 Inhibits the Formation of Benign Skin Tumors, but Enhances Progression to Invasive Spindle Carcinomas in Transgenic Mice. Cell 1996, 86, 531–542. [Google Scholar] [CrossRef]
- Massagué, J. TGFbeta in Cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef]
- Deng, Z.; Fan, T.; Xiao, C.; Tian, H.; Zheng, Y.; Li, C.; He, J. TGF-β signaling in health, disease and therapeutics. Signal Transduct. Target. Ther. 2024, 9, 61. [Google Scholar] [CrossRef]
- Ammazzalorso, F.; Pirzio, L.M.; Bignami, M.; Franchitto, A.; Pichierri, P. ATR and ATM differently regulate WRN to prevent DSBs at stalled replication forks and promote replication fork recovery. EMBO J. 2010, 29, 3156–3169. [Google Scholar] [CrossRef]
- Ueda, Y.; Richmond, A. NF-kappaB activation in melanoma. Pigment Cell Res 2006, 19, 112–124. [Google Scholar] [CrossRef]
- Msaki, A.; Sánchez, A.M.; Koh, L.F.; Barré, B.; Rocha, S.; Perkins, N.D.; Johnson, R.F. The role of RelA (p65) threonine 505 phosphorylation in the regulation of cell growth, survival, and migration. Mol. Biol. Cell 2011, 22, 3032–3040. [Google Scholar] [CrossRef]
- Moles, A.; Butterworth, J.A.; Sanchez, A.; Hunter, J.E.; Leslie, J.; Sellier, H.; Tiniakos, D.; Cockell, S.J.; Mann, D.A.; Oakley, F.; et al. A RelA(p65) Thr505 phospho-site mutation reveals an important mechanism regulating NF-κB-dependent liver regeneration and cancer. Oncogene 2016, 35, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X.; Fan, Y.; Liu, J.; Yuan, L. XCL1 Aggravates Diabetic Nephropathy-Mediated Renal Glomerular Endothelial Cell Apoptosis and Inflammatory Response via Regulating p53/Nuclear Factor-Kappa B Pathway. Nephron 2021, 146, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Dötsch, V.; Bernassola, F.; Coutandin, D.; Candi, E.; Melino, G. p63 and p73, the ancestors of p53. Cold Spring Harb. Perspect. Biol. 2010, 2, a004887. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Ye, T.; Cui, P.; Hua, Q.; Zeng, H.; Zhao, D. AP-1 transcription factor mediates VEGF-induced endothelial cell migration and proliferation. Microvasc. Res. 2016, 105, 103–108. [Google Scholar] [CrossRef]
- Khanjani, S.; Terzidou, V.; Johnson, M.R.; Bennett, P.R. NFκB and AP-1 Drive Human Myometrial IL8 Expression. Mediat. Inflamm. 2012, 2012, 504952. [Google Scholar] [CrossRef]
- Hungness, E.S.; Pritts, T.A.; Luo, G.-j.; Sun, X.; Penner, G.C.; Hasselgren, P.-O. The transcription factor activator protein-1 is activated and interleukin-6 production is increased in interleukin-1β-stimulated human enterocytes. Shock 2000, 14, 386–391. [Google Scholar] [CrossRef]
- Karin, M.; Liu, Z.-g.; Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997, 9, 240–246. [Google Scholar] [CrossRef]
- Inamdar, G.S.; Madhunapantula, S.V.; Robertson, G.P. Targeting the MAPK pathway in melanoma: Why some approaches succeed and other fail. Biochem. Pharmacol. 2010, 80, 624–637. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, N.L.L.-A.; Abdul Rahim, N.J.; Bhatt, R.; Ong, S.E.; Lim, K.Y.; Gandini, A.; Zeng, Z.; Kumari, S.; Gabrielli, B. Checkpoint Kinase 1 Inhibitor Combined with Low Dose Hydroxyurea Promotes ATM-Activated NF-κB-Dependent Pro-Inflammatory Chemokine Expression in Melanomas. Cancers 2025, 17, 1817. https://doi.org/10.3390/cancers17111817
Goh NLL-A, Abdul Rahim NJ, Bhatt R, Ong SE, Lim KY, Gandini A, Zeng Z, Kumari S, Gabrielli B. Checkpoint Kinase 1 Inhibitor Combined with Low Dose Hydroxyurea Promotes ATM-Activated NF-κB-Dependent Pro-Inflammatory Chemokine Expression in Melanomas. Cancers. 2025; 17(11):1817. https://doi.org/10.3390/cancers17111817
Chicago/Turabian StyleGoh, Nicole Lisa Li-Ann, Nur Jannah Abdul Rahim, Rituparna Bhatt, Si En Ong, Khai Yee Lim, Anastasia Gandini, Zhen Zeng, Snehlata Kumari, and Brian Gabrielli. 2025. "Checkpoint Kinase 1 Inhibitor Combined with Low Dose Hydroxyurea Promotes ATM-Activated NF-κB-Dependent Pro-Inflammatory Chemokine Expression in Melanomas" Cancers 17, no. 11: 1817. https://doi.org/10.3390/cancers17111817
APA StyleGoh, N. L. L.-A., Abdul Rahim, N. J., Bhatt, R., Ong, S. E., Lim, K. Y., Gandini, A., Zeng, Z., Kumari, S., & Gabrielli, B. (2025). Checkpoint Kinase 1 Inhibitor Combined with Low Dose Hydroxyurea Promotes ATM-Activated NF-κB-Dependent Pro-Inflammatory Chemokine Expression in Melanomas. Cancers, 17(11), 1817. https://doi.org/10.3390/cancers17111817







