Impact of Severe Postoperative Complications and P-POSSUM Score on Oncological Outcomes in Primary Retroperitoneal Sarcoma: Insights from a Tertiary Cancer Center
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Selection
2.2. Postoperative Complications and Severity Assessment
2.3. Prognostic Tools
- -
- Sarculator: A validated nomogram-based tool that integrates histology, tumor grade, size, multifocality, and completeness of resection to predict 7-year overall survival (OS) and disease-free survival (DFS). The Sarculator has been validated in high-volume centers and recalibrated to reflect recent improvements in outcomes [10].
- -
- P-POSSUM (Portsmouth Physiological and Operative Severity Score for the Enumeration of Mortality and Morbidity): This score combines 12 physiological (age, sex, cardiac signs, systolic blood pressure, pulse rate, Glasgow coma scale, serum urea, serum sodium, serum potassium, hemoglobin, white blood cell count, ECG abnormalities) and 6 surgical parameters (operative severity, number of procedures, blood loss, peritoneal contamination, presence of malignancy, elective or emergency operation) to estimate perioperative morbidity and mortality. Though originally developed for general surgery, it has been explored for oncologic surgery including sarcoma resections [15,16].
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Postoperative Outcomes and Complications
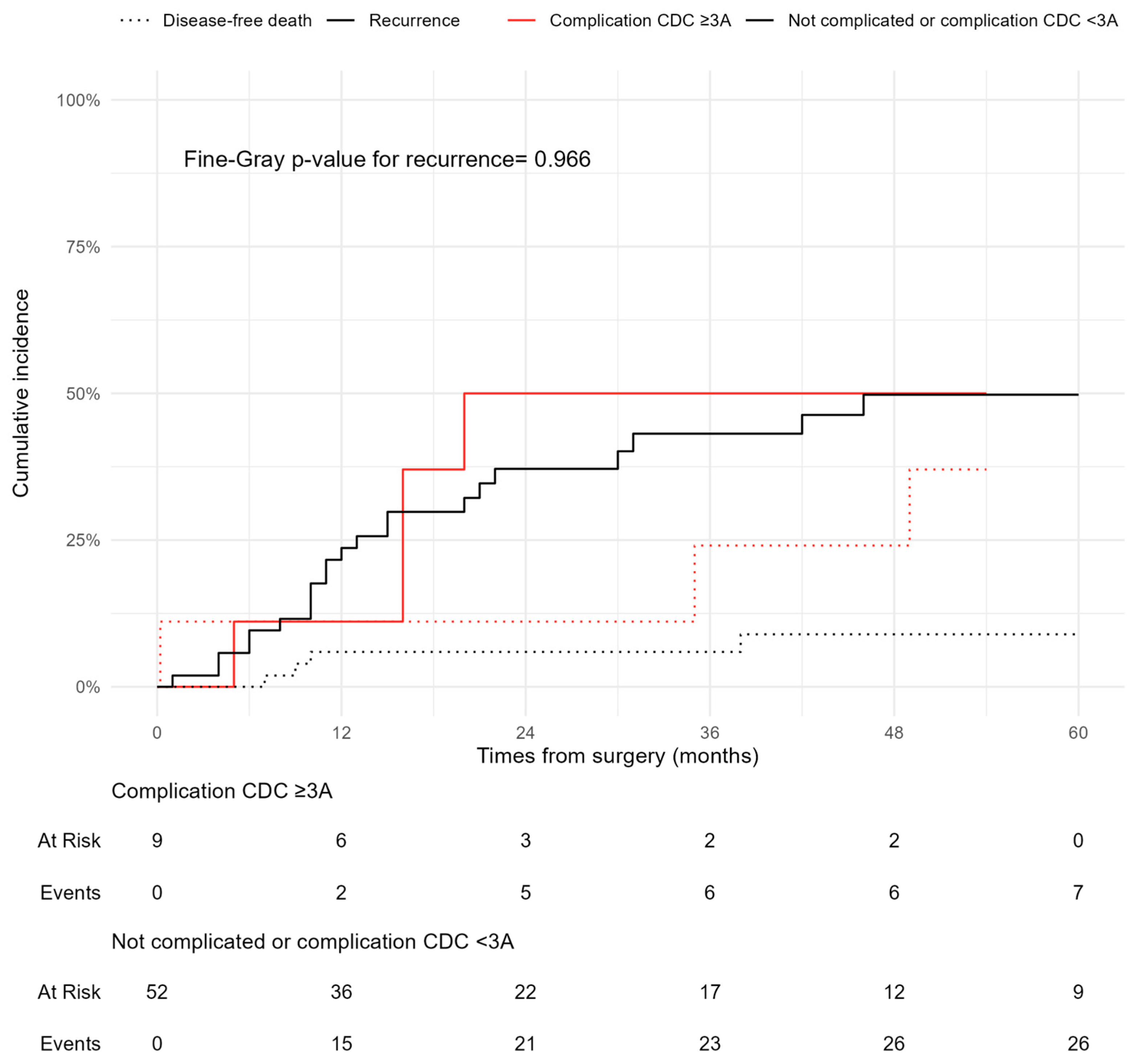
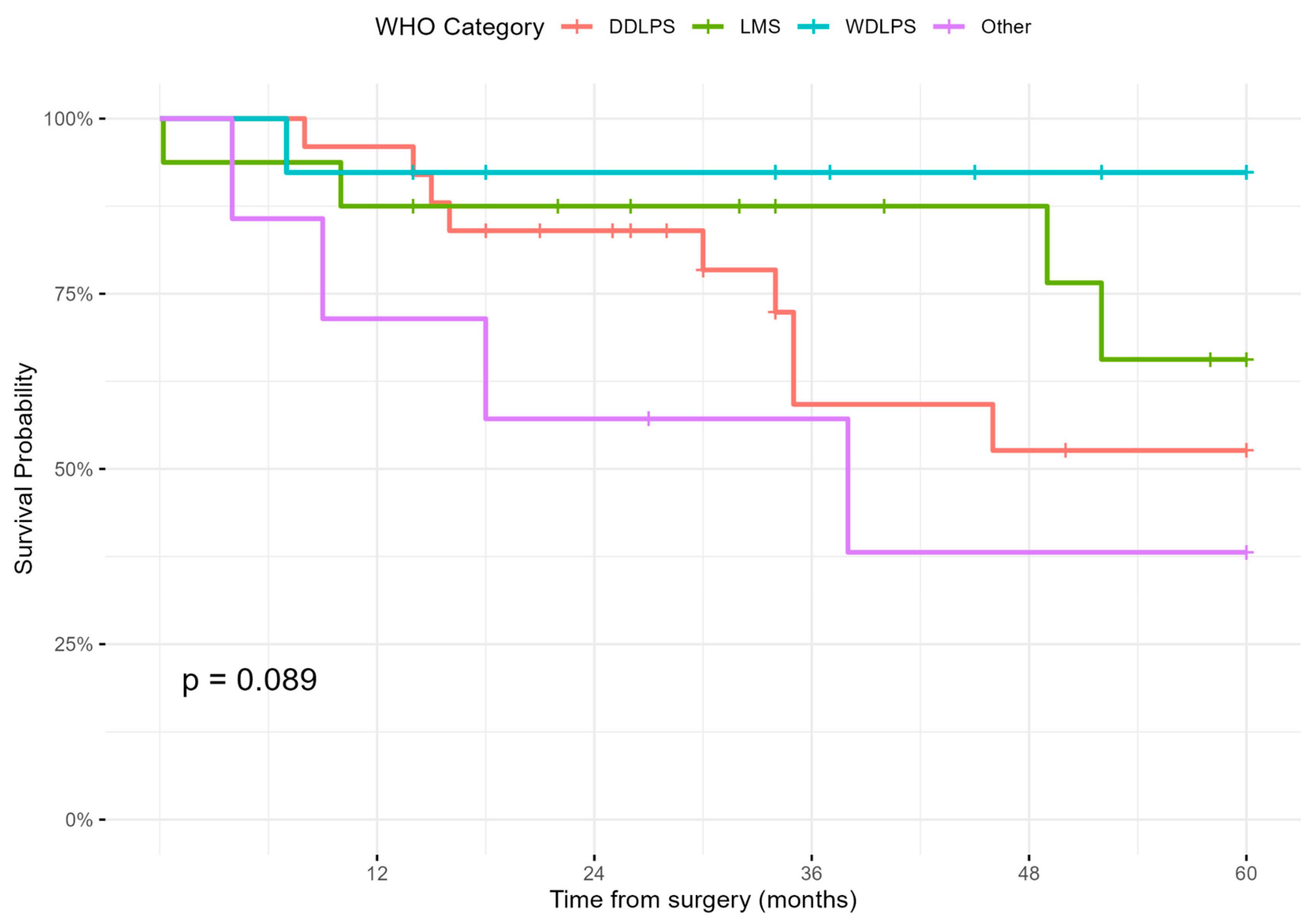
3.3. Survival and Recurrence Outcomes
3.4. Prognostic Factors for Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trama, A.; Badalamenti, G.; Baldi, G.G.; Brunello, A.; Caira, M.; Drovef, N.; Marrari, A.; Palmerini, E.; Vincenzi, B.; Dei Tos, A.P.; et al. Soft tissue sarcoma in Italy: From epidemiological data to clinical networking to improve patient care and outcomes. Cancer Epidemiol. 2019, 59, 258–264. [Google Scholar] [CrossRef] [PubMed]
- AIRTUM. I Numeri del Cancro in Italia. 2024. Available online: https://www.aiom.it/i-numeri-del-cancro-in-italia/ (accessed on 2 February 2024).
- Fairweather, M.; Gonzalez, R.J.; Strauss, D.; Raut, C.P. Current principles of surgery for retroperitoneal sarcomas. J. Surg. Oncol. 2018, 117, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Strauss, D.C.; Miceli, R.; Bonvalot, S.; Swallow, C.J.; Hohenberger, P.; Van Coevorden, F.; Rutkowski, P.; Callegaro, D.; Hayes, A.J.; et al. Variability in Patterns of Recurrence After Resection of Primary Retroperitoneal Sarcoma. Ann. Surg. 2016, 263, 1002–1009. [Google Scholar] [CrossRef]
- Bonvalot, S.; Gronchi, A.; Le Péchoux, C.; Swallow, C.J.; Strauss, D.; Meeus, P.; Van Coveorden, F.; Stoldt, S.; Stoeckle, E.; Rutkowski, P.; et al. Preoperative radiotherapy plus surgery versus surgery alone for retroperitoneal sarcoma: STRASS trial. Lancet Oncol. 2020, 21, 1366–1377. [Google Scholar] [CrossRef]
- Bonvalot, S.; Miceli, R.; Berselli, M.; Causeret, S.; Colombo, C.; Mariani, L.; Bouzaiene, H.; Le Péchoux, C.; Casali, P.G.; Le Cesne, A.; et al. Aggressive surgery in retroperitoneal soft tissue sarcoma carried out at high-volume centers is safe and is associated with improved local control. Ann. Surg. Oncol. 2010, 17, 1507–1514. [Google Scholar] [CrossRef]
- Judge, S.J.; Lata-Arias, K.; Yanagisawa, M.; Darrow, M.A.; Monjazeb, A.M.; Kirane, A.R.; Bold, R.J.; Canter, R.J.; Canter, D.J. Morbidity, mortality and temporal trends in the surgical management of retroperitoneal sarcoma: An ACS-NSQIP follow-up analysis. J. Surg. Oncol. 2019, 120, 753–760. [Google Scholar] [CrossRef]
- Nizri, E.; Netanyahu, Y.; Gerstenhaber, F.; Shamai, S.; Sher, O.; Merimsky, O.; Lahat, G.; Klausner, J.M. Severe postoperative complications are associated with impaired survival in primary but not in recurrent retroperitoneal sarcoma. Ann. Surg. Oncol. 2021, 28, 2693–2699. [Google Scholar] [CrossRef] [PubMed]
- Tirotta, F.; Parente, A.; Hodson, J.; Desai, A.; Almond, L.M.; Ford, S.J. Cumulative burden of postoperative complications in patients undergoing surgery for primary retroperitoneal sarcoma. Ann. Surg. Oncol. 2021, 28, 7939–7949. [Google Scholar] [CrossRef]
- Callegaro, D.; Barretta, F.; Raut, C.P.; Johnston, W.; Strauss, D.C.; Honoré, C.; Bonvalot, S.; Fairweather, M.; Rutkowski, P.; van Houdt, W.J.; et al. New Sarculator prognostic nomograms for patients with primary retroperitoneal sarcoma: Case volume does matter. Ann. Surg. 2023, 279, 857–865. [Google Scholar] [CrossRef]
- Hong, S.; Wang, S.; Xu, G.; Liu, J. Evaluation of the POSSUM, p-POSSUM, o-POSSUM, and APACHE II scoring systems in predicting postoperative mortality and morbidity in gastric cancer patients. Asian J. Surg. 2017, 40, 89–94. [Google Scholar] [CrossRef]
- Swallow, C.J.; Strauss, D.C.; Bonvalot, S.; Rutkowski, P.; Desai, A.; Gladdy, R.A.; Gonzalez, R.; Gyorki, D.E.; Fairweather, M.; van Houdt, W.J.; et al. Management of primary retroperitoneal sarcoma (RPS) in the adult: An updated consensus approach from the Transatlantic Australasian RPS Working Group. Ann. Surg. Oncol. 2021, 28, 7873–7888. [Google Scholar] [CrossRef] [PubMed]
- Bolliger, M.; Kroehnert, J.A.; Molineus, F.; Kandioler, D.; Schindl, M.; Riss, P. Experiences with the standardized classification of surgical complications (Clavien-Dindo) in general surgery patients. Eur. Surg. 2018, 50, 256–261. [Google Scholar] [CrossRef]
- Clavien, P.A.; Vetter, D.; Staiger, R.D.; Slankamenac, K.; Mehra, T.; Graf, R.; Puhan, M.A. The Comprehensive Complication Index (CCI®): Added value and clinical perspectives 3 years ‘down the line’. Ann. Surg. 2017, 265, 1045–1050. [Google Scholar] [CrossRef]
- Prytherch, D.R.; Whiteley, M.S.; Higgins, B.; Weaver, P.C.; Prout, W.G.; Powell, S.J. POSSUM and Portsmouth POSSUM for predicting mortality. Br. J. Surg. 1998, 85, 1217–1220. [Google Scholar] [CrossRef] [PubMed]
- Angelucci, M.; Baia, M.; Garo, M.; Alloni, R.; Callegaro, D.; Pagnoni, C.; Radaelli, S.; Colombo, C.; Pasquali, S.; Gronchi, A.; et al. Surgical outcome in retroperitoneal sarcoma surgery: Accuracy of P-POSSUM, ACS-NSQIP, and Inflammatory Biomarkers Prognostic Index (IBPI) risk-calculators for prediction of severe and overall morbidity. Ann. Surg. Oncol. 2024, 31, 7957–7966. [Google Scholar] [CrossRef] [PubMed]
- Schwarzbach, M.H.; Hormann, Y.; Hinz, U.; Leowardi, C.; Böckler, D.; Mechtersheimer, G.; Friess, H.; Büchler, M.W.; Allenberg, J.R. Clinical results of surgery for retroperitoneal sarcoma with major blood vessel involvement. J. Vasc. Surg. 2006, 44, 46–55. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, H.; Huang, B.; Xu, Y.; Huang, J. Association of perioperative allogeneic blood transfusions and long-term outcomes following radical surgery for gastric and colorectal cancers: Systematic review and meta-analysis of propensity-adjusted observational studies. BJS Open 2023, 7, zrad075. [Google Scholar] [CrossRef]
- Simões, C.M.; Carmona, M.J.C.; Hajjar, L.A.; Vincent, J.L.; Landoni, G.; Belletti, A.; Vieira, J.E.; de Almeida, J.P.; de Almeida, E.P.; Ribeiro, U., Jr.; et al. Predictors of major complications after elective abdominal surgery in cancer patients. BMC Anesthesiol. 2018, 18, 49. [Google Scholar] [CrossRef]
- Nassif, M. Predictors of postoperative complications in retroperitoneal sarcoma surgery. Saudi Surg. J. 2019, 7, 119. [Google Scholar] [CrossRef]
- Ruff, S.M.; Grignol, V.P.; Contreras, C.M.; Pollock, R.E.; Beane, J.D. Morbidity and mortality after surgery for retroperitoneal sarcoma. Curr. Oncol. 2022, 30, 492–505. [Google Scholar] [CrossRef]
- Gronchi, A.; Miceli, R.; Allard, M.A.; Callegaro, D.; Le Péchoux, C.; Fiore, M.; Honoré, C.; Sanfilippo, R.; Coppola, S.; Stacchiotti, S.; et al. Personalizing the approach to retroperitoneal soft tissue sarcoma: Histology-specific patterns of failure and postrelapse outcome after primary extended resection. Ann. Surg. Oncol. 2015, 22, 1447–1454. [Google Scholar] [CrossRef] [PubMed]
- Baiomy, T.A.; El Fattah, A.R.A.; Obaya, A.A.; Ahmed, T.Z.; Harb, O.A.; Embaby, A.; El Deen, D.S.; Osman, A.; Ibrahim, A. Surgical role, prognostic factors, and outcome prediction in primary retroperitoneal sarcoma: A prospective large cohort study. Egypt. J. Surg. 2021, 40, 2. [Google Scholar]
- Schwartz, P.B.; Stahl, C.C.; Ethun, C.; Marka, N.; Poultsides, G.A.; Roggin, K.K.; Fields, R.C.; Howard, J.H.; Clarke, C.N.; Votanopoulos, K.I.; et al. Retroperitoneal sarcoma perioperative risk stratification: A United States Sarcoma Collaborative evaluation of the ACS-NSQIP risk calculator. J. Surg. Oncol. 2020, 122, 795–802. [Google Scholar] [CrossRef]
- Pasquali, S.; Iadecola, S.; Vanzulli, A.; Infante, G.; Bologna, M.; Corino, V.; Greco, G.; Vigorito, R.; Morosi, C.; Beretta, A.; et al. Radiomic features of primary retroperitoneal sarcomas: A prognostic study. Eur. J. Cancer 2024, 213, 115120. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.X.; Koh, Y.S.; Ong, F.; Farid, M.; Tay, T.K.Y.; Teo, M. Applicability of the Sarculator and MSKCC nomograms to retroperitoneal sarcoma prognostication in an Asian tertiary center. Asian J. Surg. 2020, 43, 1078–1085. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, J.; Li, R.; Sun, L.; Sun, Q.; Wang, W.; Wang, D. Survival and prognostic factors of primary retroperitoneal sarcomas after surgery: A single-center experience. Langenbecks Arch. Surg. 2024, 409, 216. [Google Scholar] [CrossRef]
- Schenone, A.D.; Luo, J.; Montgomery, L.; Morgensztern, D.; Adkins, D.R.; Van Tine, B.A. 122P Risk stratification in soft tissue sarcoma: A single-center experience using Sarculator. ESMO Open 2025, 10, 104435. [Google Scholar]
- Sarre-Lazcano, C.; Dumitra, S.; Fiore, M. Pelvic soft tissue sarcomas. Eur. J. Surg. Oncol. 2023, 49, 1102–1110. [Google Scholar] [CrossRef]
- Ruspi, L.; Cananzi, F.C.M.; Aymerito, F.; Sicoli, F.; Samà, L.; Vanni, E.; Quagliuolo, V. Measuring the impact of complications after surgery for retroperitoneal sarcoma: Is comprehensive complication index better than Clavien-Dindo Classification? Eur. J. Surg. Oncol. 2022, 48, 978–984. [Google Scholar] [CrossRef]
- Tirotta, F.; Parente, A.; Richardson, T.; Almonib, A.; Evenden, C.; Max Almond, L.; Desai, A.; Hodson, J.; Ford, S.J. Comparison of comprehensive complication index and Clavien–Dindo classification in patients with retroperitoneal sarcoma. J. Surg. Oncol. 2021, 124, 1166–1172. [Google Scholar] [CrossRef]


| Any Complication CD ≥ 3A at 30 Days | ||||
|---|---|---|---|---|
| Characteristic | Overall N = 61 | NO N = 52 | YES N = 9 | p-Value 1 |
| Age, Mean ± SD | 63.2 ± 11.4 | 62.9 ± 11.5 | 65.1 ± 11.7 | 0.6 |
| Sex, n (%) | >0.9 | |||
| Male | 37 (60.7%) | 31 (59.6%) | 6 (66.7%) | - |
| Female | 24 (39.3) | 21 (40.4%) | 3 (33.3%) | - |
| BMI, Kg/m2, mean ± SD | 26.7 ± 4.5 | 26.8 ± 4.7 | 25.9 ± 3.2 | 0.6 |
| CCI, Median (IQR) | 5.0 (4.0, 6.0) | 5.0 (4.0, 6.0) | 5.0 (3.0, 7.0) | 0.6 |
| ECOG score, Median (IQR) | 1.0 (0.0, 1.0) | 1.0 (0.0, 1.0) | 1.0 (0.0, 1.0) | 0.9 |
| ASA score, Median (IQR) | 2.0 (2.0, 2.0) | 2.0 (2.0, 2.5) | 2.0 (2.0, 2.0) | 0.7 |
| P-POSSUM Morbidity, Median (IQR) | 24.7 (17.1, 44.4) | 23.4 (17.1, 41.7) | 49.1 (31.1, 61.5) | 0.071 |
| P-POSSUM Mortality, Median (IQR) | 4.3 (2.9, 8.7) | 4.0 (2.7, 7.5) | 10.1 (5.9, 15.4) | 0.051 |
| Disease Location, n (%) | 0.3 | |||
| Right | 33 (54%) | 30 (57%) | 3 (33.3%) | - |
| Left | 28 (46%) | 22 (43%) | 6 (66.7%) | - |
| Histology, n (%) | 0.7 | |||
| Liposarcoma (WDLPS + DDLPS) | 38 (62.3%) | 33 (63.5%) | 5 (55.6%) | - |
| Other | 23 (37.7%) | 19 (36.5%) | 4 (44.4%) | - |
| Tumor Size, mm, mean ± SD | 215.9 ± 132.3 | 223.4 ± 137.0 | 172.2 ± 95.6 | 0.3 |
| Grading (FNCLCC), n (%) | 0.4 | |||
| Low Grade (1) | 16 (26.2%) | 15 (28.8%) | 1 (11.1%) | - |
| High Grade (2–3) | 49 (73.8%) | 37 (71.2%) | 8 (88.9%) | - |
| Intraperitoneal Invasion, n (%) | >0.9 | |||
| Yes | 3 (4.9%) | 3 (5.7%) | 0 (0%) | - |
| No | 58 (95.1%) | 49 (94.3%) | 9 (100%) | - |
| Disease Multifocality, n (%) | 0.4 | |||
| Yes | 3 (4.9%) | 2 (3.8%) | 1 (11.1%) | - |
| No | 58 (95.1%) | 50 (69.2%) | 8 (88.9%) | - |
| Preoperative Chemotherapy, | 0.7 | |||
| Yes | 11 (18.0%) | 9 (17.3%) | 2 (22.2%) | - |
| No | 50 (82.0%) | 43 (82.7%) | 7 (77.8%) | - |
| Preoperative Radiotherapy, | >0.9 | |||
| Yes | 4 (6.6%) | 4 (7.7%) | 0 (0%) | - |
| No | 57 (93.4%) | 48 (92.3%) | 9 (100%) | - |
| Duration of Surgery, Min, Mean ± SD | 298.0 ± 165.9 | 280.6 ± 146.1 | 398.8 ± 238.8 | 0.048 |
| Completeness of Resection, | 0.3 | |||
| Complete R0/1 | 59 (96.7%) | 51 (98.1%) | 8 (88.9%) | - |
| Incomplete R2 | 2 (3.3%) | 1 (1.9%) | 1 (11.1%) | - |
| Number Organ Resected, Mean ± SD | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | 3.0 (2.0, 4.0) | 0.3 |
| Organ Resected, | ||||
| Small Bowel | 2 (3.28%) | 2 (3.85%) | 0 (0%) | >0.9 |
| Colon | 21 (34.4%) | 17 (32.7%) | 4 (44.4%) | 0.7 |
| Stomach | 2 (3.28%) | 0 (0%) | 2 (22.2%) | 0.020 |
| Pancreas | 3 (4.92%) | 2 (3.85%) | 1 (11.1%) | 0.4 |
| Spleen | 4 (6.56%) | 3 (5.77%) | 1 (11.1%) | 0.5 |
| Diaphragm | 4 (6.56%) | 2 (3.85%) | 2 (22.2%) | 0.10 |
| Nerve | 3 (4.92%) | 2 (3.85%) | 1 (11.1%) | 0.4 |
| Psoas | 15 (24.6%) | 12 (23.1%) | 3 (33.3%) | 0.7 |
| Vascular | 8 (13.1%) | 7 (13.5%) | 1 (11.1%) | >0.9 |
| Kidney + Adrenal gland | 30 (49.2%) | 26 (50.0%) | 4 (44.4%) | >0.9 |
| Uterus + Adnexa | 3 (4.92%) | 3 (5.77%) | 0 (0%) | >0.9 |
| Other | 6 (9.84%) | 5 (9.62%) | 1 (11.1%) | >0.9 |
| Intraoperative Transfusion, | 0.005 | |||
| Yes | 15 (24.6%) | 9 (17.3%) | 6 (66.7%) | - |
| No | 46 (75.4%) | 43 (82.7%) | 3 (33.3%) | - |
| Severe Intraoperative Complication, | 0.020 | |||
| Yes | 5 (8.2%) | 2 (3.8%) | 3 (33.3%) | - |
| No | 56 (91.8%) | 50 (96.2%) | 6 (66.7%) | - |
| Factor | N (%) |
|---|---|
| Any complications, n (%) | |
| No | 37 (60.7%) |
| Yes | 24 (39.3%) |
| Number of complications per patient, n (%) | |
| 0 | 38 (62.3%) |
| 1 | 19 (31.1%) |
| 2 | 3 (4.92%) |
| 3 | 1 (1.64%) |
| Patient with complication CD ≥ 3A, n (%) | |
| No | 52 (85.2%) |
| Yes | 9 (14.8%) |
| Type | Grade | N. |
|---|---|---|
| Acute bleeding | 3B | 2 |
| 5 | 1 | |
| Abdominal Collection | 3A | 3 |
| Bowel obstruction | 3B | 2 |
| Urinary fistula | 3A | 1 |
| Tot 9 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Complication CD ≥ 3A | 1.03 | 0.37–2.87 | 0.95 | 0.81 | 0.28–2.33 | 0.7 |
| Sarculator DFS | 0.98 | 0.96–0.99 | 0.002 | 0.97 | 0.96–0.99 | 0.004 |
| P-Possum score mortality | 1.06 | 0.99–1.14 | 0.09 | 1.057 | 0.99–1.12 | 0.09 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Complication CD ≥ 3A | 2.53 | 0.90–7.41 | 0.08 | 0.90 | 0.27–2.04 | 0.9 |
| Sarculator OS | 0.97 | 0.95–0.99 | <0.001 | 0.97 | 0.95–0.99 | 0.008 |
| P-Possum score mortality | 1.15 | 1.08–1.23 | <0.001 | 1.12 | 1.04–1.21 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abatini, C.; Barberis, L.; Lodoli, C.; Ferracci, F.; De Lorenzis, E.; D’Annibale, G.; Aulicino, M.; Quirino, M.; Di Salvatore, M.; Alfieri, S.; et al. Impact of Severe Postoperative Complications and P-POSSUM Score on Oncological Outcomes in Primary Retroperitoneal Sarcoma: Insights from a Tertiary Cancer Center. Cancers 2025, 17, 1787. https://doi.org/10.3390/cancers17111787
Abatini C, Barberis L, Lodoli C, Ferracci F, De Lorenzis E, D’Annibale G, Aulicino M, Quirino M, Di Salvatore M, Alfieri S, et al. Impact of Severe Postoperative Complications and P-POSSUM Score on Oncological Outcomes in Primary Retroperitoneal Sarcoma: Insights from a Tertiary Cancer Center. Cancers. 2025; 17(11):1787. https://doi.org/10.3390/cancers17111787
Chicago/Turabian StyleAbatini, Carlo, Lorenzo Barberis, Claudio Lodoli, Federica Ferracci, Enrico De Lorenzis, Giorgio D’Annibale, Matteo Aulicino, Michela Quirino, Mariantonietta Di Salvatore, Sergio Alfieri, and et al. 2025. "Impact of Severe Postoperative Complications and P-POSSUM Score on Oncological Outcomes in Primary Retroperitoneal Sarcoma: Insights from a Tertiary Cancer Center" Cancers 17, no. 11: 1787. https://doi.org/10.3390/cancers17111787
APA StyleAbatini, C., Barberis, L., Lodoli, C., Ferracci, F., De Lorenzis, E., D’Annibale, G., Aulicino, M., Quirino, M., Di Salvatore, M., Alfieri, S., Pacelli, F., & Santullo, F. (2025). Impact of Severe Postoperative Complications and P-POSSUM Score on Oncological Outcomes in Primary Retroperitoneal Sarcoma: Insights from a Tertiary Cancer Center. Cancers, 17(11), 1787. https://doi.org/10.3390/cancers17111787






