Impact of e-Health Interventions on Mental Health and Quality of Life in Breast Cancer Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Data Extraction
2.3. Risk of Bias and Quality Assessment
2.4. Statistical Analysis
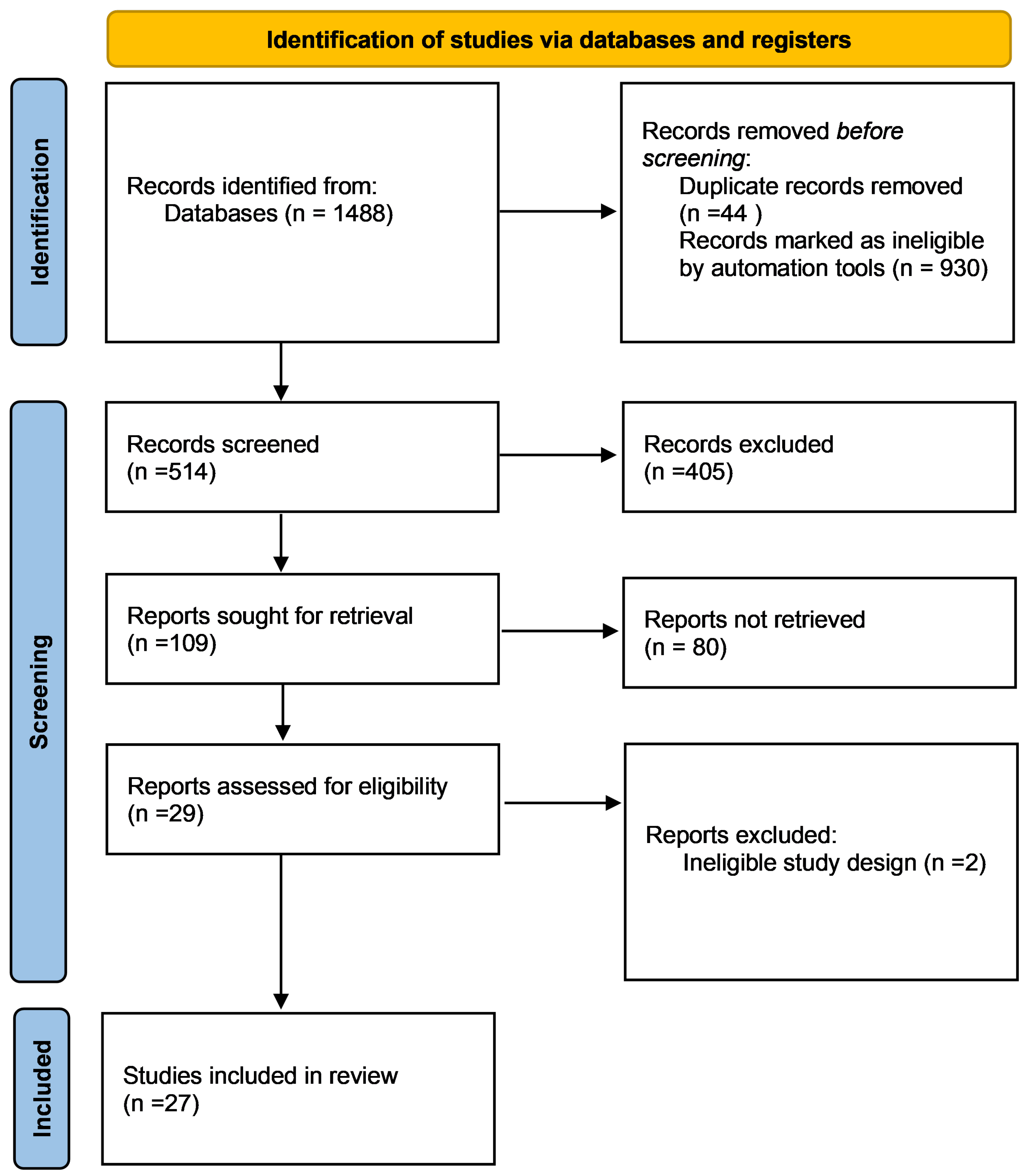
3. Results
3.1. Characteristics of Included Studies
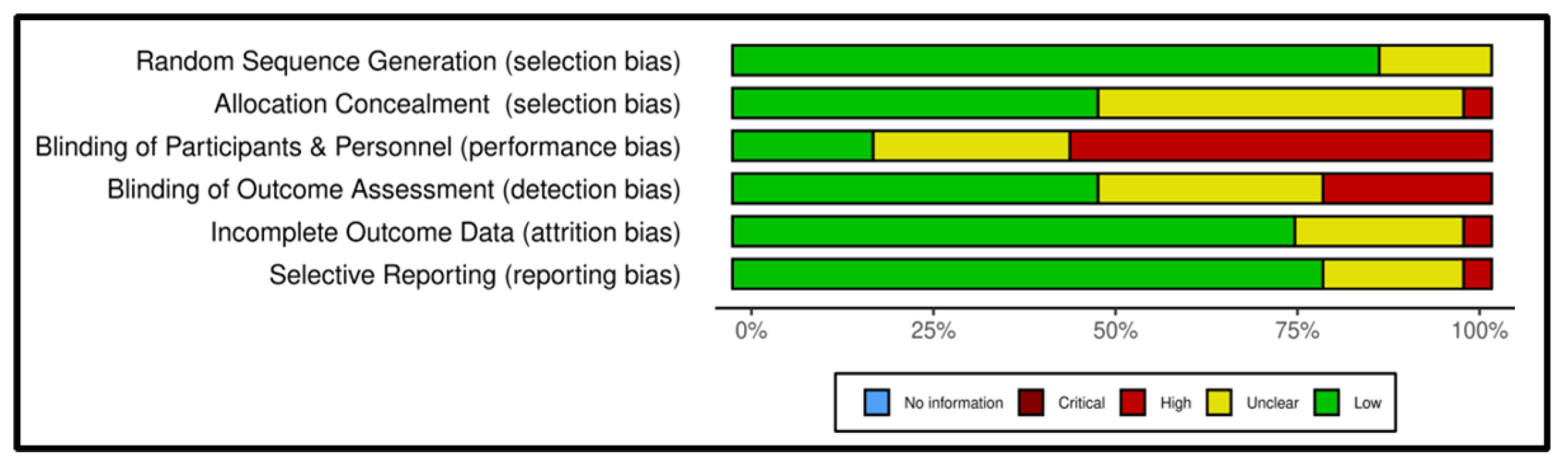
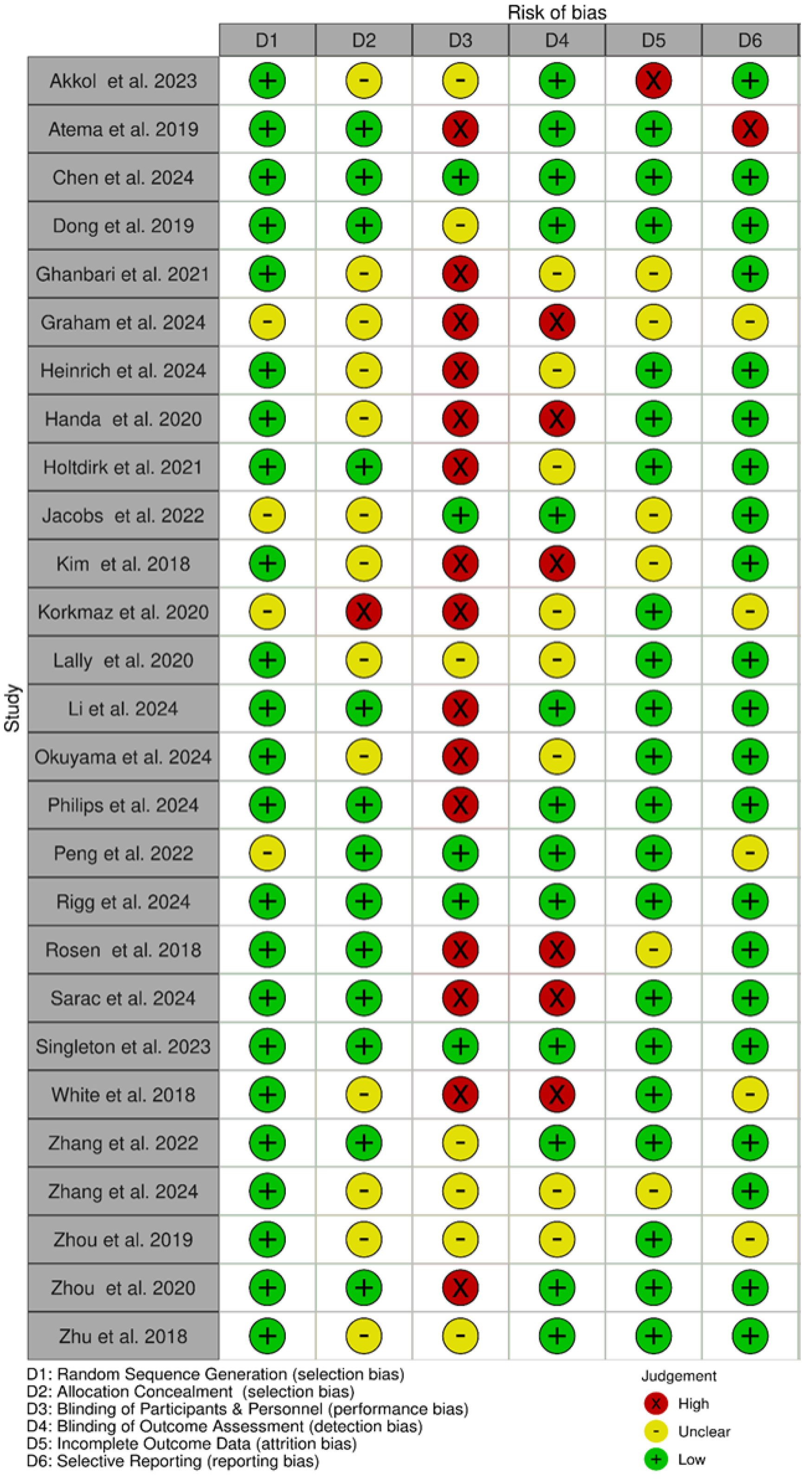
3.2. Risk of Bias Within Studies
3.3. Intervention Outcomes
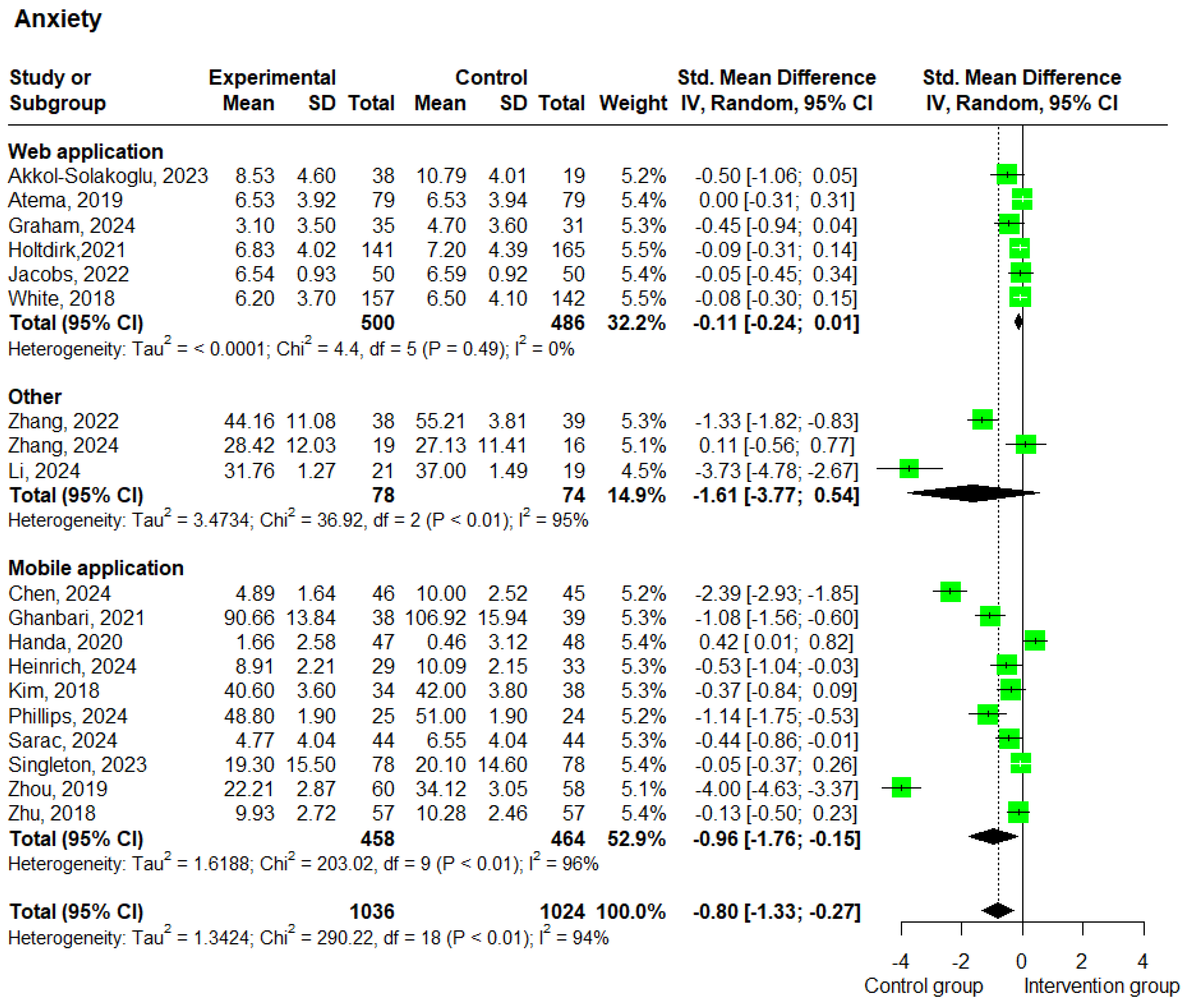
3.3.1. Anxiety
3.3.2. Depression
3.3.3. QoL
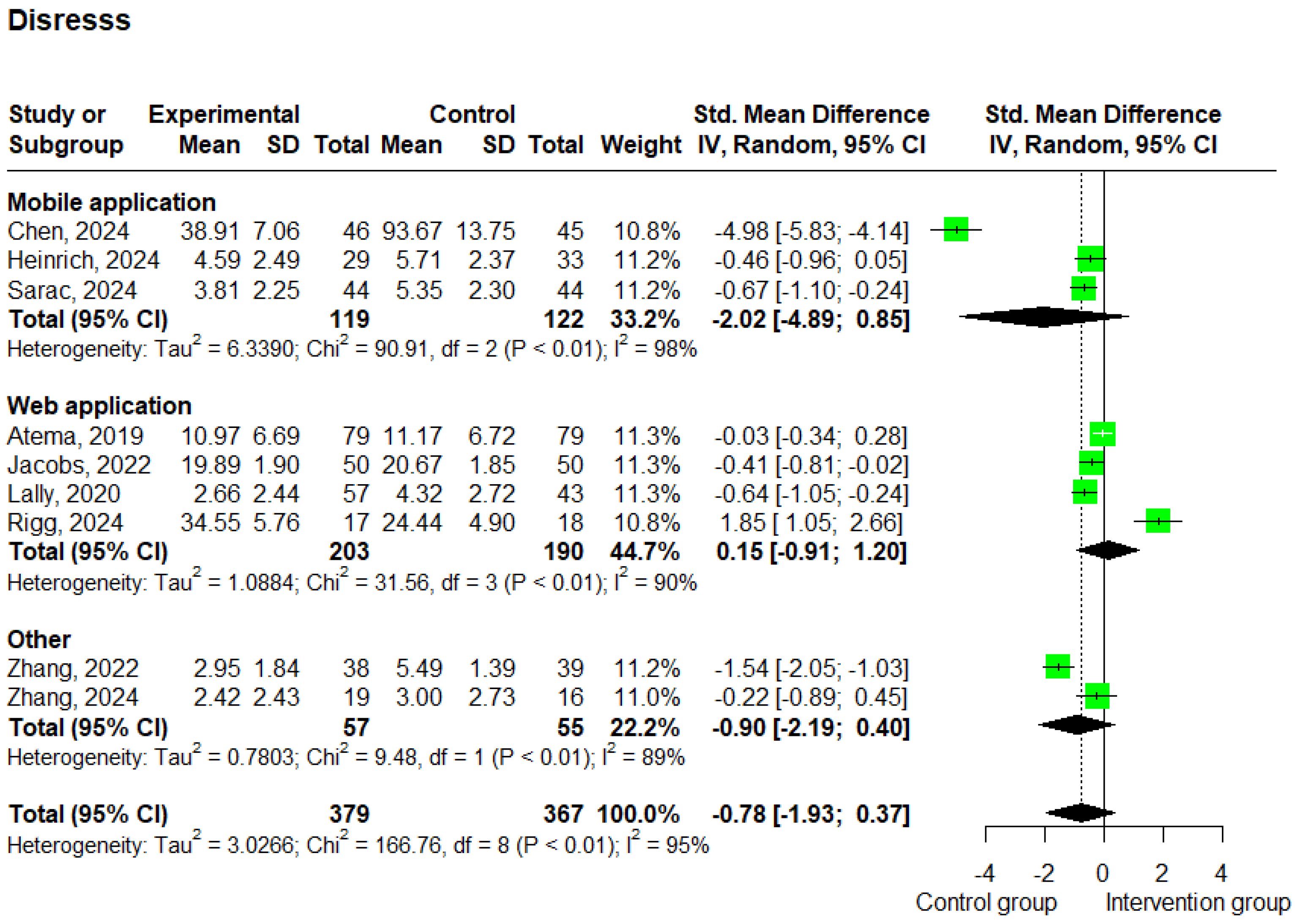
3.3.4. Distress
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| App | Application |
| BC | Breast cancer |
| E-health | Electronic health |
| HRQoL | Health-related quality of life |
| IARC | International Agency for Research on Cancer |
| PICOS | Population, Intervention, Comparator, Outcomes, Study Design |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| QoL | Quality of life |
| RCT | Randomized controlled trial |
| AI | Artificial intelligence |
| PGHD | Patient-generated health data |
| EHRs | Electronic health records |
References
- “Breast Cancer|Breast Cancer Information & Overview”. Available online: https://www.cancer.org/cancer/types/breast-cancer.html (accessed on 21 November 2024).
- Zahwe, M.; Bendahhou, K.; Eser, S.; Mukherji, D.; Fouad, H.; Fadhil, I.; Soerjomataram, I.; Znaor, A. Current and future burden of female breast cancer in the Middle East and North Africa region using estimates from GLOBOCAN 2022. Int. J. Cancer 2025, 156, 2320–2329. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Feng, Y.; Zhang, J.; Swinnen, J.; Li, Y.; Ni, Y. A Review on Curability of Cancers: More Efforts for Novel Therapeutic Options Are Needed. Cancers 2019, 11, 11782. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Gong, J.; Li, Q. The application of eHealth in cancer survivorship care: A review of web-based dyadic interventions for post-treatment cancer survivors and caregivers. Asia-Pac. J. Oncol. Nurs. 2022, 9, 100109. [Google Scholar] [CrossRef] [PubMed]
- Emre, N.; Yılmaz, S. Sleep quality, mental health, and quality of life in women with breast cancer. Indian J. Cancer 2024, 61, 299. [Google Scholar] [CrossRef]
- Nayak, M.G.; George, A.; Vidyasagar, M.S.; Mathew, S.; Nayak, S.; Nayak, B.S.; Shashidhara, Y.; Kamath, A. Quality of Life among Cancer Patients. Indian J. Palliat. Care 2017, 23, 445–450. [Google Scholar] [CrossRef]
- Zeng, Y.; Hu, C.-H.; Li, Y.-Z.; Zhou, J.-S.; Wang, S.-X.; Liu, M.-D.; Qiu, Z.-H.; Deng, C.; Ma, F.; Xia, C.-F.; et al. Association between pretreatment emotional distress and immune checkpoint inhibitor response in non-small-cell lung cancer. Nat. Med. 2024, 30, 1680–1688. [Google Scholar] [CrossRef]
- Boogerd, E.A.; Arts, T.; Engelen, L.J.; van de Belt, T.H. ‘What Is eHealth’: Time for An Update? JMIR Res. Protoc. 2015, 4, e29. [Google Scholar] [CrossRef]
- Penedo, F.J.; Oswald, L.B.; Kronenfeld, J.P.; Garcia, S.F.; Cella, D.; Yanez, B. The increasing value of eHealth in the delivery of patient-centred cancer care. Lancet Oncol. 2020, 21, e240–e251. [Google Scholar] [CrossRef]
- Gitonga, I.; Desmond, D.; Duda, N.; Maguire, R. Impact of connected health interventions on psychological wellbeing and quality of life in patients with cancer: A systematic review and meta-analysis. Psychooncology 2022, 31, 1621–1636. [Google Scholar] [CrossRef]
- Luo, X.; Gao, L.; Li, J.; Lin, Y.; Zhao, J.; Li, Q. A critical literature review of dyadic web-based interventions to support cancer patients and their caregivers, and directions for future research. Psychooncology 2020, 29, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Tang, L.; Chen, Y.; Zhou, J.; Yang, Q.; Wang, R. The effectiveness of E-health interventions promoting physical activity in cancer survivors: A systematic review and meta-analysis of randomized controlled trials. J. Cancer Res. Clin. Oncol. 2024, 150, 72. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Chapter 8: Assessing Risk of Bias in a Randomized Trial. Available online: https://training.cochrane.org/handbook/archive/v6.2/chapter-08 (accessed on 22 November 2024).
- Risk of Bias 2 (RoB 2) Tool | Cochrane Methods. Available online: https://methods.cochrane.org/risk-bias-2 (accessed on 22 November 2024).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Chen, X.; Qin, Y.; Chaimongkol, N. Effectiveness of a phone-based support program on self-care self-efficacy, psychological distress, and quality of life among women newly diagnosed with breast cancer: A randomized controlled trial. Eur. J. Oncol. Nurs. 2024, 71, 102643. [Google Scholar] [CrossRef]
- Dong, X.; Yi, X.; Gao, D.; Gao, Z.; Huang, S.; Chao, M.; Chen, W.; Ding, M. The effects of the combined exercise intervention based on internet and social media software (CEIBISMS) on quality of life, muscle strength and cardiorespiratory capacity in Chinese postoperative breast cancer patients: A randomized controlled trial. Health Qual. Life Outcomes 2019, 17, 109. [Google Scholar] [CrossRef]
- Ghanbari, E.; Yektatalab, S.; Mehrabi, M. Effects of Psychoeducational Interventions Using Mobile Apps and Mobile-Based Online Group Discussions on Anxiety and Self-Esteem in Women with Breast Cancer: Randomized Controlled Trial. JMIR Mhealth Uhealth 2021, 9, e19262. [Google Scholar] [CrossRef]
- Handa, S.; Okuyama, H.; Yamamoto, H.; Nakamura, S.; Kato, Y. Effectiveness of a Smartphone Application as a Support Tool for Patients Undergoing Breast Cancer Chemotherapy: A Randomized Controlled Trial. Clin. Breast Cancer 2020, 20, 201–208. [Google Scholar] [CrossRef]
- Heinrich, R.; Schilling, G.; Wojtyna, E.; Arnold, D.; Geisler, M.; Kley, S.; Grudzinski, P.; Księżak, M.; Schoenfelder, T. Effects of Mobile Application-Based Cognitive Behavioral Therapy on Psychological Outcomes in Women Treated for Breast Cancer: A Randomized Controlled Pilot Trial in Germany. Psycho-Oncology 2024, 33, 10. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sang, D.; Gong, L.; Wang, B.; Wang, Y.; Jia, X.; Yu, J.; Kong, Z.; Liu, H.; Zhang, Y. Improving physical and mental health in women with breast cancer undergoing anthracycline-based chemotherapy through wearable device-based aerobic exercise: A randomized controlled trial. Front. Public Health 2024, 12, 1451101. [Google Scholar] [CrossRef]
- Okuyama, H.; Takada, F.; Taira, N.; Nakamura, S. A randomized trial of the impact of symptom monitoring using an electronic patient-reported outcome app on health-related quality of life in postmenopausal breast cancer patients receiving adjuvant endocrine therapy. Breast Cancer 2024, 31, 787–797. [Google Scholar] [CrossRef]
- Phillips, S.M.; Starikovsky, J.; Solk, P.; Desai, R.; Reading, J.M.; Hasanaj, K.; Wang, S.D.; Cullather, E.; Lee, J.; Song, J.; et al. Feasibility and preliminary effects of the Fit2ThriveMB pilot physical activity promotion intervention on physical activity and patient reported outcomes in individuals with metastatic breast cancer. Breast Cancer Res. Treat. 2024, 208, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yang, Y.; Chen, M.; Xu, C.; Chen, Y.; Liu, R.; Cao, X.; Li, M. Effects of an online mindfulness-based intervention on Fear of Cancer Recurrence and quality of life among Chinese breast cancer survivors. Complement Ther. Clin. Pr. 2022, 49, 101686. [Google Scholar] [CrossRef] [PubMed]
- Rosen, K.D.; Paniagua, S.M.; Kazanis, W.; Jones, S.; Potter, J.S. Quality of life among women diagnosed with breast Cancer: A randomized waitlist controlled trial of commercially available mobile app-delivered mindfulness training. Psycho-Oncology 2018, 27, 2023–2030. [Google Scholar] [CrossRef]
- Saraç, F.S.; İlhan, S.E.; Kutun, S.; Kutlutürkan, S. The Effect of Informative Mobile App Use on Anxiety, Distress, and Quality of Life of Women with Breast Cancer. Eur. J. Breast Health 2024, 20, 207–214. [Google Scholar] [CrossRef]
- Singleton, A.C.; Raeside, R.; Partridge, S.R.; Hyun, K.K.; Tat-Ko, J.; Sum, S.C.M.; Hayes, M.; Chow, C.K.; Thiagalingam, A.; Maka, K.; et al. Supporting women’s health outcomes after breast cancer treatment comparing a text message intervention to usual care: The EMPOWER-SMS randomised clinical trial. J. Cancer Surviv. 2023, 17, 1533–1545. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, W.; Zhao, W.; Li, L.; Zhang, M.; Guo, P.; Zhou, C.; Li, M.; An, J.; Li, J.; et al. Benefits of a WeChat-based multimodal nursing program on early rehabilitation in postoperative women with breast cancer: A clinical randomized controlled trial. Int. J. Nurs. Stud. 2020, 106, 103565. [Google Scholar] [CrossRef]
- Zhu, J.; Ebert, L.; Liu, X.; Wei, D.; Chan, S.W.-C. Mobile breast cancer e-support program for chinese women with breast cancer undergoing chemotherapy (Part 2): Multicenter randomized controlled trial. JMIR MHealth UHealth 2018, 6, 9438. [Google Scholar] [CrossRef]
- Zhou, K.; Li, J.; Li, X. Effects of cyclic adjustment training delivered via a mobile device on psychological resilience, depression, and anxiety in Chinese post-surgical breast cancer patients. Breast Cancer Res. Treat. 2019, 178, 95–103. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.M.; Shin, H.; Jang, J.-S.; Kim, Y.I.; Han, D.H. A Mobile Game for Patients with Breast Cancer for Chemotherapy Self-Management and Quality-of-Life Improvement: Randomized Controlled Trial. J. Med. Internet Res. 2018, 20, e273. [Google Scholar] [CrossRef]
- Zhang, Y.; Pang, Y.; He, Y.; You, M.; Tang, L. Feasibility of online managing cancer and living meaningfully (CALM) in Chinese patients with metastatic breast cancer: A pilot randomized control trial. Sci. Rep. 2024, 14, 1. [Google Scholar] [CrossRef]
- Zhang, X.; Yao, S.; Wang, M.; Yin, X.; Bi, Z.; Jing, Y.; Cheng, H. The Impact of VR-CALM Intervention Based on VR on Psychological Distress and Symptom Management in Breast Cancer Survivors. J. Oncol. 2022, 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Atema, V.; van Leeuwen, M.; Kieffer, J.M.; Oldenburg, H.S.; van Beurden, M.; Gerritsma, M.A.; Kuenen, M.A.; Plaisier, P.W.; Cardozo, A.M.L.; van Riet, Y.E.; et al. Efficacy of Internet-Based Cognitive Behavioral Therapy for Treatment-Induced Menopausal Symptoms in Breast Cancer Survivors: Results of a Randomized Controlled Trial. J. Clin. Oncol. 2019, 37, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.D.; Ellison, R.; Hall, L.H.; Clark, J.; McNaught, E.; Green, S.M.C.; Wilkes, H.; Robson, G.; Lorentz, I.; Holmes, L.; et al. A pilot randomised controlled trial of acceptance and commitment therapy for medication decision-making and quality of life in women with breast cancer: The ACTION trial. Psycho-Oncology 2024, 33, 5. [Google Scholar] [CrossRef]
- Holtdirk, F.; Mehnert, A.; Weiss, M.; Mayer, J.; Meyer, B.; Bröde, P.; Claus, M.; Watzl, C. Results of the Optimune trial: A randomized controlled trial evaluating a novel Internet intervention for breast cancer survivors. PLoS ONE 2021, 16, 5. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Post, K.; Massad, K.; Horick, N.K.; Walsh, E.A.; Cohn, J.; Rapoport, C.S.; Clara, A.J.; Antoni, M.H.; Safren, S.A.; et al. A telehealth intervention for symptom management, distress, and adherence to adjuvant endocrine therapy: A randomized controlled trial. Cancer 2022, 128, 3541–3551. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, S.; Iyigun, E.; Tastan, S. An Evaluation of the Influence of Web-Based Patient Education on the Anxiety and Life Quality of Patients Who Have Undergone Mammaplasty: A Randomized Controlled Study. J. Cancer Educ. 2020, 35, 912–922. [Google Scholar] [CrossRef]
- Lally, R.M.; Kupzyk, K.A.; Bellavia, G.; Hydeman, J.; Gallo, S.; Helgeson, V.S.; Erwin, D.; Mills, A.C.; Brown, J.K. CaringGuidanceTM after breast cancer diagnosis eHealth psychoeducational intervention to reduce early post-diagnosis distress. Support Care Cancer 2020, 28, 2163–2174. [Google Scholar] [CrossRef]
- Rigg, A.; Kemp, E.; Koczwara, B.; Butow, P.; Girgis, A.; Hulbert-Williams, N.J.; Kaambwa, B.; Long, R.; Schofield, P.; Turner, J.; et al. Feasibility, acceptability, and preliminary efficacy of a self-directed online psychosocial intervention for women with metastatic breast cancer: Finding My Way-Advanced. Support Care Cancer 2024, 32, 744. [Google Scholar] [CrossRef] [PubMed]
- White, V.; Farrelly, A.; Pitcher, M.; Hill, D. Does access to an information-based, breast cancer specific website help to reduce distress in young women with breast cancer? Results from a randomised trial. Eur. J. Cancer Care 2018, 27, 6. [Google Scholar] [CrossRef] [PubMed]
- Akkol-Solakoglu, S.; Hevey, D. Internet-delivered cognitive behavioural therapy for depression and anxiety in breast cancer survivors: Results from a randomised controlled trial. Psycho-Oncology 2023, 32, 446–456. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Ream, M.E.; Pensak, N.; Nisotel, L.E.; Fishbein, J.N.; MacDonald, J.J.; Buzaglo, J.; Lennes, I.T.; Safren, S.A.; Pirl, W.F.; et al. Patient experiences with oral chemotherapy: Adherence, symptoms, and quality of life. JNCCN J. Natl. Compr. Cancer Netw. 2019, 17, 221–228. [Google Scholar] [CrossRef]
- Doyle-Lindrud, S. State of eHealth in Cancer Care: Review of the Benefits and Limitations of eHealth Tools. CJON 2020, 24, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Wosik, J.; Fudim, M.; Cameron, B.; Gellad, Z.F.; Cho, A.; Phinney, D.; Curtis, S.; Roman, M.; Poon, E.G.; Ferranti, J.; et al. Telehealth transformation: COVID-19 and the rise of virtual care. J. Am. Med. Inform. Assoc. 2020, 27, 957–962. [Google Scholar] [CrossRef]
- Pan, L.; Wu, X.; Lu, Y.; Zhang, H.; Zhou, Y.; Liu, X.; Liu, S.; Yan, Q. Artificial intelligence empowered digital health technologies in cancer survivorship care: A scoping review. Asia-Pac. J. Oncol. Nurs. 2022, 9, 100127. [Google Scholar] [CrossRef]
- Ye, D.; Woods, N.; Jordan; Starren, J. The role of artificial intelligence for the application of integrating electronic health records and patient-generated data in clinical decision support. AMIA Jt. Summits Transl. Sci. Proc. 2024, 2024, 459–467. [Google Scholar]
- Sun, F.; Zhang, L.; Tong, Z. Application progress of artificial intelligence in tumor diagnosis and treatment. Front. Artif. Intell. 2025, 7, 1487207. [Google Scholar] [CrossRef]
- Ruiz Sarrias, O.; Martínez del Prado, M.P.; Sala Gonzalez, M.Á.; Azcuna Sagarduy, J.; Casado Cuesta, P.; Figaredo Berjano, C.; Galve-Calvo, E.; López de San Vicente Hernández, B.; López-Santillán, M.; Nuño Escolástico, M.; et al. Leveraging Large Language Models for Precision Monitoring of Chemotherapy-Induced Toxicities: A Pilot Study with Expert Comparisons and Future Directions. Cancers 2024, 16, 2830. [Google Scholar] [CrossRef]
- Deepa, R.; Arunkumar, S.; Jayaraj, V.; Sivasamy, A. Healthcare’s new Frontier: AI-driven early cancer detection for improved well-being. AIP Adv. 2023, 13, 115331. [Google Scholar] [CrossRef]
- Nori, L.P.; Lohitha, M.; Vadapalli, R.R.; Bonthagarala, B.; Nagineni, S.R.; Kalidindi, V.R. Revolutionizing Healthcare: The Impact of AI on Precision Medicine. Int. J. Pharm. Investig. 2025, 15, 2. [Google Scholar] [CrossRef]
- Khosravi, M.; Azar, G. A systematic review of reviews on the advantages of mHealth utilization in mental health services: A viable option for large populations in low-resource settings. Camb. Prism. Glob. Ment. Health 2024, 11, e43. [Google Scholar] [CrossRef]
- Güler, K.G.; Uzun, S.; Emirza, E.G. The effectiveness of telemedicine applications in mental health services: A meta-analysis study. Ir. J. Med. Sci. 2025, 194, 233–245. [Google Scholar] [CrossRef]
- Distress-the 6th Vital Sign-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21980246/ (accessed on 5 February 2025).
- Nelson, H.D.; Pappas, M.; Cantor, A.; Haney, E.; Holmes, R. Risk Assessment, Genetic Counseling, and Genetic Testing for BRCA-Related Cancer in Women: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2019, 322, 666–685. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, Y.; Dong, N.; Zhang, L.; Zhang, S. Effect of telehealth interventions on anxiety and depression in cancer patients: A systematic review and meta-analysis of randomized controlled trials. J. Telemed. Telecare 2024, 30, 1053–1064. [Google Scholar] [CrossRef]
- Wen, T.; Chen, C.; Ren, W.; Hu, S.; Zhao, X.; Zhao, F.; Zhao, Q. Effect of electronic health (eHealth) on quality of life in women with breast cancer: A systematic review and meta-analysis of randomized controlled trials. Cancer Med. 2023, 12, 14252–14263. [Google Scholar] [CrossRef]


| Parameter | Inclusion Criteria |
|---|---|
| Population | Adult men or women (aged > 18 years old) diagnosed with BC |
| Intervention | Patient-directed e-health intervention |
| Comparator | Studies in which patients received standard care or control intervention |
| Outcomes | QoL, anxiety, depression, distress |
| Study Design | Randomized controlled trials |
| Authors | PMID/DOI | Number of Patients | Stage/Status | Therapy | Experimental Intervention | Comparison | Duration of Intervention (Weeks) | Study Outcomes (Compared to Control Group) |
|---|---|---|---|---|---|---|---|---|
| Akkol-Solakoglu, et al. [46] | 36635249 | Total = 72 (I = 49, C = 23), Mean age: 47.8 | 0, I, II, III, IV | Chemotherapy, Radiotherapy, Hormonal therapy, Surgery | Web-based cognitive behavioral therapy | Usual care | 8 | No significant effect on anxiety, depression, fear of recurrence, and QoL. |
| Atema et al. [38] | 30763176 | Total = 169 (I = 85, C = 85), Mean age: 47.4 | I, II, III, IV | Surgery, Chemotherapy, Radiation therapy, Immunotherapy, Endocrine therapy, Oophorectomy | Internet-based cognitive behavioral therapy | Waiting list | 24 | Improvements in hot flushes, sleep quality, and menopausal symptoms. |
| Chen et al. [20] | 38889503 | Total = 94 (I = 47, C = 47), Mean age: 49.3 | I, II | Chemotherapy | Phone-based support program | Usual care | 7 | Higher self-care efficacy, better QoL, less symptom distress, reduced anxiety and depression. |
| Dong et al. [21] | 31242926 | Total = 60 (I = 30, C = 30), Mean age: 49.7 | I, II, III | Chemotherapy | Internet and social media software (CEIBISMS) | Traditional rehab care | 12 | Improvements in vitality, mental health, and health transition. |
| Ghanbari et al. [22] | 34003138 | Total = 82 (I = 41, C = 41), Mean age: 46.4 | Nonmetastatic | Not reported | mHealth psychoeducational intervention | Waiting list | 5 | Lower anxiety and higher self-esteem. |
| Graham et al. [39] | 38752788 | Total = 79 (I = 40, C = 39), Mean age: 59.4 | I, II, III | Surgery, Chemotherapy, Radiation therapy, Hormone therapy | Remotely delivered one-to-one therapy | Usual care | 24 | Improvements in medication adherence, QoL, distress, and flexibility. |
| Handa et al. [23] | 32201165 | Total = 102 (I = 52, C = 50), Mean age: 49.9 | ER+, ER-, PR+, PR-, HER2+, HER2- | Chemotherapy | Smartphone app during chemotherapy | Usual care | 12 | No significant anxiety/depression change; possible enhanced care via info sharing. |
| Heinrich et al. [24] | 39439014 | Total = 70 (I = 32, C = 38), Mean age: 57.6 | Primary breast cancer | Surgery, Chemotherapy, Radiation therapy | mHealth cognitive behavioral therapy | Usual care | 12 | Improved anxiety, HRQoL, and illness perception. |
| Holtdirk et al. [40] | 33961667 | Total = 363 (I = 181, C = 182), Mean age: 49.9 | Not reported | Surgery, Chemotherapy, Radiation treatment | Website with CBT | Usual care | 12 | Improved QoL and diet; no change in exercise. |
| Jacobs et al. [41] | 35924869 | Total = 100 (I = 50, C = 50), Mean age: 56.1 | 0, I, II, III | Surgery, Chemotherapy, Radiation therapy, Endocrine therapy | Telehealth for symptom management | Medication monitoring | 12 | Less distress, better self-management, coping, mood, and QoL. |
| Kim et al. [35] | 30578205 | Total = 76 (I = 36, C = 40), Mean age: 51.0 | IV | Chemotherapy (taxanes, anthracyclines, capecitabine, platinum compounds) | mHealth game to reduce chemotherapy side effects | Conventional education group | 3 | Better drug adherence, fewer chemotherapy adverse effects, better QoL, no significant difference in depression or anxiety. |
| Authors | PMID/DOI | Number of Patients | Stage/Status | Therapy | Experimental Intervention | Comparison | Duration of Intervention (Weeks) | Study Outcomes (Compared to Control Group) |
|---|---|---|---|---|---|---|---|---|
| Korkmaz et al. [42] | 31119709 | Total = 48 (I = 24, C = 24), Mean age: 47.7 | II, III | Surgery | Web-based education program on anxiety and QoL | Routine education | 4 | Lower levels of anxiety and improvements in QoL. |
| Lally et al. [43] | 31414245 | Total = 100 (I = 57, C = 43), Mean age: 54.2 | 0, I, II | Surgery, Chemotherapy, Radiation therapy | Tailored self-management psychoeducational program | Usual care | 12 | No significant outcomes. |
| Li et al. [25] | 39363984 | Total = 44 (I = 23, C = 21), Mean age: 47.9 | I, II, III | Chemotherapy | Wearable device-based aerobic exercise for physical and mental health | Waiting list | 12 | Improvements in physical fitness, mental health, sleep quality, QoL, and fewer adverse effects. |
| Okuyama et al. [26] | 38796818 | Total = 125 (I = 61, C = 64), Mean age: 63.5 | I, II, III | Chemotherapy, Radiotherapy, endocrine therapy, Combination therapy | Electronic patient-reported outcome app | Usual care | 12 | No improvements in BC patients’ QoL. |
| Philips et al. [27] | 39014267 | Total = 49 (I = 25, C = 24), Mean age: 54.8 | IV | Chemotherapy, Radiation therapy, Immunotherapy, Targeted therapy, Hormone therapy | Physical activity promotion via mHealth intervention | Healthy lifestyle control | 12 | Improvements in activity, QoL, some PROs, social cognitive theory constructs, and functional performance. |
| Peng et al. [28] | 36347151 | Total = 60 (I = 30, C = 30), Mean age: 41.8 | I, II, III, IV | mastectomy, conservative therapy, mastectomy + breast construction | Online mindfulness-based intervention on fear of cancer recurrence and quality of life | Usual care | 6 | Lower level of fear of cancer recurrence (FCR) and an improvement in quality of life |
| Rigg et al. [44] | 39438337 | Total = 35 (I = 17, C = 18), Mean age: 57.4 | IV | Surgery, Chemotherapy, Radiotherapy, Hormonal therapy, Other treatment | Web-based self-guided psychosocial program | Usual care | 6 | Small improvements in fear of progression and global QoL, alongside some deteriorations in distress and mental QoL. |
| Rosen et al. [29] | 10.1002/pon.4764 | Total = 112 (I = 57, C = 55), Mean age: 52.2 | Not reported | Not reported | mHealth mindfulness training | Waiting list | 8 | Improvements in QoL. |
| Sarac et al. [30] | 39257013 | Total = 82 (I = 42, C = 40), Mean age: 49.0 | Not reported | Adjuvant, Neoadjuvant, Surgery (BCS + SLNB, Mastectomy + SLNB, MRM) | Informative mobile app use on anxiety, distress, and QoL | Usual care | 4 | Lower anxiety and distress levels, but no difference in overall QoL. |
| Singleton et al. [31] | 35460441 | Total = 156 (I = 78, C = 78), Mean age: 55.1 | Not reported | Surgery, Radiotherapy, Chemotherapy, Endocrine therapy, Targeted therapy | Supporting women’s health outcomes through text messages. | Usual care | 24 | No significant differences between groups for self-efficacy, adjusted mean difference, QoL, mental health, physical activity, or BMI. |
| Authors | PMID/DOI | Number of Patients | Stage/Status | Therapy | Experimental Intervention | Comparison | Duration of Intervention (Weeks) | Study Outcomes (Compared to Control Group) |
|---|---|---|---|---|---|---|---|---|
| White et al. [45] | 30137657 | Total = 337 (I = 202, C = 177), Mean age: 43.7 | I, II | Surgery, Chemotherapy, Radiotherapy, Targeted therapy, Hormonal therapy | Information-based breast cancer-specific website | Usual care | 24 | Mean level of QoL scores did not differ between groups. |
| Zhang et al. [36] | 38418478 | Total = 36 (I = 19, C = 17), Mean age: 47.2 | IV | Not reported | Virtual reality intervention for managing cancer and living meaningfully. | Waiting list | 12 | CALM therapy led to reductions in depression, distress, and attachment avoidance, as well as improvements in quality of life. |
| Zhang et al. [37] | 35712124 | Total = 90 (I = 45, C = 45), Mean age: 51.6 | I, II, III, IV | Surgery, Chemotherapy | Virtual reality intervention for psychological distress and symptom management. | Usual care | 12 | VR-CALM improves well-being in survivors. |
| Zhou et al. [32] | 32272281 | Total = 111 (I = 56, C = 55), Mean age: 49.9 | I, II, III | Surgery, Chemotherapy, Radiotherapy, Endocrine therapy | WeChat-based nursing program for postoperative BC rehabilitation | Usual care | 24 | Significant improvement in HRQoL. |
| Zhou et al. [34] | 31342310 | Total = 132 (I = 66, C = 66), Mean age: 44.5 | I, II, III | Surgery, Chemotherapy, Radiotherapy, Endocrine therapy | Mobile-based training on resilience, depression, and anxiety management | Usual care | 12 | Improvements were observed in psychological resilience, anxiety, and depression scores. |
| Zhu et al. [33] | 29712622 | Total = 114 (I = 57, C = 57), Mean age: 47.2 | I, II, III, IV | Surgery, Chemotherapy | Mobile breast cancer e-support program | Usual care | 12 | E-support + care improved self-efficacy, symptom interference, and QoL but not social support, symptom severity, anxiety, or depression. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsis, A.; Filis, P.; Karanasiou, G.; Georga, E.I.; Mauri, D.; Naka, K.K.; Constantinidou, A.; Keramida, K.; Tsekoura, D.; Mazzocco, K.; et al. Impact of e-Health Interventions on Mental Health and Quality of Life in Breast Cancer Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cancers 2025, 17, 1780. https://doi.org/10.3390/cancers17111780
Mitsis A, Filis P, Karanasiou G, Georga EI, Mauri D, Naka KK, Constantinidou A, Keramida K, Tsekoura D, Mazzocco K, et al. Impact of e-Health Interventions on Mental Health and Quality of Life in Breast Cancer Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cancers. 2025; 17(11):1780. https://doi.org/10.3390/cancers17111780
Chicago/Turabian StyleMitsis, Alexandros, Panagiotis Filis, Georgia Karanasiou, Eleni I. Georga, Davide Mauri, Katerina K. Naka, Anastasia Constantinidou, Kalliopi Keramida, Dorothea Tsekoura, Ketti Mazzocco, and et al. 2025. "Impact of e-Health Interventions on Mental Health and Quality of Life in Breast Cancer Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Cancers 17, no. 11: 1780. https://doi.org/10.3390/cancers17111780
APA StyleMitsis, A., Filis, P., Karanasiou, G., Georga, E. I., Mauri, D., Naka, K. K., Constantinidou, A., Keramida, K., Tsekoura, D., Mazzocco, K., Alexandraki, A., Kampouroglou, E., Goletsis, Y., Papakonstantinou, A., Antoniades, A., Brown, C., Bouratzis, V., Matos, E., Marias, K., ... Fotiadis, D. I. (2025). Impact of e-Health Interventions on Mental Health and Quality of Life in Breast Cancer Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cancers, 17(11), 1780. https://doi.org/10.3390/cancers17111780











