Potential of Metabolic MRI to Address Unmet Clinical Needs in Localised Kidney Cancer
Simple Summary
Abstract
1. Introduction
2. Epidemiology, Pathology, and Current Clinical Guidelines
2.1. Epidemiology and Aetiology
2.2. Histopathology
2.3. Diagnostic Evaluation
2.4. Treatment Strategies
2.5. Follow-Up
3. Metabolic Heterogeneity in Kidney Cancer
3.1. Intertumuoral Heterogeneity
3.1.1. Clear Cell RCC
3.1.2. Papillary RCC
3.1.3. SDHd-RCC and FHd-RCC
3.1.4. Chromophobe RCC and Renal Oncocytoma
3.2. Intratumoural Heterogeneity
4. Clinical Imaging of Metabolism in Kidney Cancer
4.1. Nuclear Imaging Techniques
4.1.1. Positron Emission Tomography with [18F]Fluorodeoxyglucose ([18F]FDG-PET)
4.1.2. 99Tc-Sestamibi SPECT
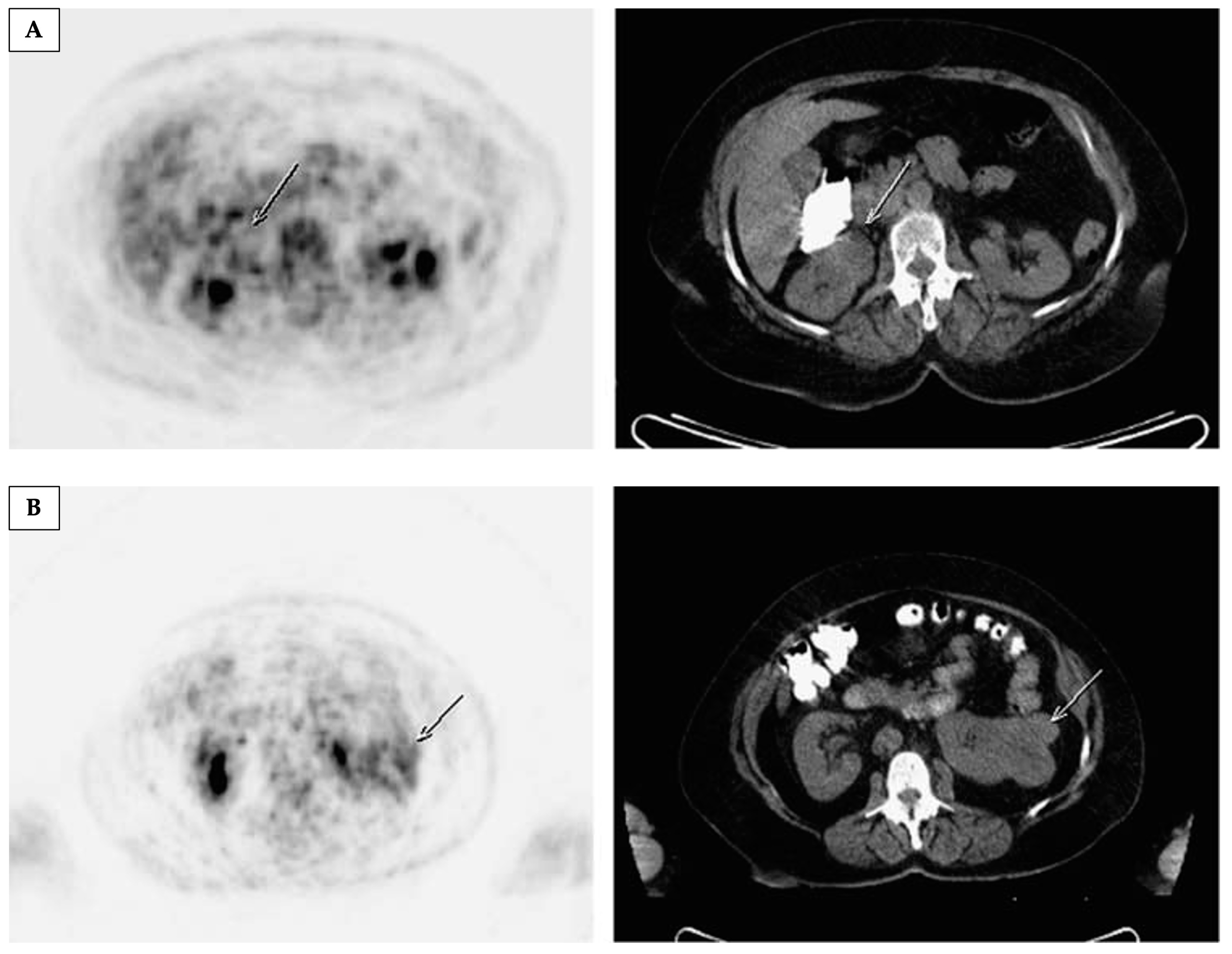
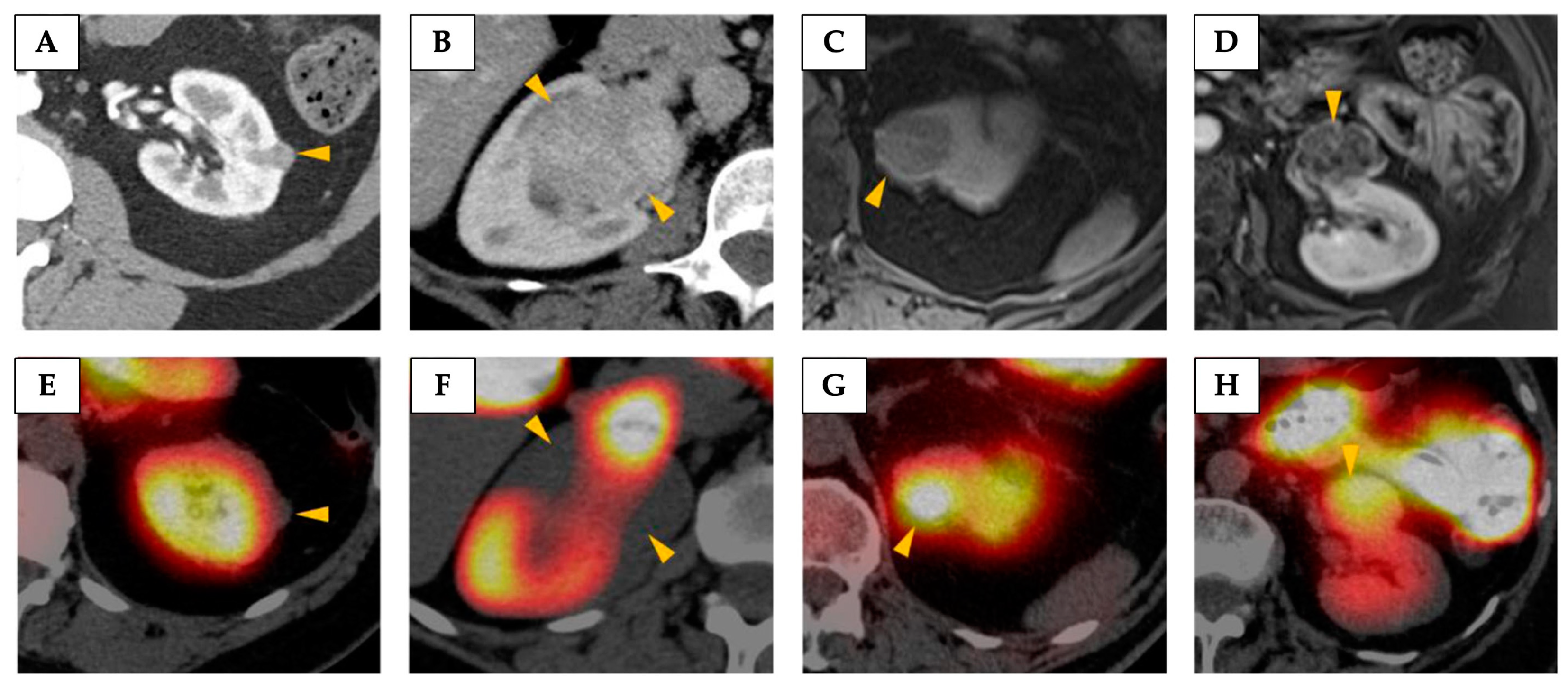
4.2. Imaging Metabolism Using Magnetic Resonance
4.2.1. Magnetic Resonance Spectroscopy (MRS)
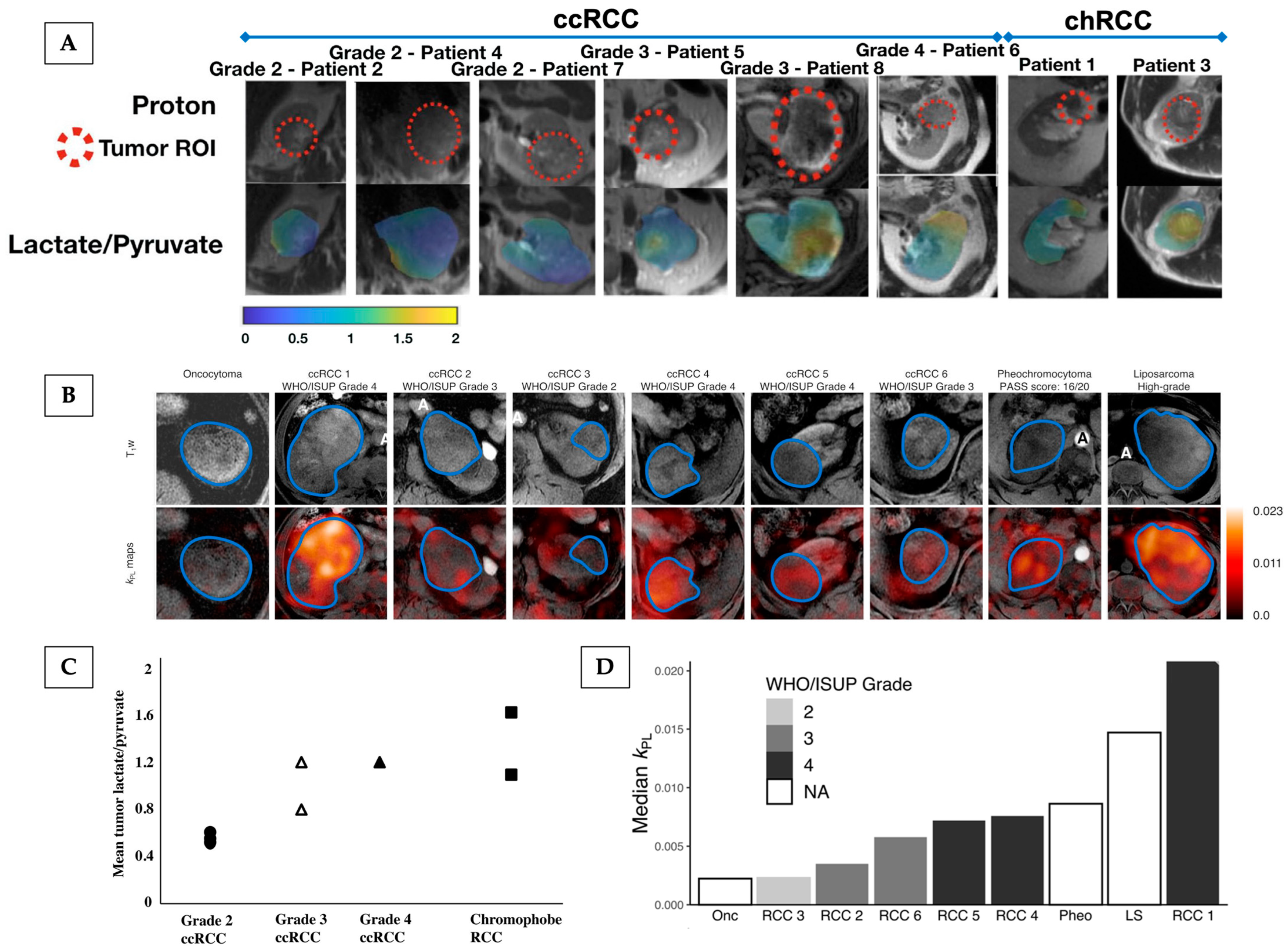
4.2.2. Hyperpolarised [1-13C]Pyruvate MRI

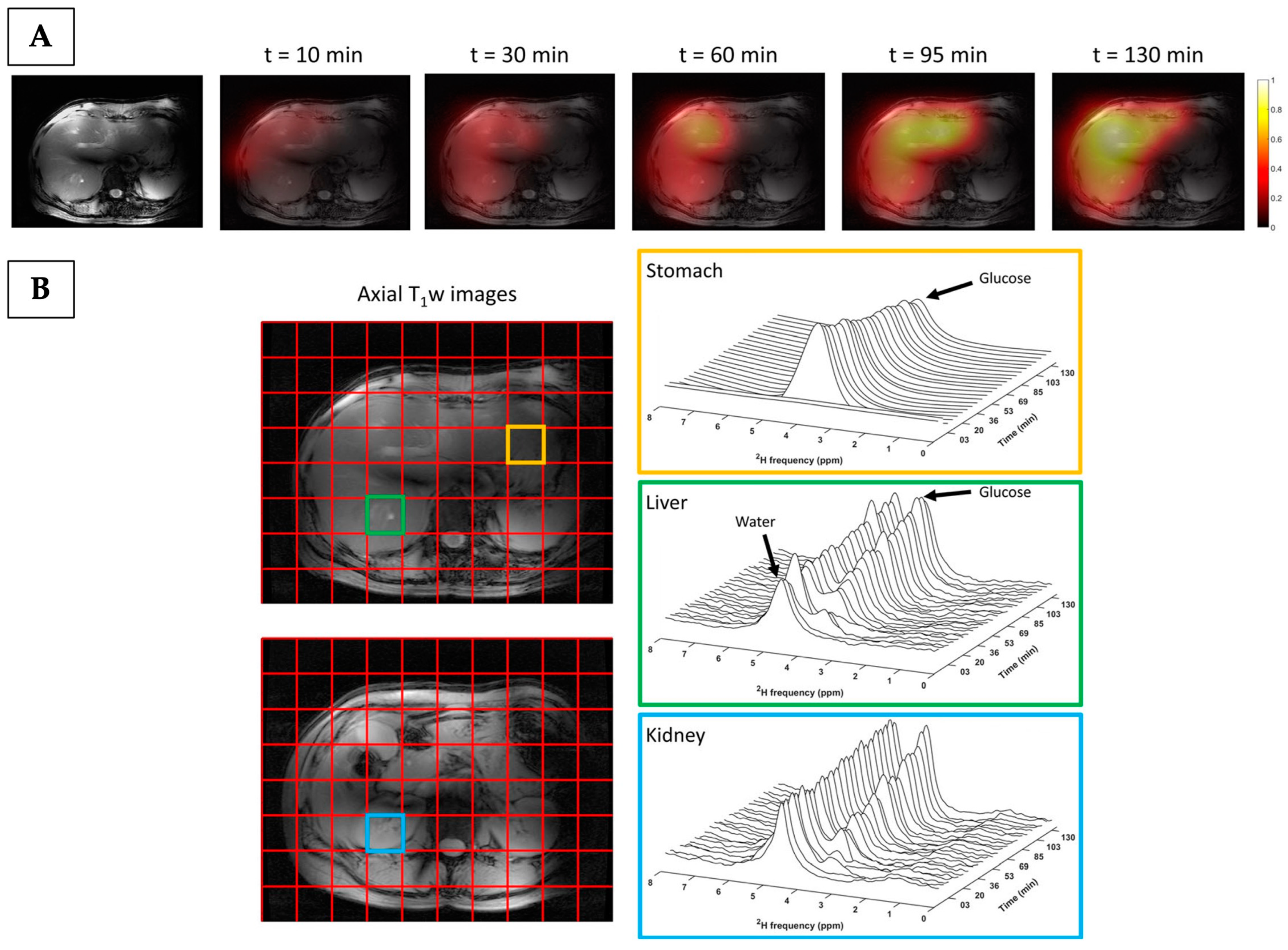
4.2.3. Deuterium Metabolic Imaging
5. Conclusion and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AS | active surveillance |
| BHD | Birt–Hogg–Dubé |
| CA | carbonic anhydrase |
| CAIX | carbonic anhydrase 9 |
| chRCC | chromophobe renal cell carcinoma |
| CSS | cancer-specific survival |
| DFS | disease-free survival |
| DMI | deuterium metabolic imaging |
| DNP | dynamic nuclear polarisation |
| DWI | diffusion-weighted imaging |
| EMA | European Medicines Agency |
| FDG-PET | fluorine-18-labelled fluorodeoxyglucose in conjunction with positron emission tomography |
| FHd-RCC | fumarate hydratase-deficient renal cell carcinoma |
| FSE | fast spin echo |
| Glx | glutamine/glutamate |
| GRE | gradient echo |
| HIF | hypoxia-inducible factor |
| HLRCC | hereditary leiomyomatosis and renal cell cancer |
| HP 13C-MRI | hyperpolarised [1-13C]pyruvate MRI |
| IHC | immunohistochemistry |
| LDHA | lactate dehydrogenase A |
| MCT1 | monocarboxylate transporter 1 |
| MPC | mitochondrial pyruvate carrier |
| MRS | magnetic resonance spectroscopy |
| mtDNA | mitochondrial DNA |
| OS | overall survival |
| OXPHOS | oxidative phosphorylation |
| PDH | pyruvate dehydrogenase |
| PFS | progression-free survival |
| pRCC | papillary renal cell carcinoma |
| pTNM | pathological tumour node metastasis staging |
| RCC | renal cell carcinoma |
| RECIST | Response Evaluation Criteria in Solid Tumours |
| RMB | renal mass biopsy |
| RO | renal oncocytoma |
| SDHd-RCC | succinate dehydrogenase-deficient renal cell carcinoma |
| SNR | signal-to-noise ratio |
| SPECT | Single-Photon Emission Computed Tomography |
| SPGR | spoiled gradient |
| SSFSE | single-shot fast spin echo |
| TCA | tricarboxylic acid |
| TKI | tyrosine kinase |
| TSC | tuberous sclerosis complex |
| VHL | von Hippel–Lindau |
| WHO | World Health Organisation |
References
- Bukavina, L.; Bensalah, K.; Bray, F.; Carlo, M.; Challacombe, B.; Karam, J.A.; Kassouf, W.; Mitchell, T.; Montironi, R.; O’Brien, T.; et al. Epidemiology of Renal Cell Carcinoma: 2022 Update. Eur. Urol. 2022, 82, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.D.; Klatte, T.; Cosmai, L.; Bex, A.; Lamb, B.W.; Moch, H.; Sala, E.; Siva, S.; Porta, C.; Gallieni, M. The Multispeciality Approach to the Management of Localised Kidney Cancer. Lancet 2022, 400, 523–534. [Google Scholar] [CrossRef]
- Gordetsky, J.; Zarzour, J. Correlating Preoperative Imaging with Histologic Subtypes of Renal Cell Carcinoma and Common Mimickers. Curr. Urol. Rep. 2016, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.H.; Blick, C.; Handforth, C.; Brown, J.E.; Stewart, G.D. Essential Research Priorities in Renal Cancer: A Modified Delphi Consensus Statement. Eur. Urol. Focus. 2020, 6, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.R.; Whitson, J.M.; Meng, M.V. Under-Grading of <4 cm Renal Masses on Renal Biopsy. BJU Int. 2012, 110, 794–797. [Google Scholar] [CrossRef]
- Patel, H.D.; Druskin, S.C.; Rowe, S.P.; Pierorazio, P.M.; Gorin, M.A.; Allaf, M.E. Surgical Histopathology for Suspected Oncocytoma on Renal Mass Biopsy: A Systematic Review and Meta-Analysis. BJU Int. 2017, 119, 661–666. [Google Scholar] [CrossRef]
- Patel, H.D.; Johnson, M.H.; Pierorazio, P.M.; Sozio, S.M.; Sharma, R.; Iyoha, E.; Bass, E.B.; Allaf, M.E. Diagnostic Accuracy and Risks of Biopsy in the Diagnosis of a Renal Mass Suspicious for Localized Renal Cell Carcinoma: Systematic Review of the Literature. J. Urol. 2016, 195, 1340–1347. [Google Scholar] [CrossRef]
- Fallara, G.; Larcher, A.; Dabestani, S.; Fossati, N.; Järvinen, P.; Nisen, H.; Gudmundsson, E.; Lam, T.B.; Marconi, L.; Fernandéz-Pello, S.; et al. Recurrence Pattern in Localized RCC: Results from a European Multicenter Database (RECUR). Urol. Oncol. Semin. Orig. Investig. 2022, 40, e11–e494. [Google Scholar] [CrossRef]
- Ko, C.-C.; Yeh, L.-R.; Kuo, Y.-T.; Chen, J.-H. Imaging Biomarkers for Evaluating Tumor Response: RECIST and Beyond. Biomark. Res. 2021, 9, 52. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Rathmell, W.K.; Rathmell, J.C.; Linehan, W.M. Metabolic Pathways in Kidney Cancer: Current Therapies and Future Directions. JCO 2018, 36, 3540–3546. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, C.J.; De Cubas, A.A.; Fan, H.; Smith, C.C.; Lang, M.; Reznik, E.; Bowlby, R.; Gibb, E.A.; Akbani, R.; Beroukhim, R.; et al. The Cancer Genome Atlas Comprehensive Molecular Characterization of Renal Cell Carcinoma. Cell Rep. 2018, 23, 313–326.e5. [Google Scholar] [CrossRef] [PubMed]
- Linehan, W.M.; Ricketts, C.J. The Cancer Genome Atlas of Renal Cell Carcinoma: Findings and Clinical Implications. Nat. Rev. Urol. 2019, 16, 539–552. [Google Scholar] [CrossRef]
- Le, A. (Ed.) The Heterogeneity of Cancer Metabolism; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2021; Volume 1311, ISBN 978-3-030-65767-3. [Google Scholar]
- Yong, C.; Stewart, G.D.; Frezza, C. Oncometabolites in Renal Cancer. Nat. Rev. Nephrol. 2020, 16, 156–172. [Google Scholar] [CrossRef]
- Linehan, W.M.; Rouault, T.A. Molecular Pathways: Fumarate Hydratase -Deficient Kidney Cancer—Targeting the Warburg Effect in Cancer. Clin. Cancer Res. 2013, 19, 3345–3352. [Google Scholar] [CrossRef]
- Xiao, Y.; Clima, R.; Busch, J.; Rabien, A.; Kilic, E.; Villegas, S.L.; Timmermann, B.; Attimonelli, M.; Jung, K.; Meierhofer, D. Decreased Mitochondrial DNA Content Drives OXPHOS Dysregulation in Chromophobe Renal Cell Carcinoma. Cancer Res. 2020, 80, 3830–3840. [Google Scholar] [CrossRef]
- Kürschner, G.; Zhang, Q.; Clima, R.; Xiao, Y.; Busch, J.F.; Kilic, E.; Jung, K.; Berndt, N.; Bulik, S.; Holzhütter, H.G.; et al. Renal oncocytoma characterized by the defective complex I of the respiratory chain boosts the synthesis of the ROS scavenger glutathione. Oncotarget 2017, 8, 105882–105904. [Google Scholar] [CrossRef]
- Karivedu, V.; Jain, A.L.; Eluvathingal, T.J.; Sidana, A. Role of Positron Emission Tomography Imaging in Metabolically Active Renal Cell Carcinoma. Curr. Urol. Rep. 2019, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.P.; Katlariwala, P.; Murad, M.H.; Abele, J.; McInnes, M.D.F.; Low, G. Diagnostic Accuracy of 99mTc-Sestamibi SPECT/CT for Detecting Renal Oncocytomas and Other Benign Renal Lesions: A Systematic Review and Meta-Analysis. Abdom. Radiol. 2020, 45, 2532–2541. [Google Scholar] [CrossRef]
- Basile, G.; Fallara, G.; Verri, P.; Uleri, A.; Chiti, A.; Gianolli, L.; Pepe, G.; Tedde, A.; Algaba, F.; Territo, A.; et al. The Role of 99mTc-Sestamibi Single-Photon Emission Computed Tomography/Computed Tomography in the Diagnostic Pathway for Renal Masses: A Systematic Review and Meta-analysis. Eur. Urol. 2024, 85, 63–71. [Google Scholar] [CrossRef]
- Zaccagna, F.; Grist, J.T.; Deen, S.S.; Woitek, R.; Lechermann, L.M.; McLean, M.A.; Basu, B.; Gallagher, F.A. Hyperpolarized Carbon-13 Magnetic Resonance Spectroscopic Imaging: A Clinical Tool for Studying Tumour Metabolism. BJR 2018, 91, 20170688. [Google Scholar] [CrossRef]
- Miller, J.J.; Grist, J.T.; Serres, S.; Larkin, J.R.; Lau, A.Z.; Ray, K.; Fisher, K.R.; Hansen, E.; Tougaard, R.S.; Nielsen, P.M.; et al. 13C Pyruvate Transport Across the Blood-Brain Barrier in Preclinical Hyperpolarised MRI. Sci. Rep. 2018, 8, 15082. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Liu, G.; Wang, J.; Pan, S.; Luo, Y.; Xu, Y.; Kong, W.; Sun, P.; Xu, J.; Xue, W.; et al. MR Spectroscopy for Detecting Fumarate Hydratase Deficiency in Hereditary Leiomyomatosis and Renal Cell Carcinoma Syndrome. Radiology 2022, 305, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Wang, J.; Liu, G.; Zhang, J.; Song, Y.; Kong, W.; Zhou, Y.; Wu, G. Factors Influencing the Detection Rate of Fumarate Peak in 1H MR Spectroscopy of Fumarate Hydratase-Deficient Renal Cell Carcinoma at 3 T MRI. Clin. Clin. Radiol. Radiol. 2024, 79, e80–e88. [Google Scholar] [CrossRef] [PubMed]
- Tognarelli, J.M.; Dawood, M.; Shariff, M.I.F.; Grover, V.P.B.; Crossey, M.M.E.; Cox, I.J.; Taylor-Robinson, S.D.; McPhail, M.J.W. Magnetic Resonance Spectroscopy: Principles and Techniques: Lessons for Clinicians. J. Clin. Exp. Hepatol. 2015, 5, 320–328. [Google Scholar] [CrossRef]
- Deen, S.S.; Rooney, C.; Shinozaki, A.; McGing, J.; Grist, J.T.; Tyler, D.J.; Serrão, E.; Gallagher, F.A. Hyperpolarized Carbon 13 MRI: Clinical Applications and Future Directions in Oncology. Radiol. Imaging Cancer 2023, 5, e230005. [Google Scholar] [CrossRef]
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of Renal Cell Carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef]
- Capitanio, U.; Montorsi, F. Renal Cancer. Lancet 2016, 387, 894–906. [Google Scholar] [CrossRef]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grünwald, V.; Gillessen, S.; Horwich, A. Renal Cell Carcinoma: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef]
- Athanazio, D.A.; Amorim, L.S.; da Cunha, I.W.; Leite, K.R.M.; da Paz, A.R.; de Paula Xavier Gomes, R.; Tavora, F.R.F.; Faraj, S.F.; Cavalcanti, M.S.; Bezerra, S.M. Classification of Renal Cell Tumors—Current Concepts and Use of Ancillary Tests: Recommendations of the Brazilian Society of Pathology. Surg. Exp. Pathol. 2021, 4, 4. [Google Scholar] [CrossRef]
- Moch, H.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Gill, A.J.; Hartmann, A.; Menon, S.; Raspollini, M.R.; Rubin, M.A.; Srigley, J.R.; et al. The 2022 World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2022, 82, 458–468. [Google Scholar] [CrossRef]
- Bhatt, N.R.; Davis, N.F.; Flynn, R.; McDermott, T.; Thornhill, J.A.; Manecksha, R.P. Dilemmas in Diagnosis and Natural History of Renal Oncocytoma and Implications for Management. Can. Urol. Assoc. J. 2015, 9, E709–E712. [Google Scholar] [CrossRef]
- Warren, A.Y.; Harrison, D. WHO/ISUP Classification, Grading and Pathological Staging of Renal Cell Carcinoma: Standards and Controversies. World J. Urol. 2018, 36, 1913–1926. [Google Scholar] [CrossRef]
- Stillebroer, A.B.; Mulders, P.F.A.; Boerman, O.C.; Oyen, W.J.G.; Oosterwijk, E. Carbonic Anhydrase IX in Renal Cell Carcinoma: Implications for Prognosis, Diagnosis, and Therapy. Eur. Urol. 2010, 58, 75–83. [Google Scholar] [CrossRef]
- Angori, S.; Lobo, J.; Moch, H. Papillary Renal Cell Carcinoma: Current and Controversial Issues. Curr. Opin. Urol. 2022, 32, 344–351. [Google Scholar] [CrossRef]
- Lindgren, D.; Sjölund, J.; Axelson, H. Tracing Renal Cell Carcinomas Back to the Nephron. Trends Cancer 2018, 4, 472–484. [Google Scholar] [CrossRef]
- Saleeb, R.M.; Brimo, F.; Farag, M.; Rompré-Brodeur, A.; Rotondo, F.; Beharry, V.; Wala, S.; Plant, P.; Downes, M.R.; Pace, K.; et al. Toward Biological Subtyping of Papillary Renal Cell Carcinoma With Clinical Implications Through Histologic, Immunohistochemical, and Molecular Analysis. Am. J. Surg. Pathol. 2017, 41, 1618–1629. [Google Scholar] [CrossRef]
- Moch, H.; Ohashi, R. Chromophobe Renal Cell Carcinoma: Current and Controversial Issues. Pathology 2021, 53, 101–108. [Google Scholar] [CrossRef]
- Wobker, S.E.; Williamson, S.R. Modern Pathologic Diagnosis of Renal Oncocytoma. J. Kidney Cancer VHL 2017, 4, 1–12. [Google Scholar] [CrossRef]
- Lobo, J.; Ohashi, R.; Amin, M.B.; Berney, D.M.; Compérat, E.M.; Cree, I.A.; Gill, A.J.; Hartmann, A.; Menon, S.; Netto, G.J.; et al. WHO 2022 Landscape of Papillary and Chromophobe Renal Cell Carcinoma. Histopathology 2022, 81, 426–438. [Google Scholar] [CrossRef]
- Vasudev, N.S.; Wilson, M.; Stewart, G.D.; Adeyoju, A.; Cartledge, J.; Kimuli, M.; Datta, S.; Hanbury, D.; Hrouda, D.; Oades, G.; et al. Challenges of Early Renal Cancer Detection: Symptom Patterns and Incidental Diagnosis Rate in a Multicentre Prospective UK Cohort of Patients Presenting with Suspected Renal Cancer. BMJ Open 2020, 10, e035938. [Google Scholar] [CrossRef]
- Powles, T.; Albiges, L.; Bex, A.; Comperat, E.; Grünwald, V.; Kanesvaran, R.; Kitamura, H.; McKay, R.; Porta, C.; Procopio, G.; et al. Renal Cell Carcinoma: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2024, 35, 692–706. [Google Scholar] [CrossRef]
- Sohaib, A.; Cook, P. Renal and adrenal tumours. In Recommendations for Cross-Sectional Imaging in Cancer Management, 2nd ed.; The Royal College of Radiologists: London, UK, 2014. [Google Scholar]
- Wang, Z.J.; Davenport, M.S.; Silverman, S.G.; Chandarana, H.; Doshi, A.; Israel, G.M.; Leyendecker, J.R.; Pedrosa, I.; Raman, S.; Remer, E.M. MRI Renal Mass Protocol v1.0. Society of Abdominal Radiology Disease Focused Panel on Renal Cell Carcinoma. Available online: https://abdominalradiology.org/wp-content/uploads/2020/11/RCC.MRIprotocolfinal-7-15-17.pdf (accessed on 21 May 2025).
- Davenport, M.S.; Chandarana, H.; Curci, N.E.; Doshi, A.; Kaffenberger, S.D.; Pedrosa, I.; Remer, E.M.; Schieda, N.; Shinagare, A.B.; Smith, A.D.; et al. Society of Abdominal Radiology Disease-Focused Panel on Renal Cell Carcinoma: Update on Past, Current, and Future Goals. Abdom. Radiol. 2018, 43, 2213–2220. [Google Scholar] [CrossRef]
- Tsili, A.C.; Moulopoulos, L.-A.; Varakarakis, I.M.; Argyropoulou, M.I. Cross-Sectional Imaging Assessment of Renal Masses with Emphasis on MRI. Acta Radiol. 2022, 63, 1570–1587. [Google Scholar] [CrossRef]
- Pedrosa, I.; Cadeddu, J.A. How We Do It: Managing the Indeterminate Renal Mass with the MRI Clear Cell Likelihood Score. Radiology 2022, 302, 256–269. [Google Scholar] [CrossRef]
- Schieda, N.; Davenport, M.S.; Silverman, S.G.; Bagga, B.; Barkmeier, D.; Blank, Z.; Curci, N.E.; Doshi, A.M.; Downey, R.T.; Edney, E.; et al. Multicenter Evaluation of Multiparametric MRI Clear Cell Likelihood Scores in Solid Indeterminate Small Renal Masses. Radiology 2022, 303, 590–599. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) BTNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2017; ISBN 978-1-119-26354-8. [Google Scholar]
- Haas, N.B.; Manola, J.; Dutcher, J.P.; Flaherty, K.T.; Uzzo, R.G.; Atkins, M.B.; DiPaola, R.S.; Choueiri, T.K. Adjuvant Treatment for High-Risk Clear Cell Renal Cancer: Updated Results of a High-Risk Subset of the ASSURE Randomized Trial. JAMA Oncol. 2017, 3, 1249. [Google Scholar] [CrossRef]
- Motzer, R.J.; Haas, N.B.; Donskov, F.; Gross-Goupil, M.; Varlamov, S.; Kopyltsov, E.; Lee, J.L.; Melichar, B.; Rini, B.I.; Choueiri, T.K.; et al. Randomized Phase III Trial of Adjuvant Pazopanib Versus Placebo After Nephrectomy in Patients With Localized or Locally Advanced Renal Cell Carcinoma. J. Clin. Oncol. 2017, 35, 3916–3923. [Google Scholar] [CrossRef]
- Gross-Goupil, M.; Kwon, T.G.; Eto, M.; Ye, D.; Miyake, H.; Seo, S.I.; Byun, S.-S.; Lee, J.L.; Master, V.; Jin, J.; et al. Axitinib versus Placebo as an Adjuvant Treatment of Renal Cell Carcinoma: Results from the Phase III, Randomized ATLAS Trial. Ann. Oncol. 2018, 29, 2371–2378. [Google Scholar] [CrossRef]
- Synold, T.W.; Plets, M.; Tangen, C.M.; Heath, E.I.; Palapattu, G.S.; Mack, P.C.; Stein, M.N.; Meng, M.V.; Lara, P.; Vogelzang, N.J.; et al. Everolimus Exposure as a Predictor of Toxicity in Renal Cell Cancer Patients in the Adjuvant Setting: Results of a Pharmacokinetic Analysis for SWOG S0931 (EVEREST), a Phase III Study (NCT01120249). Kidney Cancer 2019, 3, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Chamie, K.; Klöpfer, P.; Bevan, P.; Störkel, S.; Said, J.; Fall, B.; Belldegrun, A.S.; Pantuck, A.J. Carbonic Anhydrase-IX Score Is a Novel Biomarker That Predicts Recurrence and Survival for High-Risk, Nonmetastatic Renal Cell Carcinoma: Data from the Phase III ARISER Clinical Trial. Urol. Oncol. Semin. Orig. Investig. 2015, 33, e25–e204. [Google Scholar] [CrossRef] [PubMed]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Chang, Y.-H.; Lee, J.-L.; Sarwar, N.; et al. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N. Engl. J. Med. 2024, 390, 1359–1371. [Google Scholar] [CrossRef]
- Bex, A.; Jewett, M.; Lewis, B.; Abel, E.J.; Albiges, L.; Berg, S.A.; Bratslavsky, G.; Braun, D.; Brugarolas, J.; Choueiri, T.K.; et al. A Call for a Neoadjuvant Kidney Cancer Consortium: Lessons Learned from Other Cancer Types. Eur. Urol. 2025, 87, 385–389. [Google Scholar] [CrossRef]
- Karlsson Rosenblad, A.; Sundqvist, P.; Harmenberg, U.; Hellström, M.; Hofmann, F.; Kjellman, A.; Kröger Dahlin, B.-I.; Lindblad, P.; Lindskog, M.; Lundstam, S.; et al. Surgical Waiting Times and All-Cause Mortality in Patients with Non-Metastatic Renal Cell Carcinoma. Scand. J. Urol. 2022, 56, 383–390. [Google Scholar] [CrossRef]
- Kuusk, T.; Cullen, D.; Neves, J.B.; Campain, N.; Barod, R.; Boleti, E.; El-Sheihk, S.; Grant, L.; Kelly, J.; Marchetti, M.; et al. Impact of the First Surge of the COVID-19 Pandemic on a Tertiary Referral Centre for Kidney Cancer. BJU Int. 2021, 128, 752–758. [Google Scholar] [CrossRef] [PubMed]
- Khaleel, S.; Jiang, S.; Kotecha, R.R.; Hakimi, A.A. Neoadjuvant Systemic Therapy in Localized and Locally Advanced Renal Cell Carcinoma. Front. Urol. 2022, 2, 864778. [Google Scholar] [CrossRef]
- Stewart, G.; Jones, J.; Warren, A.; Gallagher, F.; Horvat Menih, I.; Mossop, H.; Wason, J.; Easita, F.; Thomas, M.; Welsh, S. 75 WIRE: Window of Opportunity ClinicalTrials Platform for Evaluation of NovelTreatments Strategies in Renal Cell Cancer. Oncologist 2024, 29 (Suppl. 1), S15–S16. [Google Scholar] [CrossRef]
- Marandino, L.; Raggi, D.; Necchi, A.; Capitanio, U. Neoadjuvant Treatment in Renal Cell Carcinoma: Transforming Challenges into Opportunities. Eur. Urol. 2022, 81, 574–575. [Google Scholar] [CrossRef]
- Leibovich, B.C.; Lohse, C.M.; Cheville, J.C.; Zaid, H.B.; Boorjian, S.A.; Frank, I.; Thompson, R.H.; Parker, W.P. Predicting Oncologic Outcomes in Renal Cell Carcinoma After Surgery. Eur. Urol. 2018, 73, 772–780. [Google Scholar] [CrossRef]
- Usher-Smith, J.A.; Li, L.; Roberts, L.; Harrison, H.; Rossi, S.H.; Sharp, S.J.; Coupland, C.; Hippisley-Cox, J.; Griffin, S.J.; Klatte, T.; et al. Risk Models for Recurrence and Survival after Kidney Cancer: A Systematic Review. BJU Int. 2022, 130, 562–579. [Google Scholar] [CrossRef]
- Aykan, N.F.; Özatlı, T. Objective Response Rate Assessment in Oncology: Current Situation and Future Expectations. WJCO 2020, 11, 53–73. [Google Scholar] [CrossRef]
- Beksac, A.T.; Paulucci, D.J.; Blum, K.A.; Yadav, S.S.; Sfakianos, J.P.; Badani, K.K. Heterogeneity in Renal Cell Carcinoma. Urol. Oncol. Semin. Orig. Investig. 2017, 35, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Lehuédé, C.; Dupuy, F.; Rabinovitch, R.; Jones, R.G.; Siegel, P.M. Metabolic Plasticity as a Determinant of Tumor Growth and Metastasis. Cancer Res. 2016, 76, 5201–5208. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.; McLean, M.A.; Priest, A.N.; Gill, A.B.; Scott, F.; Patterson, I.; Carmo, B.; Riemer, F.; Kaggie, J.D.; Frary, A.; et al. Multiparametric MRI of Early Tumor Response to Immune Checkpoint Blockade in Metastatic Melanoma. J. Immunother. Cancer 2021, 9, e003125. [Google Scholar] [CrossRef]
- Coffey, N.J.; Simon, M.C. Metabolic Alterations in Hereditary and Sporadic Renal Cell Carcinoma. Nat. Rev. Nephrol. 2024, 20, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The Hallmarks of Cancer Metabolism: Still Emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef]
- Okegawa, T.; Morimoto, M.; Nishizawa, S.; Kitazawa, S.; Honda, K.; Araki, H.; Tamura, T.; Ando, A.; Satomi, Y.; Nutahara, K.; et al. Intratumor Heterogeneity in Primary Kidney Cancer Revealed by Metabolic Profiling of Multiple Spatially Separated Samples within Tumors. EBioMedicine 2017, 19, 31–38. [Google Scholar] [CrossRef]
- Turajlic, S.; Xu, H.; Litchfield, K.; Rowan, A.; Chambers, T.; Lopez, J.I.; Nicol, D.; O’Brien, T.; Larkin, J.; Horswell, S.; et al. Tracking Cancer Evolution Reveals Constrained Routes to Metastases: TRACERx Renal. Cell 2018, 173, 581–594.e12. [Google Scholar] [CrossRef]
- Mankoff, D.A.; Dunnwald, L.K.; Partridge, S.C.; Specht, J.M. Blood Flow-Metabolism Mismatch: Good for the Tumor, Bad for the Patient. Clin. Cancer Res. 2009, 15, 5294–5296. [Google Scholar] [CrossRef]
- Specht, J.M.; Kurland, B.F.; Montgomery, S.K.; Dunnwald, L.K.; Doot, R.K.; Gralow, J.R.; Ellis, G.K.; Linden, H.M.; Livingston, R.B.; Allison, K.H.; et al. Tumor Metabolism and Blood Flow as Assessed by Positron Emission Tomography Varies by Tumor Subtype in Locally Advanced Breast Cancer. Clin. Cancer Res. 2010, 16, 2803–2810. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, I.; Hofheinz, F.; Buchert, R.; Steffen, I.G.; Michel, R.; Rosner, C.; Prasad, V.; Köhler, C.; Derlin, T.; Brenner, W.; et al. Combined Measurement of Tumor Perfusion and Glucose Metabolism for Improved Tumor Characterization in Advanced Cervical Carcinoma: A PET/CT Pilot Study Using [15O]Water and [18F]Fluorodeoxyglucose. Strahlenther. Onkol. 2014, 190, 575–581. [Google Scholar] [CrossRef]
- Saltveit, M.E. Respiratory Metabolism. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Elsevier: Amsterdam, The Netherlands, 2019; pp. 73–91. ISBN 978-0-12-813278-4. [Google Scholar]
- Wang, Y.; Stancliffe, E.; Fowle-Grider, R.; Wang, R.; Wang, C.; Schwaiger-Haber, M.; Shriver, L.P.; Patti, G.J. Saturation of the Mitochondrial NADH Shuttles Drives Aerobic Glycolysis in Proliferating Cells. Mol. Cell 2022, 82, 3270–3283.e9. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.D.; Bezwada, D.; Mashimo, T.; Pichumani, K.; Vemireddy, V.; Funk, A.M.; Wimberly, J.; McNeil, S.S.; Kapur, P.; Lotan, Y.; et al. Isotope Tracing of Human Clear Cell Renal Cell Carcinomas Demonstrates Suppressed Glucose Oxidation In Vivo. Cell Metab. 2018, 28, 793–800.e2. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Paffrath, V.; Clima, R.; Busch, J.F.; Rabien, A.; Kilic, E.; Villegas, S.; Timmermann, B.; Attimonelli, M.; Jung, K.; et al. Papillary Renal Cell Carcinomas Rewire Glutathione Metabolism and Are Deficient in Both Anabolic Glucose Synthesis and Oxidative Phosphorylation. Cancers 2019, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Kurelac, I.; Iommarini, L.; Vatrinet, R.; Amato, L.B.; De Luise, M.; Leone, G.; Girolimetti, G.; Umesh Ganesh, N.; Bridgeman, V.L.; Ombrato, L.; et al. Inducing Cancer Indolence by Targeting Mitochondrial Complex I Is Potentiated by Blocking Macrophage-Mediated Adaptive Responses. Nat. Commun. 2019, 10, 903. [Google Scholar] [CrossRef]
- Joshi, S.; Tolkunov, D.; Aviv, H.; Hakimi, A.A.; Yao, M.; Hsieh, J.J.; Ganesan, S.; Chan, C.S.; White, E. The Genomic Landscape of Renal Oncocytoma Identifies a Metabolic Barrier to Tumorigenesis. Cell Rep. 2015, 13, 1895–1908. [Google Scholar] [CrossRef]
- Schaeffeler, E.; Büttner, F.; Reustle, A.; Klumpp, V.; Winter, S.; Rausch, S.; Fisel, P.; Hennenlotter, J.; Kruck, S.; Stenzl, A.; et al. Metabolic and Lipidomic Reprogramming in Renal Cell Carcinoma Subtypes Reflects Regions of Tumor Origin. Eur. Urol. Focus 2019, 5, 608–618. [Google Scholar] [CrossRef]
- Bezwada, D.; Lesner, N.P.; Brooks, B.; Vu, H.S.; Wu, Z.; Cai, L.; Kasitinon, S.; Kelekar, S.; Cai, F.; Aurora, A.B.; et al. Mitochondrial Metabolism in Primary and Metastatic Human Kidney Cancers; Cancer Biology: Hong Kong, China, 2023. [Google Scholar]
- Wettersten, H.I.; Hakimi, A.A.; Morin, D.; Bianchi, C.; Johnstone, M.E.; Donohoe, D.R.; Trott, J.F.; Aboud, O.A.; Stirdivant, S.; Neri, B.; et al. Grade-Dependent Metabolic Reprogramming in Kidney Cancer Revealed by Combined Proteomics and Metabolomics Analysis. Cancer Res. 2015, 75, 2541–2552. [Google Scholar] [CrossRef]
- Clark, D.J.; Dhanasekaran, S.M.; Petralia, F.; Pan, J.; Song, X.; Hu, Y.; Da Veiga Leprevost, F.; Reva, B.; Lih, T.-S.M.; Chang, H.-Y.; et al. Integrated Proteogenomic Characterization of Clear Cell Renal Cell Carcinoma. Cell 2019, 179, 964–983.e31. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, A.A.; Reznik, E.; Lee, C.-H.; Creighton, C.J.; Brannon, A.R.; Luna, A.; Aksoy, B.A.; Liu, E.M.; Shen, R.; Lee, W.; et al. An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell 2016, 29, 104–116. [Google Scholar] [CrossRef]
- Reinfeld, B.I.; Madden, M.Z.; Wolf, M.M.; Chytil, A.; Bader, J.E.; Patterson, A.R.; Sugiura, A.; Cohen, A.S.; Ali, A.; Do, B.T.; et al. Cell-Programmed Nutrient Partitioning in the Tumour Microenvironment. Nature 2021, 593, 282–288. [Google Scholar] [CrossRef]
- Golkaram, M.; Kuo, F.; Gupta, S.; Carlo, M.I.; Salmans, M.L.; Vijayaraghavan, R.; Tang, C.; Makarov, V.; Rappold, P.; Blum, K.A.; et al. Spatiotemporal Evolution of the Clear Cell Renal Cell Carcinoma Microenvironment Links Intra-Tumoral Heterogeneity to Immune Escape. Genome Med. 2022, 14, 143. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ferdinand, J.R.; Loudon, K.W.; Bowyer, G.S.; Laidlaw, S.; Muyas, F.; Mamanova, L.; Neves, J.B.; Bolt, L.; Fasouli, E.S.; et al. Mapping Single-Cell Transcriptomes in the Intra-Tumoral and Associated Territories of Kidney Cancer. Cancer Cell 2022, 40, 1583–1599. [Google Scholar] [CrossRef] [PubMed]
- Udayakumar, D.; Zhang, Z.; Xi, Y.; Dwivedi, D.K.; Fulkerson, M.; Haldeman, S.; McKenzie, T.; Yousuf, Q.; Joyce, A.; Hajibeigi, A.; et al. Deciphering Intratumoral Molecular Heterogeneity in Clear Cell Renal Cell Carcinoma with a Radiogenomics Platform. Clin. Cancer Res. 2021, 27, 4794–4806. [Google Scholar] [CrossRef]
- Fletcher, J.W.; Djulbegovic, B.; Soares, H.P.; Siegel, B.A.; Lowe, V.J.; Lyman, G.H.; Coleman, R.E.; Wahl, R.; Paschold, J.C.; Avril, N.; et al. Recommendations on the Use of 18F-FDG PET in Oncology. J. Nucl. Med. 2008, 49, 480–508. [Google Scholar] [CrossRef]
- Nakajima, R.; Nozaki, S.; Kondo, T.; Nagashima, Y.; Abe, K.; Sakai, S. Evaluation of Renal Cell Carcinoma Histological Subtype and Fuhrman Grade Using 18F-Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography. Eur. Radiol. 2017, 27, 4866–4873. [Google Scholar] [CrossRef]
- Özülker, T.; Özülker, F.; Özbek, E.; Özpaçaci, T. A Prospective Diagnostic Accuracy Study of F-18 Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography in the Evaluation of Indeterminate Renal Masses. Nucl. Med. Commun. 2011, 32, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Caldarella, C.; Muoio, B.; Isgrò, M.A.; Porfiri, E.; Treglia, G.; Giovanella, L. The Role of Fluorine-18-Fluorodeoxyglucose Positron Emission Tomography in Evaluating the Response to Tyrosine-Kinase Inhibitors in Patients with Metastatic Primary Renal Cell Carcinoma. Radiol. Oncol. 2014, 48, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Appelbaum, D.E.; Kocherginsky, M.; Cowey, C.L.; Kimryn Rathmell, W.; McDermott, D.F.; Stadler, W.M. FDG—PET as a Predictive Biomarker for Therapy with Everolimus in Metastatic Renal Cell Cancer. Cancer Med. 2013, 2, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Yao, M.; Tateishi, U.; Minamimoto, R.; Makiyama, K.; Hayashi, N.; Sano, F.; Murakami, T.; Kishida, T.; Miura, T.; et al. Early Assessment by FDG-PET/CT of Patients with Advanced Renal Cell Carcinoma Treated with Tyrosine Kinase Inhibitors Is Predictive of Disease Course. BMC Cancer 2012, 12, 162. [Google Scholar] [CrossRef]
- Nakaigawa, N.; Kondo, K.; Kaneta, T.; Tateishi, U.; Minamimoto, R.; Namura, K.; Ueno, D.; Kobayashi, K.; Kishida, T.; Ikeda, I.; et al. FDG PET/CT after First Molecular Targeted Therapy Predicts Survival of Patients with Renal Cell Carcinoma. Cancer Chemother. Pharmacol. 2018, 81, 739–744. [Google Scholar] [CrossRef]
- Lyrdal, D.; Boijsen, M.; Suurküla, M.; Lundstam, S.; Stierner, U. Evaluation of Sorafenib Treatment in Metastatic Renal Cell Carcinoma with 2-Fluoro-2-Deoxyglucose Positron Emission Tomography and Computed Tomography. Nucl. Med. Commun. 2009, 30, 519–524. [Google Scholar] [CrossRef]
- Rowe, S.P.; Gorin, M.A.; Solnes, L.B.; Ball, M.W.; Choudhary, A.; Pierorazio, P.M.; Epstein, J.I.; Javadi, M.S.; Allaf, M.E.; Baras, A.S. Correlation of 99mTc-Sestamibi Uptake in Renal Masses with Mitochondrial Content and Multi-Drug Resistance Pump Expression. EJNMMI Res. 2017, 7, 80. [Google Scholar] [CrossRef]
- Sevcenco, S.; Krssak, M.; Javor, D.; Ponhold, L.; Kuehhas, F.E.; Fajkovic, H.; Haitel, A.; Shariat, S.F.; Baltzer, P.A. Diagnosis of Renal Tumors by in Vivo Proton Magnetic Resonance Spectroscopy. World J. Urol. 2015, 33, 17–23. [Google Scholar] [CrossRef]
- Ali, H.A.; Couch, M.J.; Menezes, R.; Evans, A.J.; Finelli, A.; Jewett, M.A.; Jhaveri, K.S. Predictive Value of In Vivo MR Spectroscopy With Semilocalization by Adiabatic Selective Refocusing in Differentiating Clear Cell Renal Cell Carcinoma From Other Subtypes. Am. J. Roentgenol. 2020, 214, 817–824. [Google Scholar] [CrossRef]
- Nurenberg, P.; Sartoni-D’Ambrosia, G.; Szczepaniak, L.S. Magnetic Resonance Spectroscopy of Renal and Other Retroperitoneal Tumors. Curr. Opin. Urol. 2002, 12, 375–380. [Google Scholar] [CrossRef]
- Casey, R.T.; McLean, M.A.; Challis, B.G.; McVeigh, T.P.; Warren, A.Y.; Mendil, L.; Houghton, R.; De Sanctis, S.; Kosmoliaptsis, V.; Sandford, R.N.; et al. Fumarate Metabolic Signature for the Detection of Reed Syndrome in Humans. Clin. Cancer Res. 2020, 26, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.H.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2018, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Woitek, R.; Gallagher, F.A. The Use of Hyperpolarised 13C-MRI in Clinical Body Imaging to Probe Cancer Metabolism. Br. J. Cancer 2021, 124, 1187–1198. [Google Scholar] [CrossRef]
- Wang, Z.J.; Ohliger, M.A.; Larson, P.E.Z.; Gordon, J.W.; Bok, A.; Slater, J.; Villanueva-Meyer, J.E.; Hess, C.P.; Vigneron, D.B. Hyperpolarized 13C MRI: State of the Art and Future Directions. Radiology 2019, 291, 12. [Google Scholar] [CrossRef]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.Z.; Harzstark, A.L.; Ferrone, M.; van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C]Pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef]
- Sriram, R.; Van Criekinge, M.; Hansen, A.; Wang, Z.J.; Vigneron, D.B.; Wilson, D.M.; Keshari, K.R.; Kurhanewicz, J. Real-Time Measurement of Hyperpolarized Lactate Production and Efflux as a Biomarker of Tumor Aggressiveness in an MR Compatible 3D Cell Culture Bioreactor: Real Time Measure of HP Lactate Production and Efflux. NMR Biomed. 2015, 28, 1141–1149. [Google Scholar] [CrossRef]
- Sriram, R.; Gordon, J.; Baligand, C.; Ahamed, F.; Delos Santos, J.; Qin, H.; Bok, R.; Vigneron, D.; Kurhanewicz, J.; Larson, P.; et al. Non-Invasive Assessment of Lactate Production and Compartmentalization in Renal Cell Carcinomas Using Hyperpolarized 13C Pyruvate MRI. Cancers 2018, 10, 313. [Google Scholar] [CrossRef]
- Sriram, R.; Van Criekinge, M.; DeLos Santos, J.; Keshari, K.R.; Wilson, D.M.; Peehl, D.; Kurhanewicz, J.; Wang, Z.J. Non-Invasive Differentiation of Benign Renal Tumors from Clear Cell Renal Cell Carcinomas Using Clinically Translatable Hyperpolarized 13C Pyruvate Magnetic Resonance. Tomography 2016, 2, 35–42. [Google Scholar] [CrossRef]
- Girgis, H.; Masui, O.; White, N.M.; Scorilas, A.; Rotondo, F.; Seivwright, A.; Gabril, M.; Filter, E.R.; Girgis, A.H.; Bjarnason, G.A.; et al. Lactate Dehydrogenase A Is a Potential Prognostic Marker in Clear Cell Renal Cell Carcinoma. Mol. Cancer 2014, 13, 101. [Google Scholar] [CrossRef]
- Cao, Y.-W.; Liu, Y.; Dong, Z.; Guo, L.; Kang, E.-H.; Wang, Y.-H.; Zhang, W.; Niu, H.-T. Monocarboxylate Transporters MCT1 and MCT4 Are Independent Prognostic Biomarkers for the Survival of Patients with Clear Cell Renal Cell Carcinoma and Those Receiving Therapy Targeting Angiogenesis. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 311.e15–311.e25. [Google Scholar] [CrossRef]
- Tran, M.; Latifoltojar, A.; Neves, J.B.; Papoutsaki, M.-V.; Gong, F.; Comment, A.; Costa, A.S.H.; Glaser, M.; Tran-Dang, M.-A.; El Sheikh, S.; et al. First-in-Human in Vivo Non-Invasive Assessment of Intra-Tumoral Metabolic Heterogeneity in Renal Cell Carcinoma. BJR|Case Rep. 2019, 5, 20190003. [Google Scholar] [CrossRef]
- Tang, S.; Meng, M.V.; Slater, J.B.; Gordon, J.W.; Vigneron, D.B.; Stohr, B.A.; Larson, P.E.Z.; Wang, Z.J. Metabolic Imaging with Hyperpolarized 13C Pyruvate Magnetic Resonance Imaging in Patients with Renal Tumors—Initial Experience. Cancer 2021, 127, 2693–2704. [Google Scholar] [CrossRef]
- Ursprung, S.; Woitek, R.; McLean, M.A.; Priest, A.N.; Crispin-Ortuzar, M.; Brodie, C.R.; Gill, A.B.; Gehrung, M.; Beer, L.; Riddick, A.C.P.; et al. Hyperpolarized 13C-Pyruvate Metabolism as a Surrogate for Tumor Grade and Poor Outcome in Renal Cell Carcinoma—A Proof of Principle Study. Cancers 2022, 14, 335. [Google Scholar] [CrossRef]
- Woitek, R.; McLean, M.A.; Gill, A.B.; Grist, J.T.; Provenzano, E.; Patterson, A.J.; Ursprung, S.; Torheim, T.; Zaccagna, F.; Locke, M.; et al. Hyperpolarized 13C MRI of Tumor Metabolism Demonstrates Early Metabolic Response to Neoadjuvant Chemotherapy in Breast Cancer. Radiol. Imaging Cancer 2020, 2, e200017. [Google Scholar] [CrossRef]
- de Kouchkovsky, I.; Chen, H.-Y.; Ohliger, M.A.; Wang, Z.J.; Bok, R.A.; Gordon, J.W.; Larson, P.E.Z.; Frost, M.; Okamoto, K.; Cooperberg, M.R.; et al. Hyperpolarized 1-[13C]-Pyruvate Magnetic Resonance Imaging Detects an Early Metabolic Response to Immune Checkpoint Inhibitor Therapy in Prostate Cancer. Eur. Urol. 2021, 81, 219. [Google Scholar] [CrossRef]
- Woitek, R.; McLean, M.A.; Ursprung, S.; Rueda, O.M.; Manzano Garcia, R.; Locke, M.J.; Beer, L.; Baxter, G.; Rundo, L.; Provenzano, E.; et al. Hyperpolarized Carbon-13 MRI for Early Response Assessment of Neoadjuvant Chemotherapy in Breast Cancer Patients. Cancer Res. 2021, 81, 6004–6017. [Google Scholar] [CrossRef] [PubMed]
- Chaumeil, M.M.; Bankson, J.A.; Brindle, K.M.; Epstein, S.; Gallagher, F.A.; Grashei, M.; Guglielmetti, C.; Kaggie, J.D.; Keshari, K.R.; Knecht, S.; et al. New Horizons in Hyperpolarized 13C MRI. Mol. Imaging Biol. 2024, 26, 222–232. [Google Scholar] [CrossRef]
- De Feyter, H.M.; Behar, K.L.; Corbin, Z.A.; Fulbright, R.K.; Brown, P.B.; McIntyre, S.; Nixon, T.W.; Rothman, D.L.; de Graaf, R.A. Deuterium Metabolic Imaging (DMI) for MRI-Based 3D Mapping of Metabolism in Vivo. Sci. Adv. 2018, 4, eaat7314. [Google Scholar] [CrossRef] [PubMed]
- Adamson, P.M.; Datta, K.; Watkins, R.; Recht, L.D.; Hurd, R.E.; Spielman, D.M. Deuterium Metabolic Imaging for 3D Mapping of Glucose Metabolism in Humans with Central Nervous System Lesions at 3T. Magn. Reson. Med. 2024, 91, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Kaggie, J.D.; Khan, A.S.; Matys, T.; Schulte, R.F.; Locke, M.J.; Grimmer, A.; Frary, A.; Menih, I.H.; Latimer, E.; Graves, M.J.; et al. Deuterium Metabolic Imaging and Hyperpolarized 13C-MRI of the Normal Human Brain at Clinical Field Strength Reveals Differential Cerebral Metabolism. NeuroImage 2022, 257, 119284. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Peterson, K.A.; Vittay, O.I.; McLean, M.A.; Kaggie, J.D.; O’Brien, J.T.; Rowe, J.B.; Gallagher, F.A.; Matys, T.; Wolfe, S. Deuterium Metabolic Imaging of Alzheimer Disease at 3-T Magnetic Field Strength: A Pilot Case-Control Study. Radiology 2024, 312, e232407. [Google Scholar] [CrossRef]
- Gursan, A.; Hendriks, A.D.; Welting, D.; De Jong, P.A.; Klomp, D.W.J.; Prompers, J.J. Deuterium Body Array for the Simultaneous Measurement of Hepatic and Renal Glucose Metabolism and Gastric Emptying with Dynamic 3D Deuterium Metabolic Imaging at 7 T. NMR Biomed. 2023, 36, e4926. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.M.; Gursan, A.; Bhogal, A.A.; Wijnen, J.P.; Klomp, D.W.J.; Prompers, J.J.; Hendriks, A.D. Deuterium echo-planar Spectroscopic Imaging (EPSI) in the Human Liver in Vivo at 7 T. Magn. Reson. Med. 2023, 90, 863–874. [Google Scholar] [CrossRef]
- Wodtke, P.; McLean, M.A.; Horvat-Menih, I.; Birchall, J.R.; Zamora-Morales, M.J.; Grimmer, A.; Latimer, E.; Wylot, M.; Schulte, R.F.; Gallagher, F.A. Deuterium Metabolic Imaging of the Human Abdomen at Clinical Field Strength. Investig. Radiol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- De Feyter, H.M.; de Graaf, R.A. Deuterium Metabolic Imaging—Back to the Future. J. Magn. Reson. 2021, 326, 106932. [Google Scholar] [CrossRef]
- van Zijl, P.C.M.; Brindle, K.M. Spectroscopic Measurements of Metabolic Fluxes. Nat. Biomed. Eng. 2020, 4, 254–256. [Google Scholar] [CrossRef]
- McLean, M.A.; Menih, I.H.; Wodtke, P.; Kaggie, J.D.; Birchall, J.R.; Schulte, R.F.; Grimmer, A.; Latimer, E.; Wylot, M.; Zamora Morales, M.J.; et al. Development and optimization of human deuterium MR spectroscopic imaging at 3 T in the abdomen. Magn. Reson. Med. 2024, 1–9. [Google Scholar] [CrossRef]
- Brindle, K.M. Imaging Cancer Metabolism Using Magnetic Resonance. NPJ Imaging 2024, 2, 1. [Google Scholar] [CrossRef]
- Horvat-Menih, I.; Casey, R.; Denholm, J.; Hamm, G.; Hulme, H.; Gallon, J.; Khan, A.S.; Kaggie, J.; Gill, A.B.; Priest, A.N.; et al. Probing Intratumoral Metabolic Compartmentalisation in Fumarate Hydratase-Deficient Renal Cancer Using Clinical Hyperpolarised13 C-MRI and Mass Spectrometry Imaging. medRxiv 2024. [Google Scholar] [CrossRef]
- Horvat-Menih, I.; Khan, A.S.; McLean, M.A.; Duarte, J.; Serrao, E.; Ursprung, S.; Kaggie, J.D.; Gill, A.B.; Priest, A.N.; Crispin-Ortuzar, M.; et al. K-Means Clustering of Hyperpolarised 13C-MRI Identifies Intratumoral Perfusion/Metabolism Mismatch in Renal Cell Carcinoma as the Best Predictor of the Highest Grade. Cancers 2025, 17, 569. [Google Scholar] [CrossRef] [PubMed]
- Ursprung, S.; Mossop, H.; Gallagher, F.A.; Sala, E.; Skells, R.; Sipple, J.A.N.; Mitchell, T.J.; Chhabra, A.; Fife, K.; Matakidou, A.; et al. The WIRE Study a Phase II, Multi-Arm, Multi-Centre, Non-Randomised Window-of-Opportunity Clinical Trial Platform Using a Bayesian Adaptive Design for Proof-of-Mechanism of Novel Treatment Strategies in Operable Renal Cell Cancer—A Study Protocol. BMC Cancer 2021, 21, 1238. [Google Scholar] [CrossRef] [PubMed]
| Renal Tumour Subtype | Characteristics |
|---|---|
| Clear cell RCC (ccRCC) |
|
| Papillary RCC (pRCC) |
|
| Chromophobe RCC (chRCC) |
|
| Renal Oncocytoma (RO) |
|
| SDHd-RCC and FHd-RCC |
|
| Sequence | Plane | Slice Thickness/Gap | Comments |
|---|---|---|---|
| 2D T2w SSFSE | Axial/Coronal | Axial: 4–5 mm/no gapCoronal: 5–6 mm/no gap | Alternative: 2D axial T2w FSE |
| 2D T1w GRE in/out phase | Axial | 5–6 mm/0.5–1 mm | Alternative: 3D Dixon, 3–4 mm/no gap |
| 3D T1w SPGR fat saturation | Axial/Coronal | 3–4 mm/no gap | |
| 3D dynamic T1w SPGR fat saturation, 0.1 mL/kg of 1M Gd contrast | Axial/Coronal | 3–4 mm/no gap | 30, 90–100, and 180–210 s, subtraction imaging; after dynamic series, obtain the other plane at 240 s |
| Optional sequences | |||
| 3D T1w SPGR fat saturation | Axial/Coronal | 3–4 mm/no gap | 5–7 min post-contrast, image in plane perpendicular to the dynamic acquisition |
| Diffusion-weighted imaging (DWI) | Axial | 5–6 mm/no gap | b-values: 0–50, 400–500, 800–1000 s/mm2 |
| Unmet Clinical Need | Possible Applications of Metabolic MRI and the Research Required to Assess These Applications |
|---|---|
| Sampling error of renal mass biopsy | Apply HP 13C-MRI to assess intratumoural metabolic variation and to enable biopsies to be targeted to the most aggressive tumour subregions [131,132] |
| Differentiating benign and malignant renal tumour subtypes | Undertake large multicentre HP 13C-MRI studies to assess metabolism in a range of renal tumour subtypes |
| Assess the role of DMI to characterise benign and malignant renal tumours | |
| Validated biomarkers for treatment response monitoring | Apply HP 13C-MRI to characterise metabolic response to neoadjuvant treatment of RCC as well as in the metastatic setting [133] |
| Biological validation of metabolic MRI | Validate metabolic MRI methods against tissue measures of metabolism to determine the biological mechanisms influencing metabolic imaging phenotypes |
| Clinical validation of metabolic MRI | Assess the added value of metabolic MRI over current standard-of-care imaging methods for probing intratumoural heterogeneity, determining tumour aggressiveness, targeting biopsies, and assessing response to therapy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Horvat-Menih, I.; Stewart, G.D.; Gallagher, F.A. Potential of Metabolic MRI to Address Unmet Clinical Needs in Localised Kidney Cancer. Cancers 2025, 17, 1773. https://doi.org/10.3390/cancers17111773
Horvat-Menih I, Stewart GD, Gallagher FA. Potential of Metabolic MRI to Address Unmet Clinical Needs in Localised Kidney Cancer. Cancers. 2025; 17(11):1773. https://doi.org/10.3390/cancers17111773
Chicago/Turabian StyleHorvat-Menih, Ines, Grant D. Stewart, and Ferdia A. Gallagher. 2025. "Potential of Metabolic MRI to Address Unmet Clinical Needs in Localised Kidney Cancer" Cancers 17, no. 11: 1773. https://doi.org/10.3390/cancers17111773
APA StyleHorvat-Menih, I., Stewart, G. D., & Gallagher, F. A. (2025). Potential of Metabolic MRI to Address Unmet Clinical Needs in Localised Kidney Cancer. Cancers, 17(11), 1773. https://doi.org/10.3390/cancers17111773







