Simple Summary
Brain connectivity and its modulation by tumor-related treatment has garnered recent interest with developments in the field of “Connectomics”, which involves integrating data from multiple advanced imaging modalities and mapping the complex brain networks, providing insights into brain disorders. In the current study, we aimed to evaluate the effect of whole brain radiotherapy (WBRT) in patients with brain metastases on human brain networks and neurocognitive outcomes. In a prospective registry, data was integrated from diffusion tensor imaging and functional magnetic resonance imaging, and individualized brain maps of 15 neuronal networks were created for each patient at different time points. We observed that the baseline anomalies due to brain metastases increased post-WBRT across multiple networks. The anomaly changes also correlated with neurocognitive decline, suggesting the importance of functional connectivity in cognitive processes. The long-term outcomes might suggest connectome-tailored radiation dose constraints to preserve cognition.
Abstract
Background/Objectives: Connectomics is an evolving branch of neuroscience that determines structural and functional connectivity in the brain. The objective of this prospective imaging study is to evaluate the effect of whole brain radiotherapy (WBRT) on the connectome. Methods: A combination of diffusion tensor imaging (DTI) and functional magnetic resonance imaging (fMRI) was used to study the structural and functional connectivity of the brain, and a machine learning algorithm trained to analyze subject-specific data was applied to create individualized brain maps with 15 neuronal networks for each patient. These brain maps were compared to normal brains from the human connectome project, producing an anomaly matrix. Connectome analysis and multi-dimensional neurocognitive testing on a web-based platform were performed at baseline and 3 months post-WBRT. The change in anomaly frequency was co-related with neurocognitive outcomes. Results: At baseline, connectome analysis revealed that the multiple demand network had the most anomalies (46%). Pre- and post-WBRT comparison revealed increases in proportional anomaly frequency across multiple networks. Pearson correlation showed correlation between neurocognitive domain decline and anomaly changes: learning and memory domain with subcortical network [Verbal recall (Pearson coefficient −0.94; p < 0.01), verbal revision (Pearson coefficient −0.89; p = 0.01), and verbal recognition (Pearson coefficient −0.94; p < 0.01)]. Conclusions: This proof-of-concept study integrated data from DTI and fMRI in the form of connectome and revealed significant changes in brain connectivity, with WBRT that also correlated with neurocognitive outcomes. Further studies in a larger cohort are underway, and correlations with white matter changes and tumor locations/numbers will be performed.
1. Introduction
The collaborative work between neuroscience and network science to characterize complex brain functionality led to a new branch called “Connectomics” that evaluates the structural and functional connectivity of the brain [1]. Diffusion tensor imaging (DTI) with tractography explores the structural connectivity of the brain and provides a detailed model of the orientation and integrity of white matter fiber bundles [2,3]. Resting-state functional magnetic resonance imaging (fMRI) evaluates the biologically plausible functional areas of the brain and measures temporal correlations between them, providing insights into the intrinsic functional architecture and connectivity patterns in various neurological conditions, including aberrations that arise in these conditions [4,5,6,7,8]. Advances in modern brain mapping techniques, including complex network analysis, integrate data from these modalities, yielding “human brain networks” [9,10,11]. To understand the brain–cognition–behavior relationship, imaging-based “parcellations” and “nodes” were defined in a network, with the functional connections termed “edges” [12,13].
The Human Connectome Project (HCP) is a large-scale research initiative that aims to map brain connectivity in a comprehensive healthy population and provide an open-access database of imaging and behavioral data [14,15]. Research using the HCP led to the recognition of a fingerprint to represent each cortical area and the creation of the first multi-model atlas of the human brain [16]. This research has been applied to guide connectome-based resections to reduce the incidence of surgery-induced neurological deficits while maintaining onco-functional balance [17,18,19]. Additionally, preliminary studies on patients with primary brain tumors receiving radiation therapy (RT) demonstrated alterations in network topology [20,21,22]. Whether these changes contribute directly to cognitive decline is yet to be demonstrated.
A prospective imaging registry trial (IRB: BHSF1701633) was initiated at our institution to develop a longitudinal imaging repository facilitating translational research. The current analysis expands on the study presented in [23,24] with the objective to evaluate the effects of whole brain radiation therapy (WBRT) on the human connectome and correlate these with changes in cognition. In this proof-of-concept study, we report the imaging findings of the first 10 patients enrolled in the registry trial and correlate the changes in connectomes with alteration in neurocognitive function.
2. Materials and Methods
Patients with brain metastases treated with WBRT were enrolled in the prospective imaging registry trial. English- or Spanish-speaking patients who consented to undergo a minimum of two magnetic resonance imaging (MRI) sequences were included.
Figure 1 depicts the workflow for the creation of brain networks. After obtaining informed consent, in addition to the standard-of-care MRI (including at least an Axial T1 weighted sequence with contrast), DTI and fMRI (resting state-blood oxygen level dependent) were acquired for each patient at each specific time point [25]. The DICOM files were uploaded to Infinitome (Omniscient Neurotechnology Pty Ltd., Sydney, Australia), a cloud-driven, HIPAA-compliant research platform where the diffusion-weighted images (DWI) were corrected for motion and gradient artifacts and co-registered to the T1 weighted sequence; the structural connectivity of white matter fibers was derived using Tournier’s method [26]. Quicktome Discovery mode™ AI brain mapping software (https://www.o8t.com/quicktome (accessed on 20 May 2025)) was used that incorporates a machine learning model trained on neuroimaging data from 178 healthy individuals obtained from Schizconnect (http://schizconnect.org) and 40 brain tumor patients enabling accurate parcellation on structurally distorted brain parenchyma and used gradient-boosted decision tree model (XGBoost) to build a statistical map between voxel level feature vector and most probable parcellation class [27]. A subject-specific HCP multi-model parcellation version 1 atlas with 15 neuronal networks for each patient at each specific time point was created. These networks are detailed in Table 1. These brain maps were then compared to normal brains from the HCP, and a connectivity-based anomaly matrix was created (Figure 2) for 379 parcellations (Quicktome Discovery mode™). A 3 standard deviation alteration from the normal brain was defined as an “anomaly”. Hyperactivation was defined as the area generating more neuronal activity compared to normal and hypoactivation when showing depressed neuronal activity. Connectome analysis was performed at baseline (pre-WBRT) and 3 months post-WBRT using the same image capturing technique, reconstruction, and processing software to ensure uniformity. The 3-month time interval for follow-up scan was chosen given the prior data on the timing of neurocognitive change after WBRT [28,29].
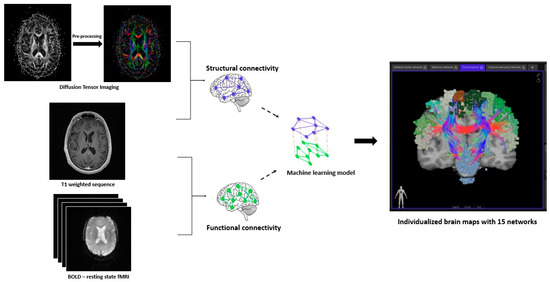
Figure 1.
Workflow for the creation of brain networks. The diffusion-weighted images (DWI) were pre-processed by correcting for motion and gradient artifacts and co-registered to the T1 weighted sequence to analyze the structural connectivity of white matter fibers. A resting-state blood oxygen level dependent (BOLD) functional MRI (fMRI) co-registered to the T1 weighted sequence gives the functional connectivity of brain. A machine learning model integrates data from these modalities and creates subject-specific neuronal networks (15) for each patient.

Table 1.
Intricacies of Brain Networks.
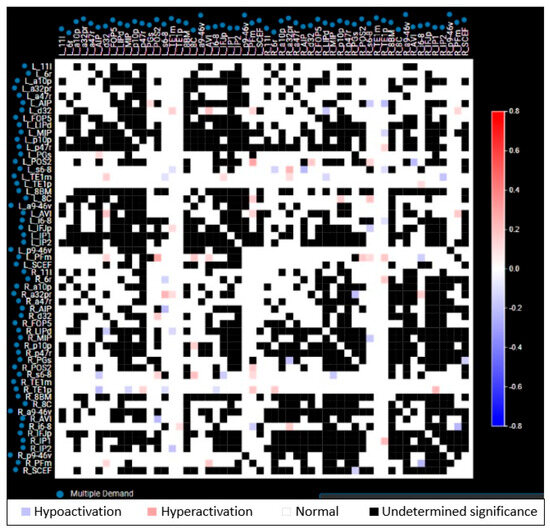
Figure 2.
Representative anomaly matrix of a single network (multiple demand network) in a patient. Each parcellation of a network is compared to the respective parcellation of template normal brain network from Human Connectome Project (HCP), and the functional status is depicted as blue (hypoactivation), red (hyperactivation), white (normal), and black (undetermined significance). Both red and blue are considered as anomalies.
As a pragmatic alternative to the traditional resource-intensive, paper-and-pencil battery-based neurocognitive assessment, a prospective clinical trial was also initiated at our institute (NCT05504681) using a novel, interactive, multi-dimensional, app-based assessment battery called “Cognition” (Brainlab AG, Munich, Germany), which was developed to evaluate patients with brain metastases [56,57]. Neurocognitive assessment included tests for the five domains: (1) learning and memory, (2) attention and speed of processing, (3) verbal fluency, (4) fine motor and speed, and (5) executive functions (details included in Table 2). A comprehensive description of the app-based testing was reported in our earlier study [58]. The patients underwent these neurocognitive evaluations prior to treatment and at 3 months after WBRT.

Table 2.
Details of Neurocognitive Assessment.
Anomaly scores were captured at baseline and post-WBRT. Descriptive statistics were used to define patient baseline characteristics and compared to the anomaly frequencies in each network between baseline and post-WBRT. Neurocognitive function change was computed using the Iverson modification of Reliable Change Index (RCI), and in accordance with the previous literature, a z-score of ±1.645 (90% confidence level) was defined as reliable change [59]. Pearson correlation was used to determine the relationship between anomaly frequency and neurocognitive decline and presented as Pearson coefficient (r). Statistical analysis was performed using SPSS version 27 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Baseline Characteristics of Study Population
Ten consecutive patients who were treated with WBRT and underwent connectome analysis pre- and post-WBRT were included in this proof-of-concept analysis. The median age was 62 years (range: 52–79 years), the majority were female (70%), and the median Karnofsky performance score (KPS) was 90% (range: 70–90%). Lung (n = 7) was the most common primary site of disease followed by breast (n = 2) and renal cell carcinoma (n = 1). Half (5/10) of the patients were treated with hippocampal-avoidant WBRT, and the other half were treated with conventional WBRT to a median dose of 30 Gy in 10 fractions. Two patients had prior resection of brain metastases, and four had prior stereotactic radiosurgery (22–24 Gy in single fraction). The median duration between prior intra-cranial treatment and WBRT was 7 months. Post-WBRT, patients received targeted therapy (n = 4), immunotherapy (n = 3), or chemotherapy (n = 1). The median duration between WBRT completion and the start of systemic therapy was two weeks.
3.2. Baseline (Pre-WBRT) Connectome Evaluation
At baseline, prior to WBRT, connectome analysis revealed significant alterations in functional connectivity across multiple networks. With a median of 10 (range: 8–14) abnormal networks per patient, the highest proportion of anomalies per network (average across all patients) was noted in the multiple demand network (MDE: 46%), followed by the paralimbic network (LIMPA: 31.5%) and the central executive network (CEN: 30.8%). Among the parcellations, p10p (posterior 10 polar, which is a part of lateral frontal lobe regions involved in episodic and working memory tasks) had the highest anomaly frequency of 15, followed by 14 in te1p (temporal area 1 posterior, part of temporal lobe, primarily related to visual pathways and visual working memory) and s6–8 (superior 6–8, part of lateral temporal lobe, involved in maintenance of spatial information).
3.3. Post-WBRT Comparison
Post-WBRT connectome analysis revealed increases in the anomaly frequencies in 8 networks, with a median of 12 (range: 6–14) abnormal networks per patient; the highest proportion of anomalies per network was in the LIMPA (47.3%), followed by the MDE (43.2%) and the CEN (34.2%). Figure 3 depicts a representative case showing alterations in connectivity post-WBRT. The highest proportional increase in anomaly frequency was observed in the limbic network (LIM: 73%), followed by LIMPA (50%), and a proportional decrease in the anomaly frequency in the default mode network (DMN: 55%), followed by visual network (VIS: 37%) (Table 3). Parcellations ip1 (intraparietal 1: part of lateral parietal lobe, involved in mental arithmetic activities), scef (supplementary and cingulate eye field: part of medial superior frontal gyrus, involved in goal-directed behavior), p9–46v (posterior 9–46 ventral: part of lateral frontal region, involved in goal-directed and higher-order cognitive processes) had the highest anomaly frequency of 14. The patients who received any prior intracranial treatment (SRS or resection) exhibited a substantial increase in the total anomaly frequency of 53% (across all networks), while those with no prior treatment had a decrease of 33%.
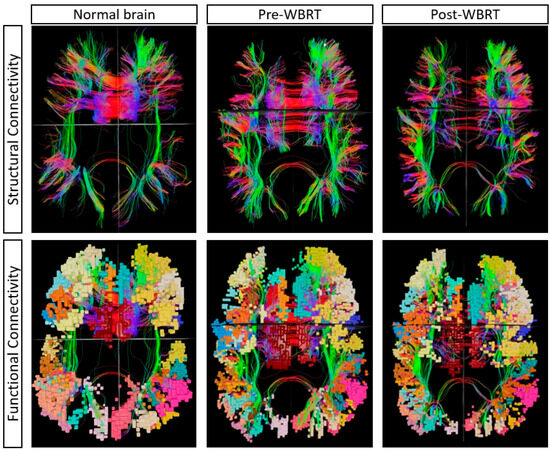
Figure 3.
A single representative case showing the changes in multiple demand network pre- and post-WBRT in comparison to a normal brain from the Human Connectome Project. The alteration in structural connectivity is visualized with decreased fiber density at baseline (pre-WBRT), which further deteriorated post-WBRT. The anomaly frequency at baseline (pre-WBRT) was 13, which increased to 32 post-WBRT.

Table 3.
Anomaly frequency changes between baseline and post-WBRT.
3.4. Neurocognitive Outcomes
The neurocognitive assessments conducted at similar intervals in six patients demonstrated a decline in learning and memory: verbal recall in 33%, verbal revision in 50%, verbal recognition in 50%, attention and speed of processing in 33%, verbal fluency in 50%, and fine motor and speed in 17% of patients compared to their own baseline assessment. Pearson correlation showed a very strong correlation between neurocognitive domain decline and anomaly changes: learning and memory domain with SCN [Verbal recall (Pearson coefficient −0.94; p < 0.01), verbal revision (Pearson coefficient −0.89; p = 0.01), and verbal recognition (Pearson coefficient −0.94; p < 0.01)] (Figure 4). The proportional anomaly frequency in SCN decreased by 11% in hippocampal avoidant WBRT (n = 5), while it increased by 133% in conventional WBRT (n = 5).
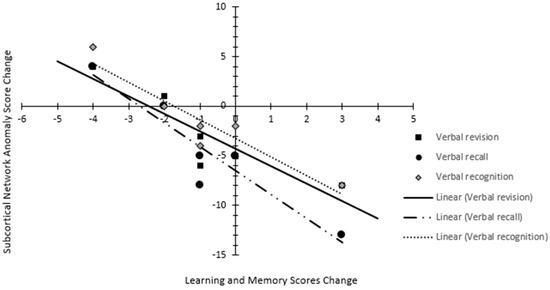
Figure 4.
Scatter plot depicting the relation between anomaly change in subcortical network and changes in learning and memory domain (verbal revision, verbal recall, and verbal recognition) score.
4. Discussion
To the best of our knowledge, this is the first study to explore the effect of WBRT on the connectome and characterize the change in anomaly frequency. Although this is a preliminary evaluation, we found that changes in the SCN correlated with a decline in learning and memory, and intriguingly, SCN anomaly frequency differed between hippocampal avoidant and conventional WBRT. Collectively, these findings suggest the relevance of brain network connectivity in cognitive functioning, which may contribute to connectome-based radiation dose constraints in the future.
In our study, network anomaly reflects hyper- or hypo-activation relative to healthy controls from the HCP. However, in this analysis, the post-WBRT anomaly frequency was compared to the patient’s own baseline value, considering that tumor-specific alterations were already abundant at baseline evaluation. During the follow-up assessment, none of the patients had disease progression in the brain, indicating the anomaly changes and cognitive decline observed during this period could not be attributed to tumor-related changes.
The key components of SCN include the intricate connections between basal ganglia, thalamus, hypothalamus, and hippocampus. Traditionally, these structures were studied as isolated anatomic loci; however, their reconceptualization as networks with coordinated neuronal activity and dynamic hubs contributing to critical cognitive functions is yielding significant clinical implications. Learning and memory processes rely extensively on the dynamic interaction between cortical and subcortical structures, with differential activity across memory encoding, consolidation, and retrieval phases [60,61]. Thalamic sub-nuclei are well known to form functional circuits with extensive reciprocal connections to cortical areas, and act as a dynamic filter of information flow. The mediodorsal thalamus enhances fronto-parietal interactions, stabilizing information during the “verbal recall” phase [62]. Concurrently, the basal ganglia reinforces verbal associations via dopaminergic signaling and is central to procedural learning [63]. Thus, “verbal revision” and “verbal recognition” require coordinated thalamocortical and basal ganglia–cortical interactions. Xu et al. compared individuals with subjective cognitive decline to healthy controls and reported significant structural and functional alterations in the cortical–subcortical circuit and the disappearance of some hubs [64]. The hippocampus binds multimodal sensory, temporal, and contextual details into coherent memories. It interacts extensively with the medial prefrontal cortex (mPFC) and plays a key role in episodic and spatial memory [65]. Dynamic causal modeling showed effective connectivity between the hippocampus and DMN during encoding and memory retrieval [66]. Although a statistical difference was not attained due to the small sample size of the current study, the difference observed in the change in SCN anomaly frequency between hippocampal avoidant and conventional WBRT reinforces the effect of RT on brain networks and might indicate a threshold dose responsible for anomalies that attain clinical significance.
Bahrami et al. previously studied the effect of RT on structural connectivity and found increased modularity along with increases in transitivity and suggested poorer communication across modules and networks after RT [20]. Sleurs et al. evaluated white matter microstructural alterations in children who received RT for infratentorial tumors and demonstrated widespread lower fiber density across the brain and lower fiber cross-section at the treated areas [22]. They also observed lower global and local efficiency across all the network costs compared to controls and identified hubs (most densely connected areas) were most significantly impacted, and demonstrated a strong correlation with intelligence scores. They suggest that sparing these hubs during RT/surgery might be of value to preserve long-term cognition. Our study analyzing both structural and functional connectivity concurs with the findings of these two studies in terms of worsening in the anomaly frequencies post-RT. The presence of brain tumors, per se, causes topological alterations in the networks, seemingly further increased by RT.
Sporns et al. suggest that brain networks demonstrate interlinked communities that form a partly decomposable modular architecture, and such architecture is of fundamental importance for understanding mental processing and cognition [67]. The ability of the brain networks to reconfigure dynamically, adapting to the environment, has been studied. A prior study reviewing functional connectomes in a longitudinal cohort of six glioblastoma patients showed a transient deterioration in network anomalies, followed by near-total recovery, 2 months post-surgery [68]. While our current study is limited by its sample size, which may affect the generalizability of results, we plan to investigate the trends in network alterations with reconfigurations over time in a larger cohort with a longer follow-up. Additionally in this study, we used a novel neurocognitive testing app in the prospective cohort to evaluate neurocognitive changes across multiple domains, which in earlier publications demonstrated results comparable to traditional paper-and-pencil battery tests [57]. This is a limitation of our findings, as the novel test employed has not been validated across diverse populations.
The current study followed a rigorous workflow constituted by several phases, specifically image acquisition, data pre-processing, brain parcellation, and anomaly matrix generation for each network at each specific time point. While the correlation between changes in anomaly frequency and neurocognitive decline is determined, the pathophysiology of radiation-induced altered connectivity has yet to be explored. As our study suggests, a threshold dose can cause the anomalies to attain clinical significance or can result from a stochastic effect, which is yet to be determined. Future efforts in this direction can yield a connectome-tailored treatment directive.
5. Conclusions
This study represents the first evaluation of the human connectome before and after WBRT. Integration of the data from DTI and fMRI can facilitate the creation of human brain networks, which reveal significant changes in functional networks, parcellations, nodes, and hubs that are not appreciated by standard follow-up MRI and correlate with neurocognitive testing on preliminary analysis. Further studies in a larger cohort are underway, and correlations with neurocognitive outcomes, white matter changes, and tumor locations/numbers will be performed.
Author Contributions
S.Y.: Data curation, formal analysis, investigation, methodology, writing—original draft. S.B.: Conceptualization, formal analysis, investigation, methodology, writing—original draft. T.K.: Methodology, writing—review and editing. J.C.: Conceptualization, writing—review and editing. T.C.R.: Data curation, writing—review and editing. M.R.M.: Data curation, writing—review and editing. M.H.: Methodology, writing—review and editing. R.P.: Methodology, writing—review and editing. Y.O.: Methodology, writing—review and editing. M.M. (Minesh Mehta): Conceptualization, methodology, writing—review and editing. M.M. (Michael McDermott): Conceptualization, writing—review and editing, supervision. R.K.: Conceptualization, methodology, writing—original draft, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Baptist Health South Florida on 27 May 2021 (approval number: BHSF1701633).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
Conflicts of Interest
Matthew Hall: Grant support from Florida Department of Health, Grant 22L01. Proton Therapy Cooperative Group of North America (PTCOG-NA) Treasurer (unpaid); Proton Therapy Cooperative Group (PTCOG) Pediatric Subcommittee Co-Chair (unpaid); Proton Collaborative Group (PCG) Executive Council Representative for Miami Cancer Institute (unpaid). Yazmin Odia: DSMB (unpaid) for Actuate Therapeutics, Oncoceutics; DSMB for GammaTile; Trial support by BMS, Cantex, Carthera, Chimerix, CNS Pharmaceuticals, Exelixis, Karyopharm, Mimivax, Novocure, VBI Therapeutics: Consultant: Pharpoint Research; Advisory Board: Modifi Bio, Novocure; No financial conflicts of interests. Minesh Mehta: Consultant: AIQ, Telix, Kazia, Novocure, Zap, Xoft; Advisory Board: Mevion Technological Advisory Board; Board of Directors: Xcision (unremunerated); Stock: Chimerix. Michael McDermott: Consultant Deinde Medical and Stryker Medical. Rupesh Kotecha: Honoraria from Accuray Inc., Elekta AB, ViewRay Inc., Novocure Inc., Elsevier Inc., Brainlab, Kazia Therapeutics, Castle Biosciences, and Ion Beam Applications and institutional research funding from Medtronic Inc., Blue Earth Diagnostics Ltd., Novocure Inc., GT Medical Technologies, AstraZeneca, Exelixis, ViewRay Inc., Brainlab, Cantex Pharmaceuticals, Kazia Therapeutics, and Ion Beam Applications. All the other authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| WBRT | Whole brain radiotherapy |
| DTI | Diffusion tensor imaging |
| fMRI | Functional magnetic resonance imaging |
| HCP | Human Connectome Project |
| MRI | Magnetic resonance imaging |
| DICOM | Digital Imaging and Communications in Medicine |
| DWI | Diffusion-weighted images |
| HIPAA | Health Insurance Portability and Accountability Act |
| BOLD | Blood oxygen level dependent |
| MDE | Multiple demand network |
| SEN | Sensorimotor network |
| DMN | Default mode network |
| CEN | Central Executive network |
| SAL | Salience network |
| LIM | Limbic network |
| LIMPA | Para limbic network |
| AUD | Auditory network |
| LAN | Language network |
| VAN | Ventral attention network |
| DAN | Dorsal attention network |
| SCN | Subcortical network |
| MT | Medial temporal network |
| VIS | Visual network |
| ACLAN | Accessory language network |
References
- Sporns, O. The Human Connectome: Origins and Challenges. NeuroImage 2013, 80, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; van Zijl, P.C.M. Fiber Tracking: Principles and Strategies—A Technical Review. NMR Biomed. 2002, 15, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Jbabdi, S.; Johansen-Berg, H. Tractography: Where Do We Go from Here? Brain Connect. 2011, 1, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Raichle, M.E. Spontaneous Fluctuations in Brain Activity Observed with Functional Magnetic Resonance Imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef]
- Cohen, A.L.; Fair, D.A.; Dosenbach, N.U.F.; Miezin, F.M.; Dierker, D.; Van Essen, D.C.; Schlaggar, B.L.; Petersen, S.E. Defining Functional Areas in Individual Human Brains Using Resting Functional Connectivity MRI. Neuroimage 2008, 41, 45–57. [Google Scholar] [CrossRef]
- Karbasforoushan, H.; Woodward, N.D. Resting-State Networks in Schizophrenia. Curr. Top. Med. Chem. 2012, 12, 2404–2414. [Google Scholar] [CrossRef]
- Burianová, H.; Faizo, N.L.; Gray, M.; Hocking, J.; Galloway, G.; Reutens, D. Altered Functional Connectivity in Mesial Temporal Lobe Epilepsy. Epilepsy Res. 2017, 137, 45–52. [Google Scholar] [CrossRef]
- Holiga, Š.; Hipp, J.F.; Chatham, C.H.; Garces, P.; Spooren, W.; D’Ardhuy, X.L.; Bertolino, A.; Bouquet, C.; Buitelaar, J.K.; Bours, C.; et al. Patients with Autism Spectrum Disorders Display Reproducible Functional Connectivity Alterations. Sci. Transl. Med. 2019, 11, eaat9223. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex Network Measures of Brain Connectivity: Uses and Interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Hagmann, P.; Cammoun, L.; Gigandet, X.; Meuli, R.; Honey, C.J.; Wedeen, V.J.; Sporns, O. Mapping the Structural Core of Human Cerebral Cortex. PLoS Biol. 2008, 6, e159. [Google Scholar] [CrossRef]
- Luppi, A.I.; Gellersen, H.M.; Liu, Z.-Q.; Peattie, A.R.D.; Manktelow, A.E.; Adapa, R.; Owen, A.M.; Naci, L.; Menon, D.K.; Dimitriadis, S.I.; et al. Systematic Evaluation of fMRI Data-Processing Pipelines for Consistent Functional Connectomics. Nat. Commun. 2024, 15, 4745. [Google Scholar] [CrossRef] [PubMed]
- Glasser, M.F.; Coalson, T.S.; Robinson, E.C.; Hacker, C.D.; Harwell, J.; Yacoub, E.; Ugurbil, K.; Andersson, J.; Beckmann, C.F.; Jenkinson, M.; et al. A Multi-Modal Parcellation of Human Cerebral Cortex. Nature 2016, 536, 171–178. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Yeo, B.T.T.; Genon, S. Imaging-Based Parcellations of the Human Brain. Nat. Rev. Neurosci. 2018, 19, 672–686. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Ugurbil, K.; Auerbach, E.; Barch, D.; Behrens, T.E.J.; Bucholz, R.; Chang, A.; Chen, L.; Corbetta, M.; Curtiss, S.W.; et al. The Human Connectome Project: A Data Acquisition Perspective. Neuroimage 2012, 62, 2222–2231. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, D.C.; Smith, S.M.; Barch, D.M.; Behrens, T.E.J.; Yacoub, E.; Ugurbil, K. The WU-Minn Human Connectome Project: An Overview. NeuroImage 2013, 80, 62–79. [Google Scholar] [CrossRef]
- Baker, C.M.; Burks, J.D.; Briggs, R.G.; Conner, A.K.; Glenn, C.A.; Sali, G.; McCoy, T.M.; Battiste, J.D.; O’Donoghue, D.L.; Sughrue, M.E. A Connectomic Atlas of the Human Cerebrum-Chapter 1: Introduction, Methods, and Significance. Oper. Neurosurg. (Hagerstown) 2018, 15, S1–S9. [Google Scholar] [CrossRef]
- Hart, M.G.; Price, S.J.; Suckling, J. Connectome Analysis for Pre-Operative Brain Mapping in Neurosurgery. Br. J. Neurosurg. 2016, 30, 506–517. [Google Scholar] [CrossRef]
- Magnani, M.; Rustici, A.; Zoli, M.; Tuleasca, C.; Chaurasia, B.; Franceschi, E.; Tonon, C.; Lodi, R.; Conti, A. Connectome-Based Neurosurgery in Primary Intra-Axial Neoplasms: Beyond the Traditional Modular Conception of Brain Architecture for the Preservation of Major Neurological Domains and Higher-Order Cognitive Functions. Life 2024, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.S.; Tait, M.J.; Ieva, A.D. Connectomics as a Prognostic Tool of Functional Outcome in Glioma Surgery of the Supplementary Motor Area: Illustrative Case. J. Neurosurg. Case Lessons 2023, 6, CASE23286. [Google Scholar] [CrossRef]
- Bahrami, N.; Seibert, T.M.; Karunamuni, R.; Bartsch, H.; Krishnan, A.; Farid, N.; Hattangadi-Gluth, J.A.; McDonald, C.R. Altered Network Topology in Patients with Primary Brain Tumors After Fractionated Radiotherapy. Brain Connect. 2017, 7, 299–308. [Google Scholar] [CrossRef]
- De Roeck, L.; Blommaert, J.; Dupont, P.; Sunaert, S.; Sleurs, C.; Lambrecht, M. Brain Network Topology and Its Cognitive Impact in Adult Glioma Survivors. Sci. Rep. 2024, 14, 12782. [Google Scholar] [CrossRef]
- Sleurs, C.; Jacobs, S.; Counsell, S.J.; Christiaens, D.; Tournier, J.-D.; Sunaert, S.; Van Beek, K.; Uyttebroeck, A.; Deprez, S.; Batalle, D.; et al. Brain Network Hubs and Cognitive Performance of Survivors of Childhood Infratentorial Tumors. Radiother. Oncol. 2021, 161, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, S. In Proceedings of the European Society for Radiotherapy and Oncology (ESTRO) 2024 Annual Meeting, Glasgow, UK, 3–7 May 2024.
- Yarlagadda, S.; Belnap, S.C.; Kutuk, T.; Candela, J.; Reyes, T.C.; Menendez, M.A.; Hall, M.D.; Press, R.H.; Mehta, M.P.; McDermott, M.W.; et al. 630: Connectome analysis & cognitive outcomes post Whole-brain RT: Prospective registry’s first analysis. Radiother. Oncol. 2024, 194, S769–S771. [Google Scholar] [CrossRef]
- Kutuk, T.; Abrams, K.J.; Tom, M.C.; Rubens, M.; Appel, H.; Sidani, C.; Hall, M.D.; Tolakanahalli, R.; Wieczorek, D.J.J.; Gutierrez, A.N.; et al. Dedicated Isotropic 3-D T1 SPACE Sequence Imaging for Radiosurgery Planning Improves Brain Metastases Detection and Reduces the Risk of Intracranial Relapse. Radiother. Oncol. 2022, 173, 84–92. [Google Scholar] [CrossRef]
- Tournier, J.-D.; Calamante, F.; Gadian, D.G.; Connelly, A. Direct Estimation of the Fiber Orientation Density Function from Diffusion-Weighted MRI Data Using Spherical Deconvolution. Neuroimage 2004, 23, 1176–1185. [Google Scholar] [CrossRef]
- Doyen, S.; Nicholas, P.; Poologaindran, A.; Crawford, L.; Young, I.M.; Romero-Garcia, R.; Sughrue, M.E. Connectivity-Based Parcellation of Normal and Anatomically Distorted Human Cerebral Cortex. Hum. Brain Mapp. 2022, 43, 1358–1369. [Google Scholar] [CrossRef]
- Palmer, J.D.; Klamer, B.; Ballman, K.V.; Brown, P.D.; Cerhan, J.H.; Anderson, K.S.; Whitton, A.C.; Greenspoon, J.; Chung, C.; Burri, S.H.; et al. Effect of Stereotactic Radiosurgery Compared to Whole-Brain Radiotherapy for Limited Brain Metastasis on Long Term Cognition and Quality of Life: A Pooled Analysis of NCCTG N107C/CEC.3 and N0574 (Alliance) Randomized Clinical Trials. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, S175–S176. [Google Scholar] [CrossRef]
- Gondi, V.; Deshmukh, S.; Brown, P.D.; Wefel, J.S.; Armstrong, T.S.; Tome, W.A.; Gilbert, M.R.; Konski, A.; Robinson, C.G.; Bovi, J.A.; et al. Sustained Preservation of Cognition and Prevention of Patient-Reported Symptoms With Hippocampal Avoidance During Whole-Brain Radiation Therapy for Brain Metastases: Final Results of NRG Oncology CC001. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 571–580. [Google Scholar] [CrossRef]
- Biswal, B.; Zerrin Yetkin, F.; Haughton, V.M.; Hyde, J.S. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-Planar Mri. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef]
- Caspers, J.; Rubbert, C.; Eickhoff, S.B.; Hoffstaedter, F.; Südmeyer, M.; Hartmann, C.J.; Sigl, B.; Teichert, N.; Aissa, J.; Turowski, B.; et al. Within- and across-Network Alterations of the Sensorimotor Network in Parkinson’s Disease. Neuroradiology 2021, 63, 2073–2085. [Google Scholar] [CrossRef]
- Raichle, M.E.; Snyder, A.Z. A Default Mode of Brain Function: A Brief History of an Evolving Idea. NeuroImage 2007, 37, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Vanhaudenhuyse, A.; Noirhomme, Q.; Tshibanda, L.J.-F.; Bruno, M.-A.; Boveroux, P.; Schnakers, C.; Soddu, A.; Perlbarg, V.; Ledoux, D.; Brichant, J.-F.; et al. Default Network Connectivity Reflects the Level of Consciousness in Non-Communicative Brain-Damaged Patients. Brain 2010, 133, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Ghumman, S.; Fortin, D.; Noel-Lamy, M.; Cunnane, S.C.; Whittingstall, K. Exploratory Study of the Effect of Brain Tumors on the Default Mode Network. J. Neurooncol. 2016, 128, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Menon, V. Large-Scale Brain Networks and Psychopathology: A Unifying Triple Network Model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Chen, A.C.; Oathes, D.J.; Chang, C.; Bradley, T.; Zhou, Z.-W.; Williams, L.M.; Glover, G.H.; Deisseroth, K.; Etkin, A. Causal Interactions between Fronto-Parietal Central Executive and Default-Mode Networks in Humans. Proc. Natl. Acad. Sci. USA 2013, 110, 19944–19949. [Google Scholar] [CrossRef]
- Chand, G.B.; Dhamala, M. Interactions Among the Brain Default-Mode, Salience, and Central-Executive Networks During Perceptual Decision-Making of Moving Dots. Brain Connect. 2016, 6, 249–254. [Google Scholar] [CrossRef]
- Menon, V. Salience Network. Brain Mapp. 2015, 2, 597–611. [Google Scholar]
- Menon, V.; Uddin, L.Q. Saliency, Switching, Attention and Control: A Network Model of Insula Function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- Enatsu, R.; Gonzalez-Martinez, J.; Bulacio, J.; Kubota, Y.; Mosher, J.; Burgess, R.C.; Najm, I.; Nair, D.R. Connections of the Limbic Network: A Corticocortical Evoked Potentials Study. Cortex 2015, 62, 20–33. [Google Scholar] [CrossRef]
- Thompson, M.; Umphred, D. The Limbic Network: Influence over Motor Control, Memory, and Learning; Mosby: Maryland Heights, MO, USA, 2019; ISBN 13: 978-0323721615. [Google Scholar]
- Kringelbach, M.L.; Rolls, E.T. The Functional Neuroanatomy of the Human Orbitofrontal Cortex: Evidence from Neuroimaging and Neuropsychology. Prog. Neurobiol. 2004, 72, 341–372. [Google Scholar] [CrossRef]
- Lou, H.C.; Gross, J.; Biermann-Ruben, K.; Kjaer, T.W.; Schnitzler, A. Coherence in Consciousness: Paralimbic Gamma Synchrony of Self-reference Links Conscious Experiences. Hum. Brain Mapp. 2010, 31, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.A.; Müller, V.I.; Fox, P.; Laird, A.R.; Hoffstaedter, F.; Kalenscher, T.; Eickhoff, S.B. Definition and Characterization of an Extended Multiple-Demand Network. Neuroimage 2018, 165, 138–147. [Google Scholar] [CrossRef]
- Kuiper, J.J.; Lin, Y.-H.; Young, I.M.; Bai, M.Y.; Briggs, R.G.; Tanglay, O.; Fonseka, R.D.; Hormovas, J.; Dhanaraj, V.; Conner, A.K.; et al. A Parcellation-Based Model of the Auditory Network. Hear. Res. 2020, 396, 108078. [Google Scholar] [CrossRef]
- Hackett, T.A. Information Flow in the Auditory Cortical Network. Hear. Res. 2011, 271, 133–146. [Google Scholar] [CrossRef]
- Ferstl, E.C.; Neumann, J.; Bogler, C.; von Cramon, D.Y. The Extended Language Network: A Meta-analysis of Neuroimaging Studies on Text Comprehension. Hum. Brain Mapp. 2007, 29, 581–593. [Google Scholar] [CrossRef]
- Asplund, C.L.; Todd, J.J.; Snyder, A.P.; Marois, R. A Central Role for the Lateral Prefrontal Cortex in Goal-Directed and Stimulus-Driven Attention. Nat. Neurosci. 2010, 13, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Shulman, G.L. Spatial Neglect and Attention Networks. Annu. Rev. Neurosci. 2011, 34, 569–599. [Google Scholar] [CrossRef] [PubMed]
- Behrens, T.E.J.; Johansen-Berg, H.; Woolrich, M.W.; Smith, S.M.; Wheeler-Kingshott, C.A.M.; Boulby, P.A.; Barker, G.J.; Sillery, E.L.; Sheehan, K.; Ciccarelli, O.; et al. Non-Invasive Mapping of Connections between Human Thalamus and Cortex Using Diffusion Imaging. Nat. Neurosci. 2003, 6, 750–757. [Google Scholar] [CrossRef]
- Ji, J.L.; Spronk, M.; Kulkarni, K.; Repovš, G.; Anticevic, A.; Cole, M.W. Mapping the Human Brain’s Cortical-Subcortical Functional Network Organization. Neuroimage 2019, 185, 35–57. [Google Scholar] [CrossRef]
- Bell, P.T.; Shine, J.M. Subcortical Contributions to Large-Scale Network Communication. Neurosci. Biobehav. Rev. 2016, 71, 313–322. [Google Scholar] [CrossRef]
- Seoane, S.; Modroño, C.; González-Mora, J.L.; Janssen, N. Medial Temporal Lobe Contributions to Resting-State Networks. Brain Struct. Funct. 2022, 227, 995–1012. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.M.; Burks, J.D.; Briggs, R.G.; Stafford, J.; Conner, A.K.; Glenn, C.A.; Sali, G.; McCoy, T.M.; Battiste, J.D.; O’Donoghue, D.L.; et al. A Connectomic Atlas of the Human Cerebrum—Chapter 9: The Occipital Lobe. Oper. Neurosurg. 2018, 15, S372. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, J.; Fan, L.; Li, H.; Zhang, W.; Hu, Q.; Jiang, T. Tractography-Based Parcellation of the Human Middle Temporal Gyrus. Sci. Rep. 2015, 5, 18883. [Google Scholar] [CrossRef] [PubMed]
- Kutuk, T.; Balda, A.; Odia, Y.; Hall, M.D.; Appel, H.; Ramos, S.; Menendez, M.R.; Mohler, A.; McDermott, M.; Ahluwalia, M.S.; et al. Implementation of an Interactive, Multi-Language, App-Based Neurocognitive Evaluation Program into Routine Stereotactic Radiosurgery Practice. Cureus J. Med. Sci. 2023, 15. Available online: https://www.cureus.com/abstracts/873-implementation-of-an-interactive-multi-language-app-based-neurocognitive-evaluation-program-into-routine-stereotactic-radiosurgery-practice (accessed on 20 May 2025).
- Leemans, K.; De Ridder, M. Cognition: Development of a Cognitive Testing Battery on the iPad for the Evaluation of Patients with Brain Mets. Acta Neurol. Belg. 2022, 122, 145–152. [Google Scholar] [CrossRef]
- Akdemir, E.Y.; Gurdikyan, S.; Reyes, T.C.; Odia, Y.; Menendez, M.A.R.; Yarlagadda, S.; Gal, O.; Hall, M.D.; Press, R.H.; Wieczorek, D.J.; et al. Integrating a Novel Tablet-Based Digital Neurocognitive Assessment Tool in Brain Metastases Patients. J. Neurooncol. 2025. [Google Scholar] [CrossRef]
- Duff, K. Evidence-Based Indicators of Neuropsychological Change in the Individual Patient: Relevant Concepts and Methods. Arch. Clin. Neuropsychol. 2012, 27, 248–261. [Google Scholar] [CrossRef]
- Sridhar, S.; Khamaj, A.; Asthana, M.K. Cognitive Neuroscience Perspective on Memory: Overview and Summary. Front. Hum. Neurosci. 2023, 17, 1217093. [Google Scholar] [CrossRef]
- Haubrich, J.; Vera, L.D.; Manahan-Vaughan, D. Cortico-Subcortical Networks That Determine Behavioral Memory Renewal Are Redefined by Noradrenergic Neuromodulation. Sci. Rep. 2025, 15, 9692. [Google Scholar] [CrossRef]
- Li, Z.; Athwal, D.; Lee, H.-L.; Sah, P.; Opazo, P.; Chuang, K.-H. Locating Causal Hubs of Memory Consolidation in Spontaneous Brain Network in Male Mice. Nat. Commun. 2023, 14, 5399. [Google Scholar] [CrossRef]
- Yoshida, J.; Oñate, M.; Khatami, L.; Vera, J.; Nadim, F.; Khodakhah, K. Cerebellar Contributions to the Basal Ganglia Influence Motor Coordination, Reward Processing, and Movement Vigor. J. Neurosci. 2022, 42, 8406–8415. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, T.; Li, W.; Li, H.; Xu, B.; Zhang, M.; Yue, L.; Wang, P.; Xiao, S. Morphological, Structural, and Functional Networks Highlight the Role of the Cortical-Subcortical Circuit in Individuals With Subjective Cognitive Decline. Front. Aging Neurosci. 2021, 13, 688113. [Google Scholar] [CrossRef] [PubMed]
- Pilly, P.K.; Grossberg, S. How Do Spatial Learning and Memory Occur in the Brain? Coordinated Learning of Entorhinal Grid Cells and Hippocampal Place Cells. J. Cogn. Neurosci. 2012, 24, 1031–1054. [Google Scholar] [CrossRef] [PubMed]
- Ushakov, V.; Sharaev, M.G.; Kartashov, S.I.; Zavyalova, V.V.; Verkhlyutov, V.M.; Velichkovsky, B.M. Dynamic Causal Modeling of Hippocampal Links within the Human Default Mode Network: Lateralization and Computational Stability of Effective Connections. Front. Hum. Neurosci. 2016, 10, 528. [Google Scholar] [CrossRef]
- Sporns, O. Network Attributes for Segregation and Integration in the Human Brain. Curr. Opin. Neurobiol. 2013, 23, 162–171. [Google Scholar] [CrossRef]
- Nenning, K.-H.; Furtner, J.; Kiesel, B.; Schwartz, E.; Roetzer, T.; Fortelny, N.; Bock, C.; Grisold, A.; Marko, M.; Leutmezer, F.; et al. Distributed Changes of the Functional Connectome in Patients with Glioblastoma. Sci. Rep. 2020, 10, 18312. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).