Skeletal Muscle Density as a Predictive Marker for Pathologic Complete Response in Triple-Negative Breast Cancer Treated with Neoadjuvant Chemoimmunotherapy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Skeletal Muscle Evaluation
2.3. Statistical Analysis
2.4. PD-L1 Expression and Combined Positive Score
3. Results
3.1. Baseline Characteristics
3.2. Association Between Muscle-Related Indices and Clinical Factors
3.3. Baseline Characteristics of Chemoimmunotherapy Group
3.4. Adverse Event and RDI
3.5. Univariate and Multivariable Regression Analysis for pCR
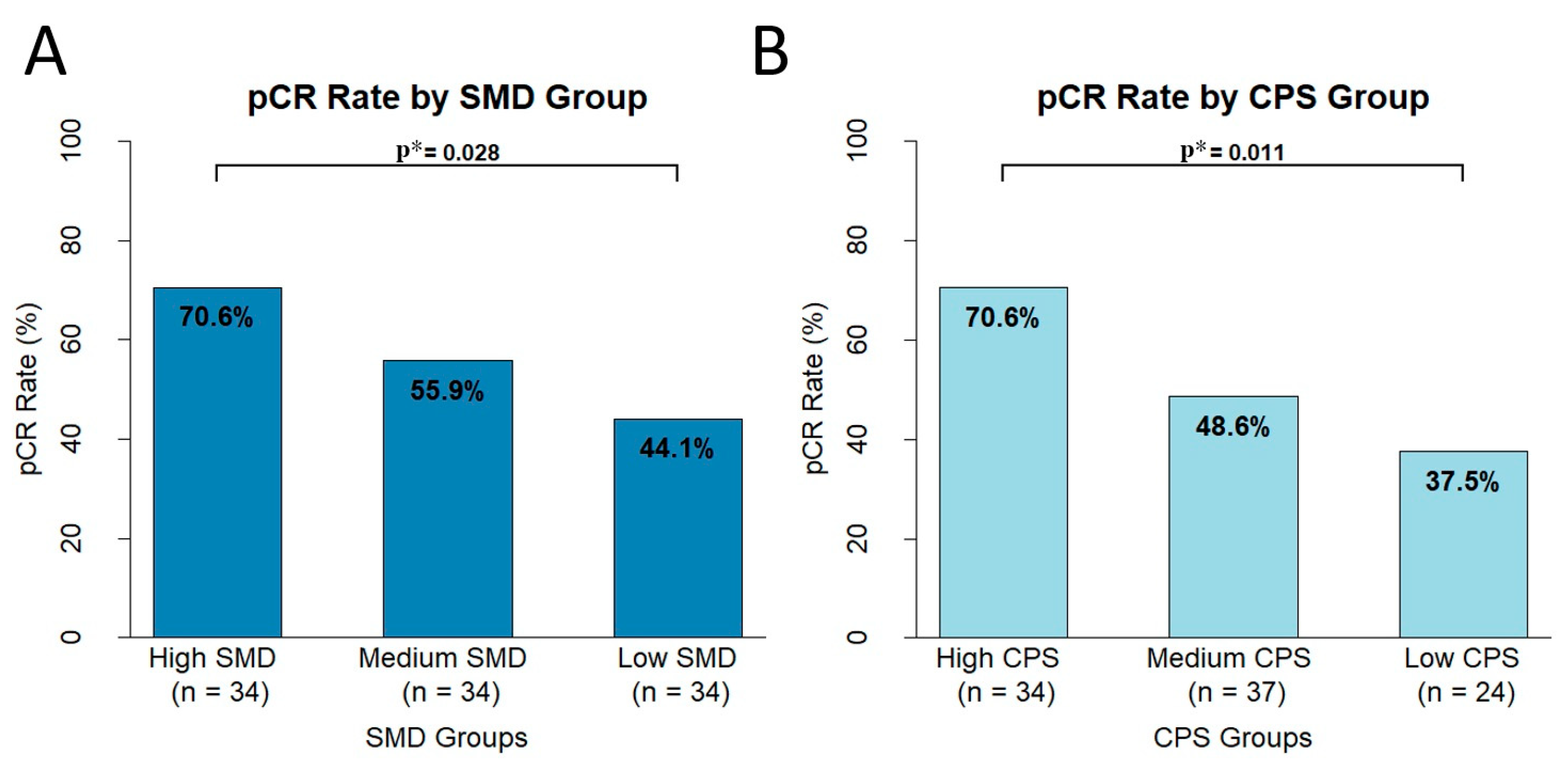
3.6. Differences in pCR Based on CPS and SMD Groups
3.7. Univariate and Multivariate Analysis for Event-Free Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TNBC | Triple-negative breast cancer |
| ER | Estrogen receptors |
| PR | Progesterone receptors |
| HER2 | Human epidermal growth factor receptor 2 |
| NAC | Neoadjuvant chemotherapy |
| pCR | Pathologic complete response |
| TIL | Tumor-infiltrating lymphocyte |
| PS | Performance status |
| SMI | Skeletal muscle index |
| SMD | Skeletal muscle density |
| KN-522 | KEYNOTE-522 |
| HU | Hounsfield units |
| NACIT | Neoadjuvant chemoimmunotherapy |
| NACT | Neoadjuvant chemotherapy only |
| EFS | Event-free survival |
| CCI | Charlson Comorbidity Index |
| RDI | Relative dose intensity |
| SLNB | Sentinel lymph node biopsy |
| SCAR | Severe cutaneous adverse reaction |
| CPS | Combined positive score |
| PFS | Progression-free survival |
| OS | Overall survival |
| ICD | Immunogenic cell death |
References
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.U.; Vanderplas, A.; Hughes, M.E.; Theriault, R.L.; Edge, S.B.; Wong, Y.N.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; Weeks, J.C. Clinicopathologic features, patterns of recurrence, and survival among women with triple-negative breast cancer in the National Comprehensive Cancer Network. Cancer 2012, 118, 5463–5472. [Google Scholar] [CrossRef] [PubMed]
- Redig, A.J.; McAllister, S.S. Breast cancer as a systemic disease: A view of metastasis. J. Intern. Med. 2013, 274, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Loi, S.; Adams, S.; Schmid, P.; Cortés, J.; Cescon, D.W.; Winer, E.P.; Toppmeyer, D.L.; Rugo, H.S.; De Laurentiis, M.; Nanda, R.; et al. Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): Results from KEYNOTE-086. Ann. Oncol. 2017, 28, v608. [Google Scholar] [CrossRef]
- Loi, S.; Michiels, S.; Adams, S.; Loibl, S.; Budczies, J.; Denkert, C.; Salgado, R. The journey of tumor-infiltrating lymphocytes as a biomarker in breast cancer: Clinical utility in an era of checkpoint inhibition. Ann. Oncol. 2021, 32, 1236–1244. [Google Scholar] [CrossRef]
- Helal, C.; Djerroudi, L.; Ramtohul, T.; Laas, E.; Vincent-Salomon, A.; Jin, M.; Seban, R.D.; Bieche, I.; Bello-Roufai, D.; Bidard, F.C.; et al. Clinico-pathological factors predicting pathological response in early triple-negative breast cancer. NPJ Breast Cancer 2025, 11, 15. [Google Scholar] [CrossRef]
- Budman, D.R.; Berry, D.A.; Cirrincione, C.T.; Henderson, I.C.; Wood, W.C.; Weiss, R.B.; Ferree, C.R.; Muss, H.B.; Green, M.R.; Norton, L.; et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. J. Natl. Cancer Inst. 1998, 90, 1205–1211. [Google Scholar] [CrossRef]
- Buonaiuto, R.; Neola, G.; Caltavituro, A.; Trasacco, P.; Mangiacotti, F.P.; Pecoraro, G.; Lambertini, M.; Pietroluongo, E.; De Placido, P.; De Placido, S.; et al. The impact of chemotherapy relative dose intensity on pathological complete response in patients with triple negative breast cancer treated with neoadjuvant chemotherapy. Cancer Res. 2024, 84, PO4-03-07. [Google Scholar] [CrossRef]
- Hryniuk, W.M. Dose Intensity—A Critique of a Critical-Review. J. Clin. Oncol. 1989, 7, 681–682. [Google Scholar] [CrossRef] [PubMed]
- Sedrak, M.S.; Sun, C.L.; Ji, J.R.; Cohen, H.J.; Gross, C.P.; Tew, W.P.; Klepin, H.D.; Wildes, T.M.; Dotan, E.; Freedman, R.A.; et al. Low-Intensity Adjuvant Chemotherapy for Breast Cancer in Older Women: Results from the Prospective Multicenter HOPE Trial. J. Clin. Oncol. 2023, 41, 316–326. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.; Noble, S.; Chester, J.; Coles, B.; Byrne, A. The assessment and impact of sarcopenia in lung cancer: A systematic literature review. BMJ Open 2014, 4, e003697. [Google Scholar] [CrossRef]
- Caan, B.J.; Cespedes Feliciano, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography with Survival in Patients with Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef]
- Ballinger, T.J.; Marques, H.S.; Xue, G.; Hoffman, R.; Gatsonis, C.; Zhao, F.; Miller, K.D.; Sparano, J.; Connolly, R.M. Impact of Muscle Measures on Outcome in Patients Receiving Endocrine Therapy for Metastatic Breast Cancer: Analysis of ECOG-ACRIN E2112. J. Natl. Compr. Canc. Netw. 2023, 21, 915–923. [Google Scholar] [CrossRef]
- Zhuang, C.L.; Wu, H.F.; Jiang, H.J.; Zhang, F.M.; Shi, H.P.; Yu, Z.; Shen, X.; Chen, X.L.; Wang, S.L. Muscle attenuation, not skeletal muscle index, is an independent prognostic factor for survival in gastric cancer patients with overweight and obesity. Nutrition 2024, 122, 112391. [Google Scholar] [CrossRef]
- Baracos, V.E.; Arribas, L. Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann. Oncol. 2018, 29, ii1–ii9. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef]
- Bozzetti, F. Forcing the vicious circle: Sarcopenia increases toxicity, decreases response to chemotherapy and worsens with chemotherapy. Ann. Oncol. 2017, 28, 2107–2118. [Google Scholar] [CrossRef]
- Shachar, S.S.; Williams, G.R.; Muss, H.B.; Nishijima, T.F. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. Eur. J. Cancer 2016, 57, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Williams, G.R.; Nyrop, K.A.; Popuri, K.; Choi, S.K.; Muss, H.B. Body Composition as a Predictor of Toxicity in Patients Receiving Anthracycline and Taxane-Based Chemotherapy for Early-Stage Breast Cancer. Clin. Cancer Res. 2017, 23, 3537–3543. [Google Scholar] [CrossRef]
- Cespedes Feliciano, E.M.; Chen, W.Y.; Lee, V.; Albers, K.B.; Prado, C.M.; Alexeeff, S.; Xiao, J.; Shachar, S.S.; Caan, B.J. Body Composition, Adherence to Anthracycline and Taxane-Based Chemotherapy, and Survival After Nonmetastatic Breast Cancer. JAMA Oncol. 2020, 6, 264–270. [Google Scholar] [CrossRef]
- Sheean, P.; Gomez-Perez, S.; Joyce, C.; O’Connor, P.; Bojko, M.; Smith, A.; Vasilopoulos, V.; Rao, R.; Sclamberg, J.; Robinson, P. Myosteatosis at diagnosis is adversely associated with 2-year survival in women with estrogen receptor-negative metastatic breast cancer. Breast Cancer Res. Treat. 2021, 190, 121–132. [Google Scholar] [CrossRef]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef] [PubMed]
- Shachar, S.S.; Deal, A.M.; Weinberg, M.; Nyrop, K.A.; Williams, G.R.; Nishijima, T.F.; Benbow, J.M.; Muss, H.B. Skeletal Muscle Measures as Predictors of Toxicity, Hospitalization, and Survival in Patients with Metastatic Breast Cancer Receiving Taxane-Based Chemotherapy. Clin. Cancer Res. 2017, 23, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, D.; Bajaj, S.; Yu, J.; Hsu, M.; Balar, A.; Pavlick, A.; Weber, J.; Osman, I.; Zhong, J. The complex relationship between body mass index and response to immune checkpoint inhibition in metastatic melanoma patients. J. Immunother. Cancer 2019, 7, 222. [Google Scholar] [CrossRef]
- Young, A.C.; Quach, H.T.; Song, H.; Davis, E.J.; Moslehi, J.J.; Ye, F.; Williams, G.R.; Johnson, D.B. Impact of body composition on outcomes from anti-PD1 +/− anti-CTLA-4 treatment in melanoma. J. Immunother. Cancer 2020, 8, e000821. [Google Scholar] [CrossRef]
- Ying, H.; Chen, Y.; Hong, Y.; Ying, K.; Li, S.; Zhang, Y.; Mei, T.; Song, X.; He, Y.; Yao, C.; et al. L3-SMI as a predictor of overall survival in oesophageal cancer patients receiving PD-1 inhibitors combined with chemotherapy. Ann. Med. 2025, 57, 2440114. [Google Scholar] [CrossRef]
- Cortellini, A.; Bersanelli, M.; Buti, S.; Cannita, K.; Santini, D.; Perrone, F.; Giusti, R.; Tiseo, M.; Michiara, M.; Di Marino, P.; et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: When overweight becomes favorable. J. Immunother. Cancer 2019, 7, 57. [Google Scholar] [CrossRef]
- Wang, Z.; Aguilar, E.G.; Luna, J.I.; Dunai, C.; Khuat, L.T.; Le, C.T.; Mirsoian, A.; Minnar, C.M.; Stoffel, K.M.; Sturgill, I.R.; et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat. Med. 2019, 25, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Bozzetti, F.; Palumbo, P.; Brocco, D.; Di Marino, P.; Tinari, N.; De Tursi, M.; Agostinelli, V.; Patruno, L.; Valdesi, C.; et al. Weighing the role of skeletal muscle mass and muscle density in cancer patients receiving PD-1/PD-L1 checkpoint inhibitors: A multicenter real-life study. Sci. Rep. 2020, 10, 1456. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Cortes, J.; Pusztai, L.; McArthur, H.; Kummel, S.; Bergh, J.; Denkert, C.; Park, Y.H.; Hui, R.; Harbeck, N.; et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, 810–821. [Google Scholar] [CrossRef]
- Litton, J.K.; Regan, M.M.; Pusztai, L.; Rugo, H.S.; Tolaney, S.M.; Garrett-Mayer, E.; Amiri-Kordestani, L.; Basho, R.K.; Best, A.F.; Boileau, J.F.; et al. Standardized Definitions for Efficacy End Points in Neoadjuvant Breast Cancer Clinical Trials: NeoSTEEP. J. Clin. Oncol. 2023, 41, 4433–4442. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic. Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Simcock, R.; Wright, J. Beyond Performance Status. Clin. Oncol. 2020, 32, 553–561. [Google Scholar] [CrossRef]
- Anderson, D.E.; D’Agostino, J.M.; Bruno, A.G.; Demissie, S.; Kiel, D.P.; Bouxsein, M.L. Variations of CT-based trunk muscle attenuation by age, sex, and specific muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 317–323. [Google Scholar] [CrossRef]
- Krishnan, J.; Patel, A.; Roy, A.M.; Alharbi, M.; Kapoor, A.; Yao, S.; Khoury, T.; Hong, C.C.; Held, N.; Chakraborty, A.; et al. Detrimental Impact of Chemotherapy Dose Reduction or Discontinuation in Early Stage Triple-Negative Breast Cancer Treated with Pembrolizumab and Neoadjuvant Chemotherapy: A Multicenter Experience. Clin. Breast Cancer 2024, 24, e701–e711. [Google Scholar] [CrossRef]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef]
- Yu, J.; Ahn, H.; Han, K.Y.; Song, W.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Park, S.H.; et al. Paradoxical Effect of Myosteatosis on the Immune Checkpoint Inhibitor Response in Metastatic Renal Cell Carcinoma. J. Cachexia Sarcopenia Muscle 2025, 16, e13758. [Google Scholar] [CrossRef]
- Yi, X.; Fu, Y.; Long, Q.; Zhao, Y.; Li, S.; Zhou, C.; Lin, H.; Liu, X.; Liu, C.; Chen, C.; et al. Myosteatosis can Predict Unfavorable Outcomes in Advanced Hepatocellular Carcinoma Patients Treated with Hepatic Artery Infusion Chemotherapy and Anti-PD-1 Immunotherapy. Front. Oncol. 2022, 12, 892192. [Google Scholar] [CrossRef] [PubMed]
- Nishioka, N.; Naito, T.; Notsu, A.; Mori, K.; Kodama, H.; Miyawaki, E.; Miyawaki, T.; Mamesaya, N.; Kobayashi, H.; Omori, S.; et al. Unfavorable impact of decreased muscle quality on the efficacy of immunotherapy for advanced non-small cell lung cancer. Cancer Med. 2021, 10, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.A.; Savas, P.; Virassamy, B.; O’Malley, M.M.R.; Kay, J.; Mueller, S.N.; Mackay, L.K.; Salgado, R.; Loi, S. Towards targeting the breast cancer immune microenvironment. Nat. Rev. Cancer 2024, 24, 554–577. [Google Scholar] [CrossRef] [PubMed]
- Van Bockstal, M.R.; Francois, A.; Altinay, S.; Arnould, L.; Balkenhol, M.; Broeckx, G.; Burgues, O.; Colpaert, C.; Dedeurwaerdere, F.; Dessauvagie, B.; et al. Interobserver variability in the assessment of stromal tumor-infiltrating lymphocytes (sTILs) in triple-negative invasive breast carcinoma influences the association with pathological complete response: The IVITA study. Mod. Pathol. 2021, 34, 2130–2140. [Google Scholar] [CrossRef]
- Reisenbichler, E.S.; Han, G.; Bellizzi, A.; Bossuyt, V.; Brock, J.; Cole, K.; Fadare, O.; Hameed, O.; Hanley, K.; Harrison, B.T.; et al. Prospective multi-institutional evaluation of pathologist assessment of PD-L1 assays for patient selection in triple negative breast cancer. Mod. Pathol. 2020, 33, 1746–1752. [Google Scholar] [CrossRef]


| Variables | High SMD (≥48) | Low SMD (<48) | ||||
|---|---|---|---|---|---|---|
| n = 68 | n = 34 | p-Value | ||||
| Pretreatment | ||||||
| Age (years) | 53 (IQR 45~61) | |||||
| <65 | 88 | 62 | 91.2% | 26 | 76.5% | 0.042 |
| ≥65 | 14 | 6 | 8.8% | 8 | 23.5% | |
| Menopausal state | ||||||
| Pre-menopausal | 55 | 43 | 63.2% | 12 | 35.3% | 0.008 |
| Post-menopausal | 47 | 25 | 36.8% | 22 | 64.7% | |
| ECOG PS | ||||||
| 0 | 93 | 63 | 92.6% | 30 | 88.2% | 0.459 |
| ≥1 | 9 | 5 | 7.4% | 4 | 11.8% | |
| CCI | ||||||
| 0 | 82 | 58 | 85.3% | 24 | 70.6% | 0.078 |
| ≥1 | 20 | 10 | 14.7% | 10 | 29.4% | |
| Stage | ||||||
| II | 60 | 39 | 57.4% | 21 | 61.8% | 0.67 |
| III | 42 | 29 | 42.6% | 13 | 38.2% | |
| Tumor size | ||||||
| T1–2 | 78 | 54 | 79.4% | 24 | 70.6% | 0.322 |
| T3–4 | 24 | 14 | 20.6% | 10 | 29.4% | |
| Node metastasis | ||||||
| Positive | 68 | 47 | 69.1% | 21 | 61.8% | 0.458 |
| Negative | 34 | 21 | 30.9% | 13 | 38.2% | |
| Differentiation | ||||||
| Grades 1–2 | 14 | 10 | 14.7% | 4 | 11.8% | 0.684 |
| Grade 3 | 88 | 58 | 85.3% | 30 | 88.2% | |
| Ki-67 | 62 (IQR 46–77) | |||||
| <20 | 3 | 1 | 1.5% | 2 | 5.9% | 0.214 |
| ≥20 | 99 | 67 | 98.5% | 32 | 94.1% | |
| Germline BRCA | ||||||
| PV/LPV | 9 | 4 | 5.9% | 5 | 14.7% | 0.062 |
| Not detected/VUS | 74 | 55 | 80.9% | 19 | 55.9% | |
| Undetermined | 19 | 9 | 13.2% | 10 | 29.4% | |
| PD-L1 (CPS) | 10 (IQR 10–25) | |||||
| <10 | 24 | 15 | 22.1% | 9 | 26.5% | 0.742 |
| ≥10 | 71 | 47 | 69.1% | 24 | 70.6% | |
| Undetermined | 7 | 6 | 8.8% | 1 | 2.9% | |
| SMD (HU) | 49.32 ± 7.18 | 53.38 ± 4.31 | 41.21 ± 4.27 | <0.001 | ||
| SMI (cm2/m2) | 39.19 ± 5.31 | 39.04 ± 5.48 | 39.50 ± 5.00 | 0.674 | ||
| BMI (kg/m2) | 23.71 ± 3.46 | 23.26 ± 3.34 | 24.62 ± 3.56 | 0.068 | ||
| Post-treatment | ||||||
| Breast Surgery | ||||||
| BCS | 89 | 60 | 88.2% | 29 | 90.6% | 0.722 |
| Mastectomy | 11 | 8 | 11.8% | 3 | 9.4% | |
| No surgery | 2 | 2 | ||||
| Axillary Surgery | ||||||
| SLNB | 90 | 64 | 94.1% | 26 | 81.3% | 0.045 |
| ALND | 10 | 4 | 5.9% | 6 | 18.8% | |
| No surgery | 2 | 2 | ||||
| Pathologic complete response (pCR) | ||||||
| pCR | 58 | 43 | 63.2% | 15 | 44.1% | 0.066 |
| Non-PCR | 44 | 25 | 36.8% | 19 | 55.9% | |
| RDI (%) | 86.9 (IQR 80.0–98.1) | 89.4 (IQR 82.4–100) | 82.5 (IQR 70.0–90.6) | 0.003 | ||
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Variables | OR | (95% CI) | p-Value | OR | (95% CI) | p-Value |
| Age | 0.98 | 0.95–1.02 | 0.34 | |||
| Menopausal state (post- vs. pre-menopausal state) | 1.55 | 0.71–3.44 | 0.275 | |||
| ECOG PS (0 vs. ≥1) | 0.50 | 0.14–1.68 | 0.264 | |||
| CCI (0 vs. ≥1) | 0.55 | 0.20–1.47 | 0.236 | |||
| Germline BRCA (ND/VUS vs. PV/LPV) | 2.88 | 0.65–20.04 | 0.201 | |||
| Stage (II vs. III) | 1.15 | 0.55–2.43 | 0.713 | |||
| T stage (T1,2 vs. T3,4) | 0.67 | 0.41–1.06 | 0.09 | 0.46 | 0.15–1.32 | 0.154 |
| Nodal status (negative vs. positive) | 1.52 | 0.66–3.50 | 0.324 | |||
| Histologic grade (grades 1–2 vs. grade 3) | 2.73 | 0.87–9.51 | 0.094 | 2.27 | 0.58–9.54 | 0.247 |
| Ki-67 (per 10% increase) | 1.20 | 0.98–1.46 | 0.076 | 1.01 | 0.99–1.04 | 0.214 |
| CPS (per 10-point increase) | 1.34 | 1.06–1.81 | 0.028 | 1.38 | 1.07–1.85 | 0.019 |
| RDI (per 10% increase) | 1.20 | 0.96–1.55 | 0.13 | |||
| BMI | 0.91 | 0.80–1.02 | 0.112 | |||
| SMI | 0.96 | 0.89–1.03 | 0.249 | |||
| SMD (per 10 HU increase) | 2.56 | 1.42–4.95 | 0.003 | 2.78 | 1.45–5.74 | 0.003 |
| SMD (per 5 HU increase) | 1.59 | 1.19–2.23 | 1.67 | 1.20–2.40 | ||
| SMD (per 1 HU increase) | 1.10 | 1.04–1.17 | 1.11 | 1.04–1.19 | ||
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variables | HR | (95% CI) | p-Value | HR | (95% CI) | p-Value |
| Age (<65 vs. ≥65) | 3.09 | 0.81–11.73 | 0.098 | 2.22 | 0.53–9.25 | 0.273 |
| Menopausal state (post- vs. pre-menopausal) | 0.43 | 0.13–1.47 | 0.177 | |||
| ECOG PS (0 vs. ≥1) | 2.02 | 0.87–4.68 | 0.102 | |||
| CCI (0 vs. ≥1) | 1.53 | 0.57–4.09 | 0.397 | |||
| Germline BRCA (ND/VUS vs. PV/LPV) | 1.13 | 0.14–8.92 | 0.906 | |||
| Stage (II vs. III) | 1.42 | 0.49–4.14 | 0.516 | |||
| T stage (T1,2 vs. T3,4) | 1.41 | 0.76–2.61 | 0.275 | |||
| Nodal status (negative vs. positive) | 1.41 | 0.37–5.33 | 0.611 | |||
| Histologic grade (grades 1–2 vs. grade 3) | 0.77 | 0.17–3.56 | 0.736 | |||
| Ki-67 (per 10% increase) | 1.00 | 0.75–1.34 | 0.993 | |||
| CPS (per 10-point increase) | 0.71 | 0.42–1.23 | 0.222 | |||
| RDI (per 10% increase) | 0.80 | 0.64–1.02 | 0.067 | 0.94 | 0.72–1.23 | 0.636 |
| BMI | 0.95 | 0.79–1.14 | 0.559 | |||
| SMI | 1.01 | 0.91–1.13 | 0.811 | |||
| SMD (per 10 HU increase) | 0.42 | 0.19–0.93 | 0.033 | 0.6 | 0.25–1.46 | 0.259 |
| SMD (per 1 HU increase) | 0.92 | 0.85–0.99 | 0.95 | 0.87–1.04 | ||
| pCR | 0.07 | 0.01–0.55 | 0.012 | 0.1 | 0.01–0.85 | 0.035 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mun, H.S.; Kim, S.H.; Lee, J.; Park, S.J.; Lee, A.; Kang, J.; Park, W.-C.; Bae, S.Y.; Choi, B.O.; Hong, J.H.; et al. Skeletal Muscle Density as a Predictive Marker for Pathologic Complete Response in Triple-Negative Breast Cancer Treated with Neoadjuvant Chemoimmunotherapy. Cancers 2025, 17, 1768. https://doi.org/10.3390/cancers17111768
Mun HS, Kim SH, Lee J, Park SJ, Lee A, Kang J, Park W-C, Bae SY, Choi BO, Hong JH, et al. Skeletal Muscle Density as a Predictive Marker for Pathologic Complete Response in Triple-Negative Breast Cancer Treated with Neoadjuvant Chemoimmunotherapy. Cancers. 2025; 17(11):1768. https://doi.org/10.3390/cancers17111768
Chicago/Turabian StyleMun, Han Song, Sung Hun Kim, Jieun Lee, Se Jun Park, Ahwon Lee, Jun Kang, Woo-Chan Park, Soo Youn Bae, Byung Ok Choi, Ji Hyun Hong, and et al. 2025. "Skeletal Muscle Density as a Predictive Marker for Pathologic Complete Response in Triple-Negative Breast Cancer Treated with Neoadjuvant Chemoimmunotherapy" Cancers 17, no. 11: 1768. https://doi.org/10.3390/cancers17111768
APA StyleMun, H. S., Kim, S. H., Lee, J., Park, S. J., Lee, A., Kang, J., Park, W.-C., Bae, S. Y., Choi, B. O., Hong, J. H., Oh, S. N., & Shin, K. (2025). Skeletal Muscle Density as a Predictive Marker for Pathologic Complete Response in Triple-Negative Breast Cancer Treated with Neoadjuvant Chemoimmunotherapy. Cancers, 17(11), 1768. https://doi.org/10.3390/cancers17111768







