Fatty Pancreas: Its Potential as a Risk Factor for Pancreatic Cancer and Clinical Implications
Simple Summary
Abstract
1. Introduction
2. Pathology
3. Physiopathology and Risk Factors
4. Prevalence
5. Diagnosis
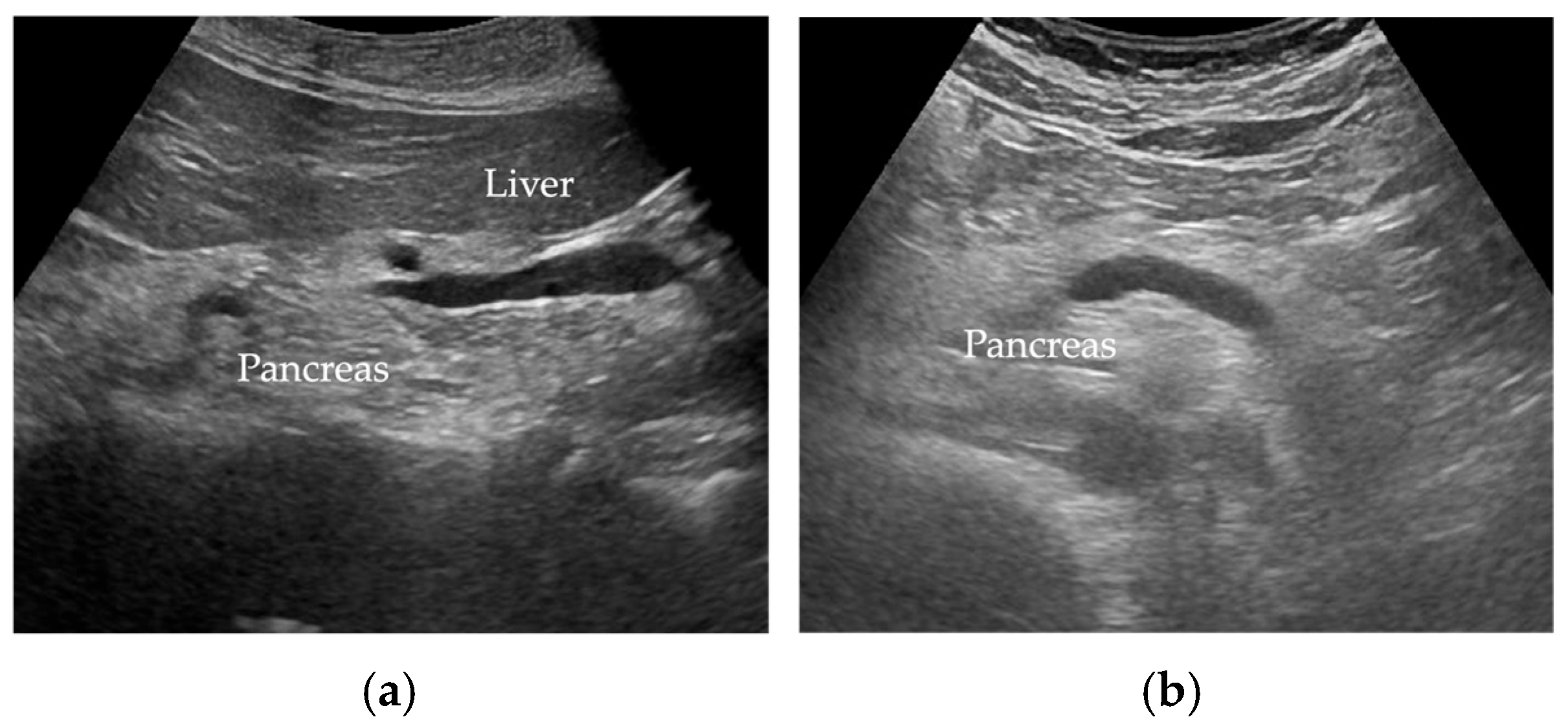
5.1. Transabdominal Ultrasonography (US)
5.2. Endoscopic Ultrasound (EUS)
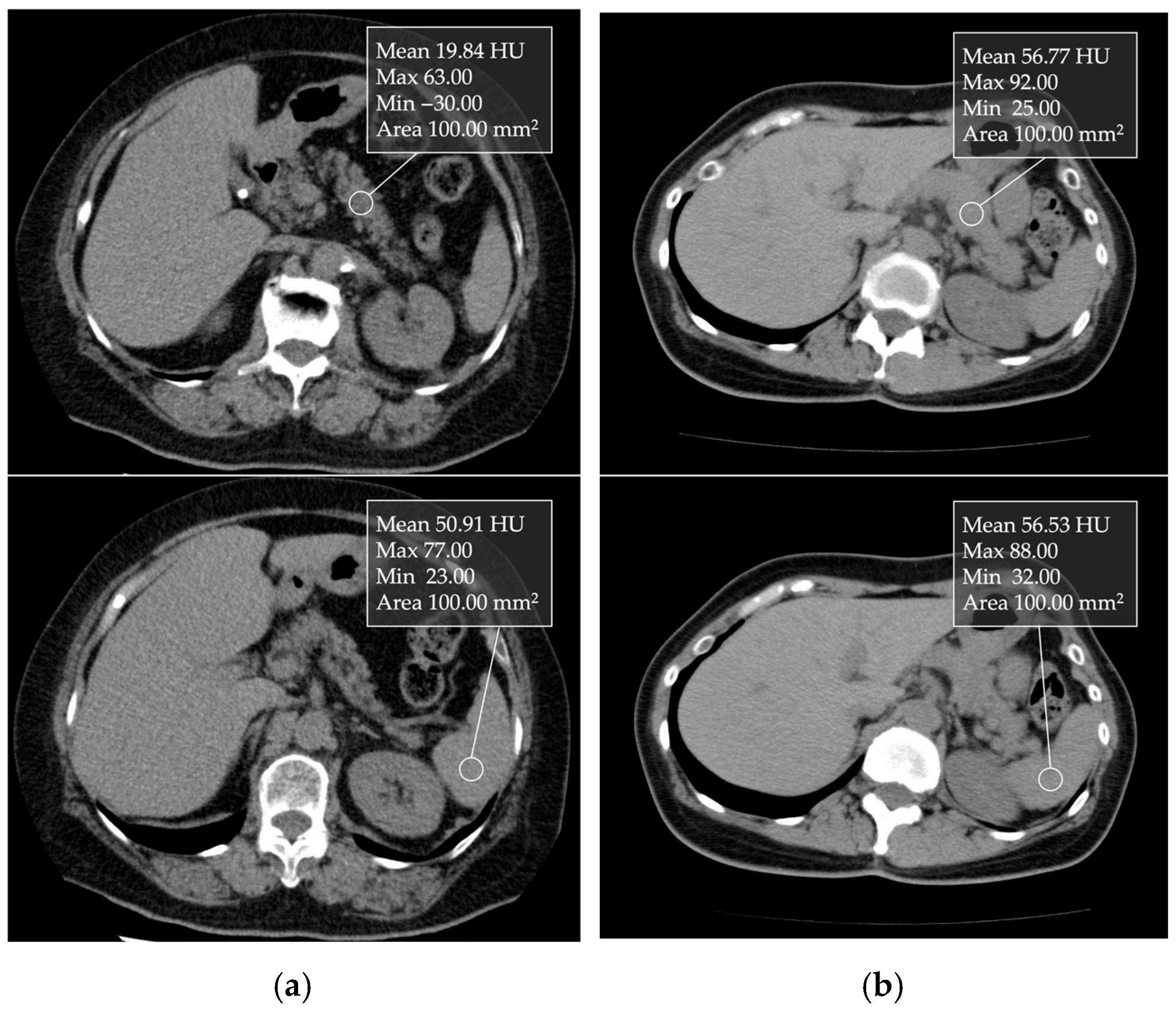
5.3. Computed Tomography (CT)
5.4. Magnetic Resonance Imaging (MRI)
5.5. Biomarkers
6. Clinical Characteristics
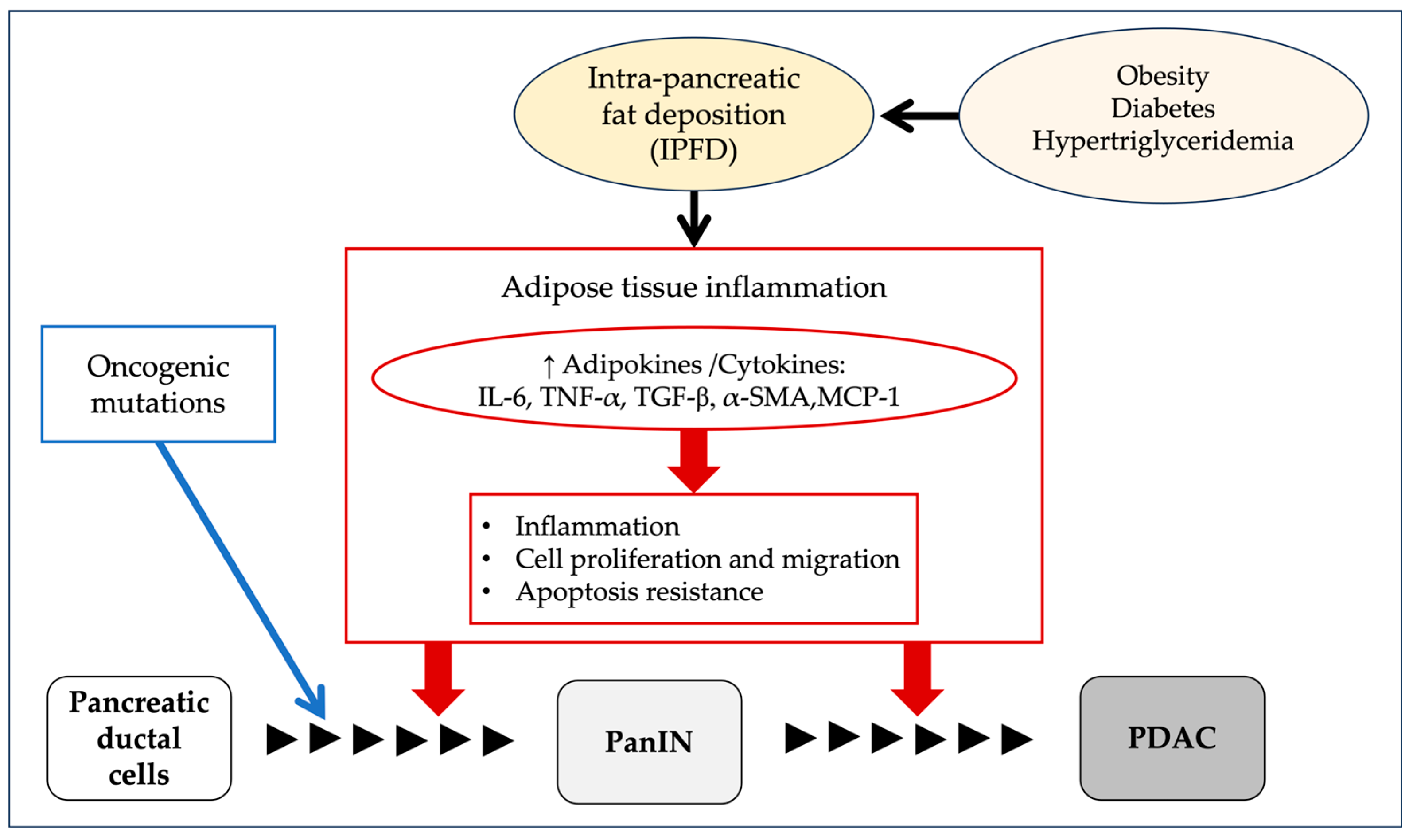
7. Carcinogenesis
8. Interventions
8.1. GLP-1 Receptor Agonists
8.2. DPP-4 Inhibitor
8.3. SGLT-2 Inhibitors
8.4. Other Medications and Treatments
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- van Geenen, E.J.; Smits, M.M.; Schreuder, T.C.; van der Peet, D.L.; Bloemena, E.; Mulder, C.J. Nonalcoholic fatty liver disease is related to nonalcoholic fatty pancreas disease. Pancreas 2010, 39, 1185–1190. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.G.; Yoon, H.D.; Wu, L.M.; Lu, J.; Plank, L.D.; Petrov, M.S. Ectopic fat accumulation in the pancreas and its clinical relevance: A systematic review, meta-analysis, and meta-regression. Metabolism 2017, 69, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.C.; Wang, C.Y. Association between non-alcoholic fatty pancreatic disease (NAFPD) and the metabolic syndrome: Case-control retrospective study. Cardiovasc. Diabetol. 2013, 12, 77. [Google Scholar] [CrossRef]
- Mizuno, S.; Isayama, H.; Nakai, Y.; Yoshikawa, T.; Ishigaki, K.; Matsubara, S.; Yamamoto, N.; Ijichi, H.; Tateishi, K.; Tada, M.; et al. Prevalence of Pancreatic Cystic Lesions Is Associated With Diabetes Mellitus and Obesity: An Analysis of 5296 Individuals Who Underwent a Preventive Medical Examination. Pancreas 2017, 46, 801–805. [Google Scholar] [CrossRef]
- Hori, M.; Takahashi, M.; Hiraoka, N.; Yamaji, T.; Mutoh, M.; Ishigamori, R.; Furuta, K.; Okusaka, T.; Shimada, K.; Kosuge, T.; et al. Association of pancreatic Fatty infiltration with pancreatic ductal adenocarcinoma. Clin. Transl. Gastroenterol. 2014, 5, e53. [Google Scholar] [CrossRef]
- Takahashi, M.; Hori, M.; Ishigamori, R.; Mutoh, M.; Imai, T.; Nakagama, H. Fatty pancreas: A possible risk factor for pancreatic cancer in animals and humans. Cancer Sci. 2018, 109, 3013–3023. [Google Scholar] [CrossRef]
- Sreedhar, U.L.; DeSouza, S.V.; Park, B.; Petrov, M.S. A Systematic Review of Intra-pancreatic Fat Deposition and Pancreatic Carcinogenesis. J. Gastrointest. Surg. 2020, 24, 2560–2569. [Google Scholar] [CrossRef] [PubMed]
- Frendi, S.; Martineau, C.; Cazier, H.; Nicolle, R.; Chassac, A.; Albuquerque, M.; Raffenne, J.; Le Faouder, J.; Paradis, V.; Cros, J.; et al. Role of the fatty pancreatic infiltration in pancreatic oncogenesis. Sci. Rep. 2024, 14, 6582. [Google Scholar] [CrossRef]
- Mizuno, S.; Nakai, Y.; Ishigaki, K.; Saito, K.; Oyama, H.; Hamada, T.; Suzuki, Y.; Inokuma, A.; Kanai, S.; Noguchi, K.; et al. Screening Strategy of Pancreatic Cancer in Patients with Diabetes Mellitus. Diagnostics 2020, 10, 572. [Google Scholar] [CrossRef]
- Oyama, H.; Tada, M.; Takagi, K.; Tateishi, K.; Hamada, T.; Nakai, Y.; Hakuta, R.; Ijichi, H.; Ishigaki, K.; Kanai, S.; et al. Long-term Risk of Malignancy in Branch-Duct Intraductal Papillary Mucinous Neoplasms. Gastroenterology 2020, 158, 226–237.e225. [Google Scholar] [CrossRef]
- Blackford, A.L.; Canto, M.I.; Dbouk, M.; Hruban, R.H.; Katona, B.W.; Chak, A.; Brand, R.E.; Syngal, S.; Farrell, J.; Kastrinos, F.; et al. Pancreatic Cancer Surveillance and Survival of High-Risk Individuals. JAMA Oncol. 2024, 10, 1087–1096. [Google Scholar] [CrossRef]
- Rebours, V.; Gaujoux, S.; d’Assignies, G.; Sauvanet, A.; Ruszniewski, P.; Lévy, P.; Paradis, V.; Bedossa, P.; Couvelard, A. Obesity and Fatty Pancreatic Infiltration Are Risk Factors for Pancreatic Precancerous Lesions (PanIN). Clin. Cancer Res. 2015, 21, 3522–3528. [Google Scholar] [CrossRef] [PubMed]
- Petrov, M.S.; Taylor, R. Intra-pancreatic fat deposition: Bringing hidden fat to the fore. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 153–168. [Google Scholar] [CrossRef]
- Otsuka, N.; Shimizu, K.; Taniai, M.; Tokushige, K. Risk factors for fatty pancreas and effects of fatty infiltration on pancreatic cancer. Front. Physiol. 2023, 14, 1243983. [Google Scholar] [CrossRef] [PubMed]
- Truong, E.; Pandol, S.; Jeon, C. Uniting epidemiology and experimental models: Pancreatic steatosis and pancreatic cancer. EBioMedicine 2022, 79, 103996. [Google Scholar] [CrossRef]
- Sasaki, M.; Nakanuma, Y.; Ando, H. Lipomatous pseudohypertrophy of the pancreas in a patient with cirrhosis due to chronic hepatitis B. Pathol. Int. 1998, 48, 566–568. [Google Scholar] [CrossRef] [PubMed]
- Uygun, A.; Kadayifci, A.; Demirci, H.; Saglam, M.; Sakin, Y.S.; Ozturk, K.; Polat, Z.; Karslioglu, Y.; Bolu, E. The effect of fatty pancreas on serum glucose parameters in patients with nonalcoholic steatohepatitis. Eur. J. Intern. Med. 2015, 26, 37–41. [Google Scholar] [CrossRef]
- Tariq, H.; Nayudu, S.; Akella, S.; Glandt, M.; Chilimuri, S. Non-Alcoholic Fatty Pancreatic Disease: A Review of Literature. Gastroenterol. Res. 2016, 9, 87–91. [Google Scholar] [CrossRef]
- Mahyoub, M.A.; Elhoumed, M.; Maqul, A.H.; Almezgagi, M.; Abbas, M.; Jiao, Y.; Wang, J.; Alnaggar, M.; Zhao, P.; He, S. Fatty infiltration of the pancreas: A systematic concept analysis. Front. Med. 2023, 10, 1227188. [Google Scholar] [CrossRef]
- Watanabe, S.; Abe, K.; Anbo, Y.; Katoh, H. Changes in the mouse exocrine pancreas after pancreatic duct ligation: A qualitative and quantitative histological study. Arch. Histol. Cytol. 1995, 58, 365–374. [Google Scholar] [CrossRef]
- Klöppel, G.; Maillet, B. Chronic pancreatitis: Evolution of the disease. Hepatogastroenterology 1991, 38, 408–412. [Google Scholar] [PubMed]
- Klöppel, G.; Maillet, B. The morphological basis for the evolution of acute pancreatitis into chronic pancreatitis. Virchows Arch. A Pathol. Anat. Histopathol. 1992, 420, 1–4. [Google Scholar] [CrossRef]
- Sepe, P.S.; Ohri, A.; Sanaka, S.; Berzin, T.M.; Sekhon, S.; Bennett, G.; Mehta, G.; Chuttani, R.; Kane, R.; Pleskow, D.; et al. A prospective evaluation of fatty pancreas by using EUS. Gastrointest. Endosc. 2011, 73, 987–993. [Google Scholar] [CrossRef]
- Ou, H.Y.; Wang, C.Y.; Yang, Y.C.; Chen, M.F.; Chang, C.J. The association between nonalcoholic fatty pancreas disease and diabetes. PLoS ONE 2013, 8, e62561. [Google Scholar] [CrossRef] [PubMed]
- Virtue, S.; Vidal-Puig, A. It’s not how fat you are, it’s what you do with it that counts. PLoS Biol. 2008, 6, e237. [Google Scholar] [CrossRef] [PubMed]
- Neeland, I.J.; Ross, R.; Després, J.P.; Matsuzawa, Y.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: A position statement. Lancet Diabetes Endocrinol. 2019, 7, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Pinte, L.; Balaban, D.V.; Băicuş, C.; Jinga, M. Non-alcoholic fatty pancreas disease—Practices for clinicians. Rom. J. Intern. Med. 2019, 57, 209–219. [Google Scholar] [CrossRef]
- Guglielmi, V.; Sbraccia, P. Type 2 diabetes: Does pancreatic fat really matter? Diabetes Metab. Res. Rev. 2018, 34, e2955. [Google Scholar] [CrossRef]
- Lupi, R.; Del Guerra, S.; Fierabracci, V.; Marselli, L.; Novelli, M.; Patanè, G.; Boggi, U.; Mosca, F.; Piro, S.; Del Prato, S.; et al. Lipotoxicity in human pancreatic islets and the protective effect of metformin. Diabetes 2002, 51 (Suppl. 1), S134–S137. [Google Scholar] [CrossRef]
- Petrov, M.S. Harnessing Analytic Morphomics for Early Detection of Pancreatic Cancer. Pancreas 2018, 47, 1051–1054. [Google Scholar] [CrossRef]
- Smits, M.M.; van Geenen, E.J. The clinical significance of pancreatic steatosis. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Petrov, M.S. The Pharmacological Landscape for Fatty Change of the Pancreas. Drugs 2024, 84, 375–384. [Google Scholar] [CrossRef]
- Singh, R.G.; Yoon, H.D.; Poppitt, S.D.; Plank, L.D.; Petrov, M.S. Ectopic fat accumulation in the pancreas and its biomarkers: A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2017, 33, e2918. [Google Scholar] [CrossRef]
- Wong, V.W.; Wong, G.L.; Yeung, D.K.; Abrigo, J.M.; Kong, A.P.; Chan, R.S.; Chim, A.M.; Shen, J.; Ho, C.S.; Woo, J.; et al. Fatty pancreas, insulin resistance, and β-cell function: A population study using fat-water magnetic resonance imaging. Am. J. Gastroenterol. 2014, 109, 589–597. [Google Scholar] [CrossRef]
- Wang, C.Y.; Ou, H.Y.; Chen, M.F.; Chang, T.C.; Chang, C.J. Enigmatic ectopic fat: Prevalence of nonalcoholic fatty pancreas disease and its associated factors in a Chinese population. J. Am. Heart Assoc. 2014, 3, e000297. [Google Scholar] [CrossRef]
- Lesmana, C.R.; Pakasi, L.S.; Inggriani, S.; Aidawati, M.L.; Lesmana, L.A. Prevalence of Non-Alcoholic Fatty Pancreas Disease (NAFPD) and its risk factors among adult medical check-up patients in a private hospital: A large cross sectional study. BMC Gastroenterol. 2015, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, M.L.; Zhang, D.D.; Lin, H.Y.; Dai, X.H.; Sun, X.L.; Li, J.T.; Song, L.Y.; Peng, H.; Wen, M.M. The correlation between pancreatic steatosis and metabolic syndrome in a Chinese population. Pancreatology 2016, 16, 578–583. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.; Cho, J.Y.; Lim, S.; Cha, K.; Lee, K.H.; Kim, Y.H.; Kim, J.H.; Yoon, Y.S.; Han, H.S.; et al. Quantitative assessment of pancreatic fat by using unenhanced CT: Pathologic correlation and clinical implications. Radiology 2014, 271, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Mori, N.; Sakurai, N.; Iizawa, H. Predicting postoperative pancreatic fistula after distal pancreatectomy by measuring the CT value ratio of future pancreas remnant to spleen (P/S ratio) in preoperative unenhanced CT images. Jpn. J. Gastroenterol. Surg. 2019, 52, 485–493. [Google Scholar] [CrossRef]
- Lingvay, I.; Esser, V.; Legendre, J.L.; Price, A.L.; Wertz, K.M.; Adams-Huet, B.; Zhang, S.; Unger, R.H.; Szczepaniak, L.S. Noninvasive quantification of pancreatic fat in humans. J. Clin. Endocrinol. Metab. 2009, 94, 4070–4076. [Google Scholar] [CrossRef]
- Hu, H.H.; Kim, H.W.; Nayak, K.S.; Goran, M.I. Comparison of fat-water MRI and single-voxel MRS in the assessment of hepatic and pancreatic fat fractions in humans. Obesity 2010, 18, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, R.S.; Begovatz, P.; Kahl, S.; Nowotny, B.; Strassburger, K.; Giani, G.; Bunke, J.; Roden, M.; Hwang, J.H. Initial clinical application of modified Dixon with flexible echo times: Hepatic and pancreatic fat assessments in comparison with (1)H MRS. MAGMA 2014, 27, 397–405. [Google Scholar] [CrossRef]
- d’Assignies, G.; Ruel, M.; Khiat, A.; Lepanto, L.; Chagnon, M.; Kauffmann, C.; Tang, A.; Gaboury, L.; Boulanger, Y. Noninvasive quantitation of human liver steatosis using magnetic resonance and bioassay methods. Eur. Radiol. 2009, 19, 2033–2040. [Google Scholar] [CrossRef]
- Dong, Z.; Luo, Y.; Zhang, Z.; Cai, H.; Li, Y.; Chan, T.; Wu, L.; Li, Z.P.; Feng, S.T. MR quantification of total liver fat in patients with impaired glucose tolerance and healthy subjects. PLoS ONE 2014, 9, e111283. [Google Scholar] [CrossRef]
- Singh, R.G.; Cervantes, A.; Kim, J.U.; Nguyen, N.N.; DeSouza, S.V.; Dokpuang, D.; Lu, J.; Petrov, M.S. Intrapancreatic fat deposition and visceral fat volume are associated with the presence of diabetes after acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G806–G815. [Google Scholar] [CrossRef]
- Tirkes, T.; Jeon, C.Y.; Li, L.; Joon, A.Y.; Seltman, T.A.; Sankar, M.; Persohn, S.A.; Territo, P.R. Association of Pancreatic Steatosis With Chronic Pancreatitis, Obesity, and Type 2 Diabetes Mellitus. Pancreas 2019, 48, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.E.; Fraser, K.; Kruger, M.C.; Sequeira, I.R.; Yip, W.; Lu, L.W.; Plank, L.D.; Murphy, R.; Cooper, G.J.S.; Martin, J.C.; et al. Untargeted metabolomics reveals plasma metabolites predictive of ectopic fat in pancreas and liver as assessed by magnetic resonance imaging: The TOFI_Asia study. Int. J. Obes. 2021, 45, 1844–1854. [Google Scholar] [CrossRef] [PubMed]
- Tushuizen, M.E.; Bunck, M.C.; Pouwels, P.J.; Bontemps, S.; van Waesberghe, J.H.; Schindhelm, R.K.; Mari, A.; Heine, R.J.; Diamant, M. Pancreatic fat content and beta-cell function in men with and without type 2 diabetes. Diabetes Care 2007, 30, 2916–2921. [Google Scholar] [CrossRef]
- Heni, M.; Machann, J.; Staiger, H.; Schwenzer, N.F.; Peter, A.; Schick, F.; Claussen, C.D.; Stefan, N.; Häring, H.U.; Fritsche, A. Pancreatic fat is negatively associated with insulin secretion in individuals with impaired fasting glucose and/or impaired glucose tolerance: A nuclear magnetic resonance study. Diabetes Metab. Res. Rev. 2010, 26, 200–205. [Google Scholar] [CrossRef]
- Rugivarodom, M.; Geeratragool, T.; Pausawasdi, N.; Charatcharoenwitthaya, P. Fatty Pancreas: Linking Pancreas Pathophysiology to Nonalcoholic Fatty Liver Disease. J. Clin. Transl. Hepatol. 2022, 10, 1229–1239. [Google Scholar] [CrossRef]
- Mak, A.L.; Wassenaar, N.; van Dijk, A.M.; Troelstra, M.; Houttu, V.; van Son, K.; Driessen, S.; Zwirs, D.; van den Berg-Faay, S.; Shumbayawonda, E.; et al. Intrapancreatic fat deposition is unrelated to liver steatosis in metabolic dysfunction-associated steatotic liver disease. JHEP Rep. 2024, 6, 100998. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, M.; Khashab, M.; Zyromski, N.; Pungpapong, S.; Wallace, M.B.; Scolapio, J.; Woodward, T.; Noh, K.; Raimondo, M. Risk factors for hyperechogenic pancreas on endoscopic ultrasound: A case-control study. Pancreas 2009, 38, 672–675. [Google Scholar] [CrossRef]
- Patel, N.S.; Peterson, M.R.; Lin, G.Y.; Feldstein, A.; Schnabl, B.; Bettencourt, R.; Seki, E.; Sirlin, C.B.; Loomba, R. Insulin Resistance Increases MRI-Estimated Pancreatic Fat in Nonalcoholic Fatty Liver Disease and Normal Controls. Gastroenterol. Res. Pract. 2013, 2013, 498296. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Iwasaki, A.; Kurita, Y.; Arimoto, J.; Yamamoto, T.; Hasegawa, S.; Sato, T.; Imajo, K.; Hosono, K.; Kobayashi, N.; et al. Three-dimensional analysis of pancreatic fat by fat-water magnetic resonance imaging provides detailed characterization of pancreatic steatosis with improved reproducibility. PLoS ONE 2019, 14, e0224921. [Google Scholar] [CrossRef] [PubMed]
- Navina, S.; Acharya, C.; DeLany, J.P.; Orlichenko, L.S.; Baty, C.J.; Shiva, S.S.; Durgampudi, C.; Karlsson, J.M.; Lee, K.; Bae, K.T.; et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci. Transl. Med. 2011, 3, 107ra110. [Google Scholar] [CrossRef]
- Park, C.H.; Chung, M.J.; Park, D.H.; Min, S.; Park, S.W. Impact of pancreatic fat on the risk of post-endoscopic retrograde cholangiopancreatography pancreatitis. Surg. Endosc. 2022, 36, 5734–5742. [Google Scholar] [CrossRef]
- Prouvot, C.; Boumaiza, M.; Maoui, K.; Peaucelle, A.S.; Mohamed, S.; Boutallaka, H.; Boutet, C.; Roblin, X.; Phelip, J.M.; Grange, R.; et al. Pancreatic steatosis is a strong risk factor for post-ERCP pancreatitis: An emerging concept. Dig. Liver Dis. 2025, 57, 542–548. [Google Scholar] [CrossRef]
- Hingorani, S.R.; Petricoin, E.F.; Maitra, A.; Rajapakse, V.; King, C.; Jacobetz, M.A.; Ross, S.; Conrads, T.P.; Veenstra, T.D.; Hitt, B.A.; et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 2003, 4, 437–450. [Google Scholar] [CrossRef]
- Guerra, C.; Schuhmacher, A.J.; Cañamero, M.; Grippo, P.J.; Verdaguer, L.; Pérez-Gallego, L.; Dubus, P.; Sandgren, E.P.; Barbacid, M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell 2007, 11, 291–302. [Google Scholar] [CrossRef]
- Khasawneh, J.; Schulz, M.D.; Walch, A.; Rozman, J.; Hrabe de Angelis, M.; Klingenspor, M.; Buck, A.; Schwaiger, M.; Saur, D.; Schmid, R.M.; et al. Inflammation and mitochondrial fatty acid beta-oxidation link obesity to early tumor promotion. Proc. Natl. Acad. Sci. USA 2009, 106, 3354–3359. [Google Scholar] [CrossRef]
- Carter, R.; Mouralidarane, A.; Soeda, J.; Ray, S.; Pombo, J.; Saraswati, R.; Novelli, M.; Fusai, G.; Rappa, F.; Saracino, C.; et al. Non-alcoholic fatty pancreas disease pathogenesis: A role for developmental programming and altered circadian rhythms. PLoS ONE 2014, 9, e89505. [Google Scholar] [CrossRef] [PubMed]
- Eibl, G.; Cruz-Monserrate, Z.; Korc, M.; Petrov, M.S.; Goodarzi, M.O.; Fisher, W.E.; Habtezion, A.; Lugea, A.; Pandol, S.J.; Hart, P.A.; et al. Diabetes Mellitus and Obesity as Risk Factors for Pancreatic Cancer. J. Acad. Nutr. Diet. 2018, 118, 555–567. [Google Scholar] [CrossRef]
- Desai, V.; Patel, K.; Sheth, R.; Barlass, U.; Chan, Y.M.; Sclamberg, J.; Bishehsari, F. Pancreatic Fat Infiltration Is Associated with a Higher Risk of Pancreatic Ductal Adenocarcinoma. Visc. Med. 2020, 36, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, K.; Minami, K.; Seino, T.; Hirata, K.; Iwasaki, E.; Inoue, N.; Iwao, Y.; Kanai, T. Pancreatic fat content may increase the risk of imaging progression in low-risk branch duct intraductal papillary mucinous neoplasm. J. Dig. Dis. 2019, 20, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Fernández-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Dutour, A.; Abdesselam, I.; Ancel, P.; Kober, F.; Mrad, G.; Darmon, P.; Ronsin, O.; Pradel, V.; Lesavre, N.; Martin, J.C.; et al. Exenatide decreases liver fat content and epicardial adipose tissue in patients with obesity and type 2 diabetes: A prospective randomized clinical trial using magnetic resonance imaging and spectroscopy. Diabetes Obes. Metab. 2016, 18, 882–891. [Google Scholar] [CrossRef]
- Vanderheiden, A.; Harrison, L.B.; Warshauer, J.T.; Adams-Huet, B.; Li, X.; Yuan, Q.; Hulsey, K.; Dimitrov, I.; Yokoo, T.; Jaster, A.W.; et al. Mechanisms of Action of Liraglutide in Patients With Type 2 Diabetes Treated With High-Dose Insulin. J. Clin. Endocrinol. Metab. 2016, 101, 1798–1806. [Google Scholar] [CrossRef]
- Smits, M.M.; Tonneijck, L.; Muskiet, M.H.; Kramer, M.H.; Pieters-van den Bos, I.C.; Vendrik, K.E.; Hoekstra, T.; Bruno, M.J.; Diamant, M.; van Raalte, D.H.; et al. Pancreatic Effects of Liraglutide or Sitagliptin in Overweight Patients With Type 2 Diabetes: A 12-Week Randomized, Placebo-Controlled Trial. Diabetes Care 2017, 40, 301–308. [Google Scholar] [CrossRef]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Choudhary, N.S.; Singh, M.K.; Wasir, J.S.; Kaur, P.; Gill, H.K.; Bano, T.; Farooqui, K.J.; et al. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: Randomised controlled trial (D-LIFT trial). Diabetologia 2020, 63, 2434–2445. [Google Scholar] [CrossRef]
- Kuriyama, T.; Ishibashi, C.; Kozawa, J.; Baden, M.Y.; Horii, T.; Niki, A.; Ozawa, H.; Hosokawa, Y.; Fujita, Y.; Sadahiro, K.; et al. Effects of liraglutide on intrapancreatic fat deposition in patients with type 2 diabetes. Clin. Nutr. ESPEN 2024, 59, 208–213. [Google Scholar] [CrossRef]
- Horii, T.; Kozawa, J.; Fujita, S.; Hosokawa, Y.; Kimura, T.; Fujita, Y.; Tokunaga, A.; Fukui, K.; Shimomura, I. Amelioration of pancreatic fat accumulation in Japanese type 2 diabetes patients treated with sodium-glucose cotransporter 2 inhibitors: A retrospective study. Obes. Sci. Pract. 2021, 7, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Gaborit, B.; Ancel, P.; Abdullah, A.E.; Maurice, F.; Abdesselam, I.; Calen, A.; Soghomonian, A.; Houssays, M.; Varlet, I.; Eisinger, M.; et al. Effect of empagliflozin on ectopic fat stores and myocardial energetics in type 2 diabetes: The EMPACEF study. Cardiovasc. Diabetol. 2021, 20, 57. [Google Scholar] [CrossRef]
- Hummel, J.; Machann, J.; Dannecker, C.; Kullmann, S.; Birkenfeld, A.L.; Häring, H.U.; Peter, A.; Fritsche, A.; Wagner, R.; Heni, M. Eight weeks of empagliflozin does not affect pancreatic fat content and insulin secretion in people with prediabetes. Diabetes Obes. Metab. 2022, 24, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Dutta, K.; Bhatt, S.P.; Gupta, R.; Tyagi, K.; Ansari, I.A.; Venugopal, V.K.; Mahajan, H.; Pandey, R.M.; Pandey, S.; et al. Dapagliflozin Improves Body Fat Patterning, and Hepatic and Pancreatic Fat in Patients With Type 2 Diabetes in North India. J. Clin. Endocrinol. Metab. 2022, 107, e2267–e2275. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, H.; Wang, W.; Zhang, X.; Liu, J.; Wang, Q.; Wang, Y.; Zhang, C.; Guo, X.; Qiao, Q.; et al. Effect of dapagliflozin on liver and pancreatic fat in patients with type 2 diabetes and non-alcoholic fatty liver disease. J. Diabetes Complicat. 2023, 37, 108610. [Google Scholar] [CrossRef]
- Souza-Mello, V.; Gregório, B.M.; Cardoso-de-Lemos, F.S.; de Carvalho, L.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Comparative effects of telmisartan, sitagliptin and metformin alone or in combination on obesity, insulin resistance, and liver and pancreas remodelling in C57BL/6 mice fed on a very high-fat diet. Clin. Sci. 2010, 119, 239–250. [Google Scholar] [CrossRef]
- Leung, P.S. The physiology of a local renin-angiotensin system in the pancreas. J. Physiol. 2007, 580, 31–37. [Google Scholar] [CrossRef]
- Tene, L.; Shelef, I.; Schwarzfuchs, D.; Gepner, Y.; Yaskolka Meir, A.; Tsaban, G.; Zelicha, H.; Bilitzky, A.; Komy, O.; Cohen, N.; et al. The effect of long-term weight-loss intervention strategies on the dynamics of pancreatic-fat and morphology: An MRI RCT study. Clin. Nutr. ESPEN 2018, 24, 82–89. [Google Scholar] [CrossRef]
- Jiang, Y.; Spurny, M.; Schübel, R.; Nonnenmacher, T.; Schlett, C.L.; von Stackelberg, O.; Ulrich, C.M.; Kaaks, R.; Kauczor, H.U.; Kühn, T.; et al. Changes in Pancreatic Fat Content Following Diet-Induced Weight Loss. Nutrients 2019, 11, 912. [Google Scholar] [CrossRef]
- Skytte, M.J.; Samkani, A.; Petersen, A.D.; Thomsen, M.N.; Astrup, A.; Chabanova, E.; Frystyk, J.; Holst, J.J.; Thomsen, H.S.; Madsbad, S.; et al. A carbohydrate-reduced high-protein diet improves HbA. Diabetologia 2019, 62, 2066–2078. [Google Scholar] [CrossRef]
- Thomsen, M.N.; Skytte, M.J.; Samkani, A.; Carl, M.H.; Weber, P.; Astrup, A.; Chabanova, E.; Fenger, M.; Frystyk, J.; Hartmann, B.; et al. Dietary carbohydrate restriction augments weight loss-induced improvements in glycaemic control and liver fat in individuals with type 2 diabetes: A randomised controlled trial. Diabetologia 2022, 65, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Della Pepa, G.; Brancato, V.; Costabile, G.; Salamone, D.; Corrado, A.; Vitale, M.; Cavaliere, C.; Mancini, M.; Salvatore, M.; Luongo, D.; et al. An Isoenergetic Multifactorial Diet Reduces Pancreatic Fat and Increases Postprandial Insulin Response in Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2022, 45, 1935–1942. [Google Scholar] [CrossRef] [PubMed]
- Heiskanen, M.A.; Motiani, K.K.; Mari, A.; Saunavaara, V.; Eskelinen, J.J.; Virtanen, K.A.; Koivumäki, M.; Löyttyniemi, E.; Nuutila, P.; Kalliokoski, K.K.; et al. Exercise training decreases pancreatic fat content and improves beta cell function regardless of baseline glucose tolerance: A randomised controlled trial. Diabetologia 2018, 61, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Petrov, M.S. Fatty change of the pancreas: The Pandora’s box of pancreatology. Lancet Gastroenterol. Hepatol. 2023, 8, 671–682. [Google Scholar] [CrossRef]


| Study | Year | Modality | No. of Healthy Individuals | Fatty Pancreas n (%) |
|---|---|---|---|---|
| Wu et al. [3] | 2013 | Ultrasonography | 557 | 72 (12.9%) |
| Uygun et al. [17] | 2014 | Ultrasonography | 35 | 5 (14.3%) |
| Wang et al. [35] | 2014 | Ultrasonography | 8097 | 1297 (16.0%) |
| Wong et al. [34] | 2014 | MRI | 685 | 110 (16.1%) |
| Lesmana et al. [36] | 2015 | Ultrasonography | 901 | 315 (35.0%) |
| Zhou et al. [37] | 2016 | Ultrasonography | 1190 | 365 (30.7%) |
| Class of Medication | Study | Year | Total No. of Individuals | Intervention | Effect on IPFD | p Value |
|---|---|---|---|---|---|---|
| GLP-1 receptor agonists | Dutour et al. [66] | 2016 | 44 | Exenatide | Decreased fat content. | “Non- significant” |
| Vanderheiden et al. [67] | 2016 | 71 | Liraglutide | Decreased median fat content. Median (IQR) −1.3 (−3.87 to 0.6) | 0.056 | |
| Smits et al. [68] | 2017 | 55 | Liraglutide | Decreased fat content. −2.4% [95%CI −6.4 to 1.6] | 0.24 | |
| Kuchay et al. [69] | 2020 | 88 | Dulaglutide | Decreased fat content. −1.4% [95%CI −3.2 to 0.3] | 0.106 | |
| Kuriyama et al. [70] | 2024 | 42 | Liraglutide | Increased CT attenuation values (pancreas-spleen, HU). Mean ± SD pre 14.3 ± 12.6 post 12.6 ± 10.9 | 0.0547 | |
| DPP-4 inhibitor | Smits et al. [68] | 2017 | 55 | Sitagliptin | Decreased fat content. −4.2% [95%CI −8.1 to −0.3] | 0.04 |
| SGLT-2 inhibitors | Horii et al. [71] | 2021 | 22 | Various SGLT-2 inhibitors (Canagliflozin, Empagliflozin, Dapagliflozin, Ipragliflozin, Luseogliflozin) | Increased CT attenuation values (pancreas-spleen, HU). Median (IQR) pre −20.8 (−34.8 to 14.3) post −14.6 (−29.5 to 7.8) | 0.041 |
| Gaborit et al. [72] | 2021 | 56 | Empagliflozin | Described as ‘no significant difference’ without further details. | - | |
| Hummel et al. [73] | 2022 | 42 | Empagliflozin | Decreased mean fat content. Mean ± SD pre 7.1 ± 4.6% post 6.2 ± 4.2% | 0.2 | |
| Ghosh et al. [74] | 2022 | 30 | Dapagliflozin | Decreased mean fat content. Mean ± SD pre 7.52 ± 5.84% post 5.99 ± 3.98% | 0.0083 | |
| Shi et al. [75] | 2023 | 84 | Dapagliflozin | Decreased median fat content. Median (IQR) −1.16 (−1.93 to −0.37) | 0.033 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otsuka, N.; Shimamatsu, Y.; Hakuta, R.; Takayama, Y.; Nakai, Y. Fatty Pancreas: Its Potential as a Risk Factor for Pancreatic Cancer and Clinical Implications. Cancers 2025, 17, 1765. https://doi.org/10.3390/cancers17111765
Otsuka N, Shimamatsu Y, Hakuta R, Takayama Y, Nakai Y. Fatty Pancreas: Its Potential as a Risk Factor for Pancreatic Cancer and Clinical Implications. Cancers. 2025; 17(11):1765. https://doi.org/10.3390/cancers17111765
Chicago/Turabian StyleOtsuka, Nao, Yutaka Shimamatsu, Ryunosuke Hakuta, Yukiko Takayama, and Yousuke Nakai. 2025. "Fatty Pancreas: Its Potential as a Risk Factor for Pancreatic Cancer and Clinical Implications" Cancers 17, no. 11: 1765. https://doi.org/10.3390/cancers17111765
APA StyleOtsuka, N., Shimamatsu, Y., Hakuta, R., Takayama, Y., & Nakai, Y. (2025). Fatty Pancreas: Its Potential as a Risk Factor for Pancreatic Cancer and Clinical Implications. Cancers, 17(11), 1765. https://doi.org/10.3390/cancers17111765






