The Definition of the Best Margin Cutoff and Related Oncological Outcomes After Liver Resection for Hepatocellular Carcinoma: A Systematic Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
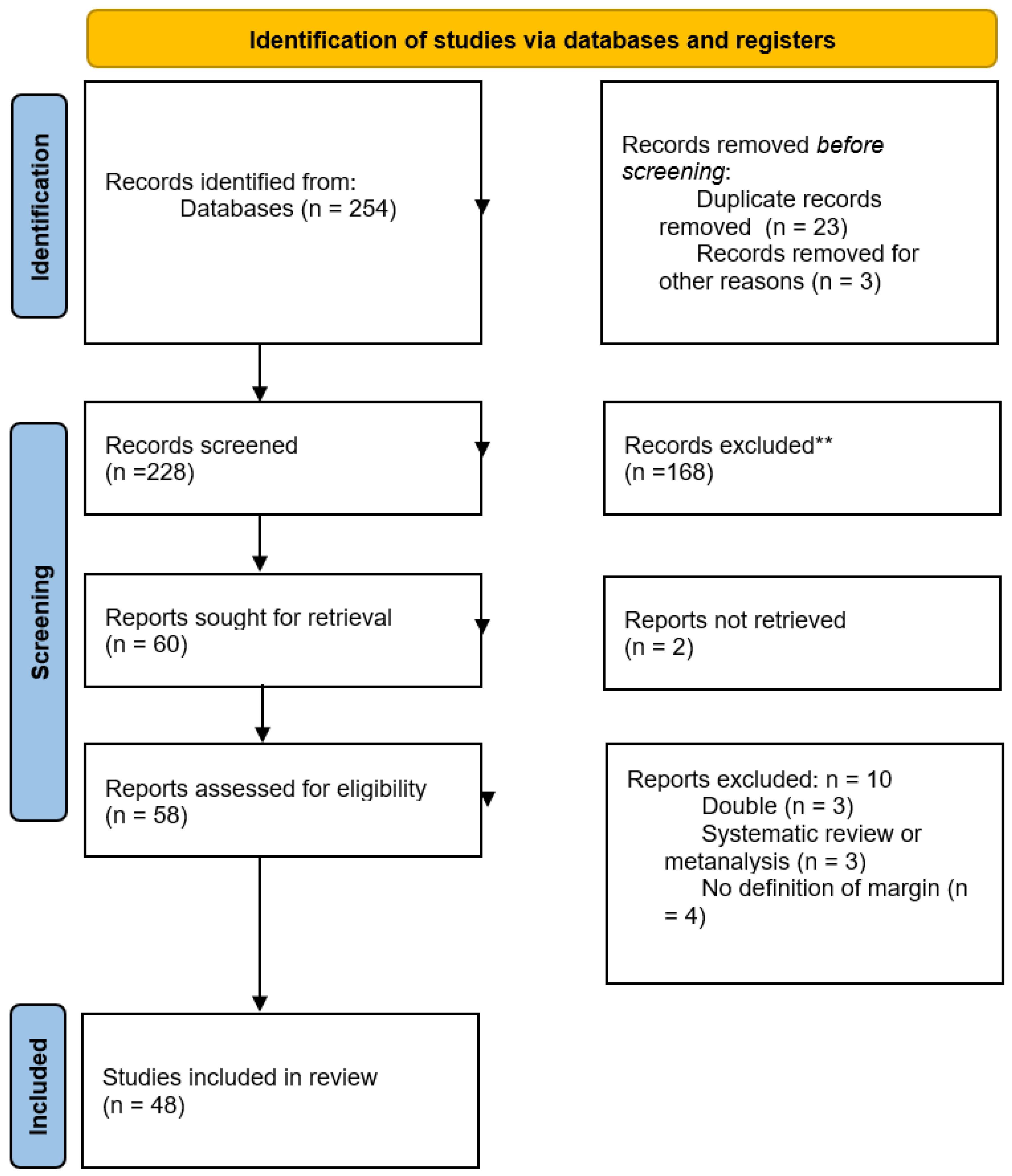
2.1. Literature Search
2.2. Data Extraction
2.3. Reported Analysis and Bias Assessment
3. Results
3.1. Definition of Surgical Margins
3.2. Impact of Surgical Margin on Long-Term Outcomes
3.2.1. Surgical Margin: 20 mm
3.2.2. Surgical Margin: 10 mm
3.2.3. Surgical Margin: 5 mm
3.2.4. Surgical Margin: 4 mm
3.2.5. Surgical Margin: 2 mm
3.2.6. Surgical Margin: 1 mm
3.3. Influence of Specific Positive Margin Cutoffs on Specific Patterns of Recurrence
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HCC | Hepatocellular carcinoma |
| DFS | Disease-free survival |
| OS | Overall survival |
| BCLC | Barcelona Clinic Liver Cancer |
| AR | Anatomical resection |
| NAR | Non-anatomical resection |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| AFP | Alpha-fetoprotein |
| NOS | Newcastle–Ottawa scale |
| ATS | Alpha-fetoprotein tumor burden score |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Y.; Zhang, S.; Fan, J.; Gao, Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 2022, 42, 2029–2041. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Serper, M.; Taddei, T.H.; Mehta, R.; D’Addeo, K.; Dai, F.; Aytaman, A.; Baytarian, M.; Fox, R.; Hunt, K.; Goldberg, D.S.; et al. Association of Provider Specialty and Multidisciplinary Care with Hepatocellular Carcinoma Treatment and Mortality. Gastroenterology 2017, 152, 1954–1964. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yopp, A.C.; Mansour, J.C.; Beg, M.S.; Arenas, J.; Trimmer, C.; Reddick, M.; Pedrosa, I.; Khatri, G.; Yakoo, T.; Meyer, J.J.; et al. Establishment of a Multidisciplinary Hepatocellular Carcinoma Clinic is Associated with Improved Clinical Outcome. Ann. Surg. Oncol. 2013, 21, 1287–1295. [Google Scholar] [CrossRef]
- Sotiropoulos, G.C.; Lang, H.; Frilling, A.; Molmenti, E.P.; Paul, A.; Nadalin, S.; Radtke, A.; I Brokalaki, E.; Saner, F.; Hilgard, P.; et al. Resectability of hepatocellular carcinoma: Evaluation of 333 consecutive cases at a single hepatobiliary specialty center and systematic review of the literature. Hepatogastroenterology 2006, 53, 322–329. [Google Scholar]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Imamura, H.; Matsuyama, Y.; Tanaka, E.; Ohkubo, T.; Hasegawa, K.; Miyagawa, S.; Sugawara, Y.; Minagawa, M.; Takayama, T.; Kawasaki, S.; et al. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J. Hepatol. 2003, 38, 200–207. [Google Scholar] [CrossRef]
- Simon, R.; Sasaki, K.; Margonis, G.A.; He, J.; Acevedo-Moreno, L.; Hazem, A.; Wolfgang, C.L.; McVey, J.; Takahashi, H.; Pitchaimuthu, M.; et al. Risk factors for very early recurrence of hepatocellular carcinoma: A retrospective review. HPB 2018, 20, S83–S84. [Google Scholar] [CrossRef][Green Version]
- Wang, W.-Q.; Li, J.; Liang, B.-Y.; Lv, X.; Zhu, R.-H.; Wang, J.-L.; Huang, Z.-Y.; Yang, S.-H.; Zhang, E.-L. Anatomical liver resection improves surgical outcomes for combined hepatocellular-cholangiocarcinoma: A propensity score matched study. Front. Oncol. 2022, 12, 980736. [Google Scholar] [CrossRef]
- Shimizu, A.; Kubota, K.; Notake, T.; Kitagawa, N.; Masuo, H.; Yoshizawa, T.; Sakai, H.; Hayashi, H.; Yamazaki, S.; Soejima, Y. Impact of anatomical liver resection for hepatocellular carcinoma in preventing early-phase local recurrence after surgery. J. Hepato-Biliary-Pancreat. Sci. 2024, 31, 513–527. [Google Scholar] [CrossRef]
- Famularo, S.; Di Sandro, S.; Giani, A.; Lauterio, A.; Sandini, M.; De Carlis, R.; Buscemi, V.; Romano, F.; Gianotti, L.; De Carlis, L. Long-term oncologic results of anatomic vs. parenchyma-sparing resection for hepatocellular carcinoma. A propensity score-matching analysis. Eur. J. Surg. Oncol. (EJSO) 2018, 44, 1580–1587. [Google Scholar] [CrossRef]
- Famularo, S.; Di Sandro, S.; Giani, A.; Lauterio, A.; Sandini, M.; De Carlis, R.; Buscemi, V.; Uggeri, F.; Romano, F.; Gianotti, L.; et al. Recurrence Patterns After Anatomic or Parenchyma-Sparing Liver Resection for Hepatocarcinoma in a Western Population of Cirrhotic Patients. Ann. Surg. Oncol. 2018, 25, 3974–3981. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting sys-tematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Pereson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 23 January 2025).
- Bai, S.; Yang, P.; Liu, J.; Xue, H.; Xia, Y.; Liu, F.; Yang, Z.; Zhang, L.; Wu, Y.; Shen, F.; et al. Surgical Margin Affects the Long-Term Prognosis of Patients with Hepatocellular Carcinoma Undergoing Radical Hepatectomy Followed by Adjuvant TACE. Oncologist 2023, 28, e633–e644. [Google Scholar] [CrossRef]
- Belli, G.; Fantini, C.; Belli, A.; Limongelli, P. Laparoscopic Liver Resection for Hepatocellular Carcinoma in Cirrhosis: Long-Term Outcomes. Dig. Surg. 2011, 28, 134–140. [Google Scholar] [CrossRef]
- Chang, W.-T.; Kao, W.-Y.; Chau, G.-Y.; Su, C.-W.; Lei, H.-J.; Wu, J.-C.; Hsia, C.-Y.; Lui, W.-Y.; King, K.-L.; Lee, S.-D. Hepatic resection can provide long-term survival of patients with non-early-stage hepatocellular carcinoma: Extending the Indication for resection? Surgery 2012, 152, 809–820. [Google Scholar] [CrossRef]
- Chen, M.; Tsai, H.; Jeng, L.; Lee, W.; Yeh, C.; Yu, M.; Hung, C. Prognostic Factors after Resection for Hepatocellular Carcinoma in Noncirrhotic Livers: Univariate and Multivariate Analysis. World J. Surg. 2003, 27, 443–447. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Zhang, X.-P.; Feng, J.-K.; Li, L.-Q.; Zhang, F.; Hu, Y.-R.; Zhong, C.-Q.; Shi, J.; Guo, W.-X.; Wu, M.-C.; et al. Actual long-term survival in hepatocellular carcinoma patients with microvascular invasion: A multicenter study from China. Hepatol. Int. 2021, 15, 642–650. [Google Scholar] [CrossRef]
- Cheng, C.-H.; Lai, Y.; Hung, H.-C.; Lee, J.-C.; Wang, Y.-C.; Wu, T.-H.; Lee, C.-F.; Wu, T.-J.; Chou, H.-S.; Chan, K.-M.; et al. Recurrence Patterns After Hepatectomy with Very Narrow Resection Margins for Hepatocellular Carcinoma. Front. Surg. 2022, 9, 926728. [Google Scholar] [CrossRef]
- Dong, S.; Wang, Z.; Wu, L.; Qu, Z. Effect of surgical margin in R0 hepatectomy on recurrence-free survival of patients with solitary hepatocellular carcinomas without macroscopic vascular invasion. Medicine 2016, 95, e5251. [Google Scholar] [CrossRef]
- Endo, Y.; Munir, M.M.; Woldesenbet, S.; Katayama, E.; Ratti, F.; Marques, H.P.; Cauchy, F.; Lam, V.; A Poultsides, G.; Kitago, M.; et al. Impact of Surgical Margin Width on Prognosis Following Resection of Hepatocellular Carcinoma Varies on the Basis of Preoperative Alpha-Feto Protein and Tumor Burden Score. Ann. Surg. Oncol. 2023, 30, 6581–6589. [Google Scholar] [CrossRef]
- Field, W.B.; Rostas, J.W.; Philps, P.; Scoggins, C.R.; McMasters, K.M.; Martin, R.C. Wide versus narrow margins after partial hepatectomy for hepatocellular carcinoma: Balancing recurrence risk and liver function. Am. J. Surg. 2017, 214, 273–277. [Google Scholar] [CrossRef]
- Han, J.; Li, Z.-L.; Xing, H.; Wu, H.; Zhu, P.; Lau, W.Y.; Zhou, Y.-H.; Gu, W.-M.; Wang, H.; Chen, T.-H.; et al. The impact of resection margin and microvascular invasion on long-term prognosis after curative resection of hepatocellular carcinoma: A multi-institutional study. HPB 2019, 21, 962–971. [Google Scholar] [CrossRef]
- Hirokawa, F.; Hayashi, M.; Miyamoto, Y.; Asakuma, M.; Shimizu, T.; Komeda, K.; Inoue, Y.; Uchiyama, K. Outcomes and predictors of microvascular invasion of solitary hepatocellular carcinoma. Hepatol. Res. 2013, 44, 846–853. [Google Scholar] [CrossRef]
- Hsiao, J.-H.; Tsai, C.-C.; Liang, T.-J.; Chiang, C.-L.; Liang, H.-L.; Chen, I.-S.; Chen, Y.-C.; Chang, P.-M.; Chou, N.-H.; Wang, B.-W. Adjuvant hepatic arterial infusion chemotherapy is beneficial for selective patients with Hepatocellular carcinoma undergoing surgical treatment. Int. J. Surg. 2017, 45, 35–41. [Google Scholar] [CrossRef]
- Huang, G.; Yang, Y.; Shen, F.; Pan, Z.-Y.; Fu, S.-Y.; Lau, W.Y.; Zhou, W.-P.; Wu, M.-C. Early Viral Suppression Predicts Good Postoperative Survivals in Patients with Hepatocellular Carcinoma with a High Baseline HBV-DNA Load. Ann. Surg. Oncol. 2012, 20, 1482–1490. [Google Scholar] [CrossRef]
- Huang, W.-J.; Jeng, Y.-M.; Lai, H.-S.; Sheu, F.-Y.B.; Lai, P.-L.; Yuan, R.-H. Tumor size is a major determinant of prognosis of resected stage I hepatocellular carcinoma. Langenbeck’s Arch. Surg. 2015, 400, 725–734. [Google Scholar] [CrossRef]
- Jeng, K.-S.; Jeng, W.-J.; Sheen, I.-S.; Lin, C.-C.; Lin, C.-K. Is less than 5 mm as the narrowest surgical margin width in central resections of hepatocellular carcinoma justified? Am. J. Surg. 2013, 206, 64–71. [Google Scholar] [CrossRef]
- Ke, Q.; Guo, Z.; He, J.; Lai, Z.; Xin, F.; Zeng, Y.; Wang, L.; Liu, J. Resection Margin Width Does Not Influence the Prognosis of Solitary Hepatocellular Carcinoma After Anatomic Resection: A Real-World Study from China. J. Hepatocell. Carcinoma 2023, 10, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Aramaki, O.; Midorikawa, Y.; Higaki, T.; Nakayama, H.; Moriguchi, M.; Takayama, T. Impact of marginal resection for hepatocellular carcinoma. Surg. Today 2020, 50, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Blanc, J.F.; Nobili, S.; Cunha, A.S.; le Bail, B.; Bioulac-Sage, P.; Balabaud, C.; Capdepont, M.; Saric, J. Prognostic Factors and Longterm Survival after Hepatic Resection for Hepatocellular Carcinoma Originating from Noncirrhotic Liver. J. Am. Coll. Surg. 2005, 201, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-S.; Sheu, J.-C.; Wang, M.; Hsu, H.-C. Long-term outcome after surgery for asymptomatic small hepatocellular carcinoma. Br. J. Surg. 1996, 83, 330–333. [Google Scholar] [CrossRef]
- Lee, S.G.; Hwang, S.; Jung, J.P.; Lee, Y.J.; Kim, K.H.; Ahn, C.S. Outcome of patients with huge hepatocellular carcinoma after primary resection and treatment of recurrent lesions. Br. J. Surg. 2007, 94, 320–326. [Google Scholar] [CrossRef]
- Lee, K.-T.; Wang, S.-N.; Su, R.-W.; Chen, H.-Y.; Shi, H.-Y.; Ker, C.-G.; Chiu, H.-C. Is wider surgical margin justified for better clinical outcomes in patients with resectable hepatocellular carcinoma? J. Formos. Med. Assoc. 2012, 111, 160–170. [Google Scholar] [CrossRef]
- Lee, W.; Han, H.-S.; Ahn, S.; Yoon, Y.-S.; Cho, J.Y.; Choi, Y. Correlation between Resection Margin and Disease Recurrence with a Restricted Cubic Spline Model in Patients with Resected Hepatocellular Carcinoma. Dig. Surg. 2018, 35, 520–531. [Google Scholar] [CrossRef]
- Lee, J.-C.; Cheng, C.-H.; Wang, Y.-C.; Wu, T.-H.; Lee, C.-F.; Wu, T.-J.; Chou, H.-S.; Chan, K.-M.; Lee, W.-C. Clinical relevance of alpha-fetoprotein in determining resection margin for hepatocellular carcinoma. Medicine 2019, 98, e14827. [Google Scholar] [CrossRef]
- Lim, C.; Goumard, C.; Casellas-Robert, M.; Lopez-Ben, S.; Lladó, L.; Busquets, J.; Salloum, C.; Albiol-Quer, M.T.; Castro-Gutiérrez, E.; Rosmorduc, O.; et al. Impact on Oncological Outcomes and Intent-to-Treat Survival of Resection Margin for Transplantable Hepatocellular Carcinoma in All-Comers and in Patients with Cirrhosis: A Multicenter Study. World J. Surg. 2020, 44, 1966–1974. [Google Scholar] [CrossRef]
- Lise, M.; Bacchetti, S.; Da Pian, P.; Nitti, D.; Pilati, P.L.; Pigato, P. Prognostic factors affecting long term outcome after liver resection for hepatocellular carcinoma. Cancer 1998, 82, 1028–1036. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.-X.; Cao, Y.; Zhang, G.; Chen, W.-B.; Jiang, C.-P. Preoperative inflammation-based markers predict early and late recurrence of hepatocellular carcinoma after curative hepatectomy. Hepatobiliary Pancreat. Dis. Int. 2016, 15, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shui, Y.; Yu, Q.; Guo, Y.; Zhang, L.; Zhou, X.; Yu, R.; Lou, J.; Wei, S.; Wei, Q. Narrow-Margin Hepatectomy Resulted in Higher Recurrence and Lower Overall Survival for R0 Resection Hepatocellular Carcinoma. Front. Oncol. 2021, 10, 610636. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, D.H.; Kim, S.H.; Kim, M.Y.; Baik, S.K.; Hong, I.S. The Clinical Implications of Liver Resection Margin Size in Patients with Hepatocellular Carcinoma in Terms of Positron Emission Tomography Positivity. World J. Surg. 2017, 42, 1514–1522. [Google Scholar] [CrossRef]
- Poon, R.T.-P.; Fan, S.-T.; Ng, I.O.-L.; Wong, J. Significance of Resection Margin in Hepatectomy for Hepatocellular Carcinoma. Ann. Surg. 2000, 231, 544–551. [Google Scholar] [CrossRef]
- Sasaki, Y.; Yamada, T.; Tanaka, H.; Ohigashi, H.; Eguchi, H.; Yano, M.; Ishikawa, O.; Imaoka, S. Risk of Recurrence in a Long-term Follow-up After Surgery in 417 Patients with Hepatitis B- or Hepatitis C-Related Hepatocellular Carcinoma. Ann. Surg. 2006, 244, 771–780. [Google Scholar] [CrossRef]
- Shapera, E.; Crespo, K.; Syblis, C.; Ross, S.; Rosemurgy, A.; Sucandy, I. Robotic liver resection for hepatocellular carcinoma: Analysis of surgical margins and clinical outcomes from a western tertiary hepatobiliary center. J. Robot. Surg. 2022, 17, 645–652. [Google Scholar] [CrossRef]
- Shi, F.; Zhou, Z.; Huang, X.; Liu, Q.; Lin, A. Is Anatomical Resection Necessary for Early Hepatocellular Carcinoma? A Single Institution Retrospective Experience. Futur. Oncol. 2019, 15, 2041–2051. [Google Scholar] [CrossRef]
- Shi, M.; Guo, R.-P.; Lin, X.-J.; Zhang, Y.-Q.; Chen, M.-S.; Zhang, C.-Q.; Lau, W.Y.; Li, J.-Q. Partial Hepatectomy with Wide Versus Narrow Resection Margin for Solitary Hepatocellular Carcinoma. Ann. Surg. 2007, 245, 36–43. [Google Scholar] [CrossRef]
- Shimada, K.; Sakamoto, Y.; Esaki, M.; Kosuge, T. Role of the width of the surgical margin in a hepatectomy for small hepatocellular carcinomas eligible for percutaneous local ablative therapy. Am. J. Surg. 2008, 195, 775–781. [Google Scholar] [CrossRef]
- Shin, S.; Kim, T.-S.; Lee, J.W.; Ahn, K.S.; Kim, Y.H.; Kang, K.J. Is the anatomical resection necessary for single hepatocellular carcinoma smaller than 3 cm?: Single-center experience of liver resection for a small HCC. Ann. Hepato-Biliary-Pancreat. Surg. 2018, 22, 326–334. [Google Scholar] [CrossRef]
- Su, C.-M.; Chou, C.-C.; Yang, T.-H.; Lin, Y.-J. Comparison of anatomic and non-anatomic resections for very early-stage hepatocellular carcinoma: The importance of surgical resection margin width in non-anatomic resection. Surg. Oncol. 2020, 36, 15–22. [Google Scholar] [CrossRef]
- Takano, S.; Oishi, H.; Kono, S.; Kawakami, S.; Nakamura, M.; Kubota, N.; Iwai, S. Retrospective analysis of type of hepatic resection for hepatocellular carcinoma. Br. J. Surg. 2000, 87, 65–70. [Google Scholar] [CrossRef]
- Torii, A.; Nonami, T.; Harada, A.; Yasui, M.; Nakao, A.; Takagi, H. Extent of hepatic resection as a prognostic factor for small, solitary hepatocellular carcinomas. J. Surg. Oncol. 1993, 54, 13–17. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Sahara, K.; Moris, D.; Hyer, J.M.; Paredes, A.Z.; Bagante, F.; Merath, K.; Farooq, A.S.; Ratti, F.; Marques, H.P.; et al. Effect of Surgical Margin Width on Patterns of Recurrence among Patients Undergoing R0 Hepatectomy for T1 Hepatocellular Carcinoma: An International Multi-Institutional Analysis. J. Gastrointest. Surg. 2019, 24, 1552–1560. [Google Scholar] [CrossRef]
- Wang, J.; Xu, L.; Liu, C.; Pang, H.; Chen, Y.; Ou, Q. Prognostic Factors and Outcome of 438 Chinese Patients with Hepatocellular Carcinoma Underwent Partial Hepatectomy in a Single Center. World J. Surg. 2010, 34, 2434–2441. [Google Scholar] [CrossRef]
- Wang, H.; Yu, H.; Qian, Y.-W.; Cao, Z.-Y.; Wu, M.-C.; Cong, W.-M. Impact of Surgical Margin on the Prognosis of Early Hepatocellular Carcinoma (≤5 cm): A Propensity Score Matching Analysis. Front. Med. 2020, 7, 139. [Google Scholar] [CrossRef]
- Li, C.; Wen, T.-F.; Yan, L.-N.; Li, B.; Wang, W.-T.; Yang, J.-Y.; Xu, M.-Q. Is Hepatectomy for Huge Hepatocellular Carcinoma (≥10 cm in Diameter) Safe and Effective? A Single-center Experience. Asian Pac. J. Cancer Prev. 2014, 15, 7069–7077. [Google Scholar] [CrossRef]
- Yang, P.; Si, A.; Yang, J.; Cheng, Z.; Wang, K.; Li, J.; Xia, Y.; Zhang, B.; Pawlik, T.M.; Lau, W.Y.; et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery 2019, 165, 721–730. [Google Scholar] [CrossRef]
- Zeng, J.; Lin, K.; Liu, H.; Huang, Y.; Guo, P.; Zeng, Y.; Zeng, J.; Liu, J. Prognosis Factors of Young Patients Undergoing Curative Resection for Hepatitis B Virus-Related Hepatocellular Carcinoma: A Multicenter Study. Cancer Manag. Res. 2020, 12, 6597–6606. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Wei, T.; Liu, X.-M.; Liu, C.; Lv, Y. Impact of Cigarette Smoking on Outcome of Hepatocellular Carcinoma after Surgery in Patients with Hepatitis B. PLoS ONE 2014, 9, e85077. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, F.; Wen, N.; Li, B.; Wei, Y. Patterns, timing, and predictors of recurrence after laparoscopic liver resection for hepatocellular carcinoma: Results from a high-volume HPB center. Surg. Endosc. 2021, 36, 1215–1223. [Google Scholar] [CrossRef]
- Zhou, K.-Q.; Sun, Y.-F.; Cheng, J.-W.; Du, M.; Ji, Y.; Wang, P.-X.; Hu, B.; Guo, W.; Gao, Y.; Yin, Y.; et al. Effect of surgical margin on recurrence based on preoperative circulating tumor cell status in hepatocellular carcinoma. EBioMedicine 2020, 62, 103107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Qi, L.; Mo, Q.; Liu, Y.; Zhou, X.; Zhou, Z.; Liang, X.; Feng, S.; Yu, H. Effect of surgical margin on postoperative prognosis in patients with solitary hepatocellular carcinoma: A propensity score matching analysis. J. Cancer 2021, 12, 4455–4462. [Google Scholar] [CrossRef]
- Nitta, H.; Allard, M.; Sebagh, M.; Golse, N.; Ciacio, O.; Pittau, G.; Vibert, E.; Cunha, A.S.; Cherqui, D.; Castaing, D.; et al. Ideal Surgical Margin to Prevent Early Recurrence After Hepatic Resection for Hepatocellular Carcinoma. World J. Surg. 2021, 45, 1159–1167. [Google Scholar] [CrossRef]
- Yoshida, Y.; Kanematsu, T.; Matsumata, T.; Takenaka, K.; Sugimachi, K. Surgical Margin and Recurrence After Resection of Hepatocellular Carcinoma in Patients with Cirrhosis. Further Evaluation of Limited Hepatic Resection. Ann. Surg. 1989, 209, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.C.S.; Ng, I.O.L.; You, K.T.; Choi, T.K.; Fan, S.T.; Mok, F.P.T.; Wong, J. Hepatectomy for large hepatocellular carcinoma: The optimal resection margin. World J. Surg. 1991, 15, 141–145. [Google Scholar] [CrossRef]
- Nonami, T.; Harada, A.; Kurokawa, T.; Nakao, A.; Takagi, H. Hepatic resection for hepatocellular carcinoma. Am. J. Surg. 1997, 173, 288–291. [Google Scholar] [CrossRef]
- Makuuchi, M.; Hasegawa, H.; Yamazaki, S. Ultrasonically Guided Subsegmentectomy. Surg. Gynecol. Obstet. 1985, 161, 346–350. [Google Scholar] [PubMed]
- Berardi, G.; Guglielmo, N.; Mariano, G.; Ettorre, G.M. Surgical Margins for Hepatocellular Carcinoma. In Hepatocellular Carcinoma; Ettorre, G.M., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 113–120. [Google Scholar] [CrossRef]
- Si, A.; Li, J.; Yang, Z.; Xia, Y.; Yang, T.; Lei, Z.; Cheng, Z.; Pawlik, T.M.; Lau, W.Y.; Shen, F. Impact of Anatomical Versus Non-anatomical Liver Resection on Short- and Long-Term Outcomes for Patients with Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2019, 26, 1841–1850. [Google Scholar] [CrossRef]
| Study | Study Type | Study Country | Patients, n° | Inclusion Period | Margin Assessed, mm | Type HCC | Type Resection | Underlying Conditions |
|---|---|---|---|---|---|---|---|---|
| Bai S. et al., 2023 [17] | Retrospective | China | 670 | 2016–2017 | 10 | - | With/without adjuvant TACE | - |
| Belli G. et al., 2011 [18] | Retrospective | Italy | 109 | 2000–2008 | 10 | - | Laparoscopic | Cirrhosis |
| Chang W.T. et al., 2012 [19] | Retrospective | Taiwan | 478 | 1991–2006 | 10 | BCLC B-C | - | - |
| Chen M.F. et al., 2003 [20] | Retrospective | Taiwan | 254 | 1986–1998 | 10 | - | - | Non-cirrhotic liver |
| Chen Z.H. et al., 2021 [21] | Retrospective | Multicentric, China | 1.517 | 2009–2012 | 10 | MVI+ | - | - |
| Cheng C.H. et al., 2022 [22] | Retrospective | Taiwan | 983 | 2003–2009 | 1 | - | - | - |
| Dong S. et al., 2016 [23] | Retrospective | China | 586 | 2001–2012 | 5 | Solitary, without macroscopical vascular invasion | - | - |
| Endo Y. et al., 2023 [24] | Retrospective | Multicentric | 782 | 2000–2020 | 5 | - | - | - |
| Field W.B.S. et al., 2017 [25] | Retrospective | USA | 3.300 | 2002–2016 | 5 | - | - | - |
| Han J. et al., 2019 [26] | Retrospective | Multicentric, China | 801 | 2007–2016 | 10 | Solitary HCC | - | - |
| Hirokawa F. et al., 2014 [27] | Retrospective | Japan | 167 | 2000–2010 | 10 | Solitary HCC | - | - |
| HsiaoJ.H. et al., 2017 [28] | Retrospective | Taiwan | 221 | 2006–2014 | 10 | - | With/without adjuvant TACE | - |
| Huang G. et al., 2013 [29] | Retrospective | China | 1.040 | 2006–2008 | 10 | - | - | High baseline HBV-DNA |
| Huang W.J. et al., 2015 [30] | Retrospective | Taiwan | 230 | 2007–2009 | 10 | Stage I HCC | - | - |
| Jeng K.S. et al., 2013 [31] | Retrospective | Taiwan | 196 | 1994–2010 | 5 | Centrally located HCC | - | - |
| Ke Q. et al., 2023 [32] | Retrospective | Multicentric, China | 1.033 | 2012–2015 | 4 | Solitary HCC | AR | - |
| Kobayashi N. et al., 2020 [33] | Retrospective | Japan | 454 | 2001–2012 | 1 | Solitary HCC | - | - |
| Laurent C. et al., 2005 [34] | Retrospective | France | 108 | 1985–2002 | 10 | - | - | Non-cirrhotic liver |
| Lee. C.S. et al., 1996 [35] | Retrospective | Taiwan | 48 | 1979–1984 | 10 | Small asymptomatic HCC | - | - |
| Lee S.G. et al., 2006 [36] | Retrospective | Korea | 100 | 1997–2003 | 10 | Huge HCC | - | - |
| Lee K.T. et al., 2012 [37] | Retrospective | Taiwan | 407 | 2000–2007 | 1–5 6–10 >10 | - | - | - |
| Lee W. et al., 2018 [38] | Retrospective | South Korea | 419 | 2004–2014 | 10 | - | - | - |
| Lee J.C. et al., 2019 [39] | Retrospective | Taiwan | 534 | 2003–2007 | <5 5–9 ≥10 | - | - | - |
| Lim C. et al., 2020 [40] | Retrospective | Multicentric, France, and Spain | 187 | 2007–2016 | 10 | Transplantable HCC | - | Cirrhosis |
| Lise M. et al., 1998 [41] | Retrospective | Italy | 100 | 1977–1995 | 10 | - | - | - |
| Liu Y. et al., 2016 [42] | Retrospective | China | 223 | 2004–2011 | 10 | - | - | - |
| Liu L. et al., 2021 [43] | Retrospective | China | 240 | 2014–2016 | 10 | - | - | - |
| Park J.H. et al., 2018 [44] | Retrospective | Korea | 92 | 2012–2015 | 10 | - | - | - |
| Poon R.T.P. et al., 2000 [45] | Retrospective | China | 288 | 1989–1997 | 10 | - | - | - |
| Sasaki Y. et al., 2006 [46] | Retrospective | Japan | 417 | 1990–1999 | 10 | - | - | HBV-or HCV-related HCC |
| Shapera E. et al., 2023 [47] | Retrospective | USA | 58 | 2016–2022 | ≤1 1.1–9.9 ≥10 | - | - | - |
| Shi F. et al., 2019 [48] | Retrospective | Japan | 276 | 2006–2015 | 10 | Early HCC | - | - |
| Shi M. et al., 2007 [49] | Prospective Randomized Trial | China | 169 | 1999–2003 | 20 vs. 10 | Solitary HCC | - | - |
| Shimada K. et al., 2008 [50] | Retrospective | Japan | 117 | 1996–2003 | 10 | Small HCC eligible for percutaneous local ablative therapy * | - | - |
| Shin S. et al., 2018 [51] | Retrospective | Korea | 116 | 2006–2015 | 10 | Solitary < 3 cm | - | - |
| Su C.M. et al., 2021 [52] | Retrospective | Taiwan | 159 | 1997–2017 | 10 | Solitary < 2 cm | - | CP A * |
| Takano S. et al., 2000 [53] | Retrospective | Japan | 300 | 1987–1997 | 10 | - | - | - |
| Torii A. et al., 1993 [54] | Retrospective | Japan | 59 | 1981–1991 | 10 | Solitary ≤ 3 cm | Minor/Major resection ** | - |
| Tsilimigras D. et al., 2020 [55] | Retrospective | Multicentric | 404 | 1998–2017 | 10 | T1 HCC | - | - |
| Wang J. et al., 2010 [56] | Retrospective | China | 438 | 1991–2004 | 20 vs. 10 | |||
| Wang H. et al., 2020 [57] | Retrospective | China | 904 | 2009–2010 | 2 | Solitary HCC ≤ 5 cm | - | - |
| Yang J. et al., 2014 [58] | Retrospective | China | 1.084 | 2006–2012 | 10 | - | - | - |
| Yang P. et al., 2019 [59] | Retrospective | China | 2.508 | 2000–2013 | 10 | - | - | HBV-related HCC |
| Zeng J. et al., 2020 [60] | Retrospective | China | 699 | 2008–2015 | 10 | - | - | HBV-related HCC, patients ≤ 40 years-old |
| Zhang X.F. et al., 2014 [61] | Retrospective | China | 302 | 2008–2011 | 10 | - | - | HBV-related HCC |
| Zhang H. et al., 2022 [62] | Retrospective | China | 425 | 2015–2018 | 10 | - | Laparoscopic | - |
| Zhou K.Q. et al., 2020 [63] | Retrospective | China | 309 | 2010–2015 | 10 | - | - | - |
| Zhou Z. et al., 2021 [64] | Retrospective | China | 217 | - | 10 | Solitary HCC | - | - |
| Study | Selection | Sample Size | Detection | Confounding | Detection |
|---|---|---|---|---|---|
| Bai S. et al., 2023 [17] | High | High | Low | Low | Unclear/High |
| Belli G. et al., 2011 [18] | High | High | Low | High | Unclear/High |
| Chang W.T. et al., 2012 [19] | Moderate | High | Low | High | Unclear/High |
| Chen M.F. et al., 2003 [20] | High | High | Low | High | Unclear/High |
| Chen Z.H. et al., 2021 [21] | High | High | Low | High | Unclear/High |
| Cheng C.H. et al., 2022 [22] | Moderate | High | Low | Low | Unclear/High |
| Dong S. et al., 2016 [23] | High | High | Low | High | Unclear/High |
| Endo Y. et al., 2023 [24] | High | High | Low | High | Unclear/High |
| Field W.B.S. et al., 2017 [25] | High | High | Low | High | Unclear/High |
| Han J. et al., 2019 [26] | High | High | Low | High | Unclear/High |
| Hirokawa F. et al., 2014 [27] | High | High | Low | High | Unclear/High |
| Hsiao J.H. et al., 2017 [28] | High | High | Low | High | Unclear/High |
| Huang G. et al., 2013 [29] | High | High | Low | High | Unclear/High |
| Huang W.J. et al., 2015 [30] | High | High | Low | High | Unclear/High |
| Jeng K.S. et al., 2013 [31] | High | High | Low | High | Unclear/High |
| Ke Q. et al., 2023 [32] | High | High | Low | Low | Unclear/High |
| Kobayashi N. et al., 2020 [33] | Moderate | High | Low | Low | Unclear/High |
| Laurent C. et al., 2005 [34] | Moderate | High | Low | High | Unclear/High |
| Lee. C.S. et al., 1996 [35] | High | High | Low | High | Unclear/High |
| Lee S.G. et al., 2007 [36] | High | High | Low | High | Unclear/High |
| Lee K.T. et al., 2012 [37] | Moderate | High | Low | High | Unclear/High |
| Lee W. et al., 2018 [38] | High | High | Low | High | Unclear/High |
| Lee J.C. et al., 2019 [39] | Moderate | High | Low | High | Unclear/High |
| Lim C. et al., 2020 [40] | Moderate | High | Low | High | Unclear/High |
| Lise M. et al., 1998 [41] | Moderate | High | Low | High | Unclear/High |
| Liu Y. et al., 2016 [42] | High | High | Low | High | Unclear/High |
| Liu L. et al., 2021 [43] | High | High | Low | High | Unclear/High |
| Park J.H. et al., 2018 [44] | High | High | Low | High | Unclear/High |
| Poon R.T.P. et al., 2000 [45] | High | High | Low | High | Unclear/High |
| Sasaki Y. et al., 2006 [46] | High | High | Low | High | Unclear/High |
| Shapera E. et al., 2023 [47] | Moderate | High | Low | High | Unclear/High |
| Shi F. et al., 2019 [48] | High | High | Low | High | Unclear/High |
| Shi M. et al., 2007 [49] | Low | Low | Low | High | Unclear/High |
| Shimada K. et al., 2008 [50] | High | High | Low | High | Unclear/High |
| Shin S. et al., 2018 [51] | High | High | Low | High | Unclear/High |
| Su C.M. et al., 2021 [52] | Moderate | High | Low | High | Moderate |
| Takano S. et al., 2000 [53] | High | High | Low | High | Unclear/High |
| Torii A. et al., 1993 [54] | High | High | Low | High | Unclear/High |
| Tsilimigras D. et al., 2020 [55] | High | High | Low | High | Unclear/High |
| Wang J. et al., 2010 [56] | High | High | Low | High | Unclear/High |
| Wang H. et al., 2020 [57] | Moderate | High | Low | Low | Unclear/High |
| Yang J. et al., 2014 [58] | High | High | Low | High | Unclear/High |
| Yang P. et al., 2019 [59] | Moderate | High | Low | Low | Unclear/High |
| Zeng J. et al., 2020 [60] | High | High | Low | High | Unclear/High |
| Zhang X.F. et al., 2014 [61] | High | High | Low | High | Unclear/High |
| Zhang H. et al., 2022 [62] | High | High | Low | High | Unclear/High |
| Zhou K.Q. et al., 2020 [63] | Moderate | High | Low | High | Unclear/High |
| Zhou Z. et al., 2021 [64] | High | High | Low | Low | Unclear/High |
| Study | Patients, n° | OS | DFS | ||||
|---|---|---|---|---|---|---|---|
| Univariate Analysis, p-Value | Multivariate Analysis, p-Value | Subgroup Analysis | Univariate Analysis, p-Value | Multivariate Analysis, p-Value | Subgroup Analysis | ||
| Margin assessed = 20 mm | |||||||
| Shi M et al., 2007 [49] | 169 | 0.008 | 0.003 | - | 0.046 | - | - |
| Wang J. et al., 2010 [56] | 438 | <0.001 | 0.011 | - | - | 0.014 | - |
| Margin assessed = 10 mm | |||||||
| Bai S. et al., 2023 [17] | 670 | 0.005 | - | - | 0.026 | - | - |
| Belli G. et al., 2011 [18] | 109 | - | - | - | 0.0014 | 0.022 | - |
| Chang WT et al., 2012 [19] | 478 | - | - | - | - | 0.042 | - |
| Chen M.F. et al., 2003 [20] | 254 | 0.0008 | - | - | 0.0823 | NI | - |
| Chen Z.H. et al., 2021 [21] | 1.517 | - | 0.006 | - | - | - | - |
| Han J.et al., 2019 [26] | 801 | <0.001 | <0.001 | Independent prognostic factor both in MVI+ and MVI− (p = < 0.001) | 0.001 | 0.001 | Independent prognostic factor both in MVI+ and MVI− (p = < 0.001) |
| Hirokawa F. et al., 2014 [27] | 167 | - | - | - | - | - | Significant only in MVI+ (p = 0.0263) |
| Huang G. et al., 2013 [29] | 1.040 | <0.001 | - | - | 0.001 | 0.001 | - |
| Hsiao J.H. et al., 2017 [28] | 221 | 0.0178 | 0.0433 | - | - | - | - |
| Huang W.J. et al., 2015 [30] | 230 | <0.001 | 0.007 | In MVI−, better RFS regardless of AR or NAR. In MVI+, AR and ≥10 mm, better RFS | <0.001 | <0.001 | In MVI−, better RFS regardless of AR or NAR. In MVI+, AR and ≥10 mm, better RFS |
| Laurent C. et al., 2005 [34] | 108 | 0.01 | - | - | 0.005 | 0.035 | - |
| Lee W. et al., 2018 [38] | 419 | 0.690 | - | - | 0.042 | 0.146 | - |
| Lee K.T. et al., 2012 [37] | 407 | NS | - | - | 0.023 | 0.010 | - |
| Lee J.C. et al., 2019 [39] | 534 | - | - | - | 0.042 | - | Significative in AFP > 200 ng/mL (p = 0.012) |
| Lee. C.-S. et al., 1996 [35] | 48 | 0.04 | 0.036 | - | - | - | - |
| Lee S.G. et al., 2007 [36] | 100 | 0.075 | - | - | 0.009 | 0.001 | - |
| Lim C. et al., 2020 [40] | 187 | 0.70 | - | - | 0.03 | - | - |
| Lise M. et al., 1998 [41] | 100 | 0.04 | 0.05 | - | 0.05 | 0.03 | - |
| Liu L. et al., 2021 [43] | 240 | <0.001 | 0.013 | - | <0.001 | 0.011 | - |
| Liu Y. et al., 2016 [42] | 223 | - | - | - | 0.005 | 0.006 | Analysis performed for the risk of recurrence |
| Park J.H. et al., 2018 [44] | 92 | 0.117 | - | Significative difference only in PET-FDG (+) HCC (p = <0.001) | 0.302 | - | Not significative both in PET-FDG (+) and (−) HCC |
| Poon R.T.P. et al., 2000 [45] | 288 | 0.495 | - | - | 0.943 | NI | - |
| Sasaki Y. et al., 2006 [46] | 406 | - | - | - | 0.002 | 0.049 | - |
| Shapera E. et al., 2023 [47] | 58 | 0.013 | - | - | - | - | - |
| Shi M. et al., 2007 [49] | 169 | 0.008 | 0.003 | - | 0.046 | - | |
| Shi F. et al., 2019 [48] | 276 | <0.001 | 0.007 | RM > 10 mm independent from AR/NAR in MVI− patients. In MVI+, both RM > 10 mm and AR are necessary | <0.001 | <0.001 | RM > 10 mm independent from AR/NAR in MVI− patients. In MVI+, both RM > 10 mm and AR are necessary |
| Shimada K. et al., 2008 [50] | 117 | - | - | - | 0.0203 | 0.034 | - |
| Shin S. et al., 2018 [51] | 116 | - | - | - | 0.453 | - | Suggested RM > 1 cm in MVI+ (p = 0.049) |
| Su C.M. et al., 2021 [52] | 159 | 0.053 | - | - | 0.096 | - | - |
| Takano S. et al., 2000 [53] | 300 | 0.0125 | - | - | - | - | - |
| Torii A. et al., 1993 [54] | 59 | <0.1 | 0.7191 | - | - | - | - |
| Tsilimigras D.I. et al., 2020 [55] | 404 | 0.047 | - | - | 0.02 | 0.017 | In AR, the RM is not an independent risk factor. In NAR, the RM is an independent risk factor |
| Yang J. et al., 2014 [58] | 1.084 | 0.005 | 0.002 | - | 0.007 | 0.011 | - |
| Yang P. et al., 2019 [59] | 2.508 | <0.001 | - | Independent prognostic factor in MVI+ (p ≤ 0.001) | <0.001 | - | Independent prognostic factor in MVI+ (p ≤ 0.001) |
| Zeng J. et al., 2020 [60] | 699 | <0.01 | <0.01 | - | <0.01 | - | - |
| Zhang X.F. et al., 2014 [61] | 302 | - | - | - | 0.048 | 0.048 | - |
| Zhang H. et al., 2022 [62] | 425 | - | - | - | 0.019 | 0.002 | - |
| Zhou K.Q. et al., 2020 [63] | 309 | - | - | Not significative in CTC > 1 (p = 0.078) | - | - | Independent risk factor when CTC > 1 (p ≤ 0.023) |
| Zhou Z. et al., 2021 [64] | 817 | 0.067 | - | - | >0.05 | - | - |
| Margin assessed = 5 mm | |||||||
| Dong S. et al., 2016 [23] | 586 | - | - | - | 0.000 | 0.001 | Suggests in NAR an RM > 5 mm (p ≤ 0.05) |
| Endo Y. et al., 2023 [24] | 782 | <0.001 | <0.01 | Especially with a high alpha-fetoprotein tumor burden score (ATS) (p ≤ 0.05) | NI | NI | Especially with a high alpha-fetoprotein tumor burden score (ATS) (p ≤ 0.05) |
| Field W.B.S. et al., 2017 [25] | 3300 | 0.23 | - | - | 0.33 | - | - |
| Lee K.T. et al., 2012 [37] | 407 | NS | - | - | 0.320 | 0.457 | - |
| Lee J.C. et al., 2019 [39] | 534 | - | - | - | 0.027 | 0.024 | Significative in AFP > 200 ng/mL (p = 0.012) |
| Jeng K.-S. et al., 2013 [31] | 196 | 0.055 | - | - | 0.066 | - | - |
| Margin assessed = 4 mm | |||||||
| Ke Q. et al., 2023 [32] | 1.033 | 0.150 | - | - | 0.470 | - | - |
| Margin assessed = 2 mm | |||||||
| Wang H. et al., 2020 [57] | 904 | <0.001 | <0.001 | Significative in MVI+ (p = 0.001) and in non-cirrhotic (p = 0.001) | <0.001 | <0.001 | Significative in MVI+ (p ≤ 0.001) and in non-cirrhotic (p ≤ 0.001) |
| Margin assessed = 1 mm | |||||||
| Cheng C.H. et al., 2022 [22] | 983 | - | - | - | 0.155 | - | - |
| Kobayashi N. et al., 2020 [33] | 454 | 0.496 | - | - | 0.375 | - | - |
| Shapera E. et al., 2023 [47] | 58 | NI | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Farai, A.; Sangiuolo, F.; Albaali, D.; Ajoub, M.; Giannone, F.; Cassese, G.; Panaro, F. The Definition of the Best Margin Cutoff and Related Oncological Outcomes After Liver Resection for Hepatocellular Carcinoma: A Systematic Review. Cancers 2025, 17, 1759. https://doi.org/10.3390/cancers17111759
Al Farai A, Sangiuolo F, Albaali D, Ajoub M, Giannone F, Cassese G, Panaro F. The Definition of the Best Margin Cutoff and Related Oncological Outcomes After Liver Resection for Hepatocellular Carcinoma: A Systematic Review. Cancers. 2025; 17(11):1759. https://doi.org/10.3390/cancers17111759
Chicago/Turabian StyleAl Farai, Abdallah, Federico Sangiuolo, Dana Albaali, Mahmoud Ajoub, Fabio Giannone, Gianluca Cassese, and Fabrizio Panaro. 2025. "The Definition of the Best Margin Cutoff and Related Oncological Outcomes After Liver Resection for Hepatocellular Carcinoma: A Systematic Review" Cancers 17, no. 11: 1759. https://doi.org/10.3390/cancers17111759
APA StyleAl Farai, A., Sangiuolo, F., Albaali, D., Ajoub, M., Giannone, F., Cassese, G., & Panaro, F. (2025). The Definition of the Best Margin Cutoff and Related Oncological Outcomes After Liver Resection for Hepatocellular Carcinoma: A Systematic Review. Cancers, 17(11), 1759. https://doi.org/10.3390/cancers17111759








