Efficacy and Safety of TROP-2-Targeting Antibody–Drug Conjugate Treatment in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Pooled Analysis of Reconstructed Patient Data
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
2.2. Risk of Bias
2.3. Outcome Measures
2.4. Data Extraction and Statistical Analysis
3. Results
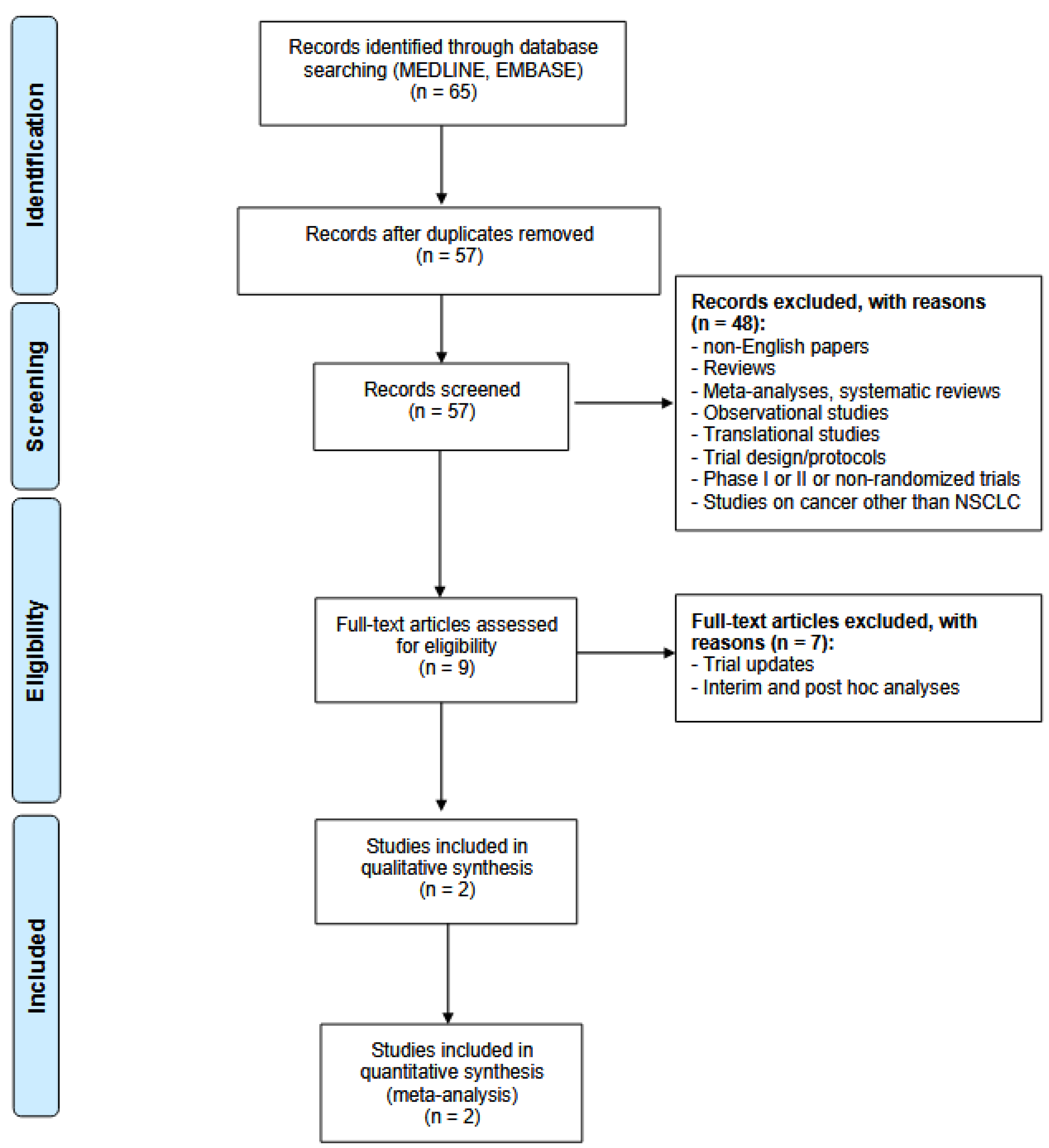
3.1. Systematic Research
3.2. Population Characteristics
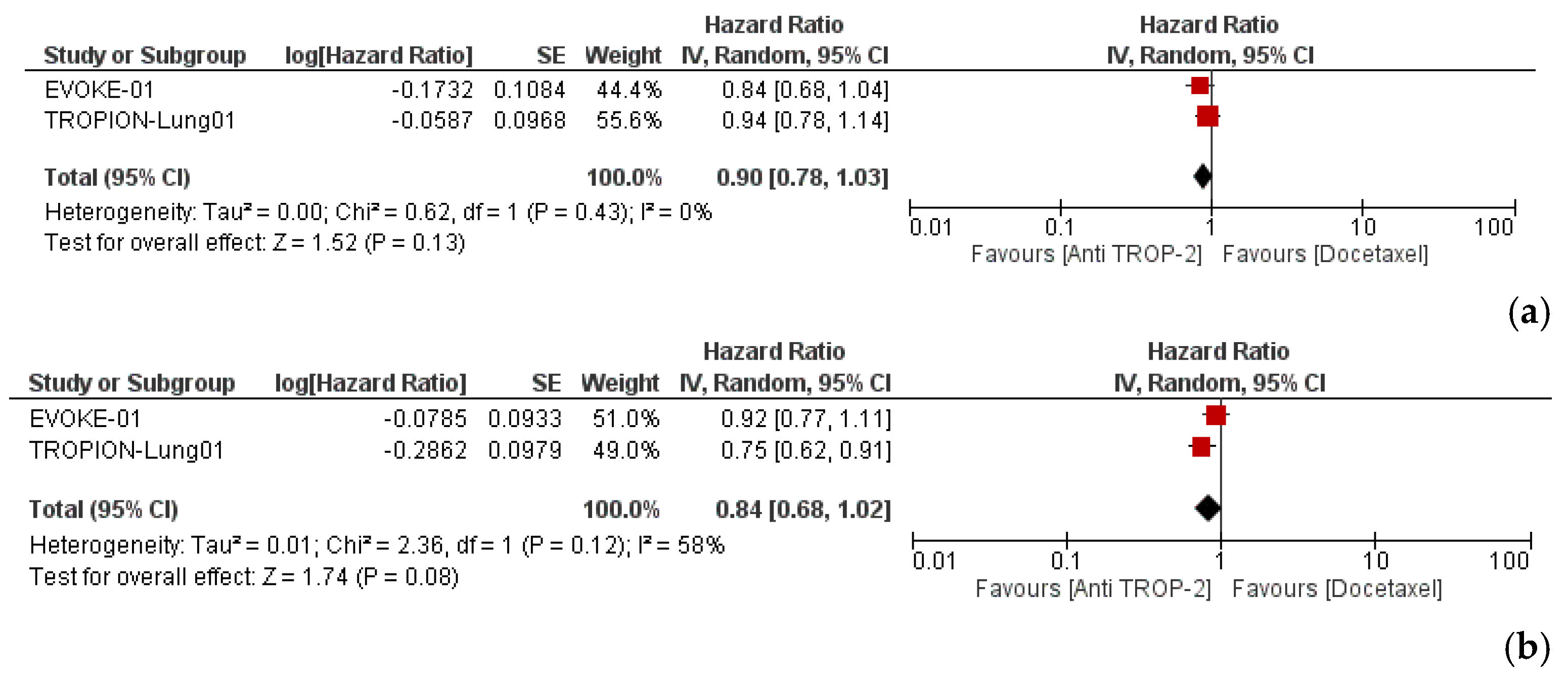
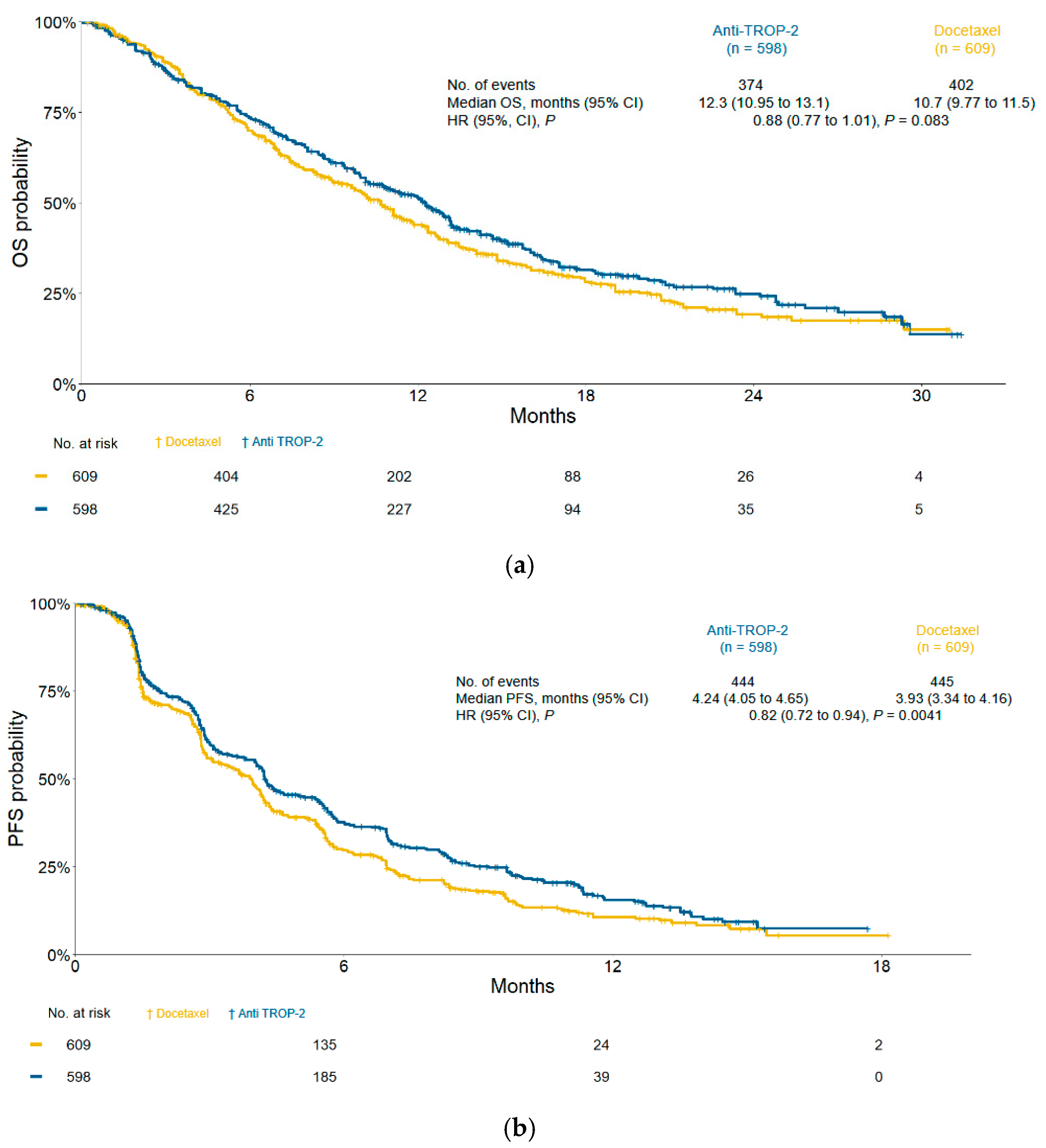
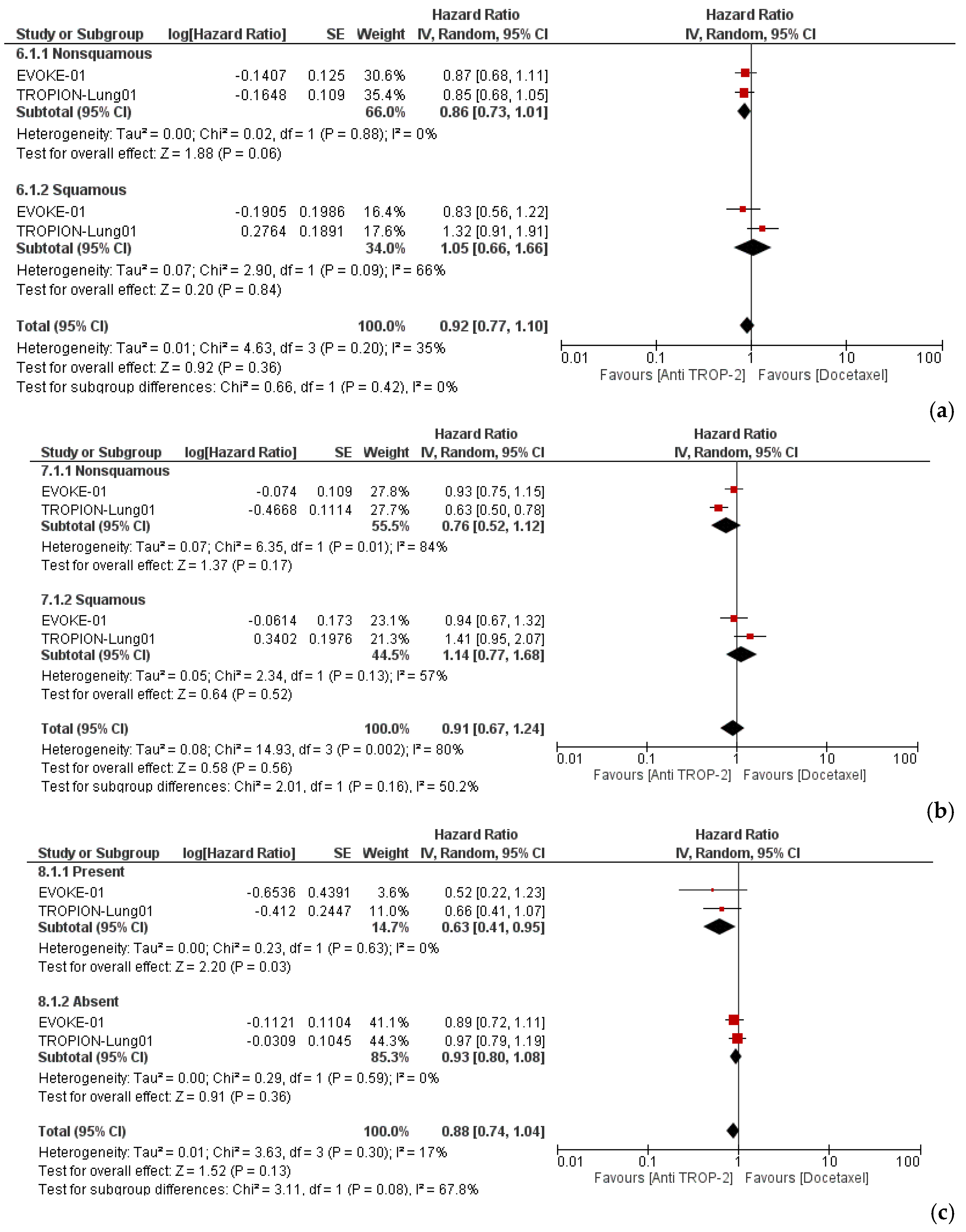
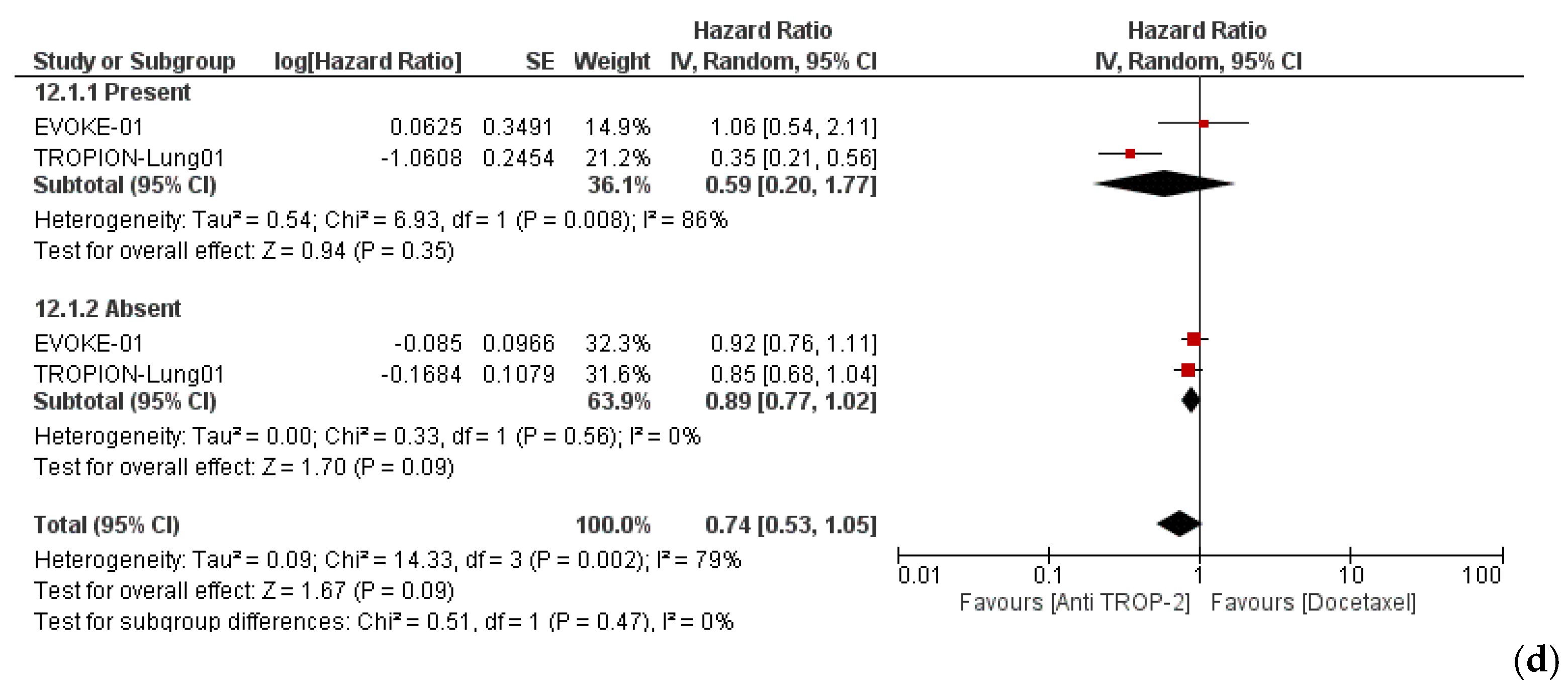
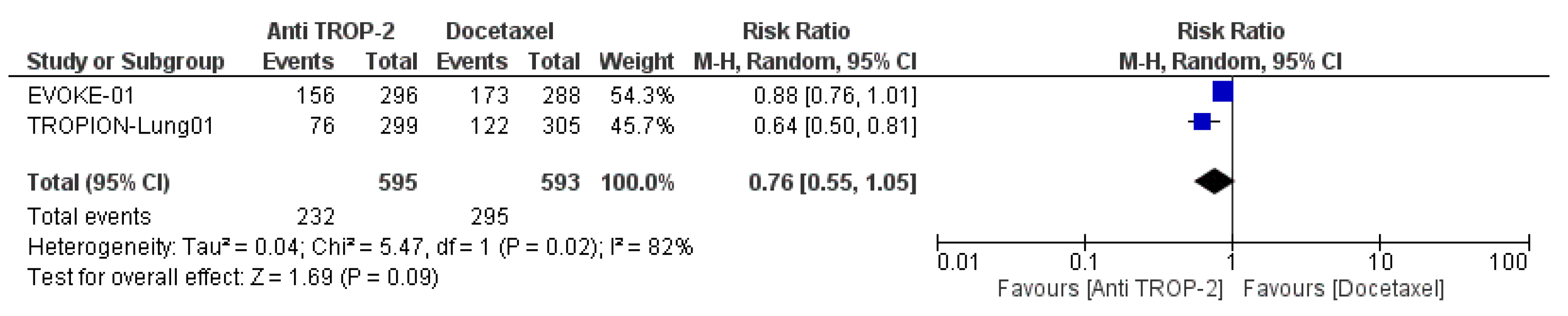
3.3. Efficacy and Subgroup Analyses
3.4. Safety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Shields, M.D.; Marin-Acevedo, J.A.; Pellini, B. Immunotherapy for Advanced Non–Small Cell Lung Cancer: A Decade of Progress. Am. Soc. Clin. Oncol. Educ. B 2021, 41, e105–e127. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, L.E.; Kerr, K.M.; Menis, J.; Mok, T.S.; Nestle, U.; Passaro, A.; Peters, S.; Planchard, D.; Smit, E.F.; Solomon, B.J.; et al. Oncogene-Addicted Metastatic Non-Small-Cell Lung Cancer: ESMO Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2023, 34, 339–357. [Google Scholar] [CrossRef]
- Fossella, F.V.; de Vore, R.; Kerr, R.N.; Crawford, J.; Natale, R.R.; Dunphy, F.; Kalman, L.; Miller, V.; Lee, J.S.; Moore, M.; et al. Randomized Phase III Trial of Docetaxel Versus Vinorelbine or Ifosfamide in Patients with Advanced Non–Small-Cell Lung Cancer Previously Treated With Platinum-Containing Chemotherapy Regimens. J. Clin. Oncol. 2000, 18, 2354–2362. [Google Scholar] [CrossRef]
- Reck, M.; Kaiser, R.; Mellemgaard, A.; Douillard, J.-Y.; Orlov, S.; Krzakowski, M.; von Pawel, J.; Gottfried, M.; Bondarenko, I.; Liao, M.; et al. Docetaxel plus Nintedanib versus Docetaxel plus Placebo in Patients with Previously Treated Non-Small-Cell Lung Cancer (LUME-Lung 1): A Phase 3, Double-Blind, Randomised Controlled Trial. Lancet Oncol. 2014, 15, 143–155. [Google Scholar] [CrossRef]
- Han, B.; Shi, Y.-K.; Feinstein, T.; Feng, D.; Mitchell, D.; Lelorier, Y.; Du, L.; Huang, L.; Mohanlal, R.; Sun, Y. LBA48 DUBLIN-3 (BPI-2358-103): A Global Phase (Ph) III Trial with the Plinabulin/Docetaxel (Plin/Doc) Combination vs. Doc in 2nd/3rd Line NSCLC Patients (Pts) with EGFR-Wild Type (Wt) Progressing on a Prior Platinum-Based Regimen. Ann. Oncol. 2021, 32, S1326. [Google Scholar] [CrossRef]
- Neal, J.; Pavlakis, N.; Kim, S.-W.; Goto, Y.; Lim, S.M.; Mountzios, G.; Fountzilas, E.; Mochalova, A.; Christoph, D.C.C.; Bearz, A.; et al. 60 CONTACT-01: Efficacy and Safety from a Phase III Study of Atezolizumab (Atezo) + Cabozantinib (Cabo) Vs Docetaxel (Doc) Monotherapy in Patients (Pts) with Metastatic NSCLC (MNSCLC) Previously Treated with Checkpoint Inhibitors and Chemotherapy. J. Thorac. Oncol. 2023, 18, S39–S40. [Google Scholar] [CrossRef]
- Borghaei, H.; de Marinis, F.; Dumoulin, D.; Reynolds, C.; Theelen, W.S.M.E.; Percent, I.; Gutierrez Calderon, V.; Johnson, M.L.; Madroszyk-Flandin, A.; Garon, E.B.; et al. SAPPHIRE: Phase III Study of Sitravatinib plus Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. Ann. Oncol. 2024, 35, 66–76. [Google Scholar] [CrossRef]
- Wen, Y.; Ouyang, D.; Zou, Q.; Chen, Q.; Luo, N.; He, H.; Anwar, M.; Yi, W. A Literature Review of the Promising Future of TROP2: A Potential Drug Therapy Target. Ann. Transl. Med. 2022, 10, 1403. [Google Scholar] [CrossRef]
- Shvartsur, A.; Bonavida, B. Trop2 and Its Overexpression in Cancers: Regulation and Clinical/Therapeutic Implications. Genes Cancer 2015, 6, 84–105. [Google Scholar] [CrossRef]
- Moon, S.-J.; Govindan, S.V.; Cardillo, T.M.; D’Souza, C.A.; Hansen, H.J.; Goldenberg, D.M. Antibody Conjugates of 7-Ethyl-10-Hydroxycamptothecin (SN-38) for Targeted Cancer Chemotherapy. J. Med. Chem. 2008, 51, 6916–6926. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, T.M.; Govindan, S.V.; Sharkey, R.M.; Trisal, P.; Arrojo, R.; Liu, D.; Rossi, E.A.; Chang, C.-H.; Goldenberg, D.M. Sacituzumab Govitecan (IMMU-132), an Anti-Trop-2/SN-38 Antibody–Drug Conjugate: Characterization and Efficacy in Pancreatic, Gastric, and Other Cancers. Bioconjug. Chem. 2015, 26, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.M.; Stein, R.; Sharkey, R.M. The Emergence of Trophoblast Cell-Surface Antigen 2 (TROP-2) as a Novel Cancer Target. Oncotarget 2018, 9, 28989–29006. [Google Scholar] [CrossRef]
- Tsao, L.-C.; Wang, J.S.; Ma, X.; Sodhi, S.; Ragusa, J.V.; Liu, B.; McBane, J.; Wang, T.; Wei, J.; Liu, C.-X.; et al. Effective Extracellular Payload Release and Immunomodulatory Interactions Govern the Therapeutic Effect of Trastuzumab Deruxtecan (T-DXd). Nat. Commun. 2025, 16, 3167. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef]
- Shimizu, T.; Sands, J.; Yoh, K.; Spira, A.; Garon, E.B.; Kitazono, S.; Johnson, M.L.; Meric-Bernstam, F.; Tolcher, A.W.; Yamamoto, N.; et al. First-in-Human, Phase I Dose-Escalation and Dose-Expansion Study of Trophoblast Cell-Surface Antigen 2-Directed Antibody-Drug Conjugate Datopotamab Deruxtecan in Non-Small-Cell Lung Cancer: TROPION-PanTumor01. J. Clin. Oncol. 2023, 41, 4678–4687. [Google Scholar] [CrossRef]
- Bardia, A.; Messersmith, W.A.; Kio, E.A.; Berlin, J.D.; Vahdat, L.; Masters, G.A.; Moroose, R.; Santin, A.D.; Kalinsky, K.; Picozzi, V.; et al. Sacituzumab Govitecan, a Trop-2-Directed Antibody-Drug Conjugate, for Patients with Epithelial Cancer: Final Safety and Efficacy Results from the Phase I/II IMMU-132-01 Basket Trial. Ann. Oncol. 2021, 32, 746–756. [Google Scholar] [CrossRef]
- Yin, Y.; Fan, Y.; Ouyang, Q.; Song, L.; Wang, X.; Li, W.; Li, M.; Yan, X.; Wang, S.; Sun, T.; et al. Sacituzumab Tirumotecan in Previously Treated Metastatic Triple-Negative Breast Cancer: A Randomized Phase 3 Trial. Nat. Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.; Jiang, S.; Yuan, F.; Zhou, F.; Jiang, K.; Zhang, X.; Li, X.; Seneviratne, L.C.; Yu, G.; Zhang, M.; et al. Efficacy and Safety of Sacituzumab Tirumotecan Monotherapy in Patients with Advanced Urothelial Carcinoma Who Progressed on or after Prior Anti-Cancer Therapies: Report from the Phase 1/2 MK-2870-001 Study. J. Clin. Oncol. 2025, 43, 796. [Google Scholar] [CrossRef]
- Paz-Ares, L.G.; Juan-Vidal, O.; Mountzios, G.S.; Felip, E.; Reinmuth, N.; de Marinis, F.; Girard, N.; Patel, V.M.; Takahama, T.; Owen, S.P.; et al. Sacituzumab Govitecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non-Small Cell Lung Cancer: The Randomized, Open-Label Phase III EVOKE-01 Study. J. Clin. Oncol. 2024, 42, 2860–2872. [Google Scholar] [CrossRef]
- Ahn, M.-J.; Tanaka, K.; Paz-Ares, L.; Cornelissen, R.; Girard, N.; Pons-Tostivint, E.; Vicente Baz, D.; Sugawara, S.; Cobo, M.; Pérol, M.; et al. Datopotamab Deruxtecan Versus Docetaxel for Previously Treated Advanced or Metastatic Non–Small Cell Lung Cancer: The Randomized, Open-Label Phase III TROPION-Lung01 Study. J. Clin. Oncol. 2024, 43, 260–272. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. PLoS Med. 2021, 18, e1003583. [Google Scholar] [CrossRef] [PubMed]
- Common Terminology Criteria for Adverse Events (CTCAE) (v 5.0); US Department of Health and Human Services: Washington, DC, USA, 2017.
- Liu, N.; Zhou, Y.; Lee, J.J. IPDfromKM: Reconstruct Individual Patient Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 2021, 21, 111. [Google Scholar] [CrossRef]
- Inamura, K.; Yokouchi, Y.; Kobayashi, M.; Ninomiya, H.; Sakakibara, R.; Subat, S.; Nagano, H.; Nomura, K.; Okumura, S.; Shibutani, T.; et al. Association of Tumor TROP2 Expression with Prognosis Varies among Lung Cancer Subtypes. Oncotarget 2017, 8, 28725–28735. [Google Scholar] [CrossRef]
- JIANG, A.; GAO, X.; ZHANG, D.; ZHANG, L.; LU, H. Expression and Clinical Significance of the Trop-2 Gene in Advanced Non-Small Cell Lung Carcinoma. Oncol. Lett. 2013, 6, 375–380. [Google Scholar] [CrossRef]
- Trabolsi, A.; Kareff, S.A.; Rodriguez, E.; Krause, H.B.; Tan, H.; Morgenstern-Kaplan, D.; Antonarakis, E.S.; Lou, E.; Nagasaka, M.; Algaze, S.; et al. The Genomic, Transcriptomic, and Immunological Landscape of TROP2 in Solid Tumors. J. Clin. Oncol. 2023, 41, 3118. [Google Scholar] [CrossRef]
- Bessede, A.; Peyraud, F.; Besse, B.; Cousin, S.; Cabart, M.; Chomy, F.; Rey, C.; Lara, O.; Odin, O.; Nafia, I.; et al. TROP2 Is Associated with Primary Resistance to Immune Checkpoint Inhibition in Patients with Advanced Non-Small Cell Lung Cancer. Clin. Cancer Res. 2024, 30, 779–785. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; Spira, A.; Ballinger, M.; Kowanetz, M.; Vansteenkiste, J.; Mazieres, J.; Park, K.; Smith, D.; Artal-Cortes, A.; Lewanski, C.; et al. Atezolizumab versus Docetaxel for Patients with Previously Treated Non-Small-Cell Lung Cancer (POPLAR): A Multicentre, Open-Label, Phase 2 Randomised Controlled Trial. Lancet 2016, 387, 1837–1846. [Google Scholar] [CrossRef]
- Bordoni, R.; Ciardiello, F.; von Pawel, J.; Cortinovis, D.; Karagiannis, T.; Ballinger, M.; Sandler, A.; Yu, W.; He, P.; Matheny, C.; et al. Patient-Reported Outcomes in OAK: A Phase III Study of Atezolizumab Versus Docetaxel in Advanced Non–Small-Cell Lung Cancer. Clin. Lung Cancer 2018, 19, 441–449.e4. [Google Scholar] [CrossRef]
- Horita, N.; Yamamoto, S.; Mizuki, Y.; Kawagoe, T.; Mihara, T.; Yamashiro, T. Minimal Clinically Important Difference (MCID) of Effect Sizes Other than Mean Difference. J. Clin. Quest. 2024, 1, 116–127. [Google Scholar] [CrossRef]
- Sands, J.; Ahn, M.; Lisberg, A.; Cho, B.C.; Blumenschein, G., Jr; Shum, E.; Tostivint, E.P.; Goto, Y.; Yoh, K.; Heist, R.; et al. Datopotamab Deruxtecan in Advanced or Metastatic Non–Small Cell Lung Cancer With Actionable Genomic Alterations: Results From the Phase II TROPION-Lung05 Study. J. Clin. Oncol. 2025, 43, 1254–1265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Cheng, Y.; Wang, Q.; Li, X.; Liao, J.; Rodon, J.; Meng, X.; Luo, Y.; Chen, Z.; Wang, W.; et al. Sacituzumab Tirumotecan in Advanced Non-Small-Cell Lung Cancer with or without EGFR Mutations: Phase 1/2 and Phase 2 Trials. Nat. Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Garassino, M.C.; Mok, T.; Mitsudomi, T. Treatment Strategies and Outcomes for Patients with EGFR-Mutant Non-Small Cell Lung Cancer Resistant to EGFR Tyrosine Kinase Inhibitors: Focus on Novel Therapies. Lung Cancer 2022, 170, 41–51. [Google Scholar] [CrossRef]
- Piper-Vallillo, A.J.; Sequist, L.V.; Piotrowska, Z. Emerging Treatment Paradigms for EGFR-Mutant Lung Cancers Progressing on Osimertinib: A Review. J. Clin. Oncol. 2020, 38, 2926–2936. [Google Scholar] [CrossRef]
- Itchins, M.; Pavlakis, N. The Quantum Leap in Therapeutics for Advanced ALK+ Non-Small Cell Lung Cancer and Pursuit to Cure with Precision Medicine. Front. Oncol. 2022, 12, 959637. [Google Scholar] [CrossRef]
- Passaro, A.; Wang, J.; Wang, Y.; Lee, S.H.; Melosky, B.; Shih, J.Y.; Azuma, K.; Juan-Vidal, O.; Cobo, M.; Felip, E.; et al. Amivantamab plus Chemotherapy with and without Lazertinib in EGFR-Mutant Advanced NSCLC after Disease Progression on Osimertinib: Primary Results from the Phase III MARIPOSA-2 Study. Ann. Oncol. 2024, 35, 77–90. [Google Scholar] [CrossRef]
- Yu, H.A.; Goto, Y.; Hayashi, H.; Felip, E.; Chih-Hsin Yang, J.; Reck, M.; Yoh, K.; Lee, S.-H.; Paz-Ares, L.; Besse, B.; et al. HERTHENA-Lung01, a Phase II Trial of Patritumab Deruxtecan (HER3-DXd) in Epidermal Growth Factor Receptor–Mutated Non–Small-Cell Lung Cancer After Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor Therapy and Platinum-Based Chemotherapy. J. Clin. Oncol. 2023, 41, 5363–5375. [Google Scholar] [CrossRef]
- Ferro, A.; Marinato, G.M.; Mulargiu, C.; Marino, M.; Pasello, G.; Guarneri, V.; Bonanno, L. The Study of Primary and Acquired Resistance to First-Line Osimertinib to Improve the Outcome of EGFR-Mutated Advanced Non-Small Cell Lung Cancer Patients: The Challenge Is Open for New Therapeutic Strategies. Crit. Rev. Oncol. Hematol. 2024, 196, 104295. [Google Scholar] [CrossRef] [PubMed]
- Okajima, D.; Yasuda, S.; Maejima, T.; Karibe, T.; Sakurai, K.; Aida, T.; Toki, T.; Yamaguchi, J.; Kitamura, M.; Kamei, R.; et al. Datopotamab Deruxtecan, a Novel TROP2-Directed Antibody–Drug Conjugate, Demonstrates Potent Antitumor Activity by Efficient Drug Delivery to Tumor Cells. Mol. Cancer Ther. 2021, 20, 2329–2340. [Google Scholar] [CrossRef]
- Santi, D.V.; Cabel, L.; Bidard, F.-C. Does Sacituzumab-Govitecan Act as a Conventional Antibody Drug Conjugate (ADC), a Prodrug of SN-38 or Both? Ann. Transl. Med. 2021, 9, 1113. [Google Scholar] [CrossRef]
- Garassino, M.C.; Sands, J.; Paz-Ares, L.; Lisberg, A.; Johnson, M.; Pérol, M.; Carroll, D.; Sade, H.; Kapil, A.; Haddad, V.; et al. PL02.11 Normalized Membrane Ratio of TROP2 by Quantitative Continuous Scoring Is Predictive of Clinical Outcomes in TROPION-Lung 01. J. Thorac. Oncol. 2024, 19, S2–S3. [Google Scholar] [CrossRef]
- Planchard, D.; Cozic, N.; Wislez, M.; Chouaid, C.; Curcio, H.; Cousin, S.; Mascaux, C.; Cadranel, J.; Geier, M.; Ghigna, M.R.; et al. ICARUS-LUNG01: A Phase 2 Study of Datopotomab Deruxtecan (Dato-DXd) in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer (NSCLC), with Sequential Tissue Biopsies and Biomarkers Analysis to Predict Treatment Outcome. J. Clin. Oncol. 2024, 42, 8501. [Google Scholar] [CrossRef]
| Anti-TROP-2 ADCs (n = 598) | Docetaxel (n = 609) | ||
|---|---|---|---|
| Age, years, median (range) | 64.5 (28.5–84) | 64 (28–85.5) | |
| Sex, No. (%) | M | 377 (63.01) | 426 (69.95) |
| F | 221 (36.99) | 183 (30.05) | |
| Ethnic group, No. (%) | White | 352 (58.86) | 342 (56.15) |
| Black | 12 (0.02) | 11 (0.02) | |
| Asian | 136 (22.74) | 146 (23.97) | |
| Other | 98 (16.38) | 110 (18.06) | |
| ECOG PS, No. (%) | 0 | 190 (31.77) | 183 (30.05) |
| ≥1 | 408 (68.23) | 424 (69.62) | |
| Histology, No. (%) | Non-squamous | 439 (73.41) | 448 (73.56) |
| Squamous | 149 (24.92) | 151 (24.79) | |
| Other | 10 (0.017) | 10 (0.016) | |
| PD-L1 status | <1% | 219 (36.62) | 243 (39.9) |
| ≥1% | 340 (56.86) | 322 (52.87) | |
| Missing/Not performed | 39 (6.52) | 44 (7.22) | |
| AGAs, No. (%) | Present | 69 (11.53) | 76 (12.71) |
| Absent | 529 (88.46) | 533 (87.52) | |
| Brain metastasis, No. (%) | Yes | 114 (19.06) | 130 (21.35) |
| No | 484 (80.94) | 479 (78.65) | |
| Previous lines of therapy, No. (%) | 1 | 334 (55.85) | 341 (55.99) |
| 2 | 211 (35.28) | 203 (33.33) | |
| ≥3 | 51 (8.53) | 64 (10.51) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stumpo, S.; Carlini, A.; Mantuano, F.; Di Federico, A.; Melotti, B.; Sperandi, F.; Favorito, V.; De Giglio, A. Efficacy and Safety of TROP-2-Targeting Antibody–Drug Conjugate Treatment in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Pooled Analysis of Reconstructed Patient Data. Cancers 2025, 17, 1750. https://doi.org/10.3390/cancers17111750
Stumpo S, Carlini A, Mantuano F, Di Federico A, Melotti B, Sperandi F, Favorito V, De Giglio A. Efficacy and Safety of TROP-2-Targeting Antibody–Drug Conjugate Treatment in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Pooled Analysis of Reconstructed Patient Data. Cancers. 2025; 17(11):1750. https://doi.org/10.3390/cancers17111750
Chicago/Turabian StyleStumpo, Sara, Andrea Carlini, Francesco Mantuano, Alessandro Di Federico, Barbara Melotti, Francesca Sperandi, Valentina Favorito, and Andrea De Giglio. 2025. "Efficacy and Safety of TROP-2-Targeting Antibody–Drug Conjugate Treatment in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Pooled Analysis of Reconstructed Patient Data" Cancers 17, no. 11: 1750. https://doi.org/10.3390/cancers17111750
APA StyleStumpo, S., Carlini, A., Mantuano, F., Di Federico, A., Melotti, B., Sperandi, F., Favorito, V., & De Giglio, A. (2025). Efficacy and Safety of TROP-2-Targeting Antibody–Drug Conjugate Treatment in Previously Treated Patients with Advanced Non-Small Cell Lung Cancer: A Systematic Review and Pooled Analysis of Reconstructed Patient Data. Cancers, 17(11), 1750. https://doi.org/10.3390/cancers17111750








