Oncology Biomarkers, Clinical Characteristics, and Survival Outcomes in Colorectal Cancer Patients with Spinal Metastases Undergoing Spinal Surgery: Insights from a Retrospective Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Considerations
2.2. Data Extraction and Outcomes of Interest
2.3. Statistical Analysis
3. Results
3.1. Patient Demographics and Clinical Characteristics
3.2. Biomarker Analysis
3.3. Surgical and Adjuvant Treatments
3.4. Neurological and Functional Outcomes
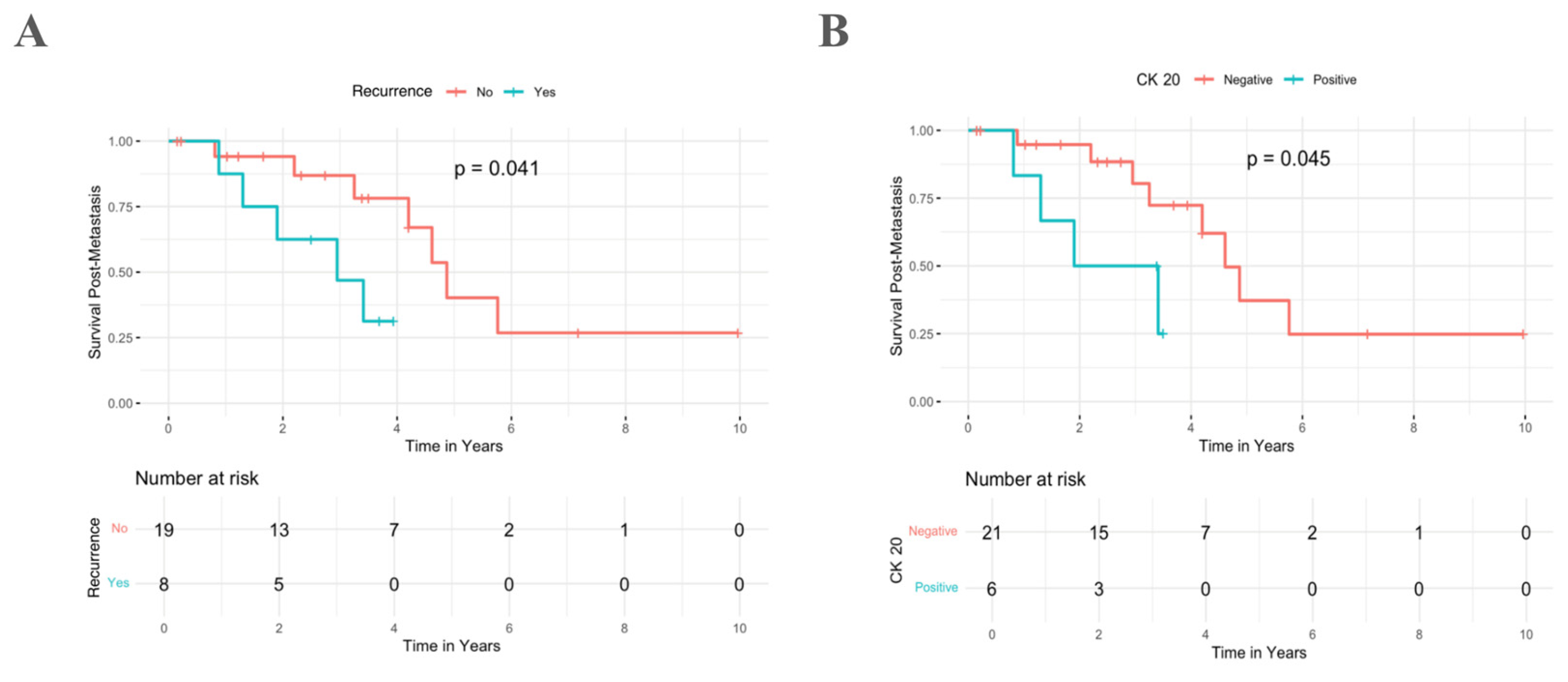
3.5. Survival Analysis
4. Discussion
Study Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colorectal Cancer. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553197/ (accessed on 1 January 2025).
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.L.; Warner, S.; Ito, K.; Raoof, M.; Wu, G.X.; Lu, W.P.; Kessler, J.; Kim, J.Y.; Fong, Y. Cytoreduction for colorectal metastases: Liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Curr. Probl. Surg. 2018, 55, 330–379. [Google Scholar]
- Genetics of Colorectal Cancer (PDQ®). Available online: https://www.ncbi.nlm.nih.gov/books/NBK126744/ (accessed on 4 January 2020).
- Sutcliffe, P.; Connock, M.; Shyangdan, D.; Court, R.; Kandala, N.B.; Clarke, A. A systematic review of evidence on malignant spinal metastases: Natural history and technologies for identifying patients at high risk of vertebral fracture and spinal cord compression. Health Technol. Assess. 2013, 17, 1–274. [Google Scholar] [CrossRef]
- Bostel, T.; Förster, R.; Schlampp, I.; Sprave, T.; Bruckner, T.; Nicolay, N.H.; Welte, S.E.; Debus, J.; Rief, H. Spinal bone metastases in colorectal cancer: A retrospective analysis of stability, prognostic factors and survival after palliative radiotherapy. Radiat. Oncol. 2017, 12, 115. [Google Scholar] [CrossRef]
- Housini, M.; Dariya, B.; Ahmed, N.; Stevens, A.; Fiadjoe, H.; Nagaraju, G.P.; Basha, R. Colorectal cancer: Genetic alterations, novel biomarkers, current therapeutic strategies and clinical trials. Gene 2024, 892, 147857. [Google Scholar] [CrossRef]
- Ravichandran, S.N.; Kumar, M.M.; Das, A.; Banerjee, A.; Veronica, S.; Sun-Zhang, A.; Zhang, H.; Anbalagan, M.; Sun, X.-F.; Pathak, S. An updated review on molecular biomarkers in diagnosis and therapy of colorectal cancer. Curr. Cancer Drug Targetss 2024, 24, 595–611. [Google Scholar] [CrossRef]
- Omran, M.M.; Fouda, M.S.; Mekkawy, S.A.; Tabll, A.A.; Abdelaziz, A.G.; Omran, A.M.; Emran, T.M. Molecular biomarkers and signaling pathways of cancer stem cells in colorectal cancer. Technol. Cancer Res. Treat. 2024, 23, 15330338241254061. [Google Scholar] [CrossRef]
- Koncina, E.; Haan, S.; Rauh, S.; Letellier, E. Prognostic and predictive molecular biomarkers for colorectal cancer: Updates and challenges. Cancers 2020, 12, 319. [Google Scholar] [CrossRef]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gøtzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef]
- Assi, R.; Mukherji, D.; Haydar, A.; Saroufim, M.; Temraz, S.; Shamseddine, A. Metastatic colorectal cancer presenting with bone marrow metastasis: A case series and review of literature. J. Gastrointest. Oncol. 2016, 7, 284–297. [Google Scholar]
- Tirumani, S.H.; Kim, K.W.; Nishino, M.; Howard, S.A.; Krajewski, K.M.; Jagannathan, J.P.; Cleary, J.M.; Ramaiya, N.H.; Shinagare, A.B. Update on the role of imaging in management of metastatic colorectal cancer. Radiographics 2014, 34, 1908–1928. [Google Scholar] [CrossRef] [PubMed]
- Roser, S.; Maharaj, M.M.; Taylor, M.A.; Kuru, R.; Hansen, M.A.; Ferch, R. Vertebrectomy in metastatic spinal tumours: A 10 year, single-centre review of outcomes and survival. J. Clin. Neurosci. 2019, 68, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Garcés-Ambrossi, G.L.; McGirt, M.J.; Witham, T.F.; Wolinsky, J.P.; Bydon, A.; Gokaslan, Z.L.; Sciubba, D.M. Thoracic vertebrectomy and spinal reconstruction via anterior, posterior, or combined approaches: Clinical outcomes in 91 consecutive patients with metastatic spinal tumors: Clinical article. J. Neurosurg. Spine 2009, 11, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Ryuk, J.P.; Choi, G.-S.; Park, J.S.; Kim, H.J.; Park, S.Y.; Yoon, G.S.; Jun, S.H.; Kwon, Y.C. Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann. Surg. Treat. Res. 2014, 86, 143–151. [Google Scholar] [CrossRef]
- Chen, L.H.; Chen, W.J.; Niu, C.C.; Shih, C.H. Anterior reconstructive spinal surgery with Zielke instrumentation for metastatic malignancies of the spine. Arch. Orthop. Trauma Surg. 2000, 120, 27–31. [Google Scholar] [CrossRef]
- Joharatnam-Hogan, N.; Wilson, W.; Shiu, K.K.; Fusai, G.K.; Davidson, B.; Hochhauser, D.; Bridgewater, J.; Khan, K. Multimodal treatment in metastatic colorectal cancer (mCRC) improves outcomes-the university college London hospital (UCLH) experience. Cancers 2020, 12, 3545. [Google Scholar] [CrossRef]
- Alamanda, V.K.; Robinson, M.M.; Kneisl, J.S.; Spector, L.R.; Patt, J.C. Survival outcomes and factors associated with revision surgery for metastatic disease of the spine. J. Oncol. 2018, 2018, 6140381. [Google Scholar] [CrossRef]
- Liu, Y.-H.; Hu, Y.-C.; Yang, X.-G.; Lun, D.-X.; Wang, F.; Yang, L.; Zhang, H.; Feng, J.-T.; Hua, K.-C. Prognostic factors of ambulatory status for patients with metastatic spinal cord compression: A systematic review and meta-analysis. World Neurosurg. 2018, 116, e278–e290. [Google Scholar] [CrossRef]
- Ning, Y.; Hanna, D.L.; Zhang, W.; Mendez, A.; Yang, D.; El-Khoueiry, R.; Matsusaka, S.; Sunakawa, Y.; Stremitzer, S.; Parekh, A.; et al. Cytokeratin-20 and Survivin-Expressing Circulating Tumor Cells Predict Survival in Metastatic Colorectal Cancer Patients by a Combined Immunomagnetic qRT-PCR Approach. Mol. Cancer Ther. 2015, 14, 2401–2408. [Google Scholar] [CrossRef]
- Hinz, S.; Hendricks, A.; Wittig, A.; Schafmayer, C.; Tepel, J.; Kalthoff, H.; Becker, T.; Röder, C. Detection of circulating tumor cells with CK20 RT-PCR is an independent negative prognostic marker in colon cancer patients-a prospective study. BMC Cancer 2017, 17, 53. [Google Scholar] [CrossRef]
- Wong, S.C.C.; Ng, S.S.M.; Cheung, M.T.; Luk, L.Y.; Chan, C.M.L.; Cheung, A.H.K.; Lee, V.H.M.; Lai, P.B.S.; Ma, B.B.Y.; Hui, E.P.; et al. Clinical significance of CDX2-positive circulating tumour cells in colorectal cancer patients. Br. J. Cancer 2011, 104, 1000–1006. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Aasebø, K.; Dragomir, A.; Sundström, M.; Mezheyeuski, A.; Edqvist, P.-H.; Eide, G.E.; Ponten, F.; Pfeiffer, P.; Glimelius, B.; Sorbye, H. CDX2: A prognostic marker in metastatic colorectal cancer defining a better BRAF mutated and a worse KRAS mutated subgroup. Front. Oncol. 2020, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- André, T.; Shiu, K.-K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Shiu, K.-K.; Kim, T.-W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab versus chemotherapy for microsatellite instability-high or mismatch repair-deficient metastatic colorectal cancer (KEYNOTE-177): Final analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 2022, 23, 659–670. [Google Scholar] [CrossRef]
- Lenz, H.J.; Van Cutsem, E.; Luisa Limon, M.; Wong, K.Y.M.; Hendlisz, A.; Aglietta, M.; García-Alfonso, P.; Neyns, B.; Luppi, G.; Cardin, D.B.; et al. First-line nivolumab plus low-dose ipilimumab for microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: The phase II CheckMate 142 study. J. Clin. Oncol. 2022, 40, 161–170. [Google Scholar] [CrossRef]
- Le, D.T.; Kim, T.W.; Van Cutsem, E.; Geva, R.; Jäger, D.; Hara, H.; Burge, M.; O’Neil, B.; Kavan, P.; Yoshino, T.; et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J. Clin. Oncol. 2020, 38, 11–19. [Google Scholar] [CrossRef]
- Slik, K.; Turkki, R.; Carpén, O.; Kurki, S.; Korkeila, E.; Sundström, J.; Pellinen, T. CDX2 loss with microsatellite stable phenotype predicts poor clinical outcome in stage II colorectal carcinoma. Am. J. Surg. Pathol. 2019, 43, 1473–1482. [Google Scholar] [CrossRef]
- Popat, S.; Hubner, R.; Houlston, R.S. Systematic review of microsatellite instability and colorectal cancer prognosis. J. Clin. Oncol. 2005, 23, 609–618. [Google Scholar] [CrossRef]
- Arrington, A.K.; Heinrich, E.L.; Lee, W.; Duldulao, M.; Patel, S.; Sanchez, J.; Garcia-Aguilar, J.; Kim, J. Prognostic and predictive roles of KRAS mutation in colorectal cancer. Int. J. Mol. Sci. 2012, 13, 12153–12168. [Google Scholar] [CrossRef]
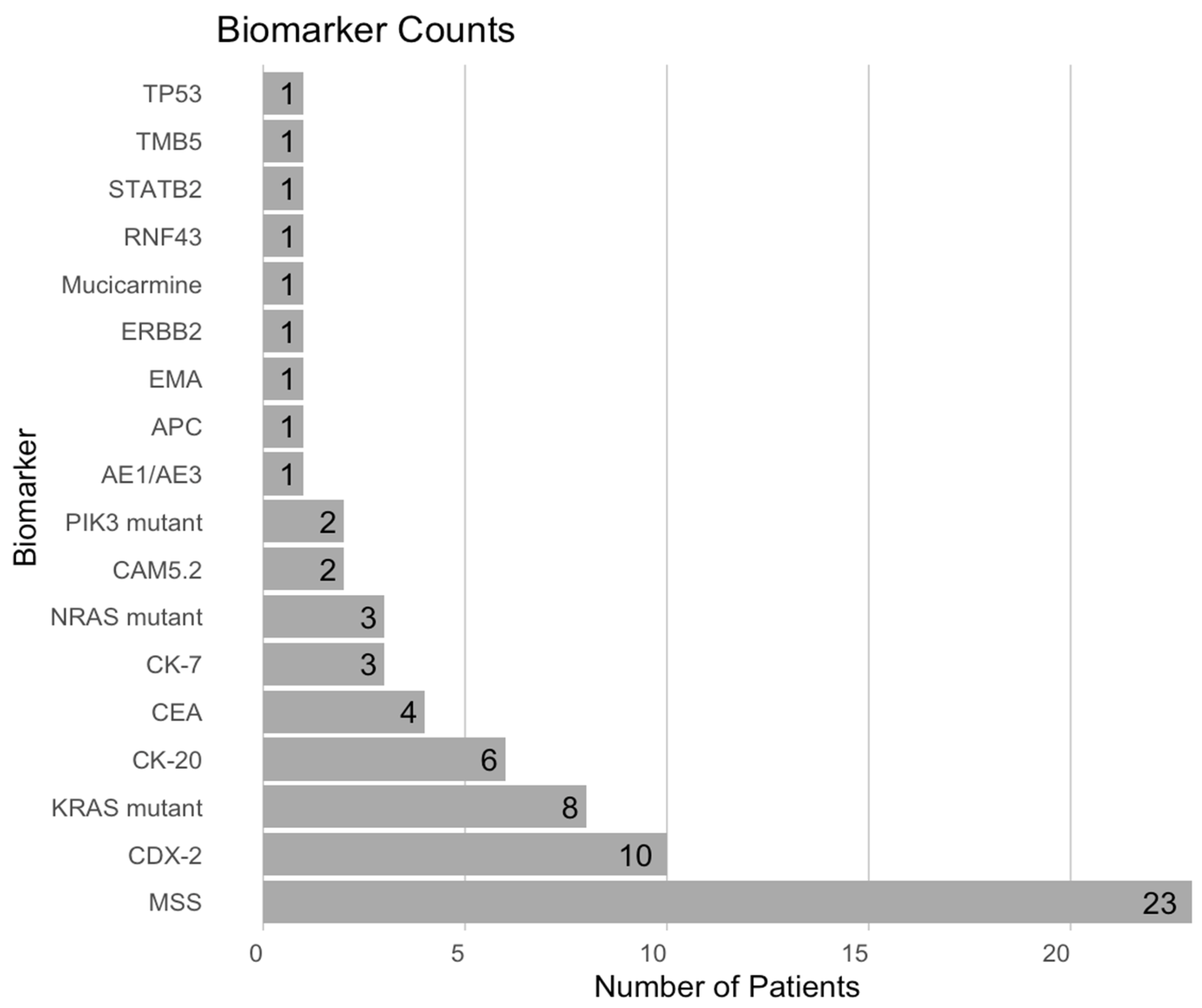
| Parameter | Description, n (%) |
|---|---|
| Age (years) | Median (IQR) = 58.0 (51.4–66.0) |
| Sex | M: 10 (37.0) F: 17 (63.0) |
| Smoking Status | Never Smoked: 15 (55.6) Ex-smokers: 12 (44.4) Current smokers: 0 |
| Mortality Rate | 15 (55.6) |
| Overall Survival | Median (IQR) = 4.9 (3.6–6.8) |
| Time to Spine Metastasis (years) | Median (IQR) = 3.7 (0.7–5.9) |
| Post-Metastasis Survival | Median (IQR) = 3.0 (1.3–4.2) |
| Post-Spinal-Metastasis Survival | Median (IQR) = 1.8 (0.3–2.6) |
| Post-Spinal-Resection Survival | Median (IQR) = 0.8 (0.2–1.6) |
| Progression Free Survival | 2 years: 19 (70.4) 5 years: 10 (37.0) 10 years: 1 (3.7) |
| Spine Level | Cervical: 1 (3.7) Thoracic: 5 (18.5) Lumbar: 5 (18.5) Sacral: 16 (59.3) |
| Other Tumors | Lung: 13 (48.1) Liver: 9 (33.3) Other Bony Lesions: 4 (14.8) Lymph Node: 3 (11.1) Pleural: 2 (7.4) Muscle: 2 (7.4) Kidney: 1 (3.7) Adrenal Gland: 1 (3.7) No Metastases: 3 (11.1) |
| Preop Frankel Score | C: 3 (11.1) D: 11 (40.7) E: 13 (48.1) |
| Postop Frankel Score | C: 3 (11.1) D: 6 (22.2) E: 18 (66.7) |
| Preop Ambulatory Status | Ambulatory: 24 (88.9) Non-Ambulatory: 3 (11.1) |
| Postop Ambulatory Status | Ambulatory: 26 (96.7) Non-Ambulatory: 1 (3.7) |
| Procedures Performed | Pre-Op Biopsy: 26 (96.3) Laminectomy: 23 (85.2) Fusion: 16 (59.3) Staged Surgery: 16 (59.3) Vertebroplasty: 6 (22.2) |
| Procedure Approach | Anterior: 2 (7.4) Posterior: 25 (92.6) |
| Treatment | Radiotherapy: 26 (96.3) Adjuvant Neoadjuvant Chemotherapy: 26 (96.3) Folfox: 12 (44.4) Capecitabine: 13 (48.1) Folfiri: 10 (37.0) Oxaliplatin: 7 (25.9) Trifluridine/Tipiracil: 5 (18.5) Fluorouracil: 2 (7.4) Leucovorin: 1 (3.7) Epirubicin: 1 (3.7) Immunotherapy: 11 (40.7) Nivolumab: 7 (25.9) CTLA4 probody: 2 (7.4) Pembrolizumab: 1 (3.7) Double checkpoint: 1 (3.7) Cetuximab: 1 (3.7) Targeted Therapy: 6 (22.2) Bevacizumab: 4 (14.8) Denosumab: 2 (7.4) Panitumumab: 2 (7.4) Panitumumab: 2 (7.4) Copanlisib: 1 (3.7) Regorafenib: 1 (3.7) Trastuzumab: 1 (3.7) |
| Postop Complications | No Complications: 20 (74.1) Complications: 7 (25.9) |
| Spine Tumor Recurrence | 8 (29.6) |
| Variable | Patients n (%) | Survival Post-Metastasis | ||
|---|---|---|---|---|
| Number of Deaths | SPM (years) (Median ± SE) | p-Value | ||
| Age at Treatment | ||||
| <58 | 13 (48.1) | 7/13 (53.8) | 4.6 ± 0.7 | 0.863 |
| ≥58 | 14 (51.9) | 5/14 (35.7) | 4.9 ± 1.3 | |
| Sex (M) | ||||
| Female | 17 (63.0) | 8/17 (47.1) | 4.2 ± 0.7 | 0.758 |
| Male | 10 (37.0) | 4/10 (40.0) | 4.6 ± 1.4 | |
| Spine Level Metastasis | ||||
| Cervical or Thoracic | 6 (22.2) | 3/6 (50.0) | 4.6 ± 0.0 | 0.507 |
| Lumbar | 5 (18.5) | 3/5 (60.0) | 1.9 ± 1.3 | |
| Sacral | 16 (59.3) | 6/16 (37.5) | 4.2 ± 1.3 | |
| Lung Metastasis | ||||
| No | 14 (51.9) | 4/14 (28.6) | 3.3 ± 2.6 | 0.185 |
| Yes | 13 (48.1) | 8/13 (61.5) | 4.2 ± 1.0 | |
| Liver Metastasis | ||||
| No | 18 (66.7) | 8/18 (44.4) | 4.9 ± 1.0 | 0.264 |
| Yes | 9 (33.3) | 4/9 (44.4) | 4.6 ± 0.0 | |
| Preop Frankel Score | ||||
| C or D | 14 (51.9) | 7/14 (50.0) | 3.4 ± 0.8 | 0.696 |
| E | 13 (48.1) | 5/13 (38.5) | 4.9 ± 0.5 | |
| Postop Frankel Score | ||||
| C or D | 9 (33.3) | 5/9 (55.5) | 4.6 ± 1.5 | 0.671 |
| E | 18 (66.6) | 7/18 (38.9) | 4.2 ± 1.0 | |
| Immunotherapy | ||||
| No | 16 (59.3) | 6/16 (37.5) | 4.2 ± 1.3 | 0.980 |
| Yes | 11 (40.7) | 6/11 (54.5) | 4.6 ± 1.2 | |
| Targeted Therapy | ||||
| No | 21 (77.8) | 10/21 (47.6) | 6.6 ± 4.6 | 0.822 |
| Yes | 6 (22.2) | 2/6 (33.3) | 4.3 ± 0.0 | |
| Postop Complications | ||||
| No | 20 (74.1) | 8/20 (40.0) | 4.9 ± 1.6 | 0.277 |
| Yes | 7 (25.9) | 4/6 (66.7) | 4.2 ± 1.5 | |
| Tumor Recurrence | ||||
| No | 19 (70.4) | 7/19 (36.8) | 4.9 ± 0.4 | 0.041 |
| Yes | 8 (29.6) | 5/8 (62.5) | 3.0 ± 0.9 | |
| MSS | ||||
| No | 10 (37.0) | 4/10 (40.0) | 5.8 ± 0.0 | 0.467 |
| Yes | 17 (63.0) | 8/17 (47.1) | 4.6 ± 0.8 | |
| CDX-2 | ||||
| No | 17 (63.0) | 7/17 (41.2) | 4.6 ± 0.5 | 0.055 |
| Yes | 10 (37.0) | 5/10 (50.0) | 2.2 ± 0.0 | |
| CK-20 | ||||
| No | 21 (77.8) | 8/21 (38.1) | 4.6 ± 0.4 | 0.045 |
| Yes | 6 (22.2) | 4/6 (66.7) | 1.9 ± 1.0 | |
| KRAS | ||||
| No | 19 (70.4) | 8/19 (42.1) | 4.9 ± 1.4 | 0.931 |
| Yes | 8 (29.6) | 4/8 (50.0) | 4.2 ± 1.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mistarehi, A.-H.; Khalilullah, T.; Ghaith, A.K.; Shafi, M.; Khalifeh, J.M.; Xia, Y.; Zaitoun, K.J.; Alnasser, A.A.; Rajasekaran, J.; Albert, A.N.; et al. Oncology Biomarkers, Clinical Characteristics, and Survival Outcomes in Colorectal Cancer Patients with Spinal Metastases Undergoing Spinal Surgery: Insights from a Retrospective Cohort Study. Cancers 2025, 17, 1739. https://doi.org/10.3390/cancers17111739
Al-Mistarehi A-H, Khalilullah T, Ghaith AK, Shafi M, Khalifeh JM, Xia Y, Zaitoun KJ, Alnasser AA, Rajasekaran J, Albert AN, et al. Oncology Biomarkers, Clinical Characteristics, and Survival Outcomes in Colorectal Cancer Patients with Spinal Metastases Undergoing Spinal Surgery: Insights from a Retrospective Cohort Study. Cancers. 2025; 17(11):1739. https://doi.org/10.3390/cancers17111739
Chicago/Turabian StyleAl-Mistarehi, Abdel-Hameed, Taha Khalilullah, Abdul Karim Ghaith, Mahnoor Shafi, Jawad M. Khalifeh, Yuanxuan Xia, Khaled J. Zaitoun, Ahmad A. Alnasser, Joseph Rajasekaran, Avi N. Albert, and et al. 2025. "Oncology Biomarkers, Clinical Characteristics, and Survival Outcomes in Colorectal Cancer Patients with Spinal Metastases Undergoing Spinal Surgery: Insights from a Retrospective Cohort Study" Cancers 17, no. 11: 1739. https://doi.org/10.3390/cancers17111739
APA StyleAl-Mistarehi, A.-H., Khalilullah, T., Ghaith, A. K., Shafi, M., Khalifeh, J. M., Xia, Y., Zaitoun, K. J., Alnasser, A. A., Rajasekaran, J., Albert, A. N., Shah, S., Theodore, N., Meyer, J., Redmond, K. J., Gearhart, S. L., & Lubelski, D. (2025). Oncology Biomarkers, Clinical Characteristics, and Survival Outcomes in Colorectal Cancer Patients with Spinal Metastases Undergoing Spinal Surgery: Insights from a Retrospective Cohort Study. Cancers, 17(11), 1739. https://doi.org/10.3390/cancers17111739





