Blood Transfusion and Survival of Children, Adolescent, and Young Adult Patients with Osteosarcoma: A Multicenter Retrospective Cohort Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
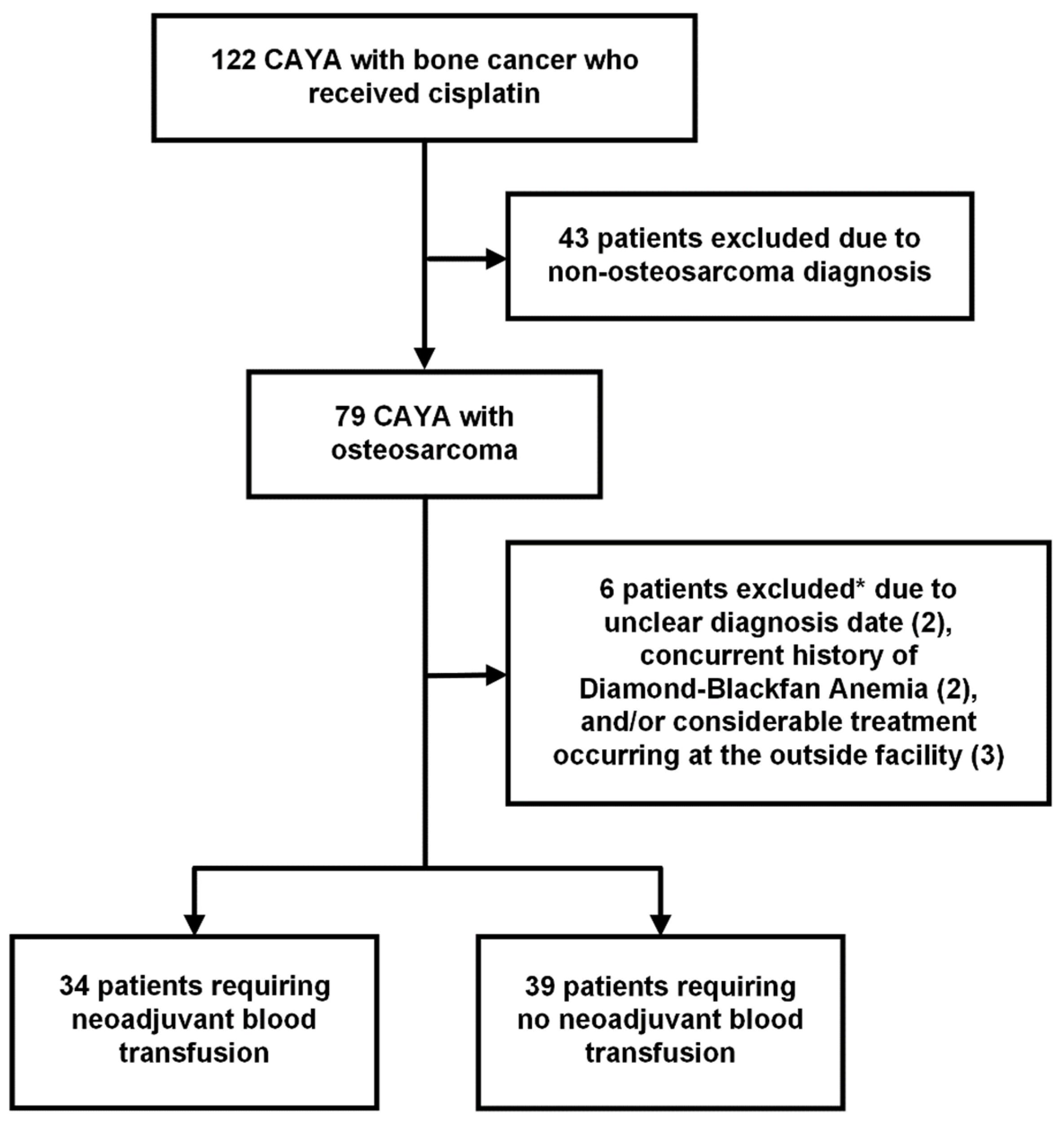
2.1. Study Design and Population
2.2. Study Exposures
2.3. Outcomes of Interest
2.4. Other Parameters of Interest
2.5. Statistical Analyses
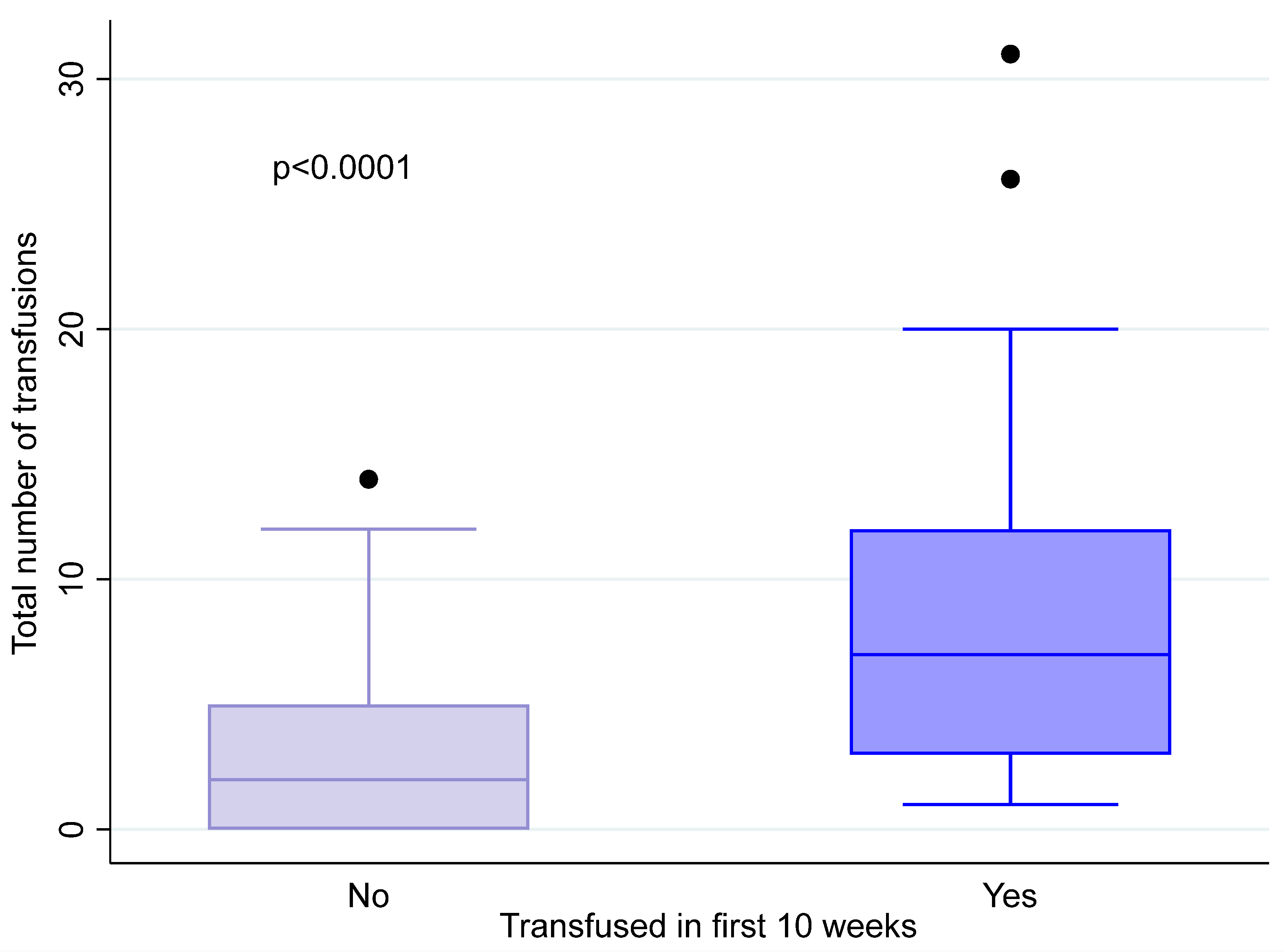
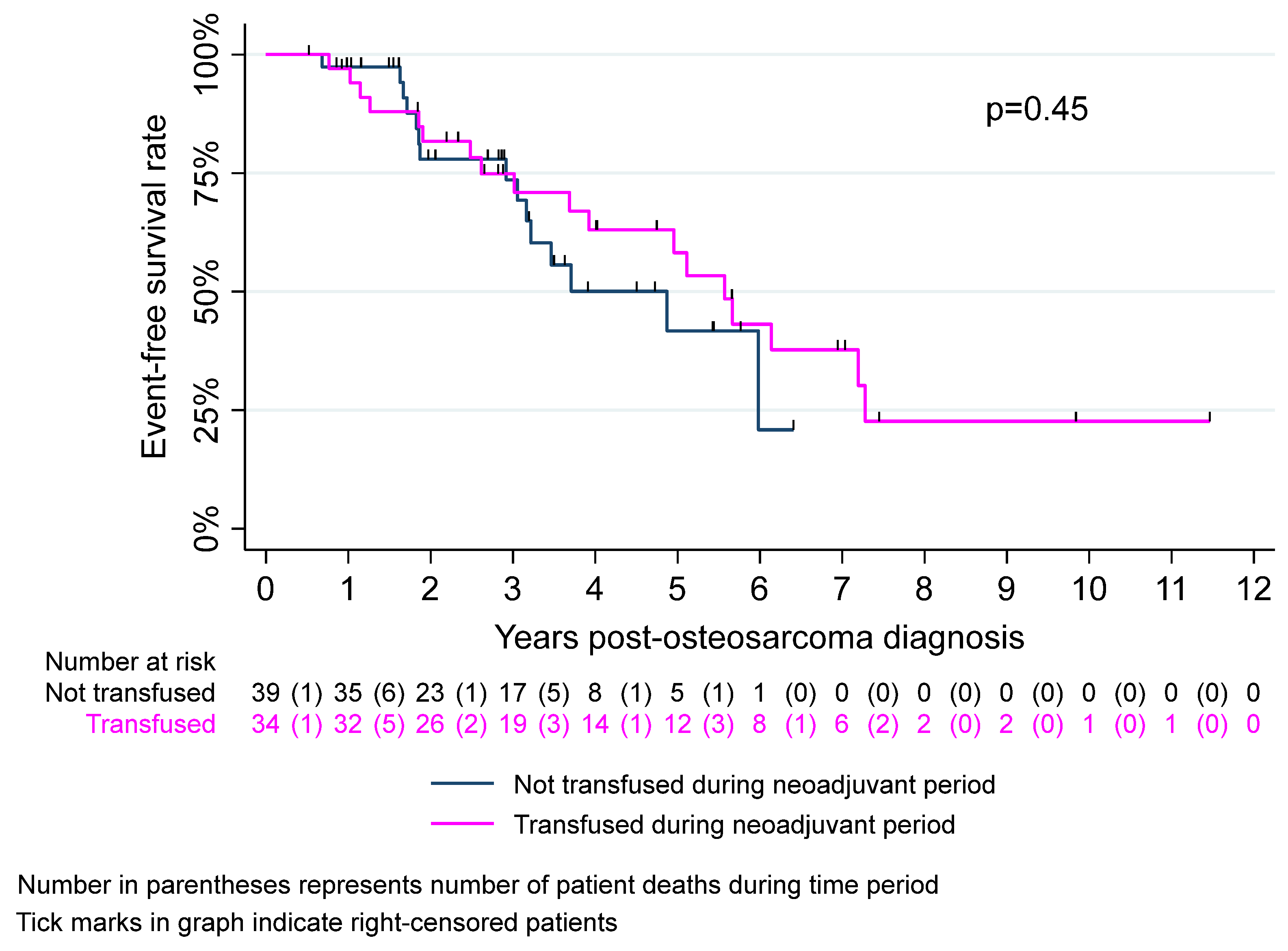
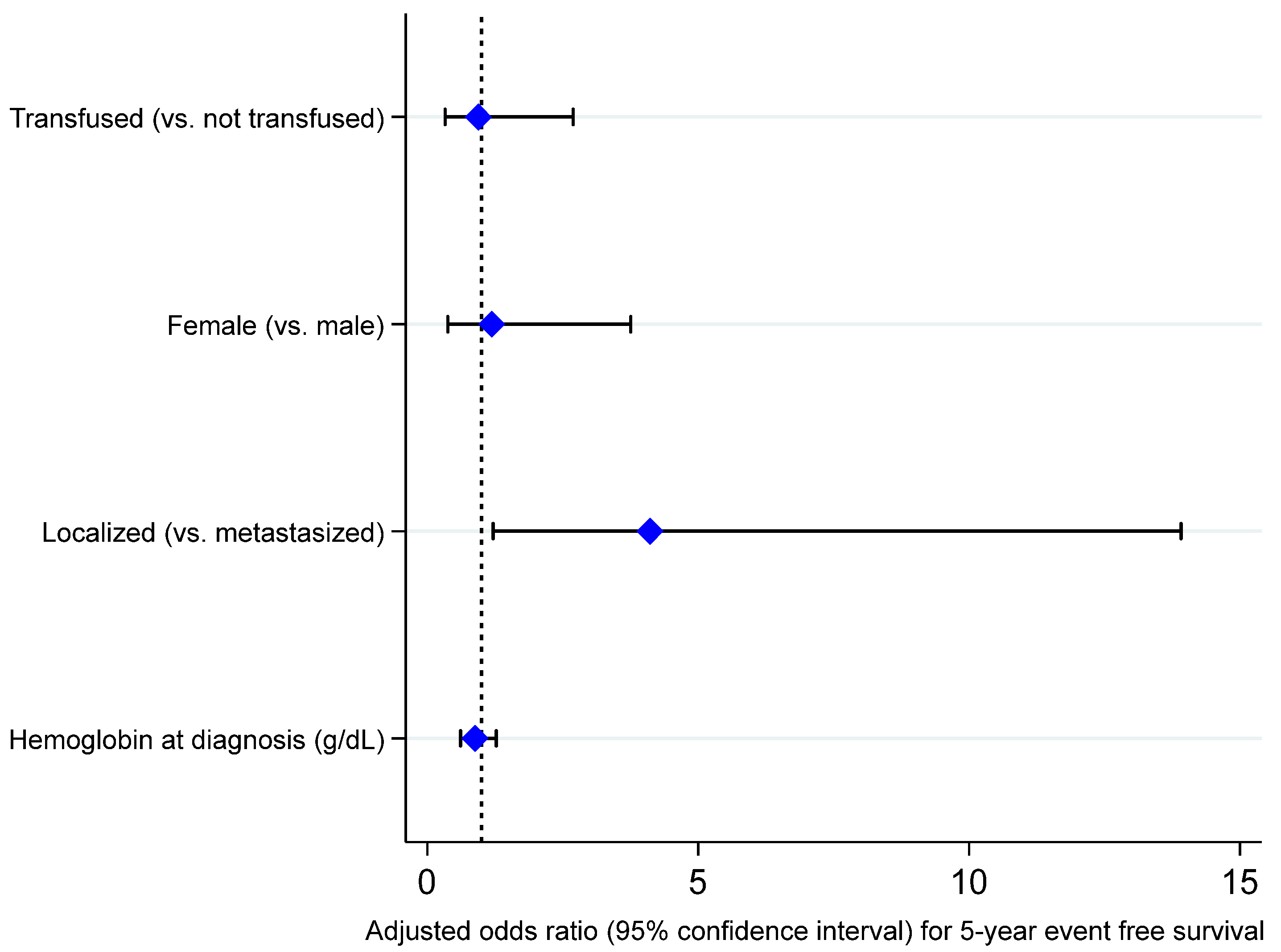
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beird, H.C.; Bielack, S.S.; Flanagan, A.M.; Gill, J.; Heymann, D.; Janeway, K.A.; Livingston, J.A.; Roberts, R.D.; Strauss, S.J.; Gorlick, R. Osteosarcoma. Nat. Rev. Dis. Primers 2022, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Gorlick, R.; Janeway, K.; Lessnick, S.; Randall, R.L.; Marina, N.; COG Bone Tumor Committee. Children’s Oncology Group’s 2013 blueprint for research: Bone tumors. Pediatr. Blood Cancer 2013, 60, 1009–1015. [Google Scholar] [CrossRef]
- Gill, J.; Gorlick, R. Advancing therapy for osteosarcoma. Nat. Rev. Clin. Oncol. 2021, 18, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, P.S.; Helman, L.J. New Horizons in the Treatment of Osteosarcoma. N. Engl. J. Med. 2021, 385, 2066–2076. [Google Scholar] [CrossRef] [PubMed]
- Bielack, S.S.; Kempf-Bielack, B.; Delling, G.; Exner, G.U.; Flege, S.; Helmke, K.; Kotz, R.; Salzer-Kuntschik, M.; Werner, M.; Winkelmann, W.; et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J. Clin. Oncol. 2002, 20, 776–790. [Google Scholar] [CrossRef] [PubMed]
- D’Avella, C.; Devarajan, K.; Edelman, M.; Geynisman, D. 180 The effect of packed red blood cell transfusions on the clinical efficacy of immunotherapy. J. Immunother. Cancer 2020, 8, A106–A107. [Google Scholar] [CrossRef]
- Opelz, G.; Terasaki, P.I. Improvement of kidney-graft survival with increased numbers of blood transfusions. N. Engl. J. Med. 1978, 299, 799–803. [Google Scholar] [CrossRef]
- Gantt, C.L. Red blood cells for cancer patients. Lancet 1981, 2, 363. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Pescatori, M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst. Rev. 2006, 2006, CD005033. [Google Scholar] [CrossRef] [PubMed]
- Harlaar, J.J.; Gosselink, M.P.; Hop, W.C.; Lange, J.F.; Busch, O.R.; Jeekel, H. Blood transfusions and prognosis in colorectal cancer: Long-term results of a randomized controlled trial. Ann. Surg. 2012, 256, 681–686, discussion 686–687. [Google Scholar] [CrossRef]
- Wu, H.L.; Tai, Y.H.; Lin, S.P.; Chan, M.Y.; Chen, H.H.; Chang, K.Y. The Impact of Blood Transfusion on Recurrence and Mortality Following Colorectal Cancer Resection: A Propensity Score Analysis of 4030 Patients. Sci. Rep. 2018, 8, 13345. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Kim, B.R.; Kim, Y.W. Association of preoperative anemia and perioperative allogenic red blood cell transfusion with oncologic outcomes in patients with nonmetastatic colorectal cancer. Curr. Oncol. 2019, 26, e357–e366. [Google Scholar] [CrossRef] [PubMed]
- Tamini, N.; Gianotti, L.; Darwish, S.; Petitto, S.; Bernasconi, D.; Oldani, M.; Uggeri, F.; Braga, M.; Nespoli, L. Do Preoperative Transfusions Impact Prognosis in Moderate to Severe Anaemic Surgical Patients with Colon Cancer? Curr. Oncol. 2021, 28, 4634–4644. [Google Scholar] [CrossRef] [PubMed]
- Rzyman, W.; Dziadziuszko, R.; Skokowski, J.; Wilimski, R.; Raiter, A.; Szymanowska, A.; Jassem, J. The influence of blood transfusion on survival in operated non-small cell lung cancer patients. J. Thorac. Cardiovasc. Surg. 2003, 126, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.J.; Tan, K.S.; Molena, D.; Huang, J.; Bott, M.J.; Park, B.J.; Adusumilli, P.S.; Rusch, V.W.; Bains, M.S.; Downey, R.J.; et al. Perioperative blood transfusion has a dose-dependent relationship with disease recurrence and survival in patients with non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2019, 157, 2469–2477.e2410. [Google Scholar] [CrossRef]
- Cho, S.; Park, J.; Lee, M.; Lee, D.; Choi, H.; Gim, G.; Kim, L.; Kang, C.Y.; Oh, Y.; Viveiros, P.; et al. Blood transfusions may adversely affect survival outcomes of patients with lung cancer: A systematic review and meta-analysis. Transl. Lung Cancer Res. 2021, 10, 1700–1710. [Google Scholar] [CrossRef]
- Kim, J.K.; Kim, H.S.; Park, J.; Jeong, C.W.; Ku, J.H.; Kim, H.H.; Kwak, C. Perioperative Blood Transfusion as a Significant Predictor of Biochemical Recurrence and Survival after Radical Prostatectomy in Patients with Prostate Cancer. PLoS ONE 2016, 11, e0154918. [Google Scholar] [CrossRef] [PubMed]
- Hsu, F.K.; Chang, W.K.; Lin, K.J.; Liu, C.Y.; Fang, W.L.; Chang, K.Y. The Associations between Perioperative Blood Transfusion and Long-Term Outcomes after Stomach Cancer Surgery. Cancers 2021, 13, 5438. [Google Scholar] [CrossRef] [PubMed]
- Murtha-Lemekhova, A.; Fuchs, J.; Ritscher, E.; Hoffmann, K. Effect of Autotransfusion in HCC Surgery on Survival and Recurrence: A Systematic Review and Meta-Analysis. Cancers 2022, 14, 4837. [Google Scholar] [CrossRef] [PubMed]
- Muller, S.D.; Both, C.P.; Sponholz, C.; Voelker, M.T.; Christiansen, H.; Niggli, F.; Schmitz, A.; Weiss, M.; Thomas, J.; Stehr, S.N.; et al. Association between Intraoperative Blood Transfusion, Regional Anesthesia and Outcome after Pediatric Tumor Surgery for Nephroblastoma. Cancers 2022, 14, 5585. [Google Scholar] [CrossRef]
- Hee, H.Z.; Chang, K.Y.; Huang, C.Y.; Chang, W.K.; Tsou, M.Y.; Lin, S.P. Perioperative Blood Transfusion Is Dose-Dependently Associated with Cancer Recurrence and Mortality after Head and Neck Cancer Surgery. Cancers 2022, 15, 99. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, J.P.; von Elm, E.; Altman, D.G.; Gotzsche, P.C.; Mulrow, C.D.; Pocock, S.J.; Poole, C.; Schlesselman, J.J.; Egger, M.; Initiative, S. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. PLoS Med. 2007, 4, e297. [Google Scholar] [CrossRef] [PubMed]
- Marina, N.M.; Smeland, S.; Bielack, S.S.; Bernstein, M.; Jovic, G.; Krailo, M.D.; Hook, J.M.; Arndt, C.; van den Berg, H.; Brennan, B.; et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): An open-label, international, randomised controlled trial. Lancet Oncol. 2016, 17, 1396–1408. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, N.; Puri, A.; Gelderblom, H. Osteosarcoma: Evolution of treatment paradigms. Sarcoma 2013, 2013, 203531. [Google Scholar] [CrossRef]
- Firth, D. Bias reduction of maximum likelihood estimates. Biometrika 1993, 80, 27–38. [Google Scholar] [CrossRef]
- Heinze, G.; Schemper, M. A solution to the problem of separation in logistic regression. Stat. Med. 2002, 21, 2409–2419. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.; Gianferante, D.M.; Zhu, B.; Mirabello, L. Osteosarcoma: A Surveillance, Epidemiology, and End Results program-based analysis from 1975 to 2017. Cancer 2022, 128, 2107–2118. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.A.; Barragan, S.; Lu, Z.; Weigel, B.J.; Spector, L.G. Sex differences in osteosarcoma survival across the age spectrum: A National Cancer Database analysis (2004–2016). Cancer Epidemiol. 2024, 92, 102565. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.B.; Gieser, P.; Goorin, A.M.; Ayala, A.; Shochat, S.J.; Ferguson, W.S.; Holbrook, T.; Link, M.P. Treatment of metastatic osteosarcoma at diagnosis: A Pediatric Oncology Group Study. J. Clin. Oncol. 1998, 16, 3641–3648. [Google Scholar] [CrossRef]
- Meyers, P.A.; Schwartz, C.L.; Krailo, M.; Kleinerman, E.S.; Betcher, D.; Bernstein, M.L.; Conrad, E.; Ferguson, W.; Gebhardt, M.; Goorin, A.M.; et al. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high-dose methotrexate. J. Clin. Oncol. 2005, 23, 2004–2011. [Google Scholar] [CrossRef] [PubMed]
- Chesi, R.; Cazzola, A.; Bacci, G.; Borghi, B.; Balladelli, A.; Urso, G. Effect of perioperative transfusions on survival in osteosarcoma treated by multimodal therapy. Cancer 1989, 64, 1727–1737. [Google Scholar] [CrossRef]
- Bassuni, W.Y.; Blajchman, M.A.; Al-Moshary, M.A. Why implement universal leukoreduction? Hematol. Oncol. Stem Cell Ther. 2008, 1, 106–123. [Google Scholar] [CrossRef]
- King, K.E.; Ness, P.M. How do we prevent transfusion-associated graft-versus-host disease in children? Transfusion 2011, 51, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, L.; Tomasulo, P.; Lipton, K.S.; Ness, P. The reintroduction of nonleukoreduced blood: Would patients and clinicians agree? Transfusion 2011, 51, 2739–2743. [Google Scholar] [CrossRef] [PubMed]
- Kopolovic, I.; Ostro, J.; Tsubota, H.; Lin, Y.; Cserti-Gazdewich, C.M.; Messner, H.A.; Keir, A.K.; DenHollander, N.; Dzik, W.S.; Callum, J. A systematic review of transfusion-associated graft-versus-host disease. Blood 2015, 126, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Kracalik, I.; Sapiano, M.R.P.; Wild, R.C.; Chavez Ortiz, J.; Stewart, P.; Berger, J.J.; Basavaraju, S.V.; Free, R.J. Supplemental findings of the 2021 National Blood Collection and Utilization Survey. Transfusion 2023, 63 (Suppl. S4), S19–S42. [Google Scholar] [CrossRef] [PubMed]
- Goubran, H.; Sheridan, D.; Radosevic, J.; Burnouf, T.; Seghatchian, J. Transfusion-related immunomodulation and cancer. Transfus. Apher. Sci. 2017, 56, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Lamora, A.; Talbot, J.; Mullard, M.; Brounais-Le Royer, B.; Redini, F.; Verrecchia, F. TGF-beta Signaling in Bone Remodeling and Osteosarcoma Progression. J. Clin. Med. 2016, 5, 96. [Google Scholar] [CrossRef]
- Tanyildiz, H.G.; Kaygusuz, G.; Unal, E.; Tacyildiz, N.; Dincaslan, H.; Yavuz, G. The prognostic importance of TGF-beta, TGF-beta receptor, and fascin in childhood solid tumors. Pediatr. Hematol. Oncol. 2017, 34, 238–253. [Google Scholar] [CrossRef] [PubMed]
- Atzil, S.; Arad, M.; Glasner, A.; Abiri, N.; Avraham, R.; Greenfeld, K.; Rosenne, E.; Beilin, B.; Ben-Eliyahu, S. Blood transfusion promotes cancer progression: A critical role for aged erythrocytes. Anesthesiology 2008, 109, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Youssef, L.A.; Spitalnik, S.L. Transfusion-related immunomodulation: A reappraisal. Curr. Opin. Hematol. 2017, 24, 551–557. [Google Scholar] [CrossRef]
- Remy, K.E.; Sun, J.; Wang, D.; Welsh, J.; Solomon, S.B.; Klein, H.G.; Natanson, C.; Cortes-Puch, I. Transfusion of recently donated (fresh) red blood cells (RBCs) does not improve survival in comparison with current practice, while safety of the oldest stored units is yet to be established: A meta-analysis. Vox Sang. 2016, 111, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Cocucci, E.; Racchetti, G.; Meldolesi, J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009, 19, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Lee, J.H.; Tai, Y.P.; Wen, C.; Wu, S.B.; Tsai, M.K.; Hsieh, D.P.; Chiang, H.C.; Hsiung, C.A.; Hsu, C.Y.; et al. High serum iron is associated with increased cancer risk. Cancer Res. 2014, 74, 6589–6597. [Google Scholar] [CrossRef] [PubMed]
- Sawicka-Zukowska, M.; Kretowska-Grunwald, A.; Topczewska, M.; Krawczuk-Rybak, M.; Grubczak, K. Is Serum Ferritin a Predictor of Blood Transfusions Outcome and Survival in Childhood Lymphomas and Solid Tumors? Cancers 2024, 16, 3742. [Google Scholar] [CrossRef]
- Haemmerle, M.; Stone, R.L.; Menter, D.G.; Afshar-Kharghan, V.; Sood, A.K. The Platelet Lifeline to Cancer: Challenges and Opportunities. Cancer Cell 2018, 33, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Stone, R.L.; Nick, A.M.; McNeish, I.A.; Balkwill, F.; Han, H.D.; Bottsford-Miller, J.; Rupairmoole, R.; Armaiz-Pena, G.N.; Pecot, C.V.; Coward, J.; et al. Paraneoplastic thrombocytosis in ovarian cancer. N. Engl. J. Med. 2012, 366, 610–618. [Google Scholar] [CrossRef]
- Pedersen, L.M.; Milman, N. Prognostic significance of thrombocytosis in patients with primary lung cancer. Eur. Respir. J. 1996, 9, 1826–1830. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Kawai, K.; Tsuno, N.H.; Sunami, E.; Kitayama, J. Impact of Preoperative Thrombocytosis on the Survival of Patients with Primary Colorectal Cancer. World J. Surg. 2012, 36, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Furukawa, H.; Imamura, H.; Shimizu, J.; Ishida, H.; Masutani, S.; Tatsuta, M.; Satomi, T. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann. Surg. Oncol. 2002, 9, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Taucher, S.; Salat, A.; Gnant, M.; Kwasny, W.; Mlineritsch, B.; Menzel, R.-C.; Schmid, M.; Smola, M.G.; Stierer, M.; Tausch, C.; et al. Impact of pretreatment thrombocytosis on survival in primary breast cancer. Thromb. Haemost. 2003, 89, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Palmerini, E.; Staals, E.; Abate, M.E.; Longhi, A.; Cesari, M.; Balladelli, A.; Pratelli, L.; Bacci, G. Sex- and age-related chemotherapy toxicity in patients with non-metastatic osteosarcoma. J. Chemother. 2009, 21, 205–210. [Google Scholar] [CrossRef] [PubMed]


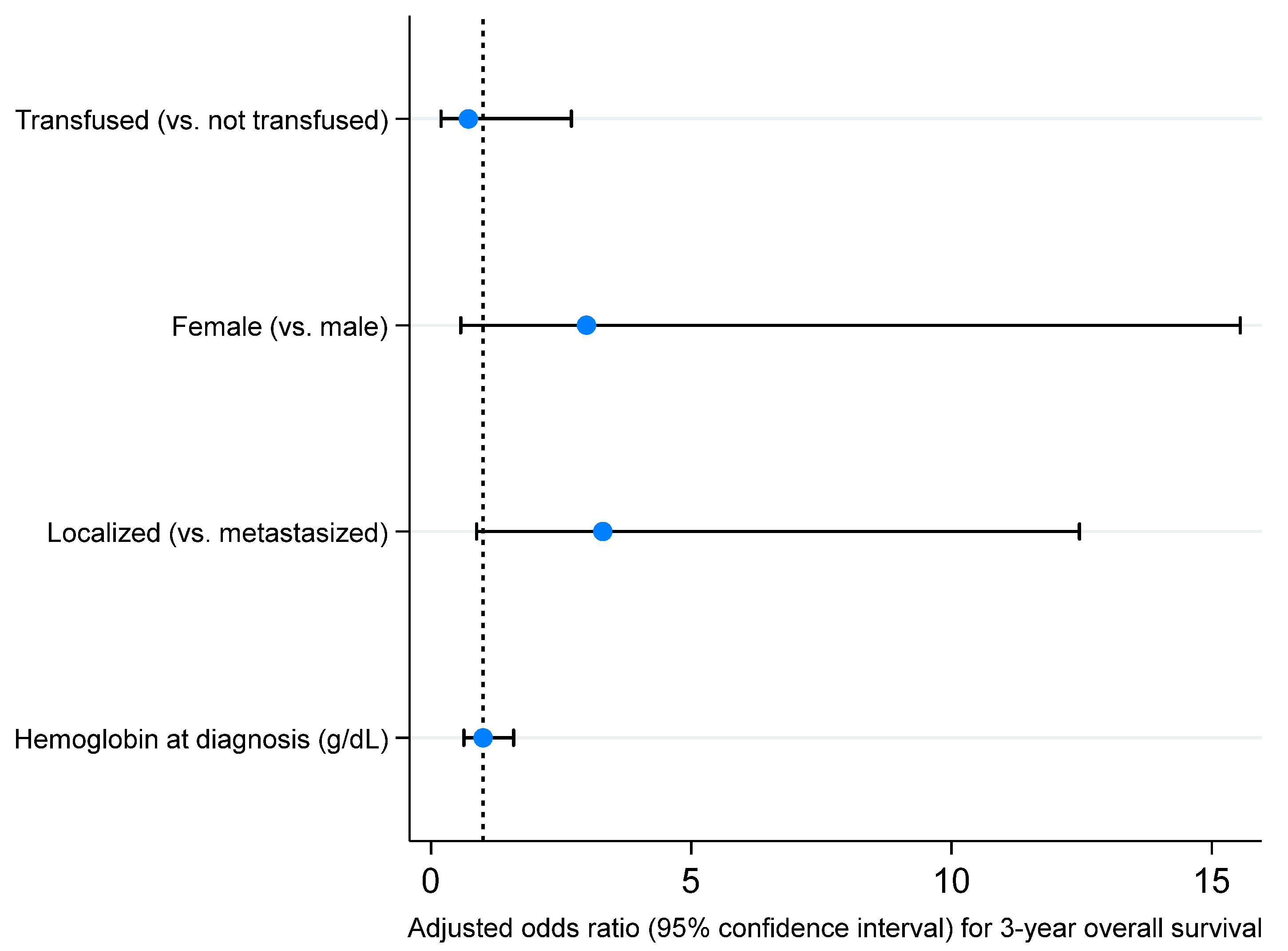

| Characteristic, Statistic | Total (n = 73) | Transfusion (n = 34) | No Transfusion (n = 39) | p-Value |
|---|---|---|---|---|
| Age at diagnosis, median (range) | 15 (4 to 29) | 14 (4 to 29) | 15 (9 to 28) | 0.50 |
| Sex, n (%) | ||||
| Male | 45 (61.6) | 16 (47.1) | 29 (74.4) | 0.02 |
| Female | 28 (38.4) | 18 (52.9) | 10 (25.6) | |
| Race, n (%) | ||||
| Black or African American | 12 (16.4) | 5 (14.7) | 7 (18.0) | 0.40 |
| White | 50 (68.5) | 27 (79.4) | 23 (59.0) | |
| Asian | 3 (4.1) | 1 (2.9) | 2 (5.1) | |
| Native Hawaiian or Other Pacific Islander | 1 (1.4) | 0 (0) | 1 (2.6) | |
| Multiracial | 2 (2.7) | 0 (0) | 2 (5.1) | |
| Unknown/Missing | 3 (4.1) | 1 (2.9) | 2 (5.1) | |
| Ethnicity, n (%) | ||||
| Hispanic/Latinx | 12 (16.4) | 4 (11.8) | 8 (20.5) | 0.52 |
| Non-Hispanic/Latinx | 39 (53.4) | 18 (52.9) | 21 (53.9) | |
| Unknown/Missing | 22 (30.1) | 12 (35.3) | 10 (25.6) | |
| Mortality during study period, n (%) | 19 (26.0) | 11 (32.4) | 8 (20.5) | 0.25 |
| Hemoglobin at diagnosis, median (range) * | 13.3 (8.8 to 16) | 12.4 (9.7 to 14.7) | 13.9 (8.8 to 16) | 0.002 |
| Ifosfamide as first-line chemotherapy, n (%) | ||||
| Yes | 4 (5.5) | 3 (8.8) | 1 (2.6) | 0.33 |
| No | 69 (94.5) | 31 (91.2) | 38 (97.4) | |
| Radiation during initial treatment, n (%) | ||||
| Yes | 3 (4.1) | 2 (5.9) | 1 (2.6) | 0.59 |
| No | 70 (95.9) | 32 (94.1) | 38 (97.4) | |
| Stage at osteosarcoma diagnosis, n (%) | ||||
| Localized | 59 (80.8) | 26 (76.5) | 33 (84.6) | 0.38 |
| Metastatic | 14 (19.2) | 8 (23.5) | 6 (15.4) | |
| Location of osteosarcoma, n (%) | ||||
| Extremity | 68 (93.2) | 31 (91.2) | 37 (94.9) | 0.66 |
| Non-extremity | 5 (6.9) | 3 (8.8) | 2 (5.1) | |
| Histologic response to neoadjuvant chemotherapy, n (%) | ||||
| Good | 36 (49.3) | 16 (47.1) | 20 (51.3) | 0.94 |
| Poor | 31 (42.5) | 15 (44.1) | 16 (41.0) | |
| Unknown/Missing | 6 (8.2) | 3 (8.8) | 3 (7.7) | |
| Secondary malignancy, n (%) | ||||
| Yes | 2 (2.7) | 0 (0) | 2 (5.1) | 0.50 |
| No | 71 (97.3) | 34 (100) | 37 (94.9) | |
| Transfusion type, n (%) | ||||
| Platelets only | - | 4 (11.8) | - | |
| Red blood cells only | - | 20 (58.2) | - | |
| Platelets and red blood cells | - | 10 (29.4) | - | |
| Transfusion instance, n (%) | ||||
| 1 | - | 17 (50) | - | |
| 2 | - | 7 (20.6) | - | |
| 3 | - | 3 (8.8) | - | |
| 4+ | - | 7 (20.6) | - | |
| Transfusion instance, median (range) | - | 1 (1 to 6) | - |
| Survival Outcome | Survival Rate (95% CI) | p-Value | ||
|---|---|---|---|---|
| Total (n = 73) | Transfusion (n = 34) | No Transfusion (n = 39) | ||
| Event-free survival | ||||
| 3-year | 74.1% (61.0% to 83.3%) | 74.9% (55.9% to 86.6%) | 73.6% (53.6% to 86%) | 0.92 |
| 5-year | 52.2% (36.9% to 65.5%) | 58.2% (37.5% to 74.2%) | 41.7% (19.7% to 62.5%) | 0.60 |
| Overall survival | ||||
| 3-year | 81.0% (68.9% to 88.8%) | 81.2% (62.7% to 91.1%) | 81.0% (62.4% to 91.0%) | 0.99 |
| 5-year | 75.7% (61.7% to 85.1%) | 76.7% (56.6% to 88.3%) | 70.8% (48.7% to 84.7%) | 0.77 |
| Survival Outcome | Survival Rate (95% CI) | p-Value | Survival Rate (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|
| RBC Transfusion (n = 30) | No RBC Transfusion (n = 43) | Platelet Transfusion (n = 14) | No Platelet Transfusion (n = 59) | |||
| Event-free survival | ||||||
| 3-year |
75.6% (55.5% to 87.6%) |
73.1% (54.3% to 85.1%) | 0.86 |
91.7% (53.9% to 98.8%) |
70.1% (55.0% to 81.0%) | 0.17 |
| 5-year |
62.3% (40.7% to 77.9%) |
37.5% (16.6% to 58.4%) | 0.35 |
61.1% (25.5% to 83.8%) |
49.1% (32.4% to 63.9%) | 0.65 |
| Overall survival | ||||||
| 3-year |
79.4% (59.7% to 90.2%) |
82.6% (65.2% to 91.8%) | 0.81 | 100% |
76.8% (62.6% to 86.2%) | 0.09 |
| 5-year |
74.5% (53.2% to 87.1%) |
73.4% (52.6% to 86.1%) | 0.96 |
90% (47.3% to 98.5%) |
70.4% (54.3% to 81.7%) | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, S.; Fierstein, J.L.; Khalaf, R.T.; Morrison, J.M.; Metts, J. Blood Transfusion and Survival of Children, Adolescent, and Young Adult Patients with Osteosarcoma: A Multicenter Retrospective Cohort Study. Cancers 2025, 17, 97. https://doi.org/10.3390/cancers17010097
Cho S, Fierstein JL, Khalaf RT, Morrison JM, Metts J. Blood Transfusion and Survival of Children, Adolescent, and Young Adult Patients with Osteosarcoma: A Multicenter Retrospective Cohort Study. Cancers. 2025; 17(1):97. https://doi.org/10.3390/cancers17010097
Chicago/Turabian StyleCho, Sukjoo, Jamie L. Fierstein, Racha T. Khalaf, John M. Morrison, and Jonathan Metts. 2025. "Blood Transfusion and Survival of Children, Adolescent, and Young Adult Patients with Osteosarcoma: A Multicenter Retrospective Cohort Study" Cancers 17, no. 1: 97. https://doi.org/10.3390/cancers17010097
APA StyleCho, S., Fierstein, J. L., Khalaf, R. T., Morrison, J. M., & Metts, J. (2025). Blood Transfusion and Survival of Children, Adolescent, and Young Adult Patients with Osteosarcoma: A Multicenter Retrospective Cohort Study. Cancers, 17(1), 97. https://doi.org/10.3390/cancers17010097





