Advancing Colorectal Cancer Prevention in Inflammatory Bowel Disease (IBD): Challenges and Innovations in Endoscopic Surveillance
Simple Summary
Abstract
1. Introduction
2. Standard Endoscopic Practice
2.1. White Light Endoscopy
2.2. Chromoendoscopy
2.3. International Guidenlines
3. Optical Diagnosis Training for IBD
3.1. Transitioning to VCE
3.2. Competence and Maintenance
3.3. Classification Systems for Dysplasia Detection
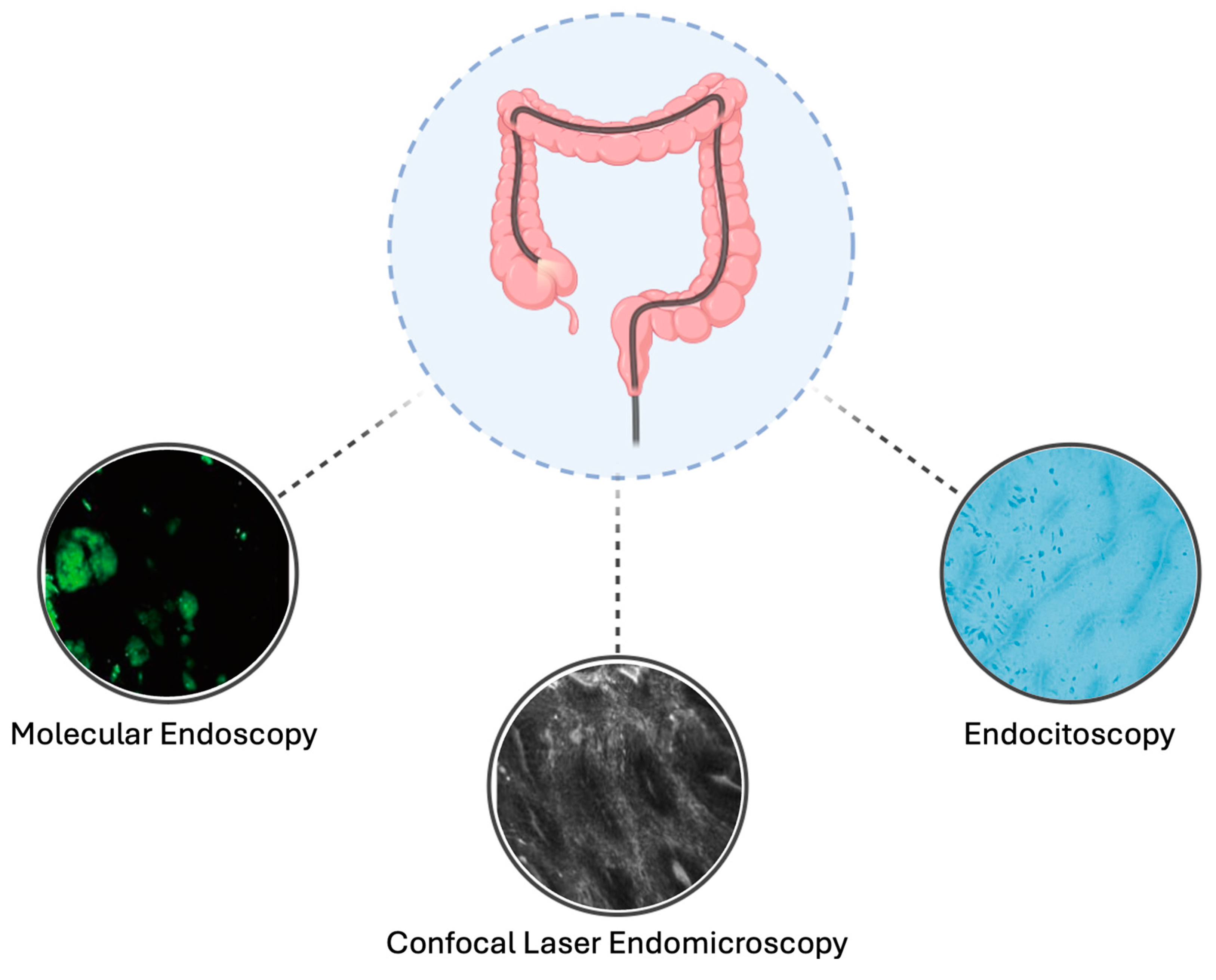
4. Advanced Endoscopic Imaging Technologies
4.1. Endocytoscopy
4.2. Confocal Laser Endomicroscopy
4.3. Molecular Endoscopy
5. Artificial Intelligence in Endoscopic Surveillance
6. Optimization of Endoscopic Surveillance Protocols
6.1. Sedation
6.2. Bowel Preparation
7. Discussion
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Laredo, V.; García-Mateo, S.; Martínez-Domínguez, S.J.; López de la Cruz, J.; Gargallo-Puyuelo, C.J.; Gomollón, F. Risk of Cancer in Patients with Inflammatory Bowel Diseases and Keys for Patient Management. Cancers 2023, 15, 871. [Google Scholar] [CrossRef] [PubMed]
- Birch, R.J.; Burr, N.; Subramanian, V.; Tiernan, J.P.; Hull, M.A.; Finan, P.; Rose, A.; Rutter, M.; Valori, R.; Downing, A.; et al. Inflammatory Bowel Disease-Associated Colorectal Cancer Epidemiology and Outcomes: An English Population-Based Study. Am. J. Gastroenterol. 2022, 117, 1858–1870. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, F.; Fasulo, E.; Jairath, V.; Paridaens, K.; Peyrin-Biroulet, L.; Danese, S. Management and Treatment Optimization of Patients with Mild to Moderate Ulcerative Colitis. Expert. Rev. Clin. Immunol. 2024, 20, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Tsujinaka, S.; Miura, T.; Kitamura, Y.; Suzuki, H.; Shibata, C. Inflammatory Bowel Disease and Colorectal Cancer: Epidemiology, Etiology, Surveillance, and Management. Cancers 2023, 15, 4154. [Google Scholar] [CrossRef]
- Dyson, J.K.; Rutter, M.D. Colorectal Cancer in Inflammatory Bowel Disease: What Is the Real Magnitude of the Risk? World J. Gastroenterol. 2012, 18, 3839–3848. [Google Scholar] [CrossRef]
- Porter, R.J.; Arends, M.J.; Churchhouse, A.M.D.; Din, S. Inflammatory Bowel Disease-Associated Colorectal Cancer: Translational Risks from Mechanisms to Medicines. J. Crohns Colitis 2021, 15, 2131–2141. [Google Scholar] [CrossRef]
- Fumery, M.; Dulai, P.S.; Gupta, S.; Prokop, L.J.; Ramamoorthy, S.; Sandborn, W.J.; Singh, S. Incidence, Risk Factors, and Outcomes of Colorectal Cancer in Patients With Ulcerative Colitis With Low-Grade Dysplasia: A Systematic Review and Meta-Analysis. Clin. Gastroenterol. Hepatol. 2017, 15, 665–674.e5. [Google Scholar] [CrossRef]
- Shahgoli, V.K.; Noorolyai, S.; Ahmadpour Youshanlui, M.; Saeidi, H.; Nasiri, H.; Mansoori, B.; Holmskov, U.; Baradaran, B. Inflammatory Bowel Disease, Colitis, and Cancer: Unmasking the Chronic Inflammation Link. Int. J. Colorectal Dis. 2024, 39, 173. [Google Scholar] [CrossRef] [PubMed]
- Al Bakir, I.; Curtius, K.; Graham, T.A. From Colitis to Cancer: An Evolutionary Trajectory That Merges Maths and Biology. Front. Immunol. 2018, 9, 2368. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Kim, J.J.; Shen, J.; Chen, B.; Dai, N. KRAS and TP53 Mutations in Inflammatory Bowel Disease-Associated Colorectal Cancer: A Meta-Analysis. Oncotarget 2017, 8, 22175–22186. [Google Scholar] [CrossRef]
- Wang, Z.; Chang, Y.; Sun, H.; Li, Y.; Tang, T. Advances in Molecular Mechanisms of Inflammatory Bowel Disease-associated Colorectal Cancer. Oncol. Lett. 2024, 27, 257. [Google Scholar] [CrossRef] [PubMed]
- Jess, T.; Rungoe, C.; Peyrin-Biroulet, L. Risk of Colorectal Cancer in Patients with Ulcerative Colitis: A Meta-Analysis of Population-Based Cohort Studies. Clin. Gastroenterol. Hepatol. 2012, 10, 639–645. [Google Scholar] [CrossRef] [PubMed]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The Risk of Colorectal Cancer in Ulcerative Colitis: A Meta-Analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [PubMed]
- Picardo, S.; Venugopal, K.; Cheng, W.; Ragunath, K. Adherence to Endoscopic Surveillance Guidelines for Patients with Inflammatory Bowel Disease: An Australian Cohort Study. J. Gastro Hepatol. 2024, 39, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Fefferman, D.S.; Farrell, R.J. Endoscopy in Inflammatory Bowel Disease: Indications, Surveillance, and Use in Clinical Practice. Clin. Gastroenterol. Hepatol. 2005, 3, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Al Bakir, I.; Kabir, M.; Yalchin, M.; Hart, A. Optimising Inflammatory Bowel Disease Surveillance and Dysplasia Management—Where Do We Stand? UEG J. 2022, 10, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- Coelho-Prabhu, N.; Lewis, J.D. Update on Endoscopic Dysplasia Surveillance in Inflammatory Bowel Disease. Am. J. Gastroenterol. 2023, 118, 1748–1755. [Google Scholar] [CrossRef]
- Gabbiadini, R.; D’Amico, F.; De Marco, A.; Terrin, M.; Zilli, A.; Furfaro, F.; Allocca, M.; Fiorino, G.; Danese, S. Colorectal Cancer Surveillance in Patients with Inflammatory Bowel Diseases: Chromoendoscopy or Non-Chromoendoscopy, That Is the Question. J. Clin. Med. 2022, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Ten Hove, J.R.; Mooiweer, E.; van der Meulen de Jong, A.E.; Dekker, E.; Ponsioen, C.Y.; Siersema, P.D.; Oldenburg, B. Clinical Implications of Low Grade Dysplasia Found during Inflammatory Bowel Disease Surveillance: A Retrospective Study Comparing Chromoendoscopy and White-Light Endoscopy. Endoscopy 2017, 49, 161–168. [Google Scholar] [CrossRef]
- Van Den Broek, F.J.C.; Stokkers, P.C.F.; Reitsma, J.B.; Boltjes, R.P.B.; Ponsioen, C.Y.; Fockens, P.; Dekker, E. Random Biopsies Taken During Colonoscopic Surveillance of Patients With Longstanding Ulcerative Colitis: Low Yield and Absence of Clinical Consequences. Am. J. Gastroenterol. 2014, 109, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Na, S.-Y.; Moon, W. Recent Advances in Surveillance Colonoscopy for Dysplasia in Inflammatory Bowel Disease. Clin. Endosc. 2022, 55, 726–735. [Google Scholar] [CrossRef]
- Iacucci, M.; Kaplan, G.G.; Panaccione, R.; Akinola, O.; Lethebe, B.C.; Lowerison, M.; Leung, Y.; Novak, K.L.; Seow, C.H.; Urbanski, S.; et al. A Randomized Trial Comparing High Definition Colonoscopy Alone With High Definition Dye Spraying and Electronic Virtual Chromoendoscopy for Detection of Colonic Neoplastic Lesions During IBD Surveillance Colonoscopy. Am. J. Gastroenterol. 2018, 113, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.-H.; Park, S.J.; Kim, H.-S.; Park, Y.S.; Park, D.I.; Lee, K.-M.; Jung, S.-A.; Choi, C.H.; Koo, J.S.; Cheon, J.H.; et al. High-Definition Chromoendoscopy Versus High-Definition White Light Colonoscopy for Neoplasia Surveillance in Ulcerative Colitis: A Randomized Controlled Trial. Am. J. Gastroenterol. 2019, 114, 1642–1648. [Google Scholar] [CrossRef] [PubMed]
- Derks, M.E.W.; Te Groen, M.; van Lierop, L.M.A.; Murthy, S.; Rubin, D.T.; Bessissow, T.; Nagtegaal, I.D.; Bemelman, W.A.; Derikx, L.A.A.P.; Hoentjen, F. Management of Colorectal Neoplasia in IBD Patients: Current Practice and Future Perspectives. J. Crohns Colitis 2024, 18, 1726–1735. [Google Scholar] [CrossRef] [PubMed]
- Wijnands, A.M.; Mahmoud, R.; Lutgens, M.W.M.D.; Oldenburg, B. Surveillance and Management of Colorectal Dysplasia and Cancer in Inflammatory Bowel Disease: Current Practice and Future Perspectives. Eur. J. Intern. Med. 2021, 93, 35–41. [Google Scholar] [CrossRef]
- Murthy, S.K.; Feuerstein, J.D.; Nguyen, G.C.; Velayos, F.S. AGA Clinical Practice Update on Endoscopic Surveillance and Management of Colorectal Dysplasia in Inflammatory Bowel Diseases: Expert Review. Gastroenterology 2021, 161, 1043–1051.e4. [Google Scholar] [CrossRef]
- Rabinowitz, L.G.; Kumta, N.A.; Marion, J.F. Beyond the SCENIC Route: Updates in Chromoendoscopy and Dysplasia Screening in Patients with Inflammatory Bowel Disease. Gastrointest. Endosc. 2022, 95, 30–37. [Google Scholar] [CrossRef]
- Resende, R.H.; Ribeiro, I.B.; de Moura, D.T.H.; Galetti, F.; Rocha, R.S. de P.; Bernardo, W.M.; Sakai, P.; de Moura, E.G.H. Surveillance in Inflammatory Bowel Disease: Is Chromoendoscopy the Only Way to Go? A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Endosc. Int. Open 2020, 8, E578–E590. [Google Scholar] [CrossRef]
- Alexandersson, B.; Hamad, Y.; Andreasson, A.; Rubio, C.A.; Ando, Y.; Tanaka, K.; Ichiya, T.; Rezaie, R.; Schmidt, P.T. High-Definition Chromoendoscopy Superior to High-Definition White-Light Endoscopy in Surveillance of Inflammatory Bowel Diseases in a Randomized Trial. Clin. Gastroenterol. Hepatol. 2020, 18, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Carballal, S.; Maisterra, S.; López-Serrano, A.; Gimeno-García, A.Z.; Vera, M.I.; Marín-Garbriel, J.C.; Díaz-Tasende, J.; Márquez, L.; Álvarez, M.A.; Hernández, L.; et al. Real-Life Chromoendoscopy for Neoplasia Detection and Characterisation in Long-Standing IBD. Gut 2018, 67, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.F.H.; Marino, D.; Elfert, K.; Beran, A.; Nayfeh, T.; Abdallah, M.A.; Sultan, S.; Shah, S.A. Dye Chromoendoscopy Outperforms High-Definition White Light Endoscopy in Dysplasia Detection for Patients With Inflammatory Bowel Disease: An Updated Meta-Analysis of Randomized Controlled Trials. Am. J. Gastroenterol. 2024, 119, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Gallinger, Z.R.; Rumman, A.; Murthy, S.K.; Nguyen, G.C. Perspectives on Endoscopic Surveillance of Dysplasia in Inflammatory Bowel Disease: A Survey of Academic Gastroenterologists. Endosc. Int. Open 2017, 5, E974–E979. [Google Scholar] [CrossRef]
- Parigi, T.L.; Nardone, O.M.; Iacucci, M. Image-Enhanced Endoscopy Surveillance of Colon and Pouch Dysplasia in IBD. Dis. Colon. Rectum 2022, 65, S119–S128. [Google Scholar] [CrossRef]
- Sinonquel, P.; Vermeire, S.; Maes, F.; Bisschops, R. Advanced Imaging in Gastrointestinal Endoscopy: A Literature Review of the Current State of the Art. GE Port. J. Gastroenterol. 2023, 30, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Shahsavari, D.; Waqar, M.; Thoguluva Chandrasekar, V. Image Enhanced Colonoscopy: Updates and Prospects-a Review. Transl. Gastroenterol. Hepatol. 2023, 8, 26. [Google Scholar] [CrossRef]
- Bisschops, R.; Bessissow, T.; Joseph, J.A.; Baert, F.; Ferrante, M.; Ballet, V.; Willekens, H.; Demedts, I.; Geboes, K.; De Hertogh, G.; et al. Chromoendoscopy versus Narrow Band Imaging in UC: A Prospective Randomised Controlled Trial. Gut 2018, 67, 1087–1094. [Google Scholar] [CrossRef]
- Shah, S.C.; Itzkowitz, S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology 2022, 162, 715–730.e3. [Google Scholar] [CrossRef] [PubMed]
- Fanizza, J.; Bencardino, S.; Allocca, M.; Furfaro, F.; Zilli, A.; Parigi, T.L.; Fiorino, G.; Peyrin-Biroulet, L.; Danese, S.; D’Amico, F. Inflammatory Bowel Disease and Colorectal Cancer. Cancers 2024, 16, 2943. [Google Scholar] [CrossRef] [PubMed]
- Fatakhova, K.; Rajapakse, R. From Random to Precise: Updated Colon Cancer Screening and Surveillance for Inflammatory Bowel Disease. Transl. Gastroenterol. Hepatol. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Laine, L.; Kaltenbach, T.; Barkun, A.; McQuaid, K.R.; Subramanian, V.; Soetikno, R.; East, J.E.; Farraye, F.A.; Feagan, B.; Ioannidis, J.; et al. SCENIC International Consensus Statement on Surveillance and Management of Dysplasia in Inflammatory Bowel Disease. Gastroenterology 2015, 148, 639–651.e28. [Google Scholar] [CrossRef]
- Gordon, H.; Biancone, L.; Fiorino, G.; Katsanos, K.H.; Kopylov, U.; Al Sulais, E.; Axelrad, J.E.; Balendran, K.; Burisch, J.; de Ridder, L.; et al. ECCO Guidelines on Inflammatory Bowel Disease and Malignancies. J. Crohns Colitis 2023, 17, 827–854. [Google Scholar] [CrossRef] [PubMed]
- Lamb, C.A.; Kennedy, N.A.; Raine, T.; Hendy, P.A.; Smith, P.J.; Limdi, J.K.; Hayee, B.; Lomer, M.C.E.; Parkes, G.C.; Selinger, C.; et al. British Society of Gastroenterology Consensus Guidelines on the Management of Inflammatory Bowel Disease in Adults. Gut 2019, 68, s1–s106. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Houwen, B.B.S.L.; Puig, I.; Bustamante-Balén, M.; Coron, E.; Dobru, D.E.; Kuvaev, R.; Neumann, H.; Johnson, G.; Pimentel-Nunes, P.; et al. Curriculum for Optical Diagnosis Training in Europe: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2020, 52, 899–923. [Google Scholar] [CrossRef] [PubMed]
- Leong, R.W.; Butcher, R.O.; Picco, M.F. Implementation of Image-Enhanced Endoscopy into Solo and Group Practices for Dysplasia Detection in Crohn’s Disease and Ulcerative Colitis. Gastrointest. Endosc. Clin. N. Am. 2014, 24, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Picco, M.F.; Pasha, S.; Leighton, J.A.; Bruining, D.; Loftus, E.V.; Thomas, C.S.; Crook, J.E.; Krishna, M.; Wallace, M. Procedure Time and the Determination of Polypoid Abnormalities with Experience: Implementation of a Chromoendoscopy Program for Surveillance Colonoscopy for Ulcerative Colitis. Inflamm. Bowel Dis. 2013, 19, 1913–1920. [Google Scholar] [CrossRef]
- Sanduleanu, S.; Rondagh, E.J.A.; Masclee, A.A.M. Development of Expertise in the Detection and Classification of Non-Polypoid Colorectal Neoplasia: Experience-Based Data at an Academic GI Unit. Gastrointest. Endosc. Clin. N. Am. 2010, 20, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbach, T.R.; Soetikno, R.M.; DeVivo, R.; Laine, L.A.; Barkun, A.; McQuaid, K.R.; Optimizing Quality of Endoscopy in IBD Group. Optimizing the Quality of Endoscopy in Inflammatory Bowel Disease: Focus on Surveillance and Management of Colorectal Dysplasia Using Interactive Image- and Video-Based Teaching. Gastrointest. Endosc. 2017, 86, 1107–1117.e1. [Google Scholar] [CrossRef] [PubMed]
- Har-Noy, O.; Katz, L.; Avni, T.; Battat, R.; Bessissow, T.; Yung, D.E.; Engel, T.; Koulaouzidis, A.; Eliakim, R.; Ben-Horin, S.; et al. Chromoendoscopy, Narrow-Band Imaging or White Light Endoscopy for Neoplasia Detection in Inflammatory Bowel Diseases. Dig. Dis. Sci. 2017, 62, 2982–2990. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Hirota, S.; Nakajima, T.; Hosobe, S.; Kusaka, H.; Kobayashi, T.; Himori, M.; Yagyuu, A. Colorectal Tumours and Pit Pattern. J. Clin. Pathol. 1994, 47, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Iacucci, M.; McQuaid, K.; Gui, X.S.; Iwao, Y.; Lethebe, B.C.; Lowerison, M.; Matsumoto, T.; Shivaji, U.N.; Smith, S.C.L.; Subramanian, V.; et al. A Multimodal (FACILE) Classification for Optical Diagnosis of Inflammatory Bowel Disease Associated Neoplasia. Endoscopy 2019, 51, 133–141. [Google Scholar] [CrossRef]
- Bisschops, R.; Bessissow, T.; Dekker, E.; East, J.E.; Para-Blanco, A.; Ragunath, K.; Bhandari, P.; Rutter, M.; Schoon, E.; Wilson, A.; et al. Pit Pattern Analysis with High-Definition Chromoendoscopy and Narrow-Band Imaging for Optical Diagnosis of Dysplasia in Patients with Ulcerative Colitis. Gastrointest. Endosc. 2017, 86, 1100–1106.e1. [Google Scholar] [CrossRef]
- Zammarchi, I.; Santacroce, G.; Iacucci, M. Next-Generation Endoscopy in Inflammatory Bowel Disease. Diagnostics 2023, 13, 2547. [Google Scholar] [CrossRef]
- Lucaciu, L.A.; Despott, E.J. Advanced Endoscopic Imaging for Detection of Dysplasia in Inflammatory Bowel Disease. Gastrointest. Endosc. Clin. N. Am. 2025, 35, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, S.; Kusaba, Y.; Tsuruta, O. Use of Endocytoscopy for Ulcerative Colitis Surveillance: A Case Study. Gastroenterology 2020, 158, e1–e2. [Google Scholar] [CrossRef]
- Misawa, M.; Kudo, S.; Ogata, N. Two Cases of Colitis-associated Neoplasia Observed with Endocytoscopy. Dig. Endosc. 2019, 31, 43–44. [Google Scholar] [CrossRef]
- Kudo, S.; Maeda, Y.; Ogata, N.; Misawa, M.; Ogawa, Y.; Takishima, K.; Ishiyama, M.; Mochizuki, K.; Minegishi, Y.; Ogura, Y.; et al. Combined Endocytoscopy with Pit Pattern Diagnosis in Ulcerative Colitis-associated Neoplasia: Pilot Study. Dig. Endosc. 2022, 34, 133–143. [Google Scholar] [CrossRef]
- Pilonis, N.D.; Januszewicz, W.; di Pietro, M. Confocal Laser Endomicroscopy in Gastro-Intestinal Endoscopy: Technical Aspects and Clinical Applications. Transl. Gastroenterol. Hepatol. 2022, 7, 7. [Google Scholar] [CrossRef]
- Kiesslich, R.; Burg, J.; Vieth, M.; Gnaendiger, J.; Enders, M.; Delaney, P.; Polglase, A.; McLaren, W.; Janell, D.; Thomas, S.; et al. Confocal Laser Endoscopy for Diagnosing Intraepithelial Neoplasias and Colorectal Cancer in Vivo. Gastroenterology 2004, 127, 706–713. [Google Scholar] [CrossRef]
- Malik, M.M.U.D.; Alqahtani, M.M.; Hadadi, I.; Kanbayti, I.; Alawaji, Z.; Aloufi, B.A. Molecular Imaging Biomarkers for Early Cancer Detection: A Systematic Review of Emerging Technologies and Clinical Applications. Diagnostics 2024, 14, 2459. [Google Scholar] [CrossRef] [PubMed]
- Kiesslich, R.; Goetz, M.; Lammersdorf, K.; Schneider, C.; Burg, J.; Stolte, M.; Vieth, M.; Nafe, B.; Galle, P.R.; Neurath, M.F. Chromoscopy-Guided Endomicroscopy Increases the Diagnostic Yield of Intraepithelial Neoplasia in Ulcerative Colitis. Gastroenterology 2007, 132, 874–882. [Google Scholar] [CrossRef] [PubMed]
- Rispo, A.; Castiglione, F.; Staibano, S.; Esposito, D.; Maione, F.; Siano, M.; Salvatori, F.; Masone, S.; Persico, M.; De Palma, G.D. Diagnostic Accuracy of Confocal Laser Endomicroscopy in Diagnosing Dysplasia in Patients Affected by Long-Standing Ulcerative Colitis. World J. Gastrointest. Endosc. 2012, 4, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Dlugosz, A.; Barakat, A.M.; Björkström, N.K.; Öst, Å.; Bergquist, A. Diagnostic Yield of Endomicroscopy for Dysplasia in Primary Sclerosing Cholangitis Associated Inflammatory Bowel Disease: A Feasibility Study. Endosc. Int. Open 2016, 4, E901–E911. [Google Scholar] [CrossRef] [PubMed]
- Hlavaty, T.; Huorka, M.; Koller, T.; Zita, P.; Kresanova, E.; Rychly, B.; Toth, J. Colorectal Cancer Screening in Patients with Ulcerative and Crohn’s Colitis with Use of Colonoscopy, Chromoendoscopy and Confocal Endomicroscopy. Eur. J. Gastroenterol. Hepatol. 2011, 23, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Wanders, L.K.; Kuiper, T.; Kiesslich, R.; Karstensen, J.G.; Leong, R.W.; Dekker, E.; Bisschops, R. Limited Applicability of Chromoendoscopy-Guided Confocal Laser Endomicroscopy as Daily-Practice Surveillance Strategy in Crohn’s Disease. Gastrointest. Endosc. 2016, 83, 966–971. [Google Scholar] [CrossRef]
- Neumann, H.; Kiesslich, R.; Wallace, M.B.; Neurath, M.F. Confocal Laser Endomicroscopy: Technical Advances and Clinical Applications. Gastroenterology 2010, 139, 388–392.e2. [Google Scholar] [CrossRef] [PubMed]
- Günther, U.; Kusch, D.; Heller, F.; Bürgel, N.; Leonhardt, S.; Daum, S.; Siegmund, B.; Loddenkemper, C.; Grünbaum, M.; Buhr, H.-J.; et al. Surveillance Colonoscopy in Patients with Inflammatory Bowel Disease: Comparison of Random Biopsy vs. Targeted Biopsy Protocols. Int. J. Colorectal Dis. 2011, 26, 667–672. [Google Scholar] [CrossRef]
- van den Broek, F.J.C.; van Es, J.A.; van Eeden, S.; Stokkers, P.C.F.; Ponsioen, C.Y.; Reitsma, J.B.; Fockens, P.; Dekker, E. Pilot Study of Probe-Based Confocal Laser Endomicroscopy during Colonoscopic Surveillance of Patients with Longstanding Ulcerative Colitis. Endoscopy 2011, 43, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.; Wang, T.D. Molecular Imaging in Gastrointestinal Endoscopy. Gastroenterology 2010, 138, 828–833.e1. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wang, T.D. Molecular Endoscopy for Targeted Imaging in the Digestive Tract. Lancet Gastroenterol. Hepatol. 2016, 1, 147–155. [Google Scholar] [CrossRef]
- Gounaris, E.; Martin, J.; Ishihara, Y.; Khan, M.W.; Lee, G.; Sinh, P.; Chen, E.Z.; Angarone, M.; Weissleder, R.; Khazaie, K.; et al. Fluorescence Endoscopy of Cathepsin Activity Discriminates Dysplasia from Colitis. Inflamm. Bowel Dis. 2013, 19, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Mitsunaga, M.; Kosaka, N.; Choyke, P.L.; Young, M.R.; Dextras, C.R.; Saud, S.M.; Colburn, N.H.; Sakabe, M.; Nagano, T.; Asanuma, D.; et al. Fluorescence Endoscopic Detection of Murine Colitis-Associated Colon Cancer by Topically Applied Enzymatically Rapid-Activatable Probe. Gut 2013, 62, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- De Palma, G.D.; Colavita, I.; Zambrano, G.; Giglio, M.C.; Maione, F.; Luglio, G.; Sarnelli, G.; Rispo, A.; Schettino, P.; D’Armiento, F.P.; et al. Detection of Colonic Dysplasia in Patients with Ulcerative Colitis Using a Targeted Fluorescent Peptide and Confocal Laser Endomicroscopy: A Pilot Study. PLoS ONE 2017, 12, e0180509. [Google Scholar] [CrossRef]
- Iacucci, M.; Santacroce, G.; Majumder, S.; Morael, J.; Zammarchi, I.; Maeda, Y.; Ryan, D.; Di Sabatino, A.; Rescigno, M.; Aburto, M.R.; et al. Opening the Doors of Precision Medicine: Novel Tools to Assess Intestinal Barrier in Inflammatory Bowel Disease and Colitis-Associated Neoplasia. Gut 2024, 73, 1749–1762. [Google Scholar] [CrossRef] [PubMed]
- Mandarino, F.V.; Danese, S.; Uraoka, T.; Parra-Blanco, A.; Maeda, Y.; Saito, Y.; Kudo, S.-E.; Bourke, M.J.; Iacucci, M. Precision Endoscopy in Colorectal Polyps’ Characterization and Planning of Endoscopic Therapy. Dig. Endosc. 2024, 36, 761–777. [Google Scholar] [CrossRef]
- Solitano, V.; Zilli, A.; Franchellucci, G.; Allocca, M.; Fiorino, G.; Furfaro, F.; D’Amico, F.; Danese, S.; Al Awadhi, S. Artificial Endoscopy and Inflammatory Bowel Disease: Welcome to the Future. JCM 2022, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kudo, S.; Ogata, N.; Misawa, M.; Mori, Y.; Mori, K.; Ohtsuka, K. Can Artificial Intelligence Help to Detect Dysplasia in Patients with Ulcerative Colitis? Endoscopy 2021, 53, E273–E274. [Google Scholar] [CrossRef]
- Diaconu, C.; State, M.; Birligea, M.; Ifrim, M.; Bajdechi, G.; Georgescu, T.; Mateescu, B.; Voiosu, T. The Role of Artificial Intelligence in Monitoring Inflammatory Bowel Disease-The Future Is Now. Diagnostics 2023, 13, 735. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kinugasa, H.; Hamada, K.; Tomiya, M.; Tanimoto, T.; Ohto, A.; Toda, A.; Takei, D.; Matsubara, M.; Suzuki, S.; et al. The Diagnostic Ability to Classify Neoplasias Occurring in Inflammatory Bowel Disease by Artificial Intelligence and Endoscopists: A Pilot Study. J. Gastro Hepatol. 2022, 37, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Vinsard, D.; Fetzer, J.R.; Agrawal, U.; Singh, J.; Damani, D.N.; Sivasubramaniam, P.; Poigai Arunachalam, S.; Leggett, C.L.; Raffals, L.E.; Coelho-Prabhu, N. Development of an Artificial Intelligence Tool for Detecting Colorectal Lesions in Inflammatory Bowel Disease. iGIE 2023, 2, 91–101.e6. [Google Scholar] [CrossRef]
- Denters, M.J.; Schreuder, M.; Depla, A.C.T.M.; Mallant-Hent, R.C.; van Kouwen, M.C.A.; Deutekom, M.; Bossuyt, P.M.; Fockens, P.; Dekker, E. Patients’ Perception of Colonoscopy: Patients with Inflammatory Bowel Disease and Irritable Bowel Syndrome Experience the Largest Burden. Eur. J. Gastroenterol. Hepatol. 2013, 25, 964–972. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Segev, M.; Reicher-Atir, R.; Steinmetz, A.; Horev, N.; Niv, Y.; Dickman, R. Perceptions of Gastroenterologists and Patients Regarding Irritable Bowel Syndrome and Inflammatory Bowel Disease. Eur. J. Gastroenterol. Hepatol. 2014, 26, 40–46. [Google Scholar] [CrossRef]
- Buisson, A.; Gonzalez, F.; Poullenot, F.; Nancey, S.; Sollellis, E.; Fumery, M.; Pariente, B.; Flamant, M.; Trang-Poisson, C.; Bonnaud, G.; et al. Comparative Acceptability and Perceived Clinical Utility of Monitoring Tools: A Nationwide Survey of Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Shingina, A.; Ou, G.; Takach, O.; Svarta, S.; Kwok, R.; Tong, J.; Donaldson, K.; Lam, E.; Enns, R. Identification of Factors Associated with Sedation Tolerance in 5000 Patients Undergoing Outpatient Colonoscopy: Canadian Tertiary Center Experience. World J. Gastrointest. Endosc. 2016, 8, 770–776. [Google Scholar] [CrossRef]
- Passi, M.; Rahman, F.; Gurram, S.; Kumar, S.; Koh, C. Identifying Who Best Tolerates Moderate Sedation: Results from a National Database of Gastrointestinal Endoscopic Outcomes. World J. Gastrointest. Endosc. 2021, 13, 97–110. [Google Scholar] [CrossRef]
- Weber, A.T.; Ather, N.; Tran, V.; Sauk, J.; Ha, C. Higher sedation requirements among inflammatory bowel disease patients undergoing colonoscopy for disease activity assessment or dysplasia surveillance. Crohn’s Colitis 360 2019, 1, otz006. [Google Scholar] [CrossRef]
- Biondi, R.B.; Salmazo, P.S.; Bazan, S.G.Z.; Hueb, J.C.; Paiva, S.A.R.D.; Sassaki, L.Y. Cardiovascular Risk in Individuals with Inflammatory Bowel Disease. CEG 2020, 13, 107–113. [Google Scholar] [CrossRef]
- Wu, H.; Hu, T.; Hao, H.; Hill, M.A.; Xu, C.; Liu, Z. Inflammatory Bowel Disease and Cardiovascular Diseases: A Concise Review. Eur. Heart J. Open 2022, 2, oeab029. [Google Scholar] [CrossRef] [PubMed]
- Sleutjes, J.A.M.; van der Woude, C.J.; Verploegh, P.J.P.; Aribas, E.; Kavousi, M.; Roeters van Lennep, J.E.; de Vries, A.C. Cardiovascular Risk Profiles in Patients with Inflammatory Bowel Disease Differ from Matched Controls from the General Population. Eur. J. Prev. Cardiol. 2023, 30, 1615–1622. [Google Scholar] [CrossRef]
- Todor, S.B.; Ichim, C.; Boicean, A.; Mihaila, R.G. Cardiovascular Risk in Philadelphia-Negative Myeloproliferative Neoplasms: Mechanisms and Implications—A Narrative Review. CIMB 2024, 46, 8407–8423. [Google Scholar] [CrossRef] [PubMed]
- Fanti, L.; Gemma, M.; Agostoni, M.; Rossi, G.; Ruggeri, L.; Azzolini, M.L.; Dabizzi, E.; Beretta, L.; Testoni, P.A. Target Controlled Infusion for Non-Anaesthesiologist Propofol Sedation during Gastrointestinal Endoscopy: The First Double Blind Randomized Controlled Trial. Dig. Liver Dis. 2015, 47, 566–571. [Google Scholar] [CrossRef]
- Agostoni, M.; Fanti, L.; Gemma, M.; Pasculli, N.; Beretta, L.; Testoni, P.A. Adverse Events during Monitored Anesthesia Care for GI Endoscopy: An 8-Year Experience. Gastrointest. Endosc. 2011, 74, 266–275. [Google Scholar] [CrossRef]
- Facciorusso, A.; Turco, A.; Barnabà, C.; Longo, G.; Dipasquale, G.; Muscatiello, N. Efficacy and Safety of Non-Anesthesiologist Administration of Propofol Sedation in Endoscopic Ultrasound: A Propensity Score Analysis. Diagnostics 2020, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Torres, J.; Barjas, E.; Nunes, J.; Glória, L.; Ferreira, R.; Rocha, M.; Pereira, S.; Dias, S.; Santos, A.; et al. Non-Anesthesiologist Administration of Propofol Sedation for Colonoscopy Is Safe in Low Risk Patients: Results of a Noninferiority Randomized Controlled Trial. Endoscopy 2016, 48, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, M.; Smarrelli, A.; Montesinos, J.C.; Carreño, S.O.; Fernandez, A.Z.; García, A.V.; Frontado, C.L.; Vidal, L.; Francesch, M.F.; Lladó, F.G.-H. Outcomes of Colonoscopy with Non-Anesthesiologist-Administered Propofol (NAAP): An Equivalence Trial. Endosc. Int. Open 2021, 9, E1070–E1076. [Google Scholar] [CrossRef] [PubMed]
- Mandarino, F.V.; Fanti, L.; Barchi, A.; Sinagra, E.; Massimino, L.; Azzolini, F.; Viale, E.; Napolitano, M.; Salmeri, N.; Agostoni, M.; et al. Safety and Tolerability Outcomes of Nonanesthesiologist-Administered Propofol Using Target-Controlled Infusion in Routine GI Endoscopy. Gastrointest. Endosc. 2024, 99, 914–923. [Google Scholar] [CrossRef]
- Steenholdt, C.; Jensen, J.T.; Brynskov, J.; Møller, A.M.; Limschou, A.C.; Konge, L.; Vilmann, P. Patient Satisfaction of Propofol Versus Midazolam and Fentanyl Sedation During Colonoscopy in Inflammatory Bowel Disease. Clin. Gastroenterol. Hepatol. 2022, 20, 559–568.e5. [Google Scholar] [CrossRef] [PubMed]
- Maida, M.; Morreale, G.C.; Sferrazza, S.; Sinagra, E.; Scalisi, G.; Vitello, A.; Vettori, G.; Rossi, F.; Catarella, D.; Di Bartolo, C.E.; et al. Effectiveness and Safety of 1L PEG-ASC Preparation for Colonoscopy in Patients with Inflammatory Bowel Diseases. Dig. Liver Dis. 2021, 53, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Shenoy, V.; Buckley, M.C.; Durbin, L.; Mackey, J.; Mone, A.; Swaminath, A. Endoscopic Disease Activity and Biologic Therapy Are Independent Predictors of Suboptimal Bowel Preparation in Patients with Inflammatory Bowel Disease Undergoing Colonoscopy. Dig. Dis. Sci. 2022, 67, 4851–4865. [Google Scholar] [CrossRef] [PubMed]
- Briot, C.; Faure, P.; Parmentier, A.L.; Nachury, M.; Trang, C.; Viennot, S.; Altwegg, R.; Bulois, P.; Thomassin, L.; Serrero, M.; et al. Efficacy, Tolerability, and Safety of Low-Volume Bowel Preparations for Patients with Inflammatory Bowel Diseases: The French Multicentre CLEAN Study. J. Crohn’s Colitis 2019, 13, 1121–1130. [Google Scholar] [CrossRef]
- Restellini, S.; Kherad, O.; Bessissow, T.; Ménard, C.; Martel, M.; Taheri Tanjani, M.; Lakatos, P.L.; Barkun, A.N. Systematic Review and Meta-Analysis of Colon Cleansing Preparations in Patients with Inflammatory Bowel Disease. World J. Gastroenterol. 2017, 23, 5994–6002. [Google Scholar] [CrossRef]
- Kim, K.O.; Jang, B.I.; Kim, E.Y.; Lee, Y.J.; Lee, H.S.; Jeon, S.W.; Kim, H.J.; Kim, S.K. Comparison of 4-L Polyethylene Glycol and 2-L Polyethylene Glycol Plus Ascorbic Acid in Patients with Inactive Ulcerative Colitis. Dig. Dis. Sci. 2017, 62, 2489–2497. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Anandasabapathy, S.; Richards-Kortum, R. Advances in Optical Gastrointestinal Endoscopy: A Technical Review. Mol. Oncol. 2021, 15, 2580–2599. [Google Scholar] [CrossRef]
- Lou, S.; Du, F.; Song, W.; Xia, Y.; Yue, X.; Yang, D.; Cui, B.; Liu, Y.; Han, P. Artificial Intelligence for Colorectal Neoplasia Detection during Colonoscopy: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. eClinicalMedicine 2023, 66, 102341. [Google Scholar] [CrossRef] [PubMed]
- Maida, M.; Marasco, G.; Maas, M.H.J.; Ramai, D.; Spadaccini, M.; Sinagra, E.; Facciorusso, A.; Siersema, P.D.; Hassan, C. Effectiveness of Artificial Intelligence Assisted Colonoscopy on Adenoma and Polyp Miss Rate: A Meta-Analysis of Tandem RCTs. Dig. Liver Dis. 2024, S1590865824010016. [Google Scholar] [CrossRef]
- Wei, M.T.; Fay, S.; Yung, D.; Ladabaum, U.; Kopylov, U. Artificial Intelligence-Assisted Colonoscopy in Real-World Clinical Practice: A Systematic Review and Meta-Analysis. Clin. Transl. Gastroenterol. 2024, 15, e00671. [Google Scholar] [CrossRef] [PubMed]
- Tontini, G.E.; Rimondi, A.; Vernero, M.; Neumann, H.; Vecchi, M.; Bezzio, C.; Cavallaro, F. Artificial Intelligence in Gastrointestinal Endoscopy for Inflammatory Bowel Disease: A Systematic Review and New Horizons. Therap Adv. Gastroenterol. 2021, 14, 17562848211017730. [Google Scholar] [CrossRef]
- Rex, D.K.; Bhandari, R.; Lorch, D.G.; Meyers, M.; Schippers, F.; Bernstein, D. Safety and Efficacy of Remimazolam in High Risk Colonoscopy: A Randomized Trial. Dig. Liver Dis. 2021, 53, 94–101. [Google Scholar] [CrossRef]
- Ichijima, R.; Ikehara, H.; Ono, H.; Hotta, K.; Yamaguchi, D.; Esaki, M.; Minoda, Y.; Nagata, Y.; Ogura, K.; Kiriyama, S.; et al. Randomized Controlled Trial of Remimazolam Compared with Placebo in Japanese Patients Undergoing Colonoscopy: A Phase III, Investigator-Initiated Trial. Digestion 2024, 105, 448–456. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Bai, N.; Li, L.; Zhang, M.; Cheng, Z.; Guo, Q. Safety and Efficacy of Remimazolam Besylate in Patients Undergoing Colonoscopy: A Multicentre, Single-Blind, Randomized, Controlled, Phase III Trial. Front. Pharmacol. 2022, 13, 900723. [Google Scholar] [CrossRef] [PubMed]

| Aspect | Requirement/Details |
|---|---|
| Pre-Adoption Requirements | - Competence in HD colonoscopy using DCE or VCE with targeted biopsies. |
| Training Steps | - Onsite Training: 1-week course with an IBD optical diagnosis expert. |
| - Self-Learning: Perform at least 20 pan-chromoendoscopy procedures with 20 targeted biopsies reviewed via histological feedback. | |
| - Incorporate random biopsies (every 10 cm) during the learning phase to ensure accuracy. | |
| Competence Assessment | - Achieve a neoplasia detection rate of ≥10% in at least 20 IBD pan-chromoendoscopy procedures with targeted biopsies only. |
| Maintaining Competence | - Perform an in vivo audit of ≥10 IBD lesions annually. |
| - If insufficient cases are performed, repeat training and learning phases. | |
| Transition to VCE | - Progress gradually from DCE to VCE. |
| - Utilize VCE after achieving competence in DCE. |
| Technology | Key Features | Advantages | Limitations | Clinical Applications |
|---|---|---|---|---|
| Endocytoscopy |
|
|
|
|
| Confocal Laser Endomicroscopy |
|
|
|
|
| Molecular Endoscopy |
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasulo, E.; D’Amico, F.; Zilli, A.; Furfaro, F.; Cicerone, C.; Parigi, T.L.; Peyrin-Biroulet, L.; Danese, S.; Allocca, M. Advancing Colorectal Cancer Prevention in Inflammatory Bowel Disease (IBD): Challenges and Innovations in Endoscopic Surveillance. Cancers 2025, 17, 60. https://doi.org/10.3390/cancers17010060
Fasulo E, D’Amico F, Zilli A, Furfaro F, Cicerone C, Parigi TL, Peyrin-Biroulet L, Danese S, Allocca M. Advancing Colorectal Cancer Prevention in Inflammatory Bowel Disease (IBD): Challenges and Innovations in Endoscopic Surveillance. Cancers. 2025; 17(1):60. https://doi.org/10.3390/cancers17010060
Chicago/Turabian StyleFasulo, Ernesto, Ferdinando D’Amico, Alessandra Zilli, Federica Furfaro, Clelia Cicerone, Tommaso Lorenzo Parigi, Laurent Peyrin-Biroulet, Silvio Danese, and Mariangela Allocca. 2025. "Advancing Colorectal Cancer Prevention in Inflammatory Bowel Disease (IBD): Challenges and Innovations in Endoscopic Surveillance" Cancers 17, no. 1: 60. https://doi.org/10.3390/cancers17010060
APA StyleFasulo, E., D’Amico, F., Zilli, A., Furfaro, F., Cicerone, C., Parigi, T. L., Peyrin-Biroulet, L., Danese, S., & Allocca, M. (2025). Advancing Colorectal Cancer Prevention in Inflammatory Bowel Disease (IBD): Challenges and Innovations in Endoscopic Surveillance. Cancers, 17(1), 60. https://doi.org/10.3390/cancers17010060







