Evaluation of Predictive Factors for Transarterial Bleomycin–Lipiodol Embolization Success in Treating Giant Hepatic Hemangiomas
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Characteristics and Diagnosis
2.2. Treatment
2.3. Predictive Factors
2.4. Statistical Analysis
3. Results
3.1. Patients Characteristics
3.2. Association Among Factors Influencing Clinical Success
3.3. Association Among Factors Influencing Volume Regression
3.4. Association Among Factors Influencing the Occurrence and Severity of Post-Embolization Syndrome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belghiti, J.; Cauchy, F.; Paradis, V.; Vilgrain, V. Diagnosis and management of solid benign liver lesions. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Isokawa, O.; Hoshiyama, K.; Hoshiyama, A.; Hoshiyama, M.; Hoshiyama, Y. Diagnosis and management of giant hepatic hemangioma: The usefulness of contrast-enhanced ultrasonography. Int. J. Hepatol. 2013, 2013, 802180. [Google Scholar] [CrossRef]
- Choi, B.Y.; Nguyen, M.H. The diagnosis and management of benign hepatic tumors. J. Clin. Gastroenterol. 2005, 39, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Chen, S.; Wu, H. Quality of life can be improved by surgical management of giant hepatic haemangioma with enucleation as the preferred option. HPB 2015, 17, 490–494. [Google Scholar] [CrossRef]
- Di Carlo, I.; Toro, A. Limiting the surgical indications for liver hemangiomas may help surgeons and patients. J. Am. Coll. Surg. 2011, 212, 1098–1099. [Google Scholar] [CrossRef]
- Özden, İ.; Poyanlı, A.; Önal, Y.; Demir, A.A.; Hoş, G.; Acunaş, B. Superselective transarterial chemoembolization as an alternative to surgery in symptomatic/enlarging liver hemangiomas. World J. Surg. 2017, 41, 2796–2803. [Google Scholar] [CrossRef]
- Kacała, A.; Dorochowicz, M.; Matus, I.; Puła, M.; Korbecki, A.; Sobański, M.; Jacków-Nowicka, J.; Patrzałek, D.; Janczak, D.; Guziński, M. Hepatic hemangioma: Review of imaging and therapeutic strategies. Medicina 2024, 60, 449. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.; Zhang, J.-L.; Duan, F.; Wang, M.-Q. Medium and Long-Term Outcome of Superselective Transcatheter Arterial Embolization with Lipiodol-Bleomycin Emulsion for Giant Hepatic Hemangiomas: Results in 241 Patients. J. Clin. Med. 2022, 11, 4762. [Google Scholar] [CrossRef]
- Torkian, P.; Li, J.; Kaufman, J.A.; Jahangiri, Y. Effectiveness of Transarterial Embolization in Treatment of Symptomatic Hepatic Hemangiomas: Systematic Review and Meta-analysis. Cardiovasc. Intervent. Radiol. 2021, 44, 80–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Z.; Tan, H.; Huang, J.; Xu, L.; Liu, L.; Si, S.; Sun, Y. Long-term result of transcatheter arterial embolization for liver hemangioma. Medicine 2017, 96, e9029. [Google Scholar] [CrossRef]
- Kacała, A.; Dorochowicz, M.; Patrzałek, D.; Janczak, D.; Guziński, M. Safety and Feasibility of Transarterial Bleomycin-Lipiodol Embolization in Patients with Giant Hepatic Hemangiomas. Medicina 2023, 59, 1358. [Google Scholar] [CrossRef]
- Oikawa, T.; Hirotani, K.; Ogasawara, H.; Katayama, T.; Ashino-Fuse, H.; Shimamura, M.; Iwaguchi, T.; Nakamura, O. Inhibition of angiogenesis by bleomycin and its copper complex. Chem. Pharm. Bull. 1990, 38, 1790–1792. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y.; Ogata, I.; Urata, J.; Takahashi, M. Cavernous hemangioma of the liver: Pathologic correlation with dynamic CT findings. Radiology 1997, 203, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Aziz, H.; Brown, Z.J.; Baghdadi, A.; Kamel, I.R.; Pawlik, T.M. A comprehensive review of hepatic hemangioma management. J. Gastrointest. Surg. 2022, 26, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Oldhafer, K.J.; Habbel, V.; Horling, K.; Makridis, G.; Wagner, K.C. Benign liver tumors. Visc. Med. 2020, 36, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Farhat, W.; Ammar, H.; Said, M.A.; Mizouni, A.; Ghabry, L.; Hammami, E.; Gupta, R.; Habiba, B.H.; Mabrouk, M.B.; Ali, A.B. Surgical management of giant hepatic hemangioma: A 10-year single center experience. Ann. Med. Surg. 2021, 69, 102542. [Google Scholar] [CrossRef] [PubMed]
- Mungovan, J.A.; Cronan, J.J.; Vacarro, J. Hepatic cavernous hemangiomas: Lack of enlargement over time. Radiology 1994, 191, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Glinkova, V.; Shevah, O.; Boaz, M.; Levine, A.; Shirin, H. Hepatic haemangiomas: Possible association with female sex hormones. Gut 2004, 53, 1352–1355. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Aggarwal, A.; Singla, R.; Kalra, N.; Chawla, Y.K. Giant hemangioma causing Budd–Chiari syndrome. J. Clin. Exp. Hepatol. 2014, 4, 380–381. [Google Scholar] [CrossRef][Green Version]
- Duxbury, M.S.; Garden, O.J. Giant haemangioma of the liver: Observation or resection? Dig. Surg. 2010, 27, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.E.; Thung, S.N.; Tsui, W.M.S.; Ferrell, L.D. Hepatic cavernous hemangioma: Underrecognized associated histologic features. Liver Int. 2006, 26, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.A.; Papaiordanou, F.; Gonçalves, J.M.; Chaib, E. Spontaneous rupture of hepatic hemangiomas: A review of the literature. World J. Hepatol. 2010, 2, 428–433. [Google Scholar]
- Yamamoto, T.; Kawarada, Y.; Yano, T.; Noguchi, T.; Mizumoto, R. Spontaneous rupture of hemangioma of the liver: Treatment with transcatheter hepatic arterial embolization. Am. J. Gastroenterol. 1991, 86, 1645–1649. [Google Scholar] [PubMed]
- Chen, Z.-Y.; Qi, Q.-H.; Dong, Z.-L. Etiology and management of hemorrhage in spontaneous liver rupture: A report of 70 cases. World J. Gastroenterol. 2002, 8, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Ramachandran, V.; Garg, R.; Pal, S.; Gamanagatti, S.R.; Srivastava, D.N. Spontaneous rupture of a giant hepatic hemangioma—Sequential management with transcatheter arterial embolization and resection. Saudi J. Gastroenterol. 2010, 16, 116–119. [Google Scholar] [PubMed]
- Xie, Q.-S.; Chen, Z.-X.; Zhao, Y.-J.; Gu, H.; Geng, X.-P.; Liu, F.-B. Outcomes of surgery for giant hepatic hemangioma. BMC Surg. 2021, 21, 186. [Google Scholar] [CrossRef] [PubMed]
- Abdel Wahab, M.; El Nakeeb, A.; Ali, M.A.; Mahdy, Y.; Shehta, A.; Abdulrazek, M.; El Desoky, M.; Abdel Wahab, R. Surgical management of giant hepatic hemangioma: Single center’s experience with 144 patients. J. Gastrointest. Surg. 2018, 22, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Yedibela, S.; Alibek, S.; Müller, V.; Aydin, U.; Langheinrich, M.; Lohmüller, C.; Hohenberger, W.; Perrakis, A. Management of hemangioma of the liver: Surgical therapy or observation? World J. Surg. 2013, 37, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Schnelldorfer, T.; Ware, A.L.; Smoot, R.; Schleck, C.D.; Harmsen, W.S.; Nagorney, D.M. Management of giant hemangioma of the liver: Resection versus observation. J. Am. Coll. Surg. 2010, 211, 724–730. [Google Scholar] [CrossRef]
- Yamagata, M.; Kanematsu, T.; Matsumata, T.; Utsunomiya, T.; Ikeda, Y.; Sugimachi, K. Management of haemangioma of the liver: Comparison of results between surgery and observation. Br. J. Surg. 1991, 78, 1223–1225. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.-Y.; Wu, T.-H.; Yu, M.-C.; Lee, W.-C.; Chao, T.-C.; Chen, M.-F. Surgical management of giant hepatic hemangiomas: Complications and review of the literature. Chang Gung Med. J. 2012, 35, 70–78. [Google Scholar] [CrossRef]
- Wang, S.; Yang, M.; Yang, X.; Xu, L.; Ke, S.; Ding, X.; Sun, W.; Gao, J. Endothelial pyroptosis underlies systemic inflammatory response following radiofrequency ablation of hepatic hemangiomas. Scand. J. Clin. Lab. Invest. 2019, 79, 619–628. [Google Scholar] [CrossRef]
- Wu, S.; Gao, R.; Yin, T.; Zhu, R.; Guo, S.; Xin, Z.; Li, A.; Kong, X.; Gao, J.; Sun, W. Complications of radiofrequency ablation for hepatic hemangioma: A multicenter retrospective analysis on 291 cases. Front. Oncol. 2021, 11, 706619. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhu, X.; Zhou, J. Ultrasound-guided percutaneous sclerotherapy versus surgical resection in the treatment of large hepatic hemangiomas: A retrospective study. BMC Surg. 2022, 22, 130. [Google Scholar] [CrossRef] [PubMed]
- Ayoobi Yazdi, N.; Mehrabinejad, M.-M.; Dashti, H.; Pourghorban, R.; Nassiri Toosi, M.; Rokni Yazdi, H. Percutaneous Sclerotherapy with Bleomycin and Ethiodized Oil: A Promising Treatment in Symptomatic Giant Liver Hemangioma. Radiology 2021, 301, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Gao, R.; Wu, S.; Shi, Y.; Yin, T.; Guo, S.; Xin, Z.; Li, A.; Kong, X.; Ma, D.; et al. Safety and efficacy of microwave versus radiofrequency ablation for large hepatic hemangioma: A multicenter retrospective study with propensity score matching. Eur. Radiol. 2022, 32, 3309–3318. [Google Scholar] [CrossRef]
- Bozkaya, H.; Cinar, C.; Besir, F.H.; Parıldar, M.; Oran, I. Minimally invasive treatment of giant haemangiomas of the liver: Embolisation with bleomycin. Cardiovasc. Intervent. Radiol. 2014, 37, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jia, Y.; Li, S.; Wang, W.; Wang, Z.; Wang, Y.; Liu, B.; Wang, W.; Chang, H.; Li, Z. Transarterial Chemoembolization of Giant Liver Haemangioma: A Multi-center Study with 836 Cases. Cell Biochem. Biophys. 2015, 73, 469–472. [Google Scholar] [CrossRef]
- Kacała, A.; Dorochowicz, M.; Korbecki, A.; Sobański, M.; Puła, M.; Patrzałek, D.; Janczak, D.; Guziński, M. Transarterial bleomycin–lipiodol chemoembolization for the treatment of giant hepatic hemangiomas: An assessment of effectiveness. Cancers 2024, 16, 380. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghpoor, S.; Torkian, P.; Golzarian, J. Transarterial Bleomycin-Lipiodol Embolization (B/LE) for Symptomatic Giant Hepatic Hemangioma. Cardiovasc. Intervent. Radiol. 2018, 41, 1674–1682. [Google Scholar] [CrossRef]
- O’Sullivan, J.M.; Huddart, R.A.; Norman, A.R.; Nicholls, J.; Dearnaley, D.P.; Horwich, A. Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann. Oncol. 2003, 14, 91–96. [Google Scholar] [CrossRef]

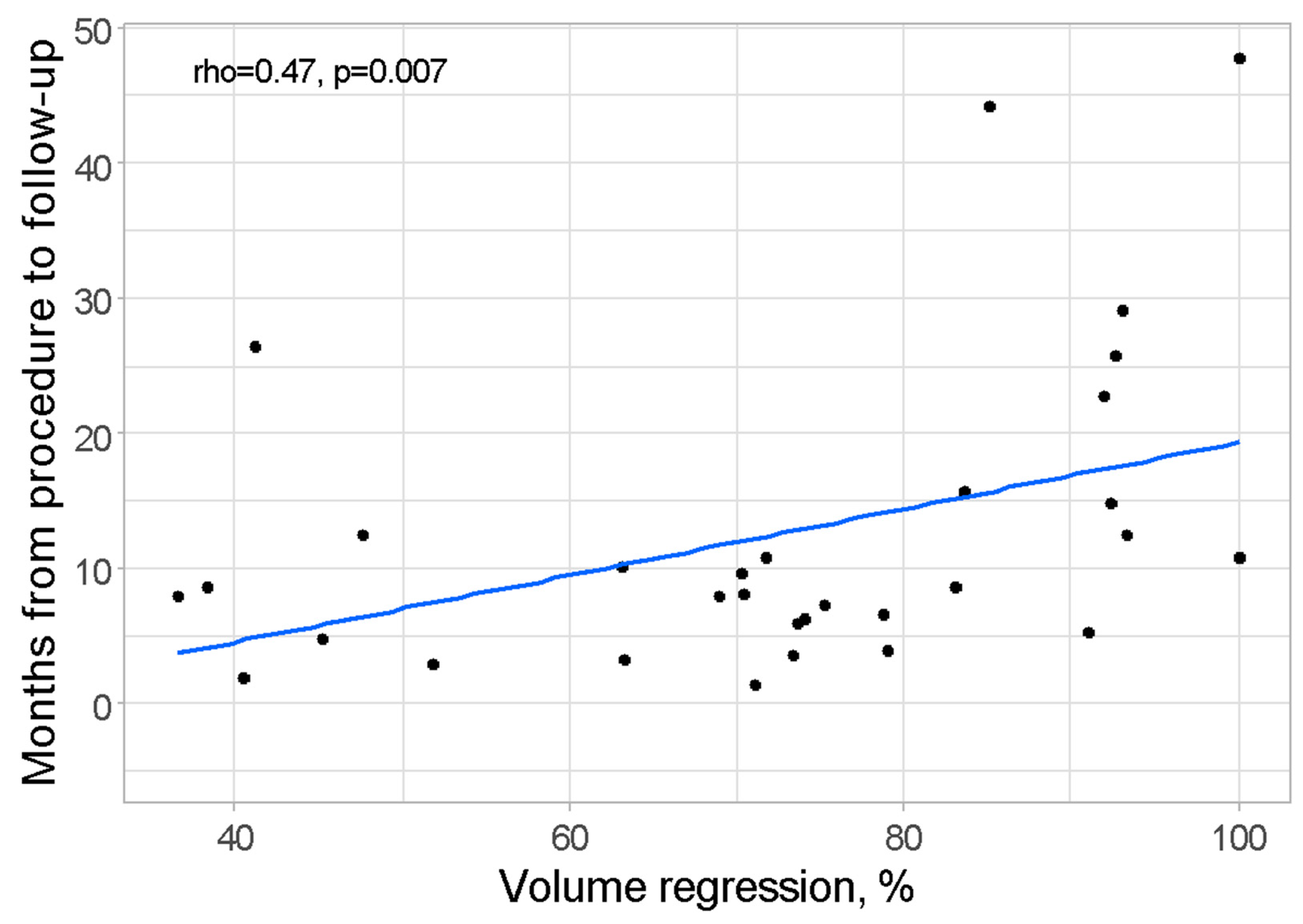

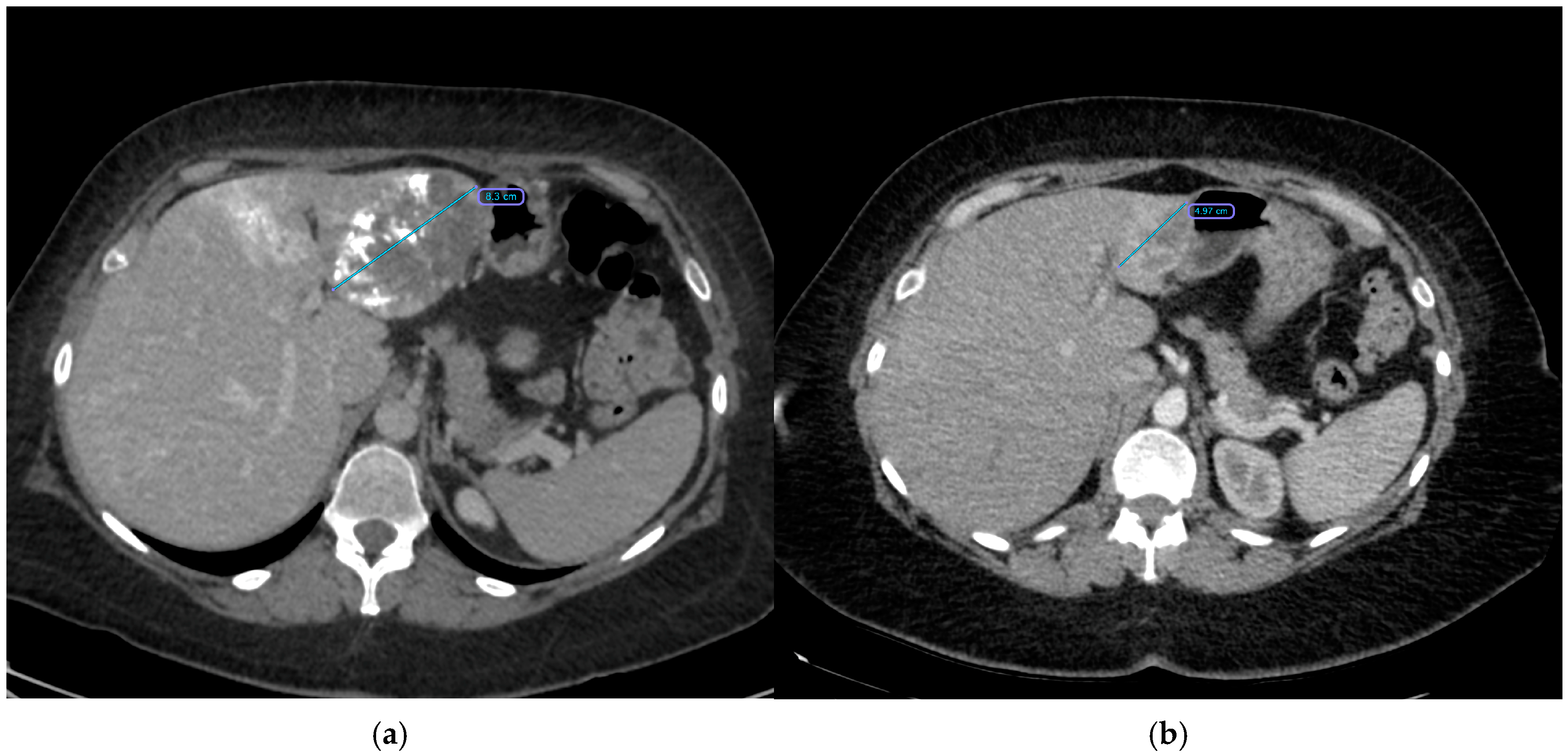
| Variable | n | Total Group |
|---|---|---|
| N | 31 | |
| Age (day of 1st procedure), years, mean ± SD | 31 | 49.39 ± 10.93 |
| Sex | 31 | |
| Female | 25 (80.6) | |
| Male | 6 (19.4) | |
| Location | 31 | |
| Left liver lobe | 13 (41.9) | |
| Right liver lobe | 17 (54.8) | |
| Both liver lobes | 1 (3.2) | |
| Diagnostics before procedure | 31 | |
| TK | 21 (67.7) | |
| MR | 5 (16.1) | |
| No information | 5 (16.1) | |
| Size before procedure, cm | ||
| x, median (IQR) | 31 | 8.70 (7.50;10.55) |
| y, median (IQR) | 31 | 7.20 (5.50;9.10) |
| z, median (IQR) | 31 | 7.40 (6.15;9.80) |
| Volume before procedure, cm3, median (IQR) | 31 | 241.04 (123.98;539.08) |
| Embolization—no of stages | 31 | |
| 1 | 14 (45.2) | |
| 2 | 10 (32.3) | |
| 3 | 5 (16.1) | |
| 4 | 2 (6.5) | |
| Embolization—no of stages, mean ± SD | 31 | 1.84 ± 0.93 |
| Bleomycin, UI, mean ± SD | 31 | 24,766.13 ± 11,856.25 |
| Lipiodol, mL, median (IQR) | 31 | 0.00 (0.00;10.00) |
| Lipiodol > 0, mL, mean ± SD | 15 | 19.60 ± 15.25 |
| Radiation, Gy, median (IQR) | 31 | 0.17 (0.00;0.59) |
| Radiation > 0, Gy, median (IQR) | 17 | 0.58 (0.25;0.85) |
| Coverage of hemangioma’s borders * | 31 | |
| <25% | 1 (3.2) | |
| 25–50% | 0 (0.0) | |
| 50–75% | 1 (3.2) | |
| 75–100% | 29 (93.5) | |
| Complication ** | 31 | 3 (9.7) |
| Post-embolization syndrome—severity, [0–3], median (IQR) *** | 31 | 0.50 (0.00;1.00) |
| Post-embolization syndrome—severity > 0, [1–3], median (IQR) *** | 22 | 1.00 (0.50;1.25) |
| Post-embolization syndrome—vomiting | 31 | 5 (16.1) |
| Hospitalization, days, median (IQR) | 31 | 6.00 (3.00;10.00) |
| Diagnostics after procedure | 31 | |
| TK | 29 (93.5) | |
| MR | 2 (6.5) | |
| Size after procedure, cm | ||
| x, mean ± SD | 31 | 5.43 ± 2.53 |
| y, median (IQR) | 31 | 4.30 (3.70;5.45) |
| z, mean ± SD | 31 | 5.19 ± 2.77 |
| Volume after procedure, cm3, median (IQR) | 31 | 59.59 (33.75;104.49) |
| Volume regression, %, mean ± SD | 31 | 72.26 ± 19.01 |
| Clinical success | 31 | 25 (80.6) |
| Days from procedure to follow-up, median (IQR) | 31 | 261.00 (170.50;417.50) |
| Months from procedure to follow-up, median (IQR) | 31 | 8.56 (5.59;13.69) |
| Variable | Clinical Success | MD (95% CI) | p | |
|---|---|---|---|---|
| Yes (n = 25) | No (n = 6) | |||
| Age (day of 1st procedure), years, mean ± SD | 48.96 ± 11.48 | 51.15 ± 8.95 | −2.19 (−12.49;8.12) 1 | 0.667 2 |
| Sex | ||||
| Female | 22 (88.0) | 3 (50.0) | - | 0.069 3 |
| Male | 3 (12.0) | 3 (50.0) | ||
| Location | ||||
| Left liver lobe | 11 (44.0) | 2 (33.3) | - | 0.736 3 |
| Right liver lobe | 13 (52.0) | 4 (66.7) | ||
| Both liver lobes | 1 (4.0) | 0 (0.0) | ||
| Volume before procedure, cm3, median (IQR) | 238.32 (139.23;534.75) | 274.44 (141.81;484.51) | −36.12 (−255.93;293.71) | 0.942 |
| Embolization—no of stages | ||||
| 1 | 9 (36.0) | 5 (83.3) | - | 0.154 3 |
| 2 | 4 (16.0) | 1 (16.7) | ||
| 3 | 10 (40.0) | 0 (0.0) | ||
| 4 | 2 (8.0) | 0 (0.0) | ||
| Bleomycin, UI, median (IQR) | 26,250.00 (15,000.00;30,000.00) | 15,000.00 (15,000.00;15,000.00) | 11,250.00 (0.00;15,000.00) | 0.108 |
| Lipiodol, mL, median (IQR) | 0.00 (0.00;10.00) | 2.50 (0.00;5.00) | −2.50 (−5.00;10.00) | 0.666 |
| Lipiodol > 0, mL, median (IQR) | 15.75 (10.00;36.25) | 5.00 (5.00;12.50) | 10.75 (−10.00;35.00) | 0.162 |
| Radiation, Gy, median (IQR) | 0.21 (0.00;0.60) | 0.00 (0.00;0.17) | 0.21 (0.00;0.58) | 0.306 |
| Radiation > 0, Gy, median (IQR) | 0.58 (0.25;0.85) | 0.67 (0.45;0.89) | −0.08 (−0.93;2.11) | >0.999 |
| Coverage of hemangioma’s borders * | ||||
| <25% | 1 (4.0) | 0 (0.0) | - | >0.999 3 |
| 25–50% | 0 (0.0) | 0 (0.0) | ||
| 50–75% | 1 (4.0) | 0 (0.0) | ||
| 75–100% | 23 (92.0) | 6 (100.0) | ||
| Days from procedure to follow-up, median (IQR) | 263.00 (180.00;453.00) | 252.50 (169.75;351.75) | 10.50 (−152.00;237.00) | 0.789 |
| Univariate Models | Multivariate Model | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% CI | p | OR | 95% CI | p |
| Age (day of 1st procedure), years | 0.98 | 0.90–1.07 | 0.655 | - | - | - |
| Sex, male (vs. female) | 0.14 | 0.02–1.01 | 0.051 | 0.08 | 0.00–1.03 | 0.072 |
| Location | ||||||
| Right liver lobe (vs. left liver lobe) | 0.59 | 0.07–3.65 | 0.583 | - | - | - |
| Both liver lobes (vs. left liver lobe) | Inf | - | 0.995 | - | - | - |
| Diagnostics before procedure, MR (vs. TK) | 0.94 | 0.10–21.10 | 0.961 | - | - | - |
| Size before procedure, cm | ||||||
| x | 1.05 | 0.79–1.52 | 0.747 | - | - | - |
| y | 1.05 | 0.81–1.48 | 0.750 | - | - | - |
| z | 1.01 | 0.80–1.37 | 0.916 | - | - | - |
| Volume before procedure, cm3 | 1.00 | 1.00–1.00 | 0.673 | - | - | - |
| Embolization—no of stages | ||||||
| 2 (vs. 1) | Inf | - | 0.996 | - | - | - |
| 3 (vs. 1) | 2.22 | 0.24–50.09 | 0.523 | - | - | - |
| 4 (vs. 1) | Inf | - | 0.998 | - | - | - |
| Embolization—no of stages | 2.85 | 0.85–18.49 | 0.161 | 6.68 | 1.13–190.28 | 0.116 |
| Bleomycin, UI | 1.00 | 1.00–1.00 | 0.211 | - | - | - |
| Lipiodol, mL | 1.04 | 0.97–1.18 | 0.409 | - | - | - |
| Lipiodol > 0, mL | 1.10 | 0.98–1.41 | 0.278 | |||
| Radiation, Gy | 2.34 | 0.49–53.03 | 0.450 | - | - | - |
| Radiation > 0, Gy | 1.24 | 0.22–44.67 | 0.849 | |||
| Coverage of hemangioma’s borders, 50–75% (vs. 75–100%) * | Inf | - | 0.995 | |||
| Hepatic artery dissection (vs. no complication) | 0.43 | 0.03–10.42 | 0.528 | - | - | - |
| Post-embolization syndrome–severity [0–3] ** | 0.50 | 0.16–1.44 | 0.190 | - | - | - |
| Post-embolization syndrome–severity > 0 [1–3] ** | 0.51 | 0.13–1.87 | 0.289 | - | - | - |
| Post-embolization syndrome—vomiting | 0.27 | 0.03–2.56 | 0.221 | 0.04 | 0.00–1.12 | 0.089 |
| Hospitalization, days | 0.98 | 0.85–1.19 | 0.829 | - | - | - |
| Diagnostics after procedurę, MR (vs. TK) | Inf | - | 0.995 | - | - | - |
| Size after procedure, cm | ||||||
| x | 0.70 | 0.42–1.02 | 0.101 | - | - | - |
| y | 0.69 | 0.45–0.97 | 0.053 | - | - | - |
| z | 0.78 | 0.54–1.08 | 0.140 | - | - | - |
| Volume after procedure, cm3 | 1.00 | 0.99–1.00 | 0.206 | - | - | - |
| Days from procedurę to follow-up | 1.00 | 1.00–1.00 | 0.610 | - | - | - |
| Months from procedurę to follow-up | 1.02 | 0.95–1.16 | 0.610 | - | - | - |
| Variable | n | Volume Regression, % | MD (95% CI) | p |
|---|---|---|---|---|
| Coverage of hemangioma’s borders (1st procedure) | ||||
| <75% | 8 | 78.12 ± 16.56 | 7.91 (−8.05;23.86) | 0.319 |
| 75–100% | 23 | 70.22 ± 19.72 | ||
| Coverage of hemangioma’s borders (maximum of procedures per patient) | ||||
| <75% | 2 | 77.00 (72.97;81.04) | 3.32 (−23.64;39.88) 1 | 0.968 2 |
| 75–100% | 29 | 73.68 (63.06;91.08) |
| Variable | n | Volume Regression, % | MD (95% CI) | p |
|---|---|---|---|---|
| Location | ||||
| Left liver lobe | 13 | 71.68 ± 18.05 | 0.15 (−14.42;14.72) | 0.986 |
| Right liver lobe | 17 | 71.54 ± 20.20 |
| Variable | n | Bleomycin, UI | MD (95% CI) | p |
|---|---|---|---|---|
| Post-embolization syndrome—vomiting | ||||
| Yes | 5 | 30,000.00 (26,250.00;37,500.00) | 13,000.00 (−3750.00;20,000.00) | 0.181 |
| No | 26 | 17,000.00 (15,000.00;30,000.00) |
| Variable | n | Lipiodol, mL | MD (95% CI) | p |
|---|---|---|---|---|
| Post-embolization syndrome—vomiting | ||||
| Yes | 3 | 21.50 (20.75;22.00) | 11.50 (−17.00;20.50) | 0.418 |
| No | 12 | 10.00 (8.75;36.25) |
| Variable | Coverage of Hemangioma’s Borders | MD (95% CI) | p | |
|---|---|---|---|---|
| <75% (n = 2) | 75–100% (n = 29) | |||
| Post-embolization syndrome—severity, [0–3] | 0.75 (0.38;1.12) | 0.50 (0.00;1.00) | 0.25 (−1.50;1.50) | >0.999 |
| Post-embolization syndrome—vomiting | 0 (0.0) | 5 (17.2) | - | >0.999 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacała, A.; Dorochowicz, M.; Korbecki, A.; Sobański, M.; Zdanowicz-Ratajczak, A.; Patrzałek, D.; Janczak, D.; Guziński, M. Evaluation of Predictive Factors for Transarterial Bleomycin–Lipiodol Embolization Success in Treating Giant Hepatic Hemangiomas. Cancers 2025, 17, 42. https://doi.org/10.3390/cancers17010042
Kacała A, Dorochowicz M, Korbecki A, Sobański M, Zdanowicz-Ratajczak A, Patrzałek D, Janczak D, Guziński M. Evaluation of Predictive Factors for Transarterial Bleomycin–Lipiodol Embolization Success in Treating Giant Hepatic Hemangiomas. Cancers. 2025; 17(1):42. https://doi.org/10.3390/cancers17010042
Chicago/Turabian StyleKacała, Arkadiusz, Mateusz Dorochowicz, Adrian Korbecki, Michał Sobański, Agata Zdanowicz-Ratajczak, Dariusz Patrzałek, Dariusz Janczak, and Maciej Guziński. 2025. "Evaluation of Predictive Factors for Transarterial Bleomycin–Lipiodol Embolization Success in Treating Giant Hepatic Hemangiomas" Cancers 17, no. 1: 42. https://doi.org/10.3390/cancers17010042
APA StyleKacała, A., Dorochowicz, M., Korbecki, A., Sobański, M., Zdanowicz-Ratajczak, A., Patrzałek, D., Janczak, D., & Guziński, M. (2025). Evaluation of Predictive Factors for Transarterial Bleomycin–Lipiodol Embolization Success in Treating Giant Hepatic Hemangiomas. Cancers, 17(1), 42. https://doi.org/10.3390/cancers17010042






