Robotic Versus Laparoscopic Adrenalectomy for Adrenal Tumors: An Up-to-Date Meta-Analysis on Perioperative Outcomes
Simple Summary
Abstract
1. Introduction
2. Methods
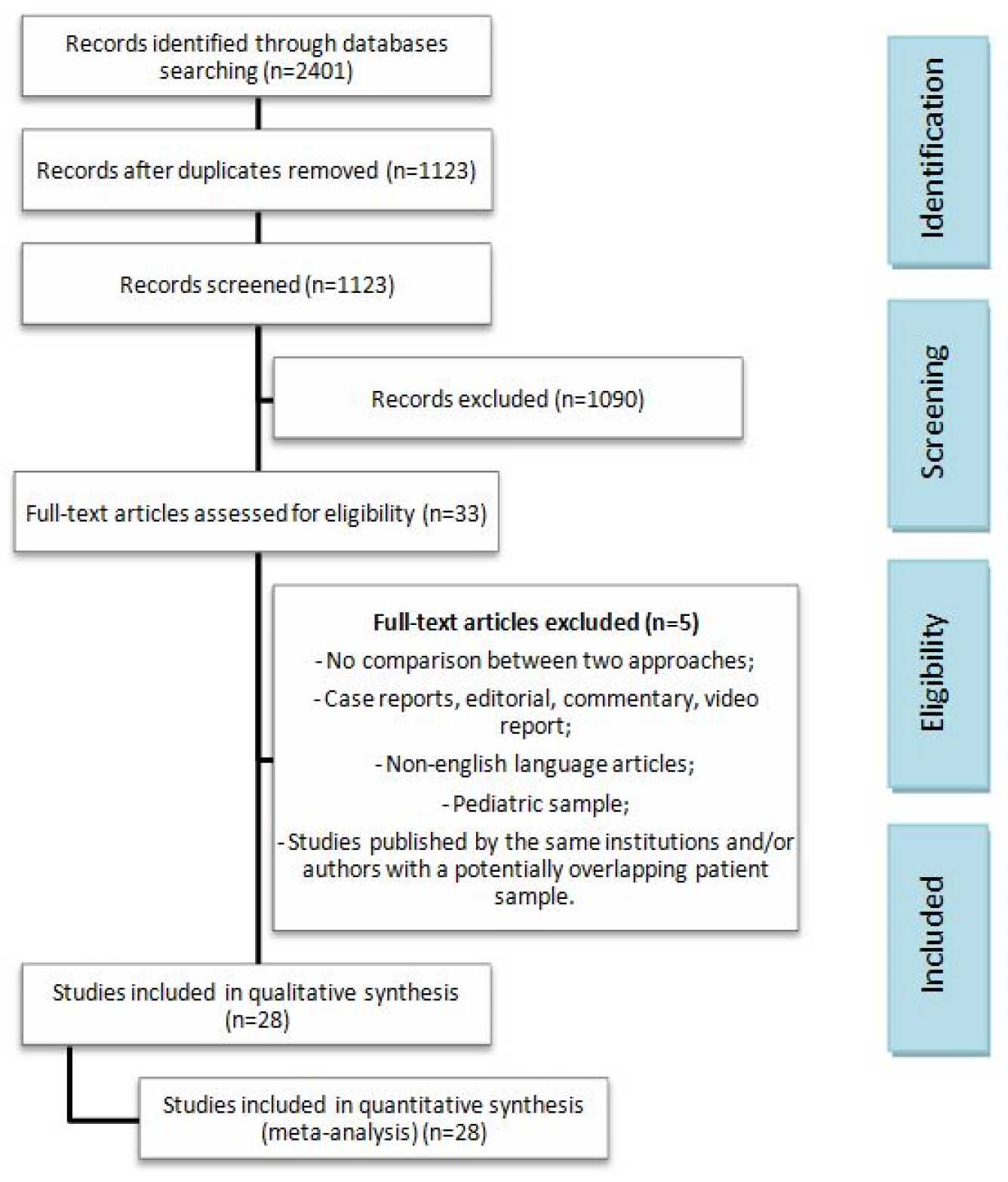
2.1. Study Design and Selection
PICOS
2.2. Eligibility Criteria
2.3. Outcomes
2.4. Quality Assessment
2.5. Statistical Analysis and Risk of Bias
3. Results
3.1. Studies and Patient Characteristics
3.2. Surgical Outcomes
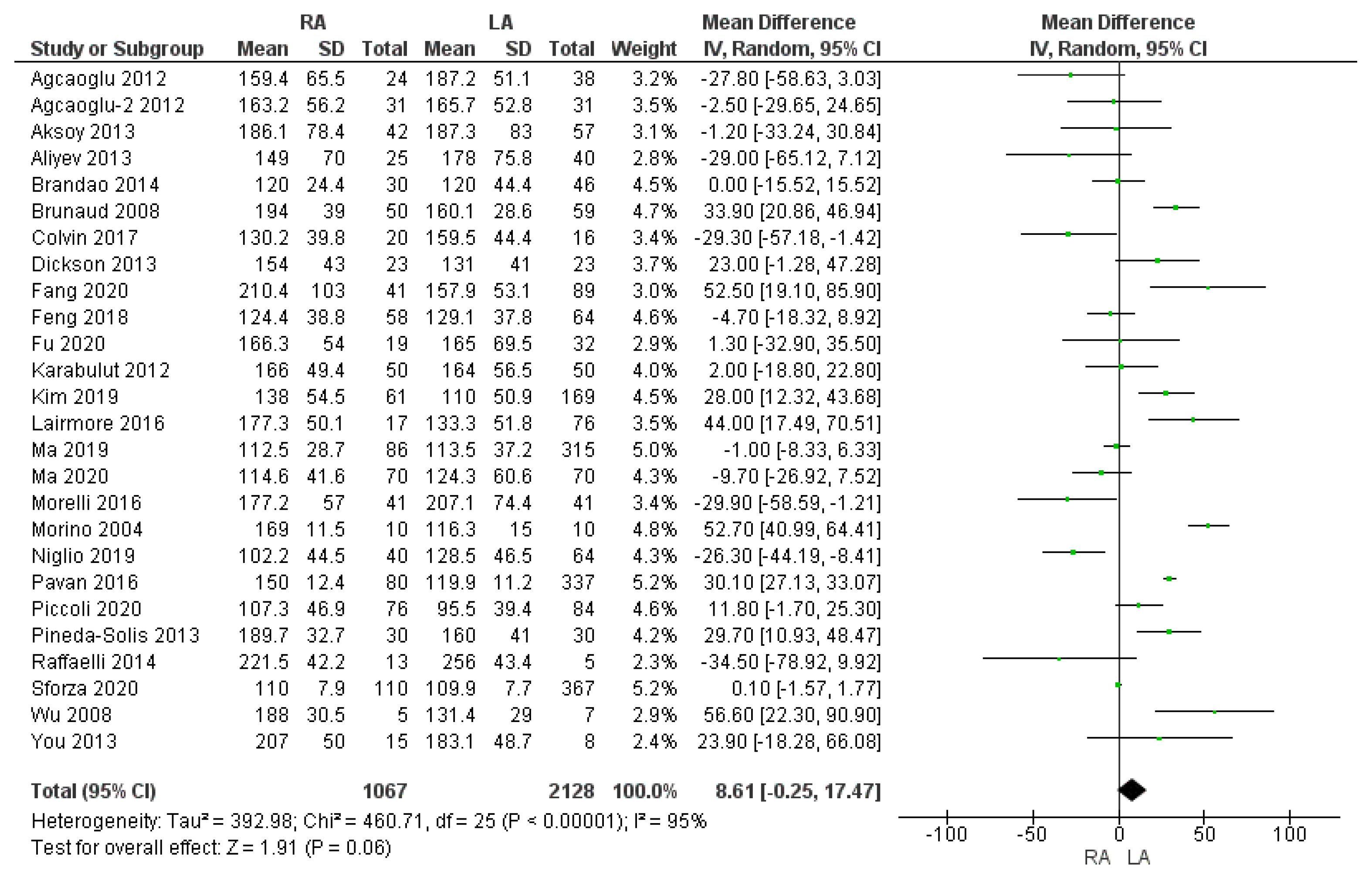
3.2.1. Operating Time
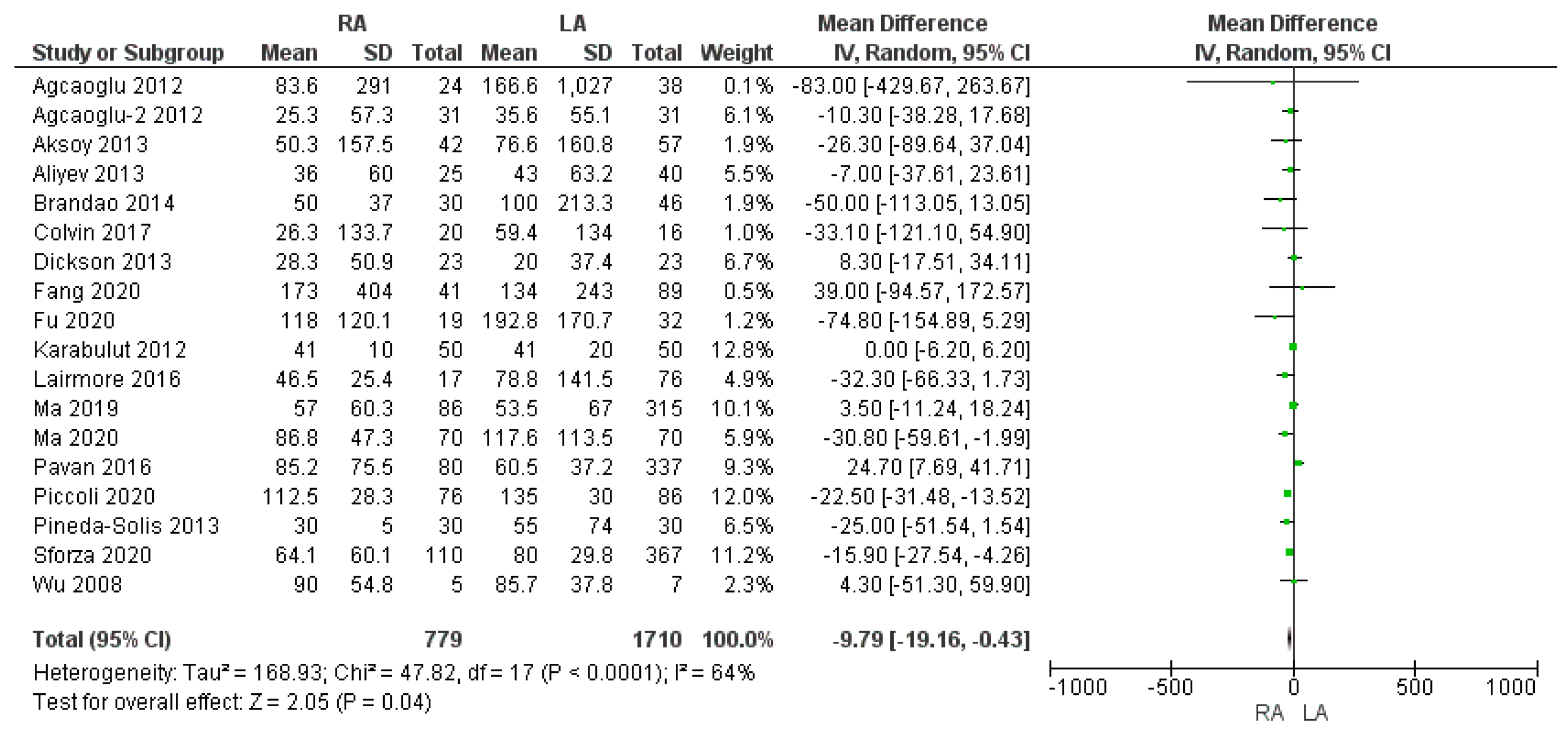
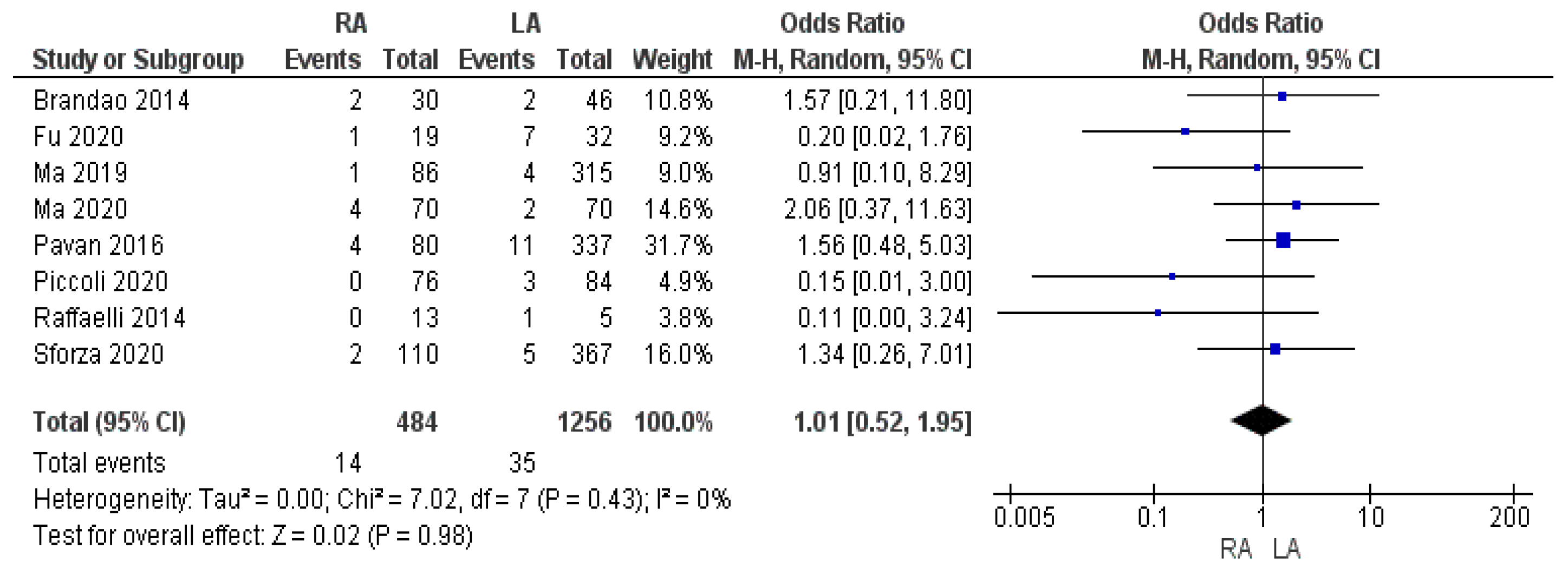
3.2.2. Intraoperative Blood Loss and Intraoperative Red Blood Cell (RBC) Transfusion Rate
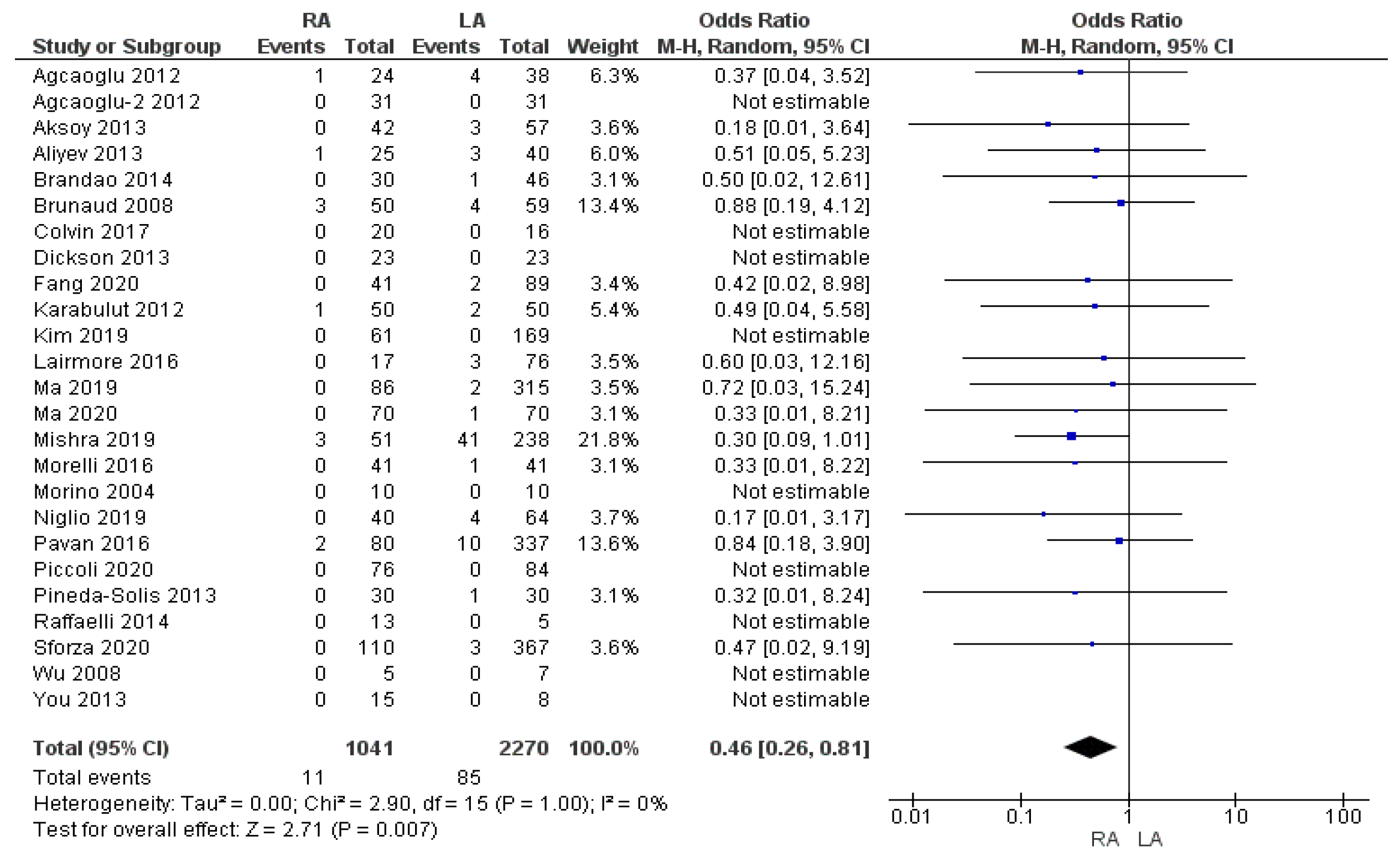
3.2.3. Conversion to Open Surgery Rate
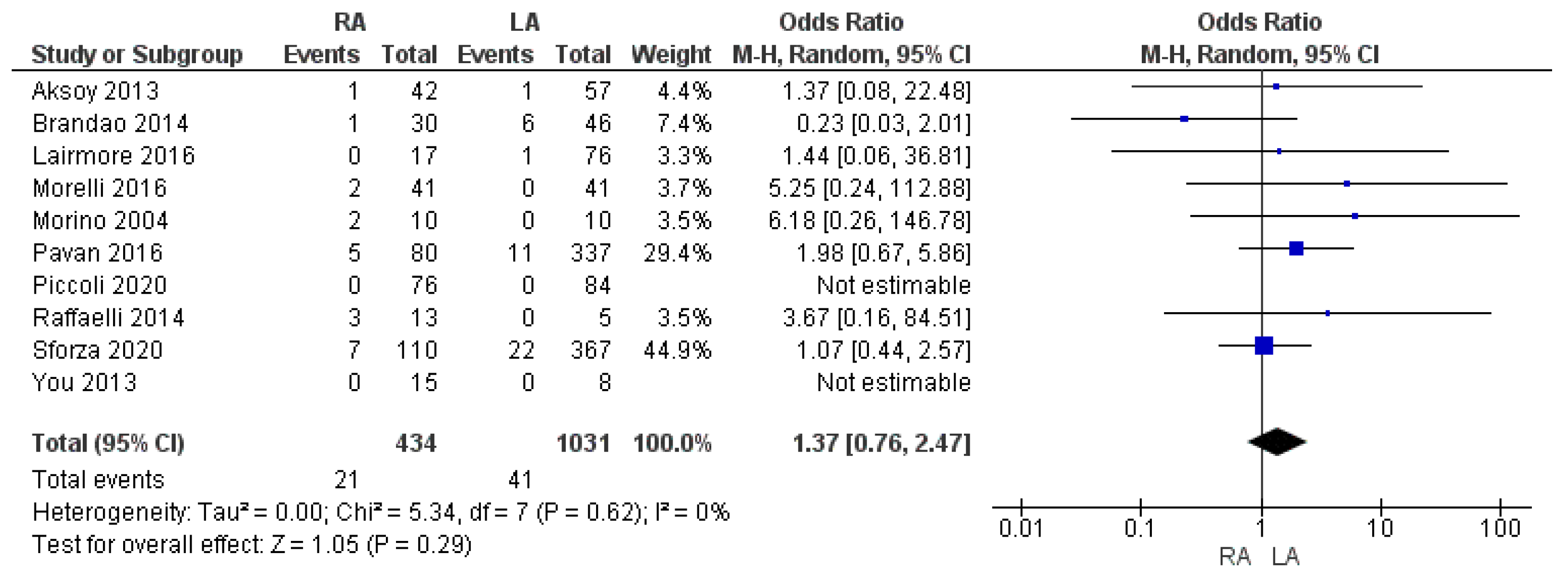
3.2.4. Intraoperative Complication Rate
3.3. Postoperative Outcomes
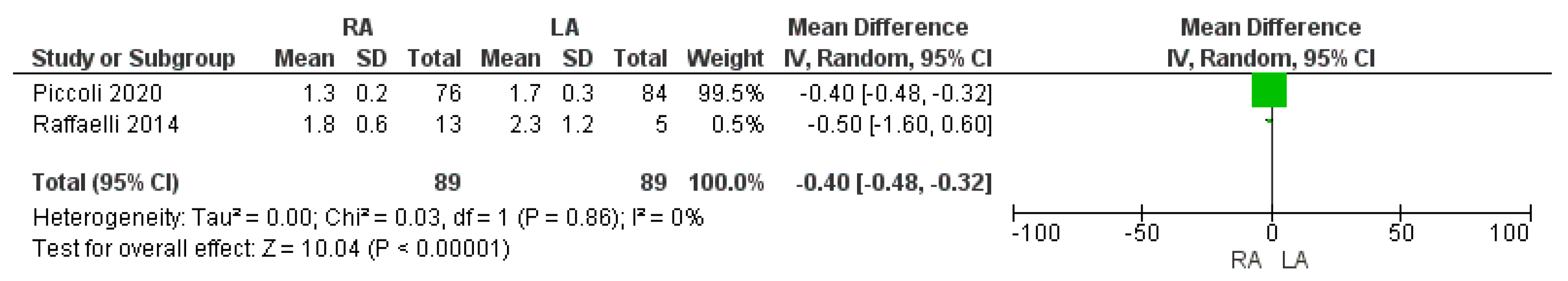
3.3.1. Time to First Flatus
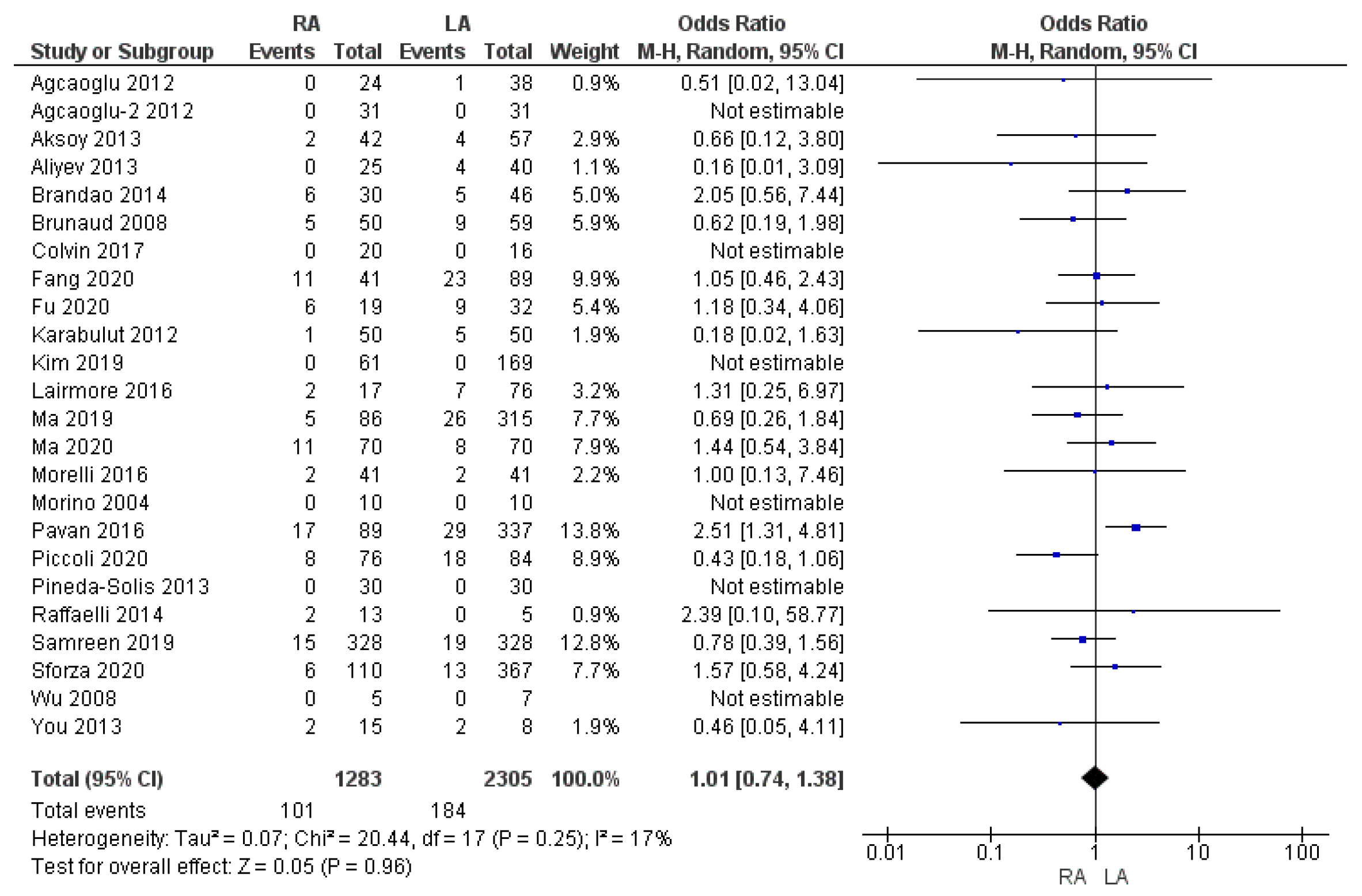
3.3.2. Overall Complication Rate
3.3.3. Complications Rate According to the Clavien–Dindo ≥ III
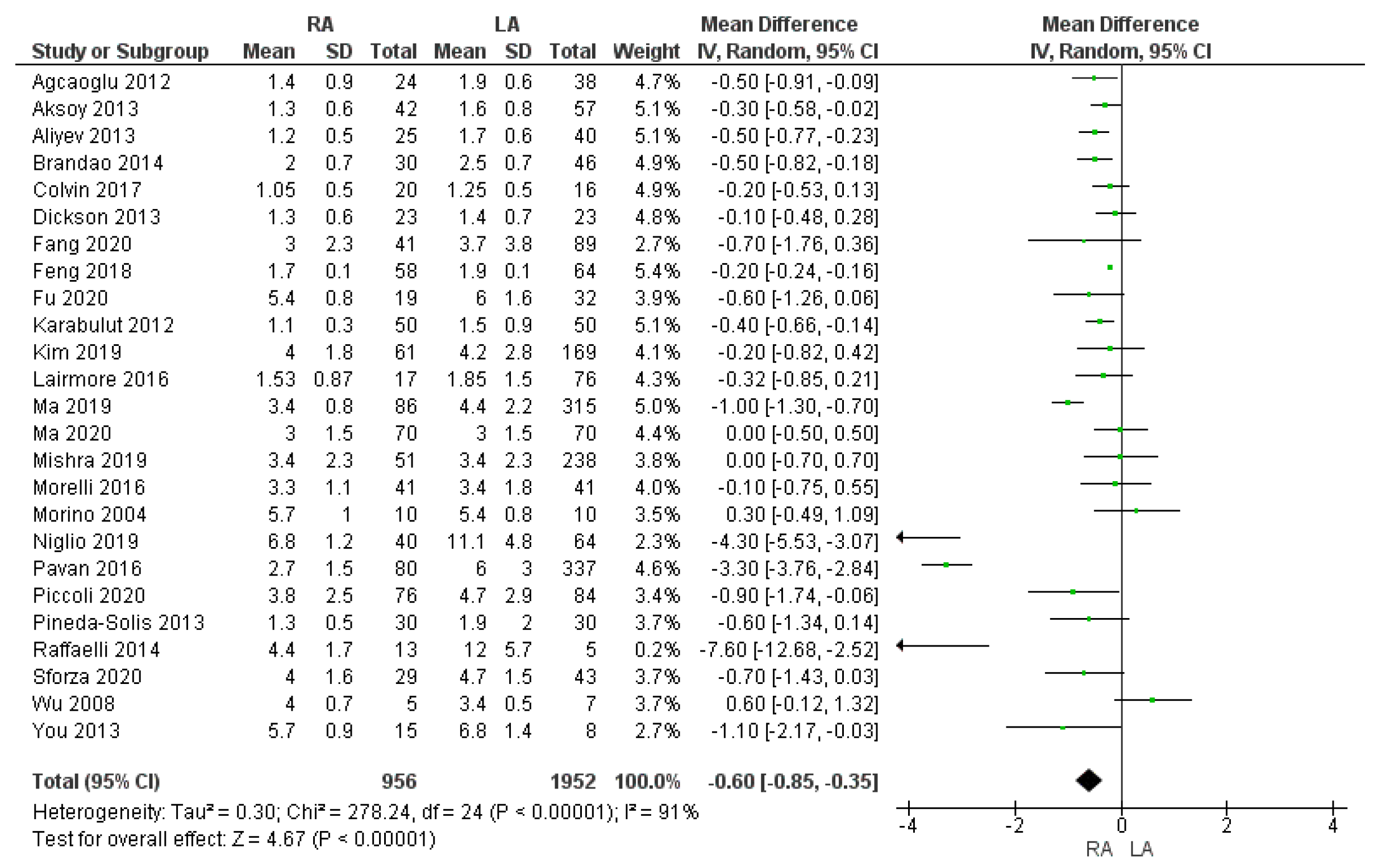
3.3.4. Length of Hospital Stay
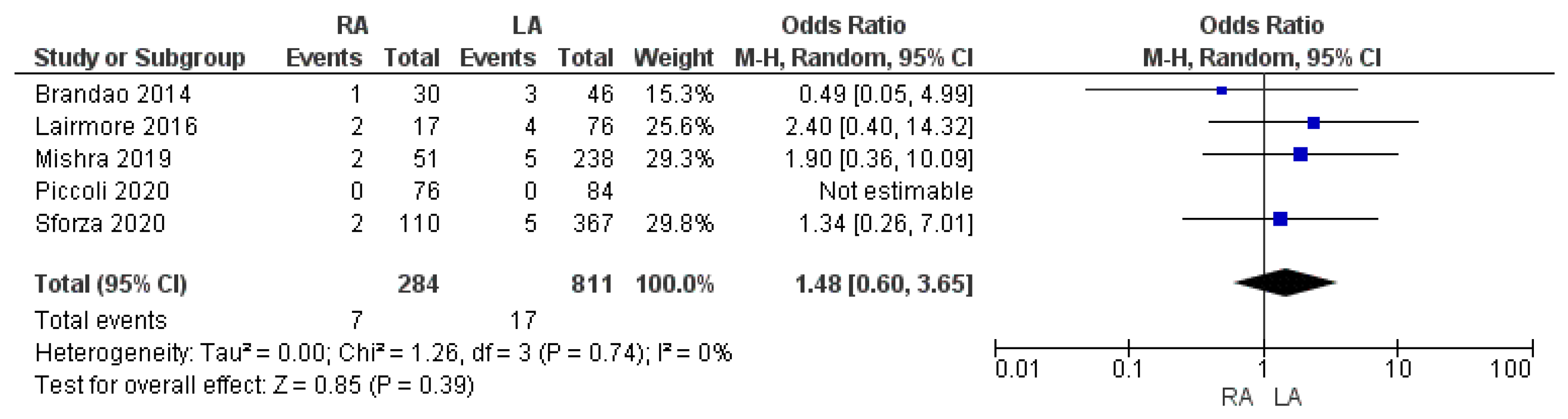
3.3.5. Re-Admission to Hospital Rate
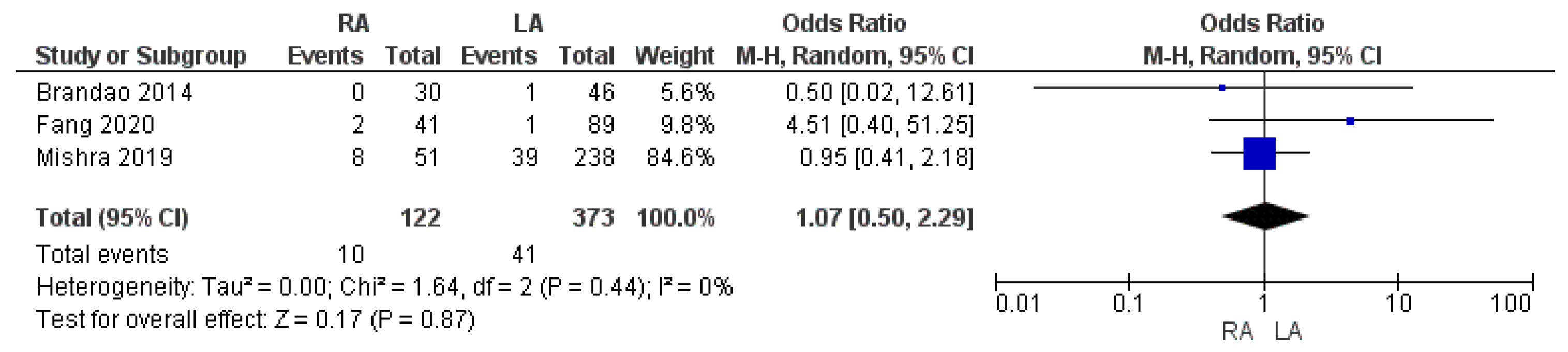
3.3.6. R1 Resection Margin Rate
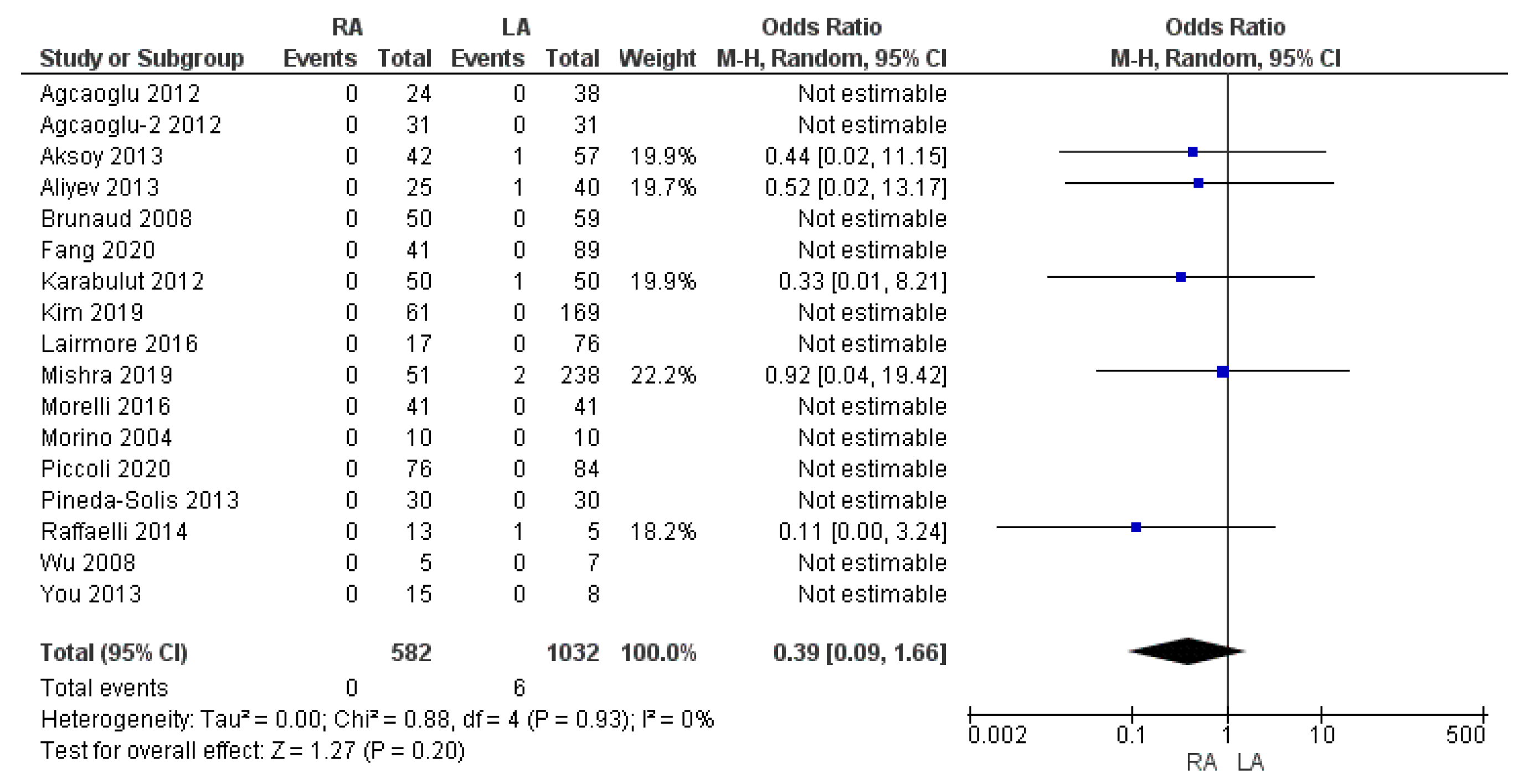
3.3.7. Thirty-Day Mortality Rate
3.3.8. Cost of the Hospitalization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gagner, M.; Lacroix, A.; Bolté, E. Laparoscopic adrenalectomy in Cushing’s syndrome and pheochromocytoma. N. Engl. J. Med. 1992, 327, 1033. [Google Scholar] [PubMed]
- Smith, C.D.; Weber, C.J.; Amerson, J.R. Laparoscopic adrenalectomy: New gold standard. World J. Surg. 1999, 23, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Germain, A.; Klein, M.; Brunaud, L. Surgical management of adrenal tumors. J. Visc. Surg. 2011, 148, e250–e261. [Google Scholar] [CrossRef]
- Economopoulos, K.P.; Mylonas, K.S.; Stamou, A.A.; Theocharidis, V.; Sergentanis, T.N.; Psaltopoulou, T.; Richards, M.L. Laparoscopic versus robotic adrenalectomy: A comprehensive meta-analysis. Int. J. Surg. 2017, 38, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Liu, Z.; Liang, J.; Tang, Y.; Zou, Z.; Zhou, C.; Zhang, F.; Lu, Y. Laparoscopic versus open adrenalectomy for localized (stage 1/2) adrenocortical carcinoma: Experience at a single, high-volumecenter. Surgery 2018, 164, 1325–1329. [Google Scholar] [CrossRef]
- Piazza, L.; Caragliano, P.; Scardilli, M.; Sgroi, A.V.; Marino, G.; Giannone, G. Laparoscopic robot-assisted right adrenalectomy and left ovariectomy (case reports). Chir. Ital. 1999, 51, 465–466. [Google Scholar]
- Piccoli, M.; Pecchini, F.; Serra, F.; Nigro, C.; Colli, G.; Gozzo, D.; Zirilli, L.; Madeo, B.; Rochira, V.; Mullineris, B. Robotic Versus Laparoscopic Adrenalectomy: Pluriannual Experience in a High-Volume Center Evaluating Indications and Results. J. Laparoendosc. Adv. Surg. Tech. A 2021, 31, 375–381. [Google Scholar] [CrossRef]
- Horgan, S.; Vanuno, D. Robots in laparoscopic surgery. J. Laparoendosc. Adv. Surg. Tech. A 2001, 11, 415–419. [Google Scholar] [CrossRef]
- Colvin, J.; Krishnamurthy, V.; Jin, J.; Shin, J.; Siperstein, A.; Berber, E. A Comparison of Robotic Versus Laparoscopic Adrenalectomy in Patients with Primary Hyperaldosteronism. Surg. Laparosc. Endosc. Percutan. Tech. 2017, 27, 391–393. [Google Scholar] [CrossRef]
- Stylopoulos, N.; Rattner, D. Robotics and ergonomics. Surg. Clin. N. Am. 2003, 83, 1321–1337. [Google Scholar] [CrossRef] [PubMed]
- Morris, L.F.; Perrier, N.D. Advances in robotic adrenalectomy. Curr. Opin. Oncol. 2012, 24, 1–6. [Google Scholar] [CrossRef]
- Nehs, M.A.; Ruan, D.T. Minimally invasive adrenal surgery: An update. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 193–197. [Google Scholar] [CrossRef]
- Aksoy, E.; Taskin, H.E.; Aliyev, S.; Mitchell, J.; Siperstein, A.; Berber, E. Robotic versus laparoscopic adrenalectomy in obese patients. Surg. Endosc. 2013, 27, 1233–1236. [Google Scholar] [CrossRef]
- Simone, G.; Anceschi, U.; Tuderti, G.; Misuraca, L.; Celia, A.; De Concilio, B. Robot-assisted Partial Adrenalectomy for the Treatment of Conn’s Syndrome: Surgical Technique, and Perioperative and Functional Outcomes. Eur. Urol. 2019, 75, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Anceschi, U.; Tuderti, G.; Fiori, C.; Zappalà, O.; Ferriero, M.C.; Brassetti, A. Minimally Invasive Partial Versus Total Adrenalectomy for the Treatment of Primary Aldosteronism: Results of a Multicenter Series According to the PASO Criteria. Eur. Urol. Focus 2021, 7, 1418–1423. [Google Scholar] [CrossRef]
- Perivoliotis, K.; Baloyiannis, I.; Sarakatsianou, C.; Tzovaras, G. Comparing the efficacy and safety of laparoscopic and robotic adrenalectomy: A meta-analysis and trial sequential analysis. Langenbeck’s Arch. Surg. 2020, 405, 125–135. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Goossen, K.; Tenckhoff, S.; Probst, P.; Grummich, K.; Mihaljevic, A.L.; Buechler, M.W.; Diener, M.K. Optimal literature search for systematic reviews in surgery. Langenbeck’s Arch. Surg. 2018, 403, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024); Cochrane Collaboration: London, UK; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2024. [Google Scholar]
- Brunaud, L.; Bresler, L.; Ayav, A.; Zarnegar, R.; Raphoz, A.L.; Levan, T.; Weryha, G.; Boisselet, P. Robotic-assisted adrenalectomy: What advantages compared to lateral transperitoneal laparoscopic adrenalectomy? Am. J. Surg. 2008, 195, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.H.; Wu, H.S.; Lin, M.S.; Chou, D.A.; Huang, M.H. Comparison of robot-assisted laparoscopic adrenalectomy with traditional laparoscopic adrenalectomy—1 year follow-up. Surg. Endosc. 2008, 22, 463–466. [Google Scholar] [CrossRef]
- Agcaoglu, O.; Aliyev, S.; Karabulut, K.; Siperstein, A.; Berber, E. Robotic vs. laparoscopic posterior retroperitoneal adrenalectomy. Arch. Surg. 2012, 147, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Agcaoglu, O.; Aliyev, S.; Karabulut, K.; Mitchell, J.; Siperstein, A.; Berber, E. Robotic versus laparoscopic resection of large adrenal tumors. Ann. Surg. Oncol. 2012, 19, 2288–2294. [Google Scholar] [CrossRef]
- Karabulut, K.; Agcaoglu, O.; Aliyev, S.; Siperstein, A.; Berber, E. Comparison of intraoperative time use and perioperative outcomes for robotic versus laparoscopic adrenalectomy. Surgery 2012, 151, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Aliyev, S.; Karabulut, K.; Agcaoglu, O.; Wolf, K.; Mitchell, J.; Siperstein, A.; Berber, E. Robotic versus laparoscopic adrenalectomy for pheochromocytoma. Ann. Surg. Oncol. 2013, 20, 4190–4194. [Google Scholar] [CrossRef]
- Dickson, P.V.; Alex, G.C.; Grubbs, E.G.; Jimenez, C.; Lee, J.E.; Perrier, N.D. Robotic-assisted retroperitoneoscopic adrenalectomy: Making a good procedure even better. Am. Surg. 2013, 79, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Solís, K.; Medina-Franco, H.; Heslin, M.J. Robotic versus laparoscopic adrenalectomy: A comparative study in a high-volume center. Surg. Endosc. 2013, 27, 599–602. [Google Scholar] [CrossRef] [PubMed]
- Lairmore, T.C.; Folek, J.; Govednik, C.M.; Snyder, S.K. Improving Minimally Invasive Adrenalectomy: Selection of Optimal Approach and Comparison of Outcomes. World J. Surg. 2016, 40, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; Tartaglia, D.; Bronzoni, J.; Palmeri, M.; Guadagni, S.; Di Franco, G.; Gennai, A.; Bianchini, M.; Bastiani, L.; Moglia, A.; et al. Robotic assisted versus pure laparoscopic surgery of the adrenal glands: A case-control study comparing surgical techniques. Langenbeck’s Arch. Surg. 2016, 401, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Pavan, N.; Autorino, R.; Lee, H.; Porpiglia, F.; Sun, Y.; Greco, F.; Chueh, S.J.; Han, D.H.; Cindolo, L.; Ferro, M.; et al. Impact of novel techniques on minimally invasive adrenal surgery: Trends and outcomes from a contemporary international large series in urology. World J. Urol. 2016, 34, 1473–1479. [Google Scholar] [CrossRef] [PubMed]
- You, J.Y.; Lee, H.Y.; Son, G.S.; Lee, J.B.; Bae, J.W.; Kim, H.Y. Comparison of robotic adrenalectomy with traditional laparoscopic adrenalectomy with a lateral transperitoneal approach: A single-surgeon experience. Int. J. Med. Robot. 2013, 9, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Brandao, L.F.; Autorino, R.; Zargar, H.; Krishnan, J.; Laydner, H.; Akca, O.; Mir, M.C.; Samarasekera, D.; Stein, R.; Kaouk, J. Robot-assisted laparoscopic adrenalectomy: Step-by-step technique and comparative outcomes. Eur. Urol. 2014, 66, 898–905. [Google Scholar] [CrossRef]
- Raffaelli, M.; Brunaud, L.; De Crea, C.; Hoche, G.; Oragano, L.; Bresler, L.; Bellantone, R.; Lombardi, C.P. Synchronous bilateral adrenalectomy for Cushing’s syndrome: Laparoscopic versus posterior retroperitoneoscopic versus robotic approach. World J. Surg. 2014, 38, 709–715. [Google Scholar] [CrossRef]
- Kim, W.W.; Lee, Y.M.; Chung, K.W.; Hong, S.J.; Sung, T.Y. Comparison of Robotic Posterior Retroperitoneal Adrenalectomy over Laparoscopic Posterior Retroperitoneal Adrenalectomy: A Single Tertiary Center Experience. Int. J. Endocrinol. 2019, 2019, 9012910. [Google Scholar] [CrossRef]
- Feng, Z.; Feng, M.P.; Feng, D.P.; Rice, M.J.; Solórzano, C.C. A cost-conscious approach to robotic adrenalectomy. J. Robot. Surg. 2018, 12, 607–611. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Mao, Y.; Dai, J.; Alimu, P.; Zhuo, R.; He, W.; Zhao, J.; Xu, D.; Sun, F. Propensity Score Matched Analysis Comparing Robotic-Assisted with Laparoscopic Posterior Retroperitoneal Adrenalectomy. J. Investig. Surg. 2020, 34, 1248–1253. [Google Scholar] [CrossRef]
- Mishra, K.; Maurice, M.J.; Bukavina, L.; Abouassaly, R. Comparative Efficacy of Laparoscopic Versus Robotic Adrenalectomy for Adrenal Malignancy. Urology 2019, 123, 146–150. [Google Scholar] [CrossRef]
- Niglio, A.; Grasso, M.; Costigliola, L.; Zenone, P.; De Palma, M. Laparoscopic and robot-assisted transperitoneal lateral adrenalectomy: A large clinical series from a single center. Updates Surg. 2020, 72, 193–198. [Google Scholar] [CrossRef]
- Samreen, S.; Fluck, M.; Hunsinger, M.; Wild, J.; Shabahang, M.; Blansfield, J.A. Laparoscopic versus robotic adrenalectomy: A review of the national inpatient sample. J. Robot. Surg. 2019, 13, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Fang, A.M.; Rosen, J.; Saidian, A.; Bae, S.; Tanno, F.Y.; Chambo, J.L.; Bloom, J.; Gordetsky, J.; Srougi, V.; Phillips, J.; et al. Perioperative outcomes of laparoscopic, robotic, and open approaches to pheochromocytoma. J. Robot. Surg. 2020, 14, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Q.; Zhuang, C.S.; Yang, X.R.; Xie, W.J.; Gong, B.B.; Liu, Y.F.; Liu, J.; Sun, T.; Ma, M. Comparison of robot-assisted retroperitoneal laparoscopic adrenalectomy versus retroperitoneal laparoscopic adrenalectomy for large pheochromocytoma: A single-centre retrospective study. BMC Surg. 2020, 20, 227. [Google Scholar] [CrossRef]
- Sforza, S.; Minervini, A.; Tellini, R.; Ji, C.; Bergamini, C.; Giordano, A.; Lu, Q.; Chen, W.; Zhang, F.; Ji, H.; et al. Perioperative outcomes of robotic and laparoscopic adrenalectomy: A large international multicenter experience. Surg. Endosc. 2021, 35, 1801–1807. [Google Scholar] [CrossRef]
- Morino, M.; Benincà, G.; Giraudo, G.; Del Genio, G.M.; Rebecchi, F.; Garrone, C. Robot-assisted vs. laparoscopic adrenalectomy: A prospective randomized controlled trial. Surg Endosc. 2004, 18, 1742–1746. [Google Scholar] [CrossRef]
- Ma, W.; Mao, Y.; Zhuo, R.; Dai, J.; Fang, C.; Wang, C.; Zhao, J.; He, W.; Zhu, Y.; Xu, D.; et al. Surgical outcomes of a randomized controlled trial compared robotic versus laparoscopic adrenalectomy for pheochromocytoma. Eur. J. Surg. Oncol. 2020, 46, 1843–1847. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Peng, L.; Li, J.; Meng, C.; Li, K.; Wu, J.; Zhang, Z.; Li, Y. Comparison of the effectiveness and safety of robotic-assisted and laparoscopic in adrenalectomy: A systematic review and meta-analysis. Int. J. Surg. 2022, 105, 106853. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
| Author | Region | Year | Study Period | Institution | Study Design | Sample Size | Surgical Approach | MINORS (Quality) | |
|---|---|---|---|---|---|---|---|---|---|
| Single or Multi | RA | LA | |||||||
| Morino [48] | Italy | 2004 | 2002–2002 | Single center | RCT | 10 | 10 | ANT | NA |
| Brunaud [25] | France | 2008 | 1996–2005 | Single center | OCS (P) | 50 | 59 | ANT | 22 |
| Wu [5] | Taiwan | 2008 | 2003–2005 | Single center | OCS (P) | 5 | 7 | ANT | 20 |
| Agcaoglu [28] | USA | 2012 | 2000–2011 | Single center | OCS (P) | 24 | 38 | POST | 22 |
| Agcaoglu-2 [27] | USA | 2012 | 2009–2011 | Single center | OCS (P) | 31 | 31 | POST | 23 |
| Karabulut [29] | USA | 2012 | 2008–2011 | Single center | OCS (P) | 50 | 50 | ANT + POST | 23 |
| Aksoy [13] | USA | 2013 | 2003–2012 | Single center | OCS (P) | 42 | 57 | ANT + POST | 23 |
| Aliyev [30] | USA | 2013 | 2008–2012 | Single center | OCS (P) | 25 | 40 | ANT + POST | 22 |
| Dickson [31] | USA | 2013 | 2009–2011 | Single center | OCS (P) | 23 | 23 | POST | 23 |
| Pineda-Solis [32] | USA | 2013 | NA | Single center | OCS (R) | 30 | 30 | ANT | 21 |
| You [36] | Korea | 2013 | 2009–2012 | Single center | OCS (R) | 15 | 8 | ANT | 22 |
| Brandao [37] | USA | 2014 | 2004–2013 | Single center | OCS (P) | 30 | 46 | ANT | 22 |
| Raffaelli [38] | Italy | 2014 | 1999–2012 | Multi center | OCS (P) | 13 | 5 | ANT | 22 |
| Lairmore [33] | USA | 2016 | 2005–2015 | Single center | OCS (P) | 17 | 76 | POST | 23 |
| Morelli [16] | Italy | 2016 | 1994–2014 | Single center | OCS (R) | 41 | 41 | ANT | 22 |
| Pavan [35] | Italy | 2016 | 2008–2013 | Multi center | OCS (R) | 80 | 337 | ANT + POST | 22 |
| Colvin [9] | USA | 2017 | 2000–2014 | Single center | OCS (P) | 20 | 16 | ANT + POST | 23 |
| Feng [40] | USA | 2018 | 2010–2017 | Single center | OCS (R) | 58 | 64 | ANT + POST | 22 |
| Kim [39] | Korea | 2019 | 2014–2017 | Single center | OCS (R) | 61 | 169 | POST | 22 |
| Ma [41] | China | 2019 | 2013–2018 | Single center | OCS (R) | 86 | 315 | POST | 23 |
| Mishra [42] | USA | 2019 | 2010–2013 | Multi center | OCS (R) | 51 | 238 | NA | 22 |
| Niglio [43] | Italy | 2019 | 2011–2018 | Single center | OCS (R) | 40 | 64 | ANT | 22 |
| Smreen [44] | USA | 2019 | 2009–2012 | Multi center | OCS (R) | 328 | 328 | NA | 22 |
| Fang [45] | USA | 2020 | 2000–2017 | Multi center | OCS (R) | 41 | 89 | NA | 22 |
| Fu [46] | China | 2020 | 2016–2019 | Single center | OCS (R) | 19 | 32 | POST | 23 |
| Ma [49] | China | 2020 | 2016–2019 | Single center | RCT | 70 | 70 | ANT + POST | NA |
| Sforza [47] | Italy | 2020 | 2008–2018 | Multi center | OCS (R) | 110 | 367 | ANT + POST | 22 |
| Piccoli [7] | Italy | 2021 | 2006–2019 | Single center | OCS (R) | 76 | 84 | ANT | 22 |
| RA | LA | Studies (n) | |
|---|---|---|---|
| Total patients included (n.) (range) | 1446 (5–328) | 2694 (5–367) | 4140 (28) |
| Follow-up (months) (range) | 28.3 (4.5–96) | 38.6 (12–96) | 7 |
| Age (years) (range) | 51.7 (38.5–62) | 51.9 (40.3–60) | 27 |
| BMI (range) | 28.3 (21.9–35.3) | 29 (22.8–52.9) | 26 |
| Mean size of lesion (cm) (range) | 4.17 (1.7–8) | 4.01 (1.3–7.7) | 26 |
| Previous abdominal surgery (%) | 201/607 (33.1%) | 389/1166 (33.4%) | 13 |
| ASA score I/II (%) | 208/374 (55.6%) | 647/889 (72.8%) | 7 |
| ASA score ≥ III (%) | 166/374 (44.4%) | 242/889 (27.2%) | 7 |
| Side of lesion/surgery (n.) | 961 | 1950 | 23 |
| Right (%) | 422 (43.9%) | 924 (47.4%) | |
| Left (%) | 523 (54.4%) | 1005 (51.5%) | |
| Bilateral (%) | 16 (1.7%) | 21 (1.1%) | |
| Surgical approach (n.) | 976 | 2039 | 25 |
| Anterior (%) | 683 (66.6%) | 1040 (51%) | |
| Posterior (%) | 343 (33.3%) | 999 (49%) | |
| Tumor histology (n.) | 1429 | 2618 | 27 |
| Malignant (%) | 132 (9.2%) | 456 (17.4%) | |
| Benign (%) | 1297 (90.8%) | 2162 (82.6%) |
| Surgical Outcome | Type of Surgery | Observations (n) | Mean or % | Studies Included (n) | p Value |
|---|---|---|---|---|---|
| Operating time (min) | RA | 1067 | 157.2 | 26 | 0.06 |
| LA | 2128 | 149.6 | |||
| Intraoperative blood loss (mL) | RA | 779 | 66.9 | 18 | 0.04 * |
| LA | 1710 | 85.3 | |||
| RBC transfusion rate | RA | 14/484 | 2.9% | 8 | 0.98 |
| LA | 35/1256 | 2.8% | |||
| Conversion to open surgery rate | RA | 11/1041 | 1.05% | 25 | 0.007 * |
| LA | 85/2270 | 3.7% | |||
| Intraoperative complication rate | RA | 21/434 | 4.8% | 10 | 0.29 |
| LA | 41/1031 | 3.9% | |||
| Time to first flatus (day) | RA | 89 | 1.5 | 2 | 0.00001 * |
| LA | 89 | 2 | |||
| Overall complication rate | RA | 101/1283 | 7.8% | 24 | 0.96 |
| LA | 184/2305 | 8% | |||
| Clavien–Dindo ≥ III complication rate | RA | 11/607 | 1.8% | 12 | 0.45 |
| LA | 16/1488 | 1.1% | |||
| Length of hospital stay (days) | RA | 956 | 3.06 | 25 | 0.00001 * |
| LA | 1952 | 4 | |||
| Readmission rate | RA | 7/284 | 2.5% | 5 | 0.39 |
| LA | 17/811 | 2.1% | |||
| R1 resection margin rate | RA | 10/122 | 8.2% | 3 | 0.87 |
| LA | 41/373 | 11% | |||
| 30-day mortality rate | RA | 0/582 | 0% | 17 | 0.20 |
| LA | 6/1032 | 0.6% | |||
| Cost of hospitalization (USD) | RA | 156 | 8695.45 | 2 | 0.00001 * |
| LA | 385 | 4560.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, G.; Mullineris, B.; Colli, G.; Curia, S.; Piccoli, M. Robotic Versus Laparoscopic Adrenalectomy for Adrenal Tumors: An Up-to-Date Meta-Analysis on Perioperative Outcomes. Cancers 2025, 17, 150. https://doi.org/10.3390/cancers17010150
Esposito G, Mullineris B, Colli G, Curia S, Piccoli M. Robotic Versus Laparoscopic Adrenalectomy for Adrenal Tumors: An Up-to-Date Meta-Analysis on Perioperative Outcomes. Cancers. 2025; 17(1):150. https://doi.org/10.3390/cancers17010150
Chicago/Turabian StyleEsposito, Giuseppe, Barbara Mullineris, Giovanni Colli, Serena Curia, and Micaela Piccoli. 2025. "Robotic Versus Laparoscopic Adrenalectomy for Adrenal Tumors: An Up-to-Date Meta-Analysis on Perioperative Outcomes" Cancers 17, no. 1: 150. https://doi.org/10.3390/cancers17010150
APA StyleEsposito, G., Mullineris, B., Colli, G., Curia, S., & Piccoli, M. (2025). Robotic Versus Laparoscopic Adrenalectomy for Adrenal Tumors: An Up-to-Date Meta-Analysis on Perioperative Outcomes. Cancers, 17(1), 150. https://doi.org/10.3390/cancers17010150