Effects on the Physical Functioning of Two Exercise Interventions in Patients with Multiple Myeloma: A Pilot Feasibility Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Setting
2.2. Intervention
2.3. Safety
2.4. Physical Therapy Assessment
2.5. Endpoints
2.6. Statistical Methods
2.7. Data Availability
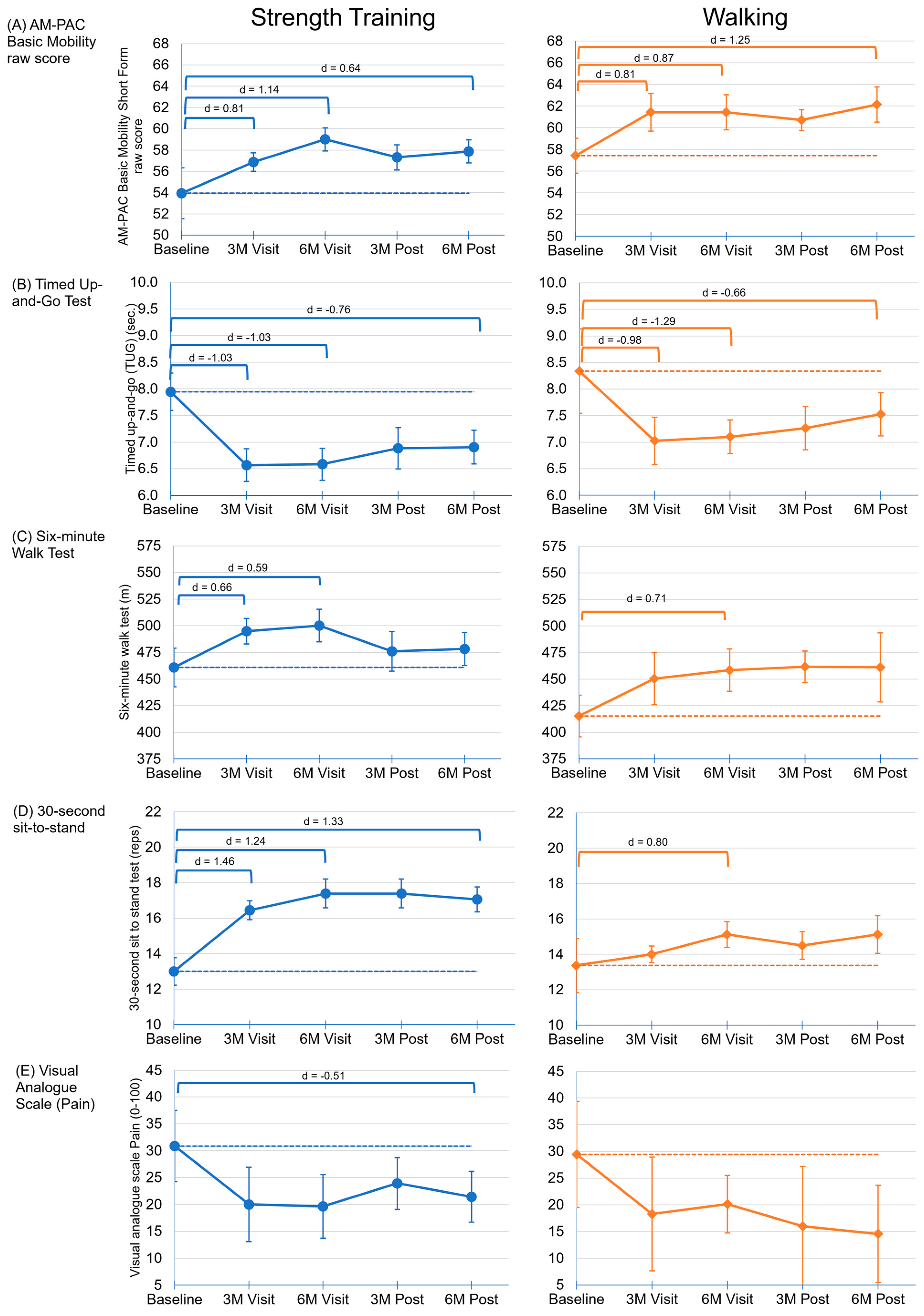
3. Results
3.1. Descriptive Characteristics
3.2. Feasibility and Adherence
3.3. Safety
3.4. Physical Functioning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Raje, N.; Roodman, G.D. Advances in the biology and treatment of bone disease in multiple myeloma. Clin. Cancer Res. 2011, 17, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Abramson, H.N. Recent Advances in the Applications of Small Molecules in the Treatment of Multiple Myeloma. Int. J. Mol. Sci. 2023, 24, 2645. [Google Scholar] [CrossRef]
- Cowan, A.J.; Green, D.J.; Kwok, M.; Lee, S.; Coffey, D.G.; Holmberg, L.A.; Tuazon, S.; Gopal, A.K.; Libby, E.N. Diagnosis and Management of Multiple Myeloma: A Review. JAMA 2022, 327, 464–477. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, N.; Usmani, S.Z.; Yong, K. CAR T-cell therapy for multiple myeloma: State of the art and prospects. Lancet Haematol. 2021, 8, e446–e461. [Google Scholar] [CrossRef]
- Swan, D.; Routledge, D.; Harrison, S. The evolving status of immunotherapies in multiple myeloma: The future role of bispecific antibodies. Br. J. Haematol. 2022, 196, 488–506. [Google Scholar] [CrossRef]
- Kumar, S.K.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Pandey, S.; Kapoor, P.; Dingli, D.; Hayman, S.R.; Leung, N.; et al. Continued improvement in survival in multiple myeloma: Changes in early mortality and outcomes in older patients. Leukemia 2014, 28, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Weller, S.; Hart, N.H.; Bolam, K.A.; Mansfield, S.; Santa Mina, D.; Winters-Stone, K.M.; Campbell, A.; Rosenberger, F.; Wiskemann, J.; Quist, M.; et al. Exercise for individuals with bone metastases: A systematic review. Crit. Rev. Oncol./Hematol. 2021, 166, 103433. [Google Scholar] [CrossRef]
- Avancini, A.; Benato, G.; Borsati, A.; Oliviero, L.; Belluomini, L.; Sposito, M.; Tregnago, D.; Trestini, I.; Insolda, J.; Zacchi, F.; et al. Exercise and Bone Health in Cancer: Enemy or Ally? Cancers 2022, 14, 6078. [Google Scholar] [CrossRef]
- Joseph, J.M.; Hillengass, M.; Sweeney, N.W.; Molina, T.H.; Ahlstrom, J.M.; Moysich, K.; Cannioto, R.; Hillengass, J. Physical Activity and Patient-Reported Outcomes in Monoclonal Plasma Cell Disorders. Med. Sci. Sports Exerc. 2023, 55, 1952–1960. [Google Scholar] [CrossRef]
- Waszczuk-Gajda, A.; Penack, O.; Sbianchi, G.; Koster, L.; Blaise, D.; Reményi, P.; Russell, N.; Ljungman, P.; Trneny, M.; Mayer, J.; et al. Complications of Autologous Stem Cell Transplantation in Multiple Myeloma: Results from the CALM Study. J. Clin. Med. 2022, 11, 3541. [Google Scholar] [CrossRef]
- Zhou, X.; Rasche, L.; Kortüm, K.M.; Danhof, S.; Hudecek, M.; Einsele, H. Toxicities of Chimeric Antigen Receptor T Cell Therapy in Multiple Myeloma: An Overview of Experience From Clinical Trials, Pathophysiology, and Management Strategies. Front. Immunol. 2020, 11, 620312. [Google Scholar] [CrossRef]
- Davis, J.A.; Dima, D.; Ahmed, N.; DeJarnette, S.; McGuirk, J.; Jia, X.; Raza, S.; Khouri, J.; Valent, J.; Anwer, F.; et al. Impact of Frailty on Outcomes after Chimeric Antigen Receptor T Cell Therapy for Patients with Relapsed/Refractory Multiple Myeloma. Transplant. Cell Ther. 2024, 30, 298–305. [Google Scholar] [CrossRef]
- St Bernard, R.; Chodirker, L.; Masih-Khan, E.; Jiang, H.; Franke, N.; Kukreti, V.; Tiedemann, R.; Trudel, S.; Reece, D.; Chen, C.I. Efficacy, toxicity and mortality of autologous SCT in multiple myeloma patients with dialysis-dependent renal failure. Bone Marrow Transplant. 2015, 50, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Fischer, L.; Grieb, N.; Born, P.; Weiss, R.; Seiffert, S.; Boldt, A.; Fricke, S.; Franz, P.; Heyn, S.; Kubasch, A.S.; et al. Cellular dynamics following CAR T cell therapy are associated with response and toxicity in relapsed/refractory myeloma. Leukemia 2024, 38, 372–382. [Google Scholar] [CrossRef]
- Mian, H.; McCurdy, A.; Giri, S.; Grant, S.; Rochwerg, B.; Winks, E.; Rosko, A.E.; Engelhardt, M.; Pawlyn, C.; Cook, G.; et al. The prevalence and outcomes of frail older adults in clinical trials in multiple myeloma: A systematic review. Blood Cancer J. 2023, 13, 6. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Bringhen, S.; Mateos, M.V.; Larocca, A.; Facon, T.; Kumar, S.K.; Offidani, M.; McCarthy, P.; Evangelista, A.; Lonial, S.; et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood 2015, 125, 2068–2074. [Google Scholar] [CrossRef]
- Aguirre, L.E.; Villareal, D.T. Physical Exercise as Therapy for Frailty. Nestle Nutr. Inst. Workshop Ser. 2015, 83, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, D.; Lai, J.K.L.; Wareing, H.; Ren, Y.; Li, T.; Swain, C.T.V.; Smith, D.P.; Adams, D.; Martiniuk, A.; David, M. Effect of exercise interventions on hospital length of stay and admissions during cancer treatment: A systematic review and meta-analysis. Br. J. Sports Med. 2024, 58, 97–109. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) 2017. Available online: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf (accessed on 11 April 2024).
- Boston University. Activity Measure for Post-Acute Care. Available online: https://am-pac.com/ (accessed on 1 November 2023).
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the six-minute walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Shirley Ryan AbilityLab. 30 Second Sit to Stand Test. Available online: https://www.sralab.org/rehabilitation-measures/30-second-sit-stand-test (accessed on 20 May 2013).
- Shirley Ryan AbilityLab. Timed Up and Go. Available online: https://www.sralab.org/rehabilitation-measures/timed-and-go (accessed on 6 November 2013).
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15 (Suppl. S1), S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Shirley Ryan AbilityLab. Hand-Held Dynamometer/Grip Strength. Available online: https://www.sralab.org/rehabilitation-measures/hand-held-dynamometer-grip-strength (accessed on 7 February 2014).
- Haley, S.M.; Coster, W.J.; Andres, P.L.; Ludlow, L.H.; Ni, P.; Bond, T.L.; Sinclair, S.J.; Jette, A.M. Activity outcome measurement for postacute care. Med. Care 2004, 42, I49–I61. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.M.; Hillengass, M.; Jacobson, H.; Wallace, P.K.; Tario, J.D.; Attwood, K.; Groman, A.; Cannioto, R.; Wittmeyer, B.; Mohammadpour, H.; et al. T cell exhaustion markers in multiple myeloma patients are lower after physical activity intervention. Clin. Lymphoma Myeloma Leuk. 2024; in press. [Google Scholar]
- Lacroix, A.; Hortobágyi, T.; Beurskens, R.; Granacher, U. Effects of Supervised vs. Unsupervised Training Programs on Balance and Muscle Strength in Older Adults: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 2341–2361. [Google Scholar] [CrossRef] [PubMed]
- Nicol, J.L.; Chong, J.E.; McQuilten, Z.K.; Mollee, P.; Hill, M.M.; Skinner, T.L. Safety, Feasibility, and Efficacy of Exercise Interventions for People With Multiple Myeloma: A Systematic Review. Clin. Lymphoma Myeloma Leuk. 2023, 23, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Hillengass, M.; Joseph, J.; McCarthy, J.; Hillengass, J. Physical Activity in Multiple Myeloma: A Review of the Current Literature. J. Adv. Pract. Oncol. 2023, 14, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Coleman, E.A.; Coon, S.K.; Kennedy, R.L.; Lockhart, K.D.; Stewart, C.B.; Anaissie, E.J.; Barlogie, B. Effects of exercise in combination with epoetin alfa during high-dose chemotherapy and autologous peripheral blood stem cell transplantation for multiple myeloma. Oncol. Nurs. Forum 2008, 35, E53–E61. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.F.; Jarden, M.; Minet, L.R.; Frølund, U.C.; Abildgaard, N. Supervised and home-based physical exercise in patients newly diagnosed with multiple myeloma—A randomized controlled feasibility study. Pilot. Feasibility Stud. 2019, 5, 130. [Google Scholar] [CrossRef] [PubMed]
- Persoon, S.; Chin, A.M.J.M.; Buffart, L.M.; Liu, R.D.K.; Wijermans, P.; Koene, H.R.; Minnema, M.C.; Lugtenburg, P.J.; Marijt, E.W.A.; Brug, J.; et al. Randomized controlled trial on the effects of a supervised high intensity exercise program in patients with a hematologic malignancy treated with autologous stem cell transplantation: Results from the EXIST study. PLoS ONE 2017, 12, e0181313. [Google Scholar] [CrossRef]
- Coleman, E.A.; Goodwin, J.A.; Kennedy, R.; Coon, S.K.; Richards, K.; Enderlin, C.; Stewart, C.B.; McNatt, P.; Lockhart, K.; Anaissie, E.J. Effects of exercise on fatigue, sleep, and performance: A randomized trial. Oncol. Nurs. Forum 2012, 39, 468–477. [Google Scholar] [CrossRef]
- Coleman, E.A.; Coon, S.; Hall-Barrow, J.; Richards, K.; Gaylor, D.; Stewart, B. Feasibility of exercise during treatment for multiple myeloma. Cancer Nurs. 2003, 26, 410–419. [Google Scholar] [CrossRef]

| All Participants (n = 42) | Strength (n = 24) | Walking (n = 18) | p-Value 1 | |
|---|---|---|---|---|
| Mean (SD) | ||||
| Age at consent (years) | 63.2 (7.7) | 63.9 (5.9) | 62.2 (9.6) | 0.52 |
| Body mass index, baseline (BMI) (kg/m2) | 30.3 (6.3) | 30.9 (6.5) | 29.5 (6.1) | 0.49 |
| n (%) † | ||||
| Sex | ||||
| Female | 26 (61.9) | 14 (58.3) | 12 (66.7) | 0.58 |
| Male | 16 (38.1) | 10 (41.7) | 6 (33.3) | |
| Race and ethnicity | ||||
| Non-Hispanic White | 36 (85.7) | 21 (87.5) | 15 (83.3) | 0.66 |
| Non-Hispanic Black or African-American | 3 (7.1) | 1 (4.2) | 2 (11.1) | |
| Other or unknown race/ethnicity | 3 (7.1) | 2 (8.3) | 1 (5.6) | |
| ECOG, baseline | ||||
| 0 Fully active | 26 (61.9) | 17 (70.8) | 9 (50.0) | 0.26 |
| 1 Restricted in physically strenuous activity | 15 (35.7) | 7 (29.2) | 8 (44.4) | |
| Disease response, baseline | ||||
| Complete response (CR) | 26 (61.9) | 16 (66.7) | 10 (55.6) | 0.44 |
| <Complete response | 15 (35.7) | 8 (33.3) | 7 (38.9) | |
| Disease status | ||||
| Active disease | 4 (9.5) | 1 (4.2) | 3 (16.7) | 0.17 |
| Non-active disease | 38 (90.5) | 23 (95.8) | 15 (83.3) | |
| Treatment at baseline 2 | ||||
| None | 6 (14.3) | 4 (16.7) | 2 (11.1) | 0.11 |
| Immunomodulatory drugs (IMiDs) | 19 (45.2) | 14 (58.3) | 5 (27.8) | |
| IMiD + monoclonal antibody (MoAb) | 7 (16.7) | 1 (4.2) | 6 (33.3) | |
| IMiD + proteosome inhibitor (PI) | 5 (11.9) | 3 (12.5) | 2 (11.1) | |
| MoAb | 1 (2.4) | 1 (4.2) | 0 (0.0) | |
| PI | 3 (7.1) | 1 (4.2) | 2 (11.1) | |
| PI + MoAb | 1 (2.4) | 0 (0.0) | 1 (5.6) | |
| Total Adverse Events | Grade 3+ 1 | Related 2 | |
|---|---|---|---|
| Category | N (%) | N (%) | N (%) |
| Musculoskeletal and connective tissue disorders | 42 (38.5) | 0 (0) | 18 (16.5) |
| Infections and infestations | 18 (16.5) | 1 (0.9) | 0 (0) |
| Gastrointestinal disorders | 9 (8.3) | 0 (0) | 1 (0.9) |
| Nervous system disorders | 9 (8.3) | 0 (0) | 4 (3.7) |
| Injury, poisoning, and procedural complications | 8 (7.3) | 0 (0) | 0 (0) |
| Respiratory, thoracic, and mediastinal disorders | 8 (7.3) | 0 (0) | 0 (0) |
| General disorders and administration site conditions | 6 (5.5) | 0 (0) | 0 (0) |
| Surgical and medical procedures | 3 (2.8) | 1 (0.9) | 0 (0) |
| Vascular disorders | 3 (2.8) | 0 (0) | 1 (0.9) |
| Skin and subcutaneous tissue disorders | 1 (0.9) | 0 (0) | 0 (0) |
| Psychiatric disorders | 1 (0.9) | 0 (0) | 0 (0) |
| Renal and urinary disorders | 1 (0.9) | 0 (0) | 0 (0) |
| All Categories | 109 (100.0) | 2 (1.8) | 24 (22.0) |
| Category | Description of Serious Adverse Event | Severity | Relatedness |
|---|---|---|---|
| Gastrointestinal disorders | Gastrointestinal hemorrhage | 5 (Death) | 1 (Unrelated) |
| Infections and infestations | Appendicitis | 3 (Severe) | 1 (Unrelated) |
| Infections and infestations | Pneumonia, hospitalization | 3 (Severe) | 2 (Unlikely) |
| Infections and infestations | COVID-19 pneumonia | 3 (Severe) | 1 (Unrelated) |
| Infections and infestations | COVID-19 pneumonia (shortness of breath), further hospital admission | 3 (Severe) | 1 (Unrelated) |
| Surgical and medical procedures | Prolonged hospitalization after CAR-T cell therapy | 3 (Severe) | 1 (Unrelated) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hillengass, J.; Hillengass, M.; Joseph, J.M.; Attwood, K.; Cannioto, R.; Jacobson, H.; Miller, C.; Wittmeyer, B.; Moysich, K. Effects on the Physical Functioning of Two Exercise Interventions in Patients with Multiple Myeloma: A Pilot Feasibility Study. Cancers 2024, 16, 1774. https://doi.org/10.3390/cancers16091774
Hillengass J, Hillengass M, Joseph JM, Attwood K, Cannioto R, Jacobson H, Miller C, Wittmeyer B, Moysich K. Effects on the Physical Functioning of Two Exercise Interventions in Patients with Multiple Myeloma: A Pilot Feasibility Study. Cancers. 2024; 16(9):1774. https://doi.org/10.3390/cancers16091774
Chicago/Turabian StyleHillengass, Jens, Michaela Hillengass, Janine M. Joseph, Kristopher Attwood, Rikki Cannioto, Hillary Jacobson, Carolyn Miller, Bryan Wittmeyer, and Kirsten Moysich. 2024. "Effects on the Physical Functioning of Two Exercise Interventions in Patients with Multiple Myeloma: A Pilot Feasibility Study" Cancers 16, no. 9: 1774. https://doi.org/10.3390/cancers16091774
APA StyleHillengass, J., Hillengass, M., Joseph, J. M., Attwood, K., Cannioto, R., Jacobson, H., Miller, C., Wittmeyer, B., & Moysich, K. (2024). Effects on the Physical Functioning of Two Exercise Interventions in Patients with Multiple Myeloma: A Pilot Feasibility Study. Cancers, 16(9), 1774. https://doi.org/10.3390/cancers16091774






