Simple Summary
Sex differences in cancer are well-established. However, less is known about sex differences in the diagnosis of brain metastasis and outcomes among patients with advanced melanoma. Using the nationwide Flatiron Health electronic health record-derived de-identified database, this study showed that males had greater odds of brain metastasis, and poorer real-world overall survival compared to females among those with brain metastasis, while there were no sex differences in clinical outcomes for those with advanced melanoma without brain metastasis.
Abstract
Sex differences in cancer are well-established. However, less is known about sex differences in diagnosis of brain metastasis and outcomes among patients with advanced melanoma. Using a United States nationwide electronic health record-derived de-identified database, we evaluated patients diagnosed with advanced melanoma from 1 January 2011–30 July 2022 who received an oncologist-defined rule-based first line of therapy (n = 7969, 33% female according to EHR, 35% w/documentation of brain metastases). The odds of documented brain metastasis diagnosis were calculated using multivariable logistic regression adjusted for age, practice type, diagnosis period (pre/post-2017), ECOG performance status, anatomic site of melanoma, group stage, documentation of non-brain metastases prior to first-line of treatment, and BRAF positive status. Real-world overall survival (rwOS) and progression-free survival (rwPFS) starting from first-line initiation were assessed by sex, accounting for brain metastasis diagnosis as a time-varying covariate using the Cox proportional hazards model, with the same adjustments as the logistic model, excluding group stage, while also adjusting for race, socioeconomic status, and insurance status. Adjusted analysis revealed males with advanced melanoma were 22% more likely to receive a brain metastasis diagnosis compared to females (adjusted odds ratio [aOR]: 1.22, 95% confidence interval [CI]: 1.09, 1.36). Males with brain metastases had worse rwOS (aHR: 1.15, 95% CI: 1.04, 1.28) but not worse rwPFS (adjusted hazard ratio [aHR]: 1.04, 95% CI: 0.95, 1.14) following first-line treatment initiation. Among patients with advanced melanoma who were not diagnosed with brain metastases, survival was not different by sex (rwOS aHR: 1.06 [95% CI: 0.97, 1.16], rwPFS aHR: 1.02 [95% CI: 0.94, 1.1]). This study showed that males had greater odds of brain metastasis and, among those with brain metastasis, poorer rwOS compared to females, while there were no sex differences in clinical outcomes for those with advanced melanoma without brain metastasis.
1. Introduction
Despite aggressive multi-modal therapies, the survival rates for cancer patients with brain metastases (BrM) remains generally poor. Over the last decade, tremendous progress has been made in uncovering the molecular genetics of malignant primary brain tumors, allowing for identification of key hallmark alterations associated with accuracy of diagnosis and prognosis. This same progress has not been made in BrM. BrM continues to be one of the major complications of advanced melanoma and is the most common cause of melanoma deaths [1,2]. The median overall survival of a patient with melanoma that has developed BrM is less than 2 years.
Sex is an important factor which has been shown to influence disease development, progression, and clinical outcomes. Sexual differentiation results in sex differences in cellular and systems biology [3,4,5]. These sex-specific differences produce sexually dimorphic traits, including metabolism and disease risk [6,7]. Sex disparities in cancer incidence, survival, and prevalence have been well established for a variety of cancers, including brain tumors. Not only do males develop cancers 20% more often than females, males also have poorer responses to therapy, as measured by poorer progression-free and overall survival determinations compared to females [8,9]. Our group has shown that the incidence of glioblastoma (GBM) is higher in males through all stages of life [10]. In addition, females with GBM have a statistically significant overall survival advantage after adjustment for age, Karnofsky performance status, extent of resection, and receipt of standard of care [11]. Additionally, sex differences in melanoma have been well-documented, in which similarly, males have higher incidence and worse survival outcomes compared to females [8,12,13,14]. The exact biological mechanisms underlying these sex differences remain to be determined.
While sex differences in primary tumors, such as GBM and melanoma, have been well established, less is known about sex differences in the diagnosis of BrM and clinical outcomes among patients with advanced melanoma. This study aims to evaluate potential sex differences in the odds of brain metastasis and survival after brain metastasis among patients with advanced melanoma.
2. Methods
Study Design: This study was a retrospective observational cohort study using the nationwide Flatiron Health electronic health record (EHR)-derived de-identified database. Institutional Review Board approval of the study protocol was obtained prior to the conducting of the study and included a waiver of informed consent. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. The Flatiron Health database is a longitudinal database, comprising de-identified patient-level structured and unstructured data, curated via technology-enabled abstraction [15,16].
Patient Selection: The study period was 1 January 2011 through 31 December 2022. During this time period, the de-identified patient data originated from approximately 280 cancer clinics (~800 sites of care) in the United States (US). Care sites consisted of community oncology and academic medical center clinics. The study population included patients diagnosed with advanced melanoma from 1 January 2011 through 1 July 2022 who had at least two documented EHR visits on or after 1 January 2011, documented treatment with a first-line therapy, and a notation of their sex associated with the record. Individuals were selected based on ICD codes (ICD-9: 172.x, ICD-10: C43x, or D03x) and a determination of pathologic stages III or IV at initial diagnosis or at locoregional or distant recurrence, and diagnosis of advanced melanoma was confirmed via abstraction. Diagnosis date was limited to six months prior to data cutoff (31 December 2022), in order to allow for a potential minimum of six months’ patient observation.
Study Variables and Endpoints: The primary predictors of interest in this study were sex and BrM status. Sex was typically documented by clinicians upon patient intake and is a structured data element available in the data; it is assumed to be the sex assigned at birth. BrM status was determined through abstraction based on clinical and pathologic statements that confirm the metastatic site and date of advanced melanoma diagnosis. The date of BrM diagnosis was determined as the earliest date that a patient showed evidence of BrM from a radiology scan, pathology report, or physician statement. Date of BrM diagnosis was recorded at the month-level; thus, estimates of times to events using BrM diagnosis were also at the month-level.
Patient-level clinical and demographic characteristics were identified using both structured and unstructured data. Technology-enabled abstraction was used to extract relevant data from unstructured data. Clinical and demographic data included age at advanced melanoma diagnosis, SES quintile at the Census block group level, anatomic site of melanoma as determined by structured diagnosis codes, group stage at initial melanoma diagnosis, diagnosis of non-brain metastases prior to the first line of systemic treatment, and BRAF mutation status (positive/negative; 30 days prior, or up to 60 days after advanced diagnosis date). Additionally, Eastern Cooperative Oncology Group (ECOG) performance status (PS) was determined from the structured data and extracted using natural language processing, as determined at around the time of first-line treatment (30 days prior to up to 7 days after first-line initiation). Age at advanced melanoma diagnosis was subject to de-identification requirements such that the earliest recorded birth year was 85 years prior to data cutoff year of 1937.
Endpoints included real-world overall survival (rwOS) and real-world progression-free survival (rwPFS) [17]. Mortality was identified using structured and unstructured EHR documentation supplemented with external commercial and U.S. Social Security Death Index data. This composite mortality endpoint has been validated with high agreement and accuracy against the National Death Index [18].
Analyses: Descriptive statistics of baseline characteristics were summarized across the overall population, as well as stratified by sex and BrM diagnosis. Differences in distributions were assessed with an χ2 test. Logistic regression was used to estimate the adjusted and unadjusted odds of BrM diagnosis by sex in the overall population.
Cox proportional hazards regression was used to estimate the adjusted and unadjusted hazard of mortality, as well as the hazard of disease progression or mortality, as indexed to first-line of therapy start date among all patients. The rwOS was defined as ending on date of death, censoring at last confirmed activity. The rwPFS was defined as ending on the first clinician documentation of disease progression or date of death, censoring at the date of the last clinic note [19].
For each adjusted model, adjusted covariates were identified using forward stepwise variable selection, measured by change in Akaike information criterion (AIC), for which candidate covariates included the following: age at diagnosis (categorical: 0–34, 35–49, 50–64, 65–74, and 75+), race/ethnicity (Asian, Black or African American, Hispanic or Latino, White, Other, or Unknown), practice type (academic or community), year of advanced melanoma diagnosis (before 2017, during or after 2017), insurance coverage at advanced diagnosis (a year prior to, or up to 30 days after advanced diagnosis date), SES index (quintile), ECOG PS (0, 1, 2+, or unknown), anatomic site of melanoma per ICD codes, group stage at initial diagnosis, record of metastases other than brain prior to the start of the first line of treatment, and BRAF biomarker status. Year of advanced melanoma diagnosis was included as a categorical variable to account for potential era effects associated with the use of checkpoint inhibitor therapy for advanced melanoma. For the logistic regression analysis, candidate covariates that were dropped from the final model were race, insurance at advanced diagnosis, and SES index. For the Cox proportional hazards models assessing rwOS, the candidate covariate dropped from the model was group stage at initial diagnosis, for both those with and those without documented BrM. For the Cox proportional hazard models assessing rwPFS, candidate covariates that were dropped from the final model for those with documented BrM included race, practice type, SES index, anatomic site of melanoma (ever), group stage at initial diagnosis, and history of metastases other than brain prior to 1L start, while covariates dropped from the final model for those without documented BrM included practice type, as well as history of BRAF status. Cox regression models estimating rwOS and rwPFS for patients with documented BrM additionally included a BrM diagnosis as a time-varying covariate.
A reverse Kaplan–Meier analysis was performed, in which loss to follow-up was recorded as the ‘event’ and death as ‘censoring’ to evaluate differences in follow-up time between those who did and did not develop BrM.
3. Results
Overall, there were 7969 patients with advanced melanoma identified, with 2794 (35%) having documentation of BrM, and there were a higher percentage of male patients with BrM, compared to those without BrM (69% vs. 66% respectively, p = 0.003) (Supplemental Table S1). Descriptive statistics stratified by sex, presented separately for patients with BrM and without documented BrM, are described in Table 1. There was a notable difference in age distribution by sex, with a higher proportion of older individuals among the male patients with BrM (65–74 yrs: 31% male vs. 24% female, 75+ yrs: 21% male vs. 15% female, p < 0.001) and among male patients without BrM (65–74: 29% male vs. 27% female, 75+: 35% male vs. 29% female, p < 0.001). Additionally, sex differences in Medicare coverage were also observed, in that males were more likely to have Medicare coverage than were females, both among patients with BrM (18% male vs. 15% female, p = 0.036) and among those with no documented BrM (22% male vs. 20% female, p = 0.034). There was a significant difference in the distribution of SES index by sex among non-BrM patients, with a higher percentage of lowest-SES individuals among females (12%) compared to males (9.3%, p = 0.009). Additionally, there was a sex difference in the distribution of patients with metastases outside of the brain prior to the start of first line of treatment among non-BrM patients (74% male vs. 71% female, p = 0.012). Across both groups, the BRAF mutation was more common in males than females (BrM: 39% male vs. 36% female, p = 0.003, non-BrM: 29% male vs. 25% female, p < 0.001).

Table 1.
Distribution of demographic and clinical characteristics for patients with advanced melanoma according to documentation of brain metastases and sex (2011–2022).
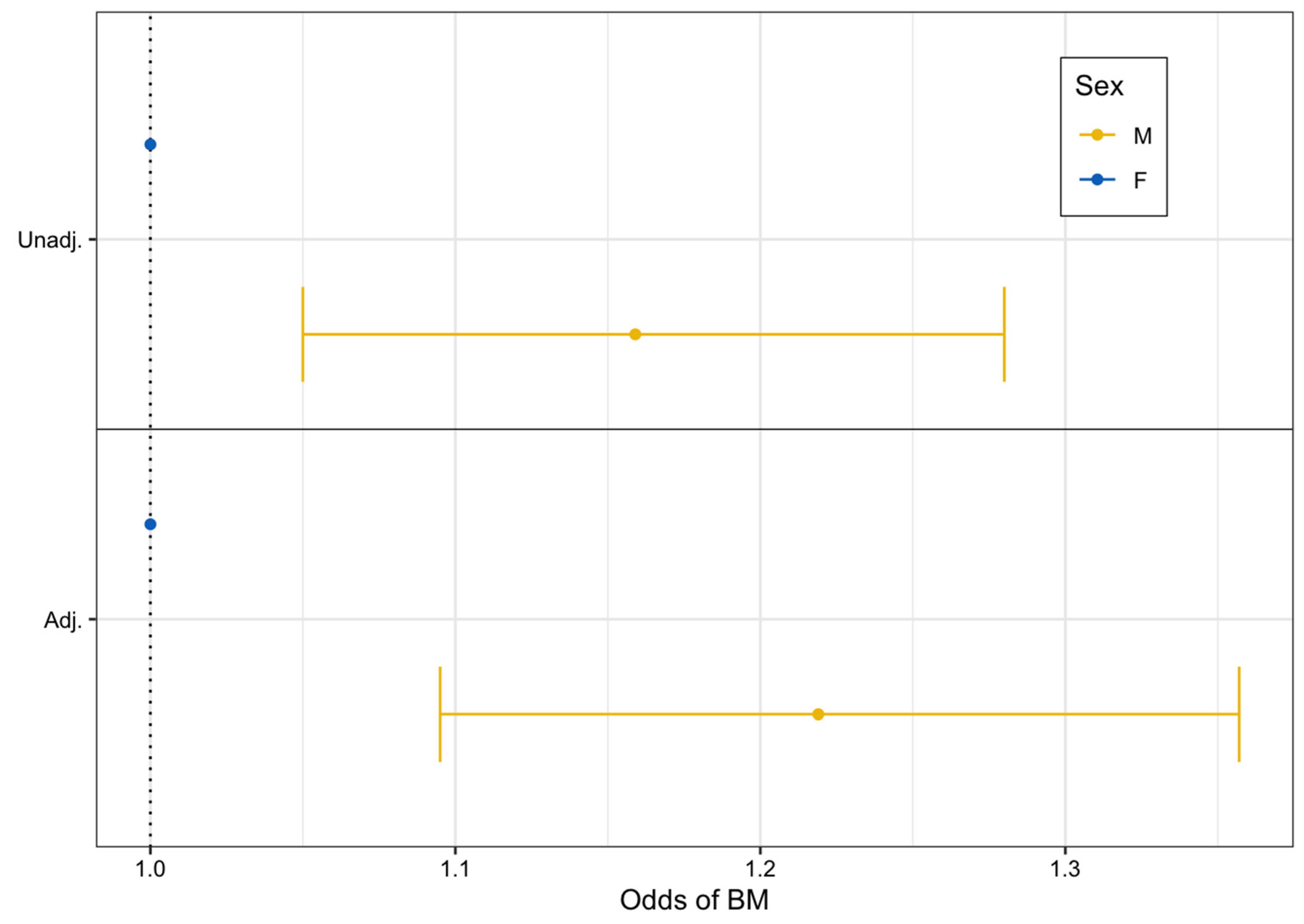
From unadjusted logistic regression modeling, we found that males had 16% higher odds of developing BrM compared to females (unadjusted OR: 1.16, 95% CI: 1.05–1.28, p = 0.003). After adjustment for additional factors, males still had statistically significantly higher odds of BrM development compared to females (adjusted OR: 1.22, 95% CI: 1.10–1.36, p < 0.001) (Figure 1).
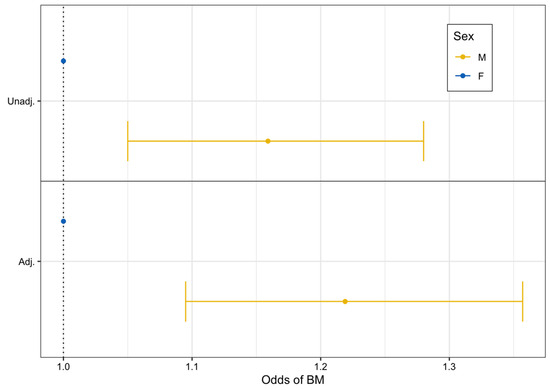
Figure 1.
Unadjusted and adjusted logistic regression forest plot assessing the odds of brain metastasis after advanced melanoma diagnosis by sex (2011–2022).
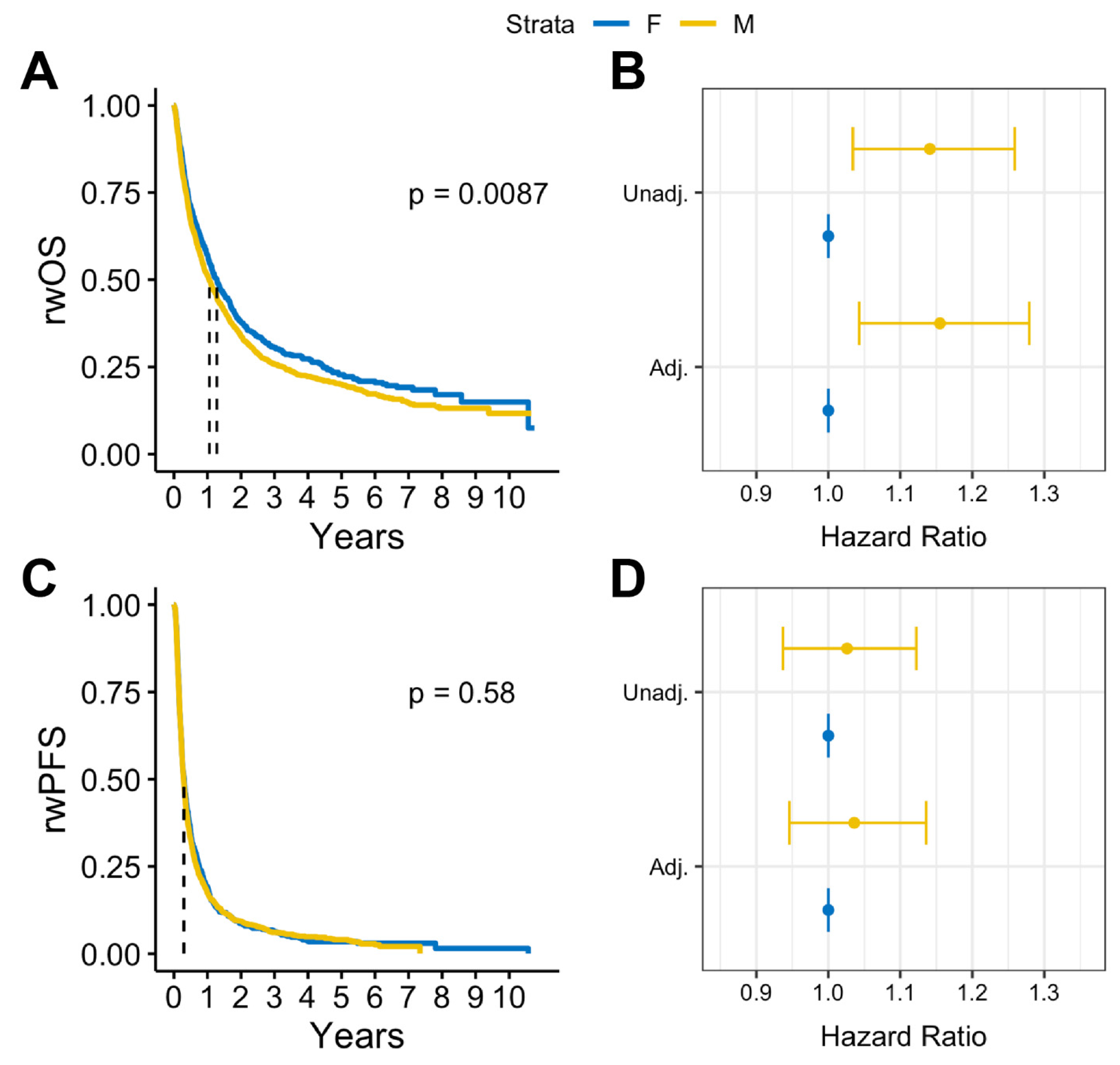
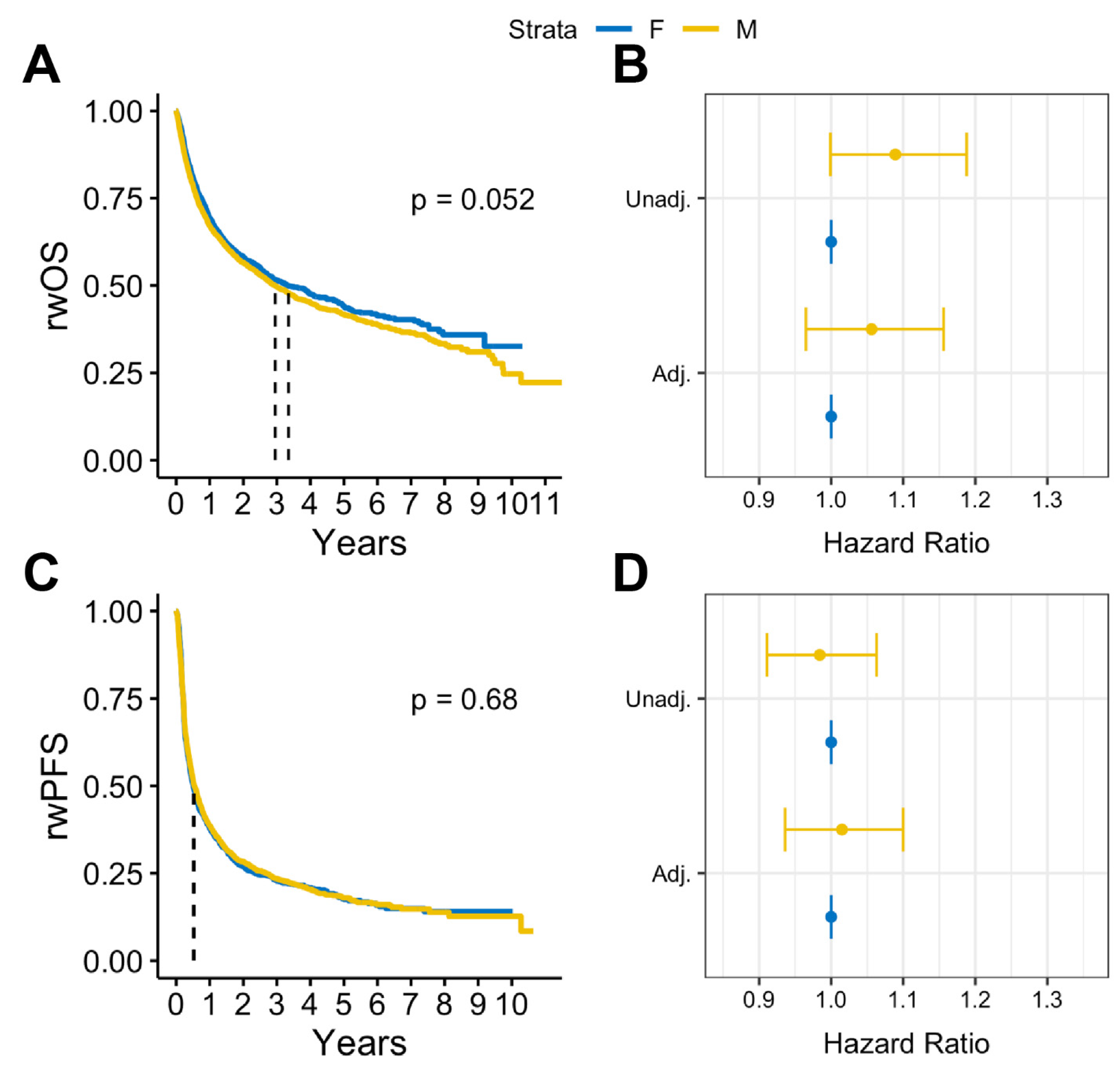
In survival analysis, we found that among patients with BrM, males had statistically significantly lower median rwOS than females (13 months vs. 15 months, log rank p = 0.009), but there was no observed difference in rwPFS (log rank p = 0.58) (Figure 2A,B). Similar results were observed after adjusting for covariates. Among patients with BrM, males had worse rwOS compared to females (adjusted HR: 1.18, 95% CI:1.05-1.32, p = 0.005) when adjusted for select covariates (Figure 2C). No statistically significant differences by sex were observed on rwPFS (Figure 2D). Overall, in patients with advanced melanoma without documented BrM, there were no statistically significant sex differences in either rwOS or rwPFS observed (Figure 3).
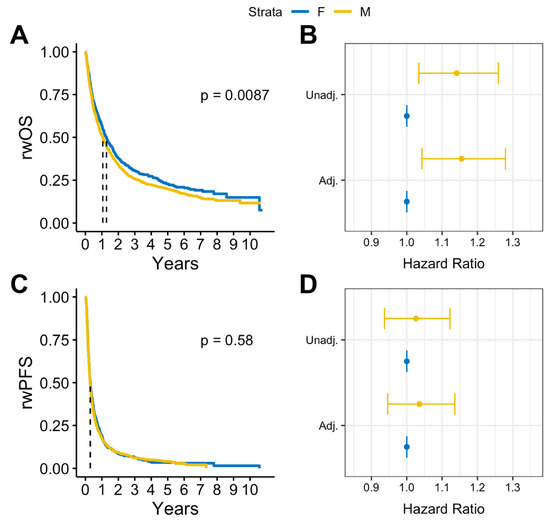
Figure 2.
(A) Kaplan–Meier survival curves and (B) Cox proportional hazards forest plots for real-world overall survival and (C) Kaplan-Meier survival curves and (D) Cox proportional hazard forest plots for progression-free survival for advanced melanoma cases with brain metastases (2011–2022).
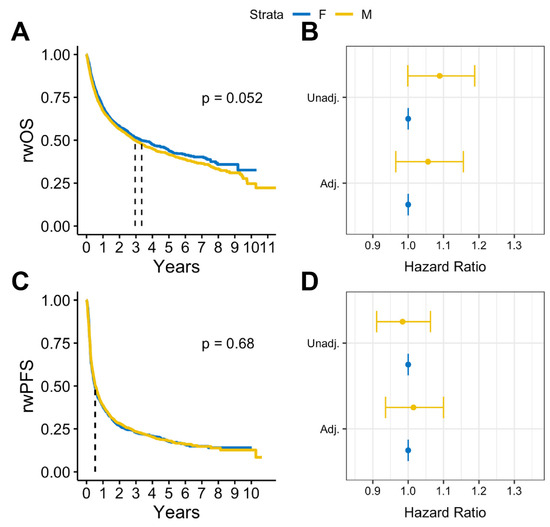
Figure 3.
(A) Kaplan Meier survival curves and (B) Cox proportional hazards forest plots for real-world overall survival and (C) Kaplan-Meier survival curves and (D) Cox proportional hazards forest plots for progression-free survival for advanced melanoma cases without brain metastases (2011–2022).
From the reverse Kaplan–Meier analysis, it was found that individuals who developed BrM were 24% less likely to be lost to follow-up at any given time compared to those who did not develop BrM (HR: 0.76, 95% CI: 0.71–0.82, p < 0.001).
4. Discussion
In this study, we report that among patients with advanced melanoma, males are more likely to develop BrM, as compared to females. Further, among patients diagnosed with BrM, males have worse rwOS outcomes compared to females. In contrast, there were no observed differences in rwPFS. Our results suggest that among patients with advanced melanoma, there may be sex disparities in the development of BrM and survival after BrM development.
The prevalent use of tanning beds, and subsequent UV exposure, most commonly observed in young, adolescent females, has been implicated in increased melanoma incidence in younger women [20]. Indeed, indoor tanning has been linked as a contributing factor for the increased melanoma rates in younger women when compared to men [21]. Given the increased risk of metastases at a younger primary melanoma diagnosis, and higher female incidence of melanoma in this earlier age demographic, one may expect BrM risk to be greater in females. However, based on our findings, the odds of BrM development are significantly greater for males than females, when adjusted for risk factors like age at advanced melanoma diagnosis, which may indicate potential variations in behaviors or underlying biological mechanisms that could be driving this difference. Males tend to utilize primary care less than females do, allowing tumors time to grow and deepen [22,23]. Further, skin awareness, a known activity associated with the discovery of thinner melanoma lesions and a significant decrease in melanoma mortality [24], is more prevalent in females than males [25,26,27]. These health behaviors in males have thus been suggested to contribute to the prevalence of later-tumor-stage melanoma tumors in males.
Sex differences were also observed in overall survival, with males having significantly worse rwOS than females among patients with advanced melanoma who developed BrM. Survival differences by sex have been well documented in melanoma [8,12,13]. However, the majority of these studies do not focus on advanced melanoma or analysis relevant to the metastasis to the brain. A study by Enniga et al. found that sex differences in melanoma survival have variations by stage, with significant differences occurring more often in earlier stages (I–II) [14]. A small proportion of the patients in our study were classified as stage II or lower at initial diagnosis (26%), and at the time of the study had recurrent disease with a pathological stage of III or IV, which may explain the lack of significant sex difference in survival among patients who did not develop a BM. The possibility that males are diagnosed later in the course of disease could contribute to a “lead time bias” that suggests males have shorter survival, whether overall or progression-free, despite potentially there being no such difference when indexing to comparable points in the natural history of disease. Additionally, the observed median survival differences by sex among those with BrM, while statistically significant, are small (2 months). However, given the aggressive prognosis of metastatic melanoma, even incremental changes in overall survival are clinically relevant.
Several factors analyzed in this study have been previously reported to impact the development of BrM, including BRAF mutation status. The BRAF mutation has been shown to be associated with distant metastases in advanced melanoma and other cancers [28,29,30]. Earlier works by others have demonstrated that advanced melanoma patients with BRAF mutations not only have an increased risk of developing BrM, but also have a shorter disease-free interval from primary diagnosis to diagnosis of BrM [31]. Similar results were found here with regard to advanced melanoma patients who progressed to BrM, as patients with BRAF mutation had a higher proportion of BrM development than those without a mutation. While advanced melanoma patients with BRAF mutations have increased risk for BrM, those patients with BrM who are BRAF mutation-positive have a better survival than BrM patients who are mutation-negative. This is likely due to the availability of targeted therapies [32]. The most common BRAF mutation is V600E [28]; however, the V600K mutation has been associated with males and those patients of older age [33]. While we did observe a sex bias in the distribution of BRAF status, with males having a higher percentage of mutation-positive cases, when adjusted for BRAF status sex was still found to be a significant predictor of BrM development. Further, advanced melanoma patients with the BRAF V6000K mutation have poorer survival when compared to those with the V600E mutation [33]. Thus, it may be plausible to postulate given this dataset that perhaps the observation of poorer survival in males is related to a higher percentage of patients having the V600K mutation. Due to limitations within the data, we are unable to adjust for BRAF mutation type.
Sex-specific signatures in the tumor microenvironment have been reported globally [34] as well as within the brain [35,36,37,38]. In primary brain tumors, research has shown that sex differences in the tumor microenvironment, particularly in immune cell composition, contribute to sex differences in survival [39]. Additionally, transcriptomic analysis has demonstrated differences in transcription differences between males and females that correspond to survival [39,40]. While these studies have all been performed in primary brain tumors, it remains a possibility that similar sex differences in the metastatic brain microenvironment contribute to the significant survival differences observed among patients who developed BrM. This remains to be investigated and is beyond the scope of this analysis.
To our knowledge, this study is one of the largest contemporary studies assessing the association between sex and BrM, in addition to subsequent survival outcomes among real-world patients with advanced melanoma in the US. We leveraged a large nationwide cohort of patients with curated and harmonized data which included abstraction of confirmed advanced melanoma diagnosis, demographic and clinical risk factors, and progression. This allowed us to not only evaluate diagnoses of BrM, but also both rwPFS and rwOS outcomes. Long-term longitudinal follow-up and assessment data throughout the patient treatment journey made this study feasible.
The findings of our work must be considered within the context of the study’s limitations. First, sex is documented as structured data in the EHR data, and likely reflects the patient’s sex at birth. Second, although we were able to adjust for several demographic and clinical factors, as is common with observational studies utilizing real-world data, certain relevant characteristics were not available, such as UV exposure level. The results from this study may be impacted by unmeasured clinical, demographic, or other confounding factors. Additionally, individuals with documented BrM were followed up longer than those without BrM documentation, which suggests that patients without BrM might have been more likely to be lost to follow-up, resulting in misclassification of BrM among those patients. Lastly, this study is U.S.-based, and our results may not be generalizable to patients who received care outside the U.S.
This study aimed to investigate potential sex differences in BrM development, and survival after BrM development, among patients with advanced melanoma. It was observed that males have higher odds of BrM development, and a higher risk of death after BrM development, compared to females, as adjusted for known risk factors such as age and BRAF mutation status. Studies such as these provide crucial insights on differences in disease burden, which can help drive efforts to improve patient outcomes.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers16091771/s1. Supplemental Table S1: Distribution of demographic and clinical characteristics for patients with advanced melanoma by documentation of brain metastases (2011–2022).
Author Contributions
Conceptualization, G.C., K.A.W., M.D., X.W., T.J.R., G.S.C., T.W., C.G.R., B.D.K. and J.S.B.-S.; methodology, J.S.B.-S., G.C., M.S.A., X.W. and B.D.K.; validation, J.S.B.-S., G.C., M.S.A., K.A.W., X.W., T.J.R., G.S.C., T.W., C.G.R. and B.D.K.; software, M.S.A., X.W., T.J.R. and G.S.C.; methodology, J.S.B.-S., G.C., M.S.A., X.W., T.J.R. and G.S.C.; and B.D.K.; formal analysis, G.C., M.S.A. and X.W.; investigation, J.S.B.-S., G.C., M.S.A., K.A.W., M.D., X.W., T.J.R., G.S.C., T.W., C.G.R. and B.D.K.; resources, T.J.R., G.S.C., B.D.K. and J.S.B.-S.; data curation, M.S.A., G.C. and X.W.; writing—original draft preparation, G.C., K.A.W. and M.S.A.; writing—review and editing, J.S.B.-S., G.C., M.S.A., K.A.W., M.D., X.W., T.J.R., G.S.C., T.W., C.G.R. and B.D.K.; visualization, J.S.B.-S., G.C., M.S.A., K.A.W., M.D., X.W., T.J.R., G.S.C., T.W., C.G.R. and B.D.K.; supervision, J.S.B.-S., T.J.R., G.S.C. and B.D.K.; project administration, K.A.W., X.W., T.J.R. and G.S.C.; funding acquisition, J.S.B.-S., T.J.R. and G.S.C. All authors have read and agreed to the published version of the manuscript.
Funding
Jill Barnholtz-Sloan is a full-time employee of the National Cancer Institute/National Institutes of Health and receives intramural funding for her research. This research was supported in part by an appointment to G.C. to the National Cancer Institute (NCI) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the National Institute of Health. ORISE is managed by ORAU under DOE contract number DE-SC0014664. All opinions expressed in this paper are the author’s and do not necessarily reflect the policies and views of NIH, NCBI, DOE, or ORAU/ORISE.
Institutional Review Board Statement
Institutional Review Board (IRB) approval by the WCG IRB (The Flatiron Health Real-World Evidence Parent Protocol, Tranck #FLI118044, approval date 7 February 2018) of the study protocol was obtained prior to the conducting of the study and included a waiver of informed consent. Patient consent was waived as the protocol contained de-identified patient data.
Data Availability Statement
The data that support the findings of this study were originated by Flatiron Health, Inc. Requests for data sharing by license or by permission for the specific purpose of replicating results in this manuscript can be submitted to PublicationsDataAccess@flatiron.com.
Conflicts of Interest
J.S.B.-S. is a full-time, paid employee of the NIH/NCI. G.C. and K.A.W. are full-time, paid contractors of the NIH/NCI. M.D. is a full-time fellow of the NIH/NCI. This research was supported in part by an appointment to G.C. to the National Cancer Institute (NCI) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the National Institute of Health. ORISE is managed by ORAU under DOE contract number DE-SC0014664. All opinions expressed in this paper are the author’s and do not necessarily reflect the policies and views of NIH, NCBI, DOE, or ORAU/ORISE. M.S.A., X.W., T.J.R. and G.S.C. report employment with Flatiron Health, which is an independent member of the Roche Group, and stock ownership in Roche. G.S.C. reports research funding unrelated to this study awarded to the University of Illinois Chicago from Pfizer.
References
- Budman, D.R.; Camacho, E.; Wittes, R.E. The current causes of death in patients with malignant melanoma. Eur. J. Cancer (1965) 1978, 14, 327–330. [Google Scholar] [CrossRef]
- Sampson, J.H.; Carter, J.H., Jr.; Friedman, A.H.; Seigler, H.F. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J. Neurosurg. 1998, 88, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Plutynski, A.; Ward, S.; Rubin, J.B. An integrative view on sex differences in brain tumors. Cell. Mol. Life Sci. 2015, 72, 3323–3342. [Google Scholar] [CrossRef] [PubMed]
- Knoedler, J.R.; Shah, N.M. Molecular mechanisms underlying sexual differentiation of the nervous system. Curr. Opin. Neurobiol. 2018, 53, 192–197. [Google Scholar] [CrossRef] [PubMed]
- VanRyzin, J.W.; Pickett, L.A.; McCarthy, M.M. Microglia: Driving critical periods and sexual differentiation of the brain. Dev. Neurobiol. 2018, 78, 580–592. [Google Scholar] [CrossRef]
- Zore, T.; Palafox, M.; Reue, K. Sex differences in obesity, lipid metabolism, and inflammation-A role for the sex chromosomes? Mol. Metab. 2018, 15, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Dearden, L.; Bouret, S.G.; Ozanne, S.E. Sex and gender differences in developmental programming of metabolism. Mol. Metab. 2018, 15, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Goldman, N.; A Glei, D.; Weinstein, M. What Matters Most for Predicting Survival? A Multinational Population-Based Cohort Study. PLoS ONE 2016, 11, e0159273. [Google Scholar] [CrossRef]
- Wang, G.-M.; Cioffi, G.; Patil, N.; A Waite, K.; Lanese, R.; Ostrom, Q.T.; Kruchko, C.; E Berens, M.; Connor, J.R.; Lathia, J.D.; et al. Importance of the intersection of age and sex to understand variation in incidence and survival for primary malignant gliomas. Neuro-Oncology 2022, 24, 302–310. [Google Scholar] [CrossRef]
- Gittleman, H.; Ostrom, Q.T.; Stetson, L.C.; Waite, K.; Hodges, T.R.; Wright, C.H.; Wright, J.; Rubin, J.B.; E Berens, M.; Lathia, J.; et al. Sex is an important prognostic factor for glioblastoma but not for nonglioblastoma. Neuro-Oncol. Pract. 2019, 6, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Joosse, A.; de Vries, E.; Eckel, R.; Nijsten, T.; Eggermont, A.M.; Hölzel, D.; Coebergh, J.W.W.; Engel, J. Gender differences in melanoma survival: Female patients have a decreased risk of metastasis. J. Investig. Dermatol. 2011, 131, 719–726. [Google Scholar] [CrossRef]
- Gupta, S.; Artomov, M.; Goggins, W.; Daly, M.; Tsao, H. Gender Disparity and Mutation Burden in Metastatic Melanoma. JNCI J. Natl. Cancer Inst. 2015, 107, djv221. [Google Scholar] [CrossRef] [PubMed]
- Enninga, E.A.L.; Moser, J.C.; Weaver, A.L.; Markovic, S.N.; Brewer, J.D.; Leontovich, A.A.; Hieken, T.J.; Shuster, L.; Kottschade, L.A.; Olariu, A.; et al. Survival of cutaneous melanoma based on sex, age, and stage in the United States, 1992–2011. Cancer Med. 2017, 6, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Long, L.; Moon, S.; Adamson, B.J.S.; Baxi, S.S. Comparison of Population Characteristics in Real-World Clinical Oncology Databases in the US: Flatiron Health, SEER, and NPCR. medRxiv 2023. medRxiv:2020.03.16.20037143. [Google Scholar]
- Birnbaum, B.; Nussbaum, N.; Seidl-Rathkopf, K.; Agrawal, M.; Estevez, M.; Estola, E.; Haimson, J.; He, L.; Larson, P.; Richardson, P. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv 2020, arXiv:2001.09765. [Google Scholar]
- Torres, A.Z.; Nussbaum, N.C.; Parrinello, C.M.; Bourla, A.B.; Bowser, B.E.; Wagner, S.; Tabano, D.C.; George, D.; Miksad, R.A. Analysis of a Real-World Progression Variable and Related Endpoints for Patients with Five Different Cancer Types. Adv. Ther. 2022, 39, 2831–2849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Gossai, A.; Monroe, S.; Nussbaum, N.C.; Parrinello, C.M. Validation analysis of a composite real-world mortality endpoint for patients with cancer in the United States. Health Serv. Res. 2021, 56, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Griffith, S.D.; Tucker, M.; Bowser, B.; Calkins, G.; Chang, C.-H.; Guardino, E.; Khozin, S.; Kraut, J.; You, P.; Schrag, D.; et al. Generating Real-World Tumor Burden Endpoints from Electronic Health Record Data: Comparison of RECIST, Radiology-Anchored, and Clinician-Anchored Approaches for Abstracting Real-World Progression in Non-Small Cell Lung Cancer. Adv. Ther. 2019, 36, 2122–2136. [Google Scholar] [CrossRef]
- Dessinioti, C.; Stratigos, A.J. An Epidemiological Update on Indoor Tanning and the Risk of Skin Cancers. Curr. Oncol. 2022, 29, 8886–8903. [Google Scholar] [CrossRef]
- Lazovich, D.; Vogel, R.I.; Weinstock, M.A.; Nelson, H.H.; Ahmed, R.L.; Berwick, M. Association Between Indoor Tanning and Melanoma in Younger Men and Women. JAMA Dermatol. 2016, 152, 268–275. [Google Scholar] [CrossRef]
- Cleary, P.D.; Mechanic, D.; Greenley, J.R. Sex differences in medical care utilization: An empirical investigation. J. Health Soc. Behav. 1982, 23, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Galdas, P.M.; Cheater, F.; Marshall, P. Men and health help-seeking behaviour: Literature review. J. Adv. Nurs. 2005, 49, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Berwick, M.; Armstrong, B.K.; Ben-Porat, L.; Fine, J.; Kricker, A.; Eberle, C.; Barnhill, R. Sun exposure and mortality from melanoma. J. Natl. Cancer Inst. 2005, 97, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Swetter, S.M.; Johnson, T.M.; Miller, D.R.; Layton, C.J.; Brooks, K.R.; Geller, A.C. Melanoma in middle-aged and older men: A multi-institutional survey study of factors related to tumor thickness. Arch. Dermatol. 2009, 145, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Paddock, L.E.; Lu, S.E.; Bandera, E.V.; Rhoads, G.G.; Fine, J.; Paine, S.; Barnhill, R.; Berwick, M. Skin self-examination and long-term melanoma survival. Melanoma Res. 2016, 26, 401–408. [Google Scholar] [CrossRef]
- Brady, M.S.; Oliveria, S.A.; Christos, P.J.; Berwick, M.; Coit, D.G.; Katz, J.; Halpern, A.C. Patterns of detection in patients with cutaneous melanoma. Cancer 2000, 89, 342–347. [Google Scholar] [CrossRef]
- Colombino, M.; Capone, M.; Lissia, A.; Cossu, A.; Rubino, C.; De Giorgi, V.; Massi, D.; Fonsatti, E.; Staibano, S.; Nappi, O.; et al. BRAF/NRAS mutation frequencies among primary tumors and metastases in patients with melanoma. J. Clin. Oncol. 2012, 30, 2522–2529. [Google Scholar] [CrossRef] [PubMed]
- Capper, D.; Berghoff, A.S.; Magerle, M.; Ilhan, A.; Wöhrer, A.; Hackl, M.; Pichler, J.; Pusch, S.; Meyer, J.; Habel, A.; et al. Immunohistochemical testing of BRAF V600E status in 1,120 tumor tissue samples of patients with brain metastases. Acta Neuropathol. 2012, 123, 223–233. [Google Scholar] [CrossRef]
- Ramanujam, S.; Schadendorf, D.; Long, G.V. Systemic therapies for melanoma brain metastases: Which drug for whom and when? Chin. Clin. Oncol. 2015, 4, 25. [Google Scholar]
- Long, G.V.; Menzies, A.M.; Nagrial, A.M.; Haydu, L.E.; Hamilton, A.L.; Mann, G.J.; Hughes, T.M.; Thompson, J.F.; Scolyer, R.A.; Kefford, R.F. Prognostic and Clinicopathologic Associations of Oncogenic BRAF in Metastatic Melanoma. J. Clin. Oncol. 2011, 29, 1239–1246. [Google Scholar] [CrossRef]
- Frinton, E.; Tong, D.; Tan, J.; Read, G.; Kumar, V.; Kennedy, S.; Lim, C.; Board, R.E. Metastatic melanoma: Prognostic factors and survival in patients with brain metastases. J. Neuro-Oncol. 2017, 135, 507–512. [Google Scholar] [CrossRef]
- Bucheit, A.D.; Syklawer, E.; Jakob, J.A.; Bassett, R.L.; Curry, J.L.; Gershenwald, J.E.; Kim, K.B.; Hwu, P.; Lazar, A.J.; Davies, M.A. Clinical characteristics and outcomes with specific BRAF and NRAS mutations in patients with metastatic melanoma. Cancer 2013, 119, 3821–3829. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yang, Y.; Li, X.; Wu, J.; Sheng, Y.; Qiu, J.; Wang, Q.; Li, J.; He, Y.; Cheng, L.; et al. Pan-cancer analysis reveals sex-specific signatures in the tumor microenvironment. Mol. Oncol. 2022, 16, 2153–2173. [Google Scholar] [CrossRef]
- Krawczyk, M.C.; Haney, J.R.; Pan, L.; Caneda, C.; Khankan, R.R.; Reyes, S.D.; Chang, J.W.; Morselli, M.; Vinters, H.V.; Wang, A.C.; et al. Human Astrocytes Exhibit Tumor Microenvironment-, Age-, and Sex-Related Transcriptomic Signatures. J. Neurosci. 2022, 42, 1587–1603. [Google Scholar] [CrossRef]
- Bayik, D.; Zhou, Y.; Park, C.; Hong, C.; Vail, D.; Silver, D.J.; Lauko, A.; Roversi, G.; Watson, D.C.; Lo, A.; et al. Myeloid-Derived Suppressor Cell Subsets Drive Glioblastoma Growth in a Sex-Specific Manner. Cancer Discov. 2020, 10, 1210–1225. [Google Scholar] [CrossRef]
- Lathia, J.D.; Li, M.; Sinyuk, M.; Alvarado, A.G.; Flavahan, W.A.; Stoltz, K.; Rosager, A.M.; Hale, J.; Hitomi, M.; Gallagher, J.; et al. High-throughput flow cytometry screening reveals a role for junctional adhesion molecule a as a cancer stem cell maintenance factor. Cell Rep. 2014, 6, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Nesterova, D.S.; Midya, V.; E Zacharia, B.; A Proctor, E.; Lee, S.Y.; Stetson, L.C.; Lathia, J.D.; Rubin, J.B.; A Waite, K.; E Berens, M.; et al. Sexually dimorphic impact of the iron-regulating gene, HFE, on survival in glioblastoma. Neuro-Oncol. Adv. 2020, 2, vdaa001. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Warrington, N.M.; Taylor, S.J.; Whitmire, P.; Carrasco, E.; Singleton, K.W.; Wu, N.; Lathia, J.D.; Berens, M.E.; Kim, A.H.; et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci. Transl. Med. 2019, 11, eaao5253. [Google Scholar] [CrossRef]
- Johansen, M.L.; Stetson, L.C.; Vadmal, V.; Waite, K.; E Berens, M.; Connor, J.R.; Lathia, J.; Rubin, J.B.; Barnholtz-Sloan, J.S. Gliomas display distinct sex-based differential methylation patterns based on molecular subtype. Neuro-Oncol. Adv. 2020, 2, vdaa002. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).