The Impact of the COVID-19 Pandemic on Time to Treatment in Surgical Oncology: A National Registry Study in The Netherlands
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
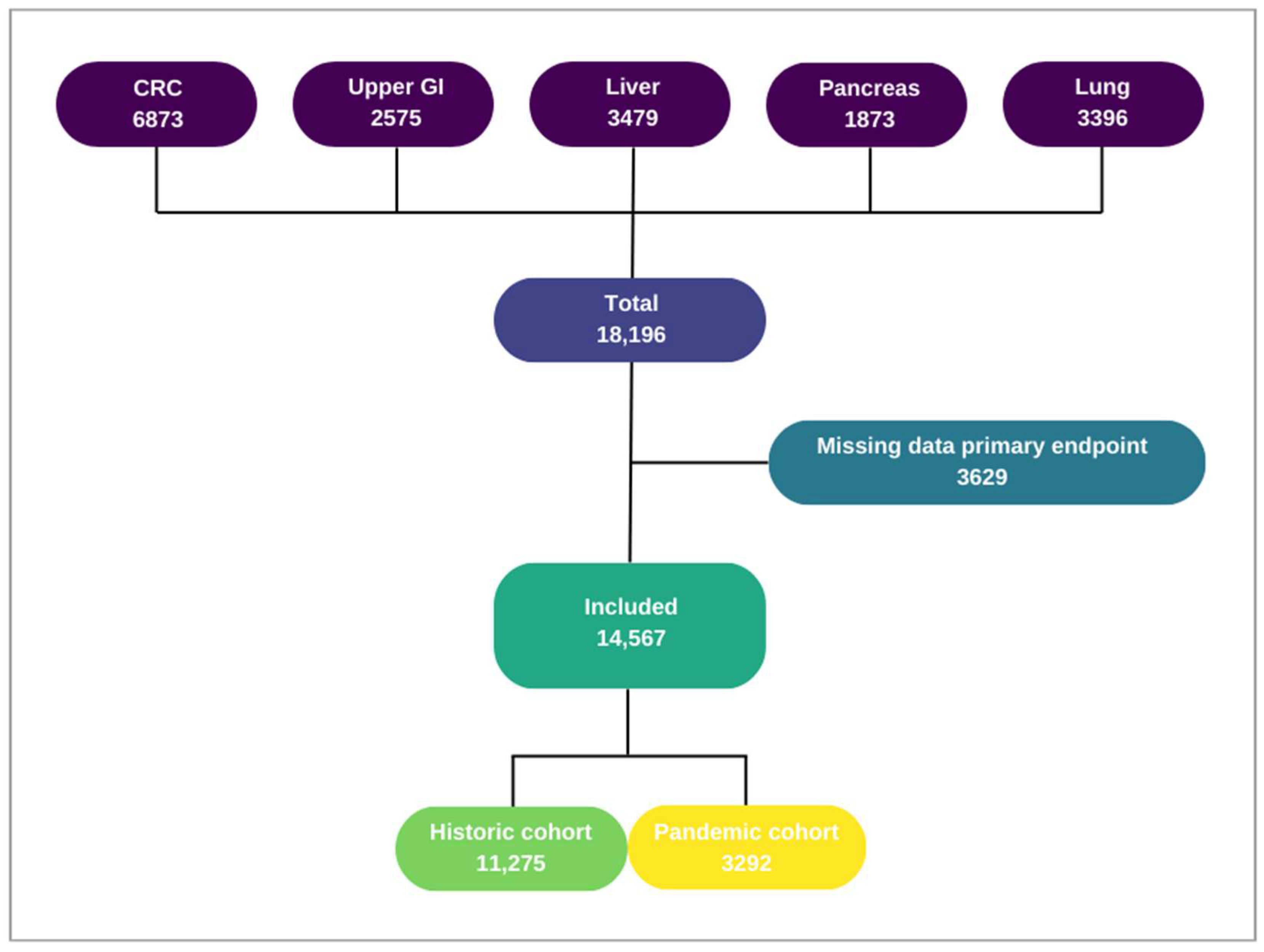
2.2. Study Population
2.3. Outcomes
2.4. Variables
2.5. COVID-19 Waves
2.6. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics
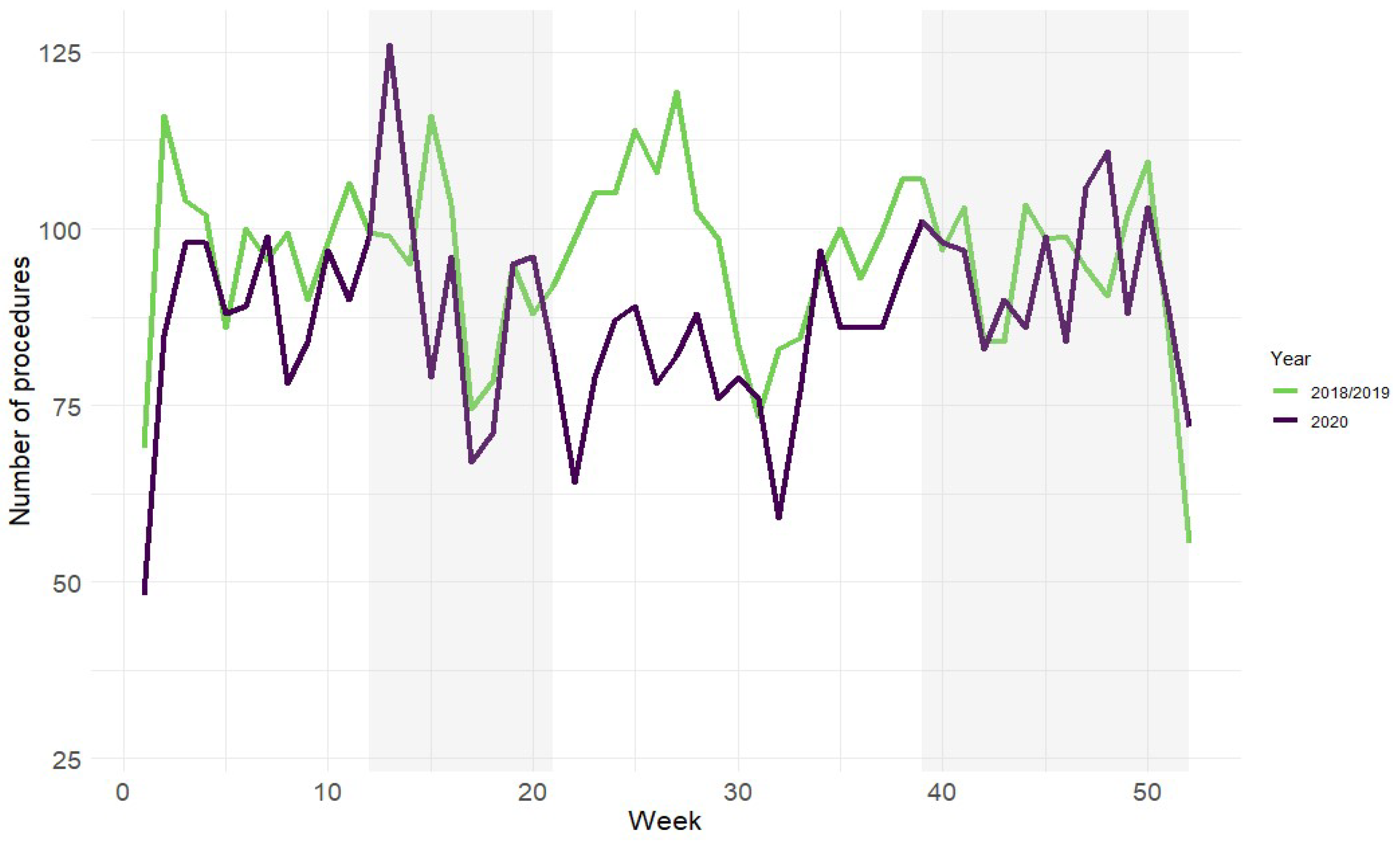
3.2. Time to Treatment
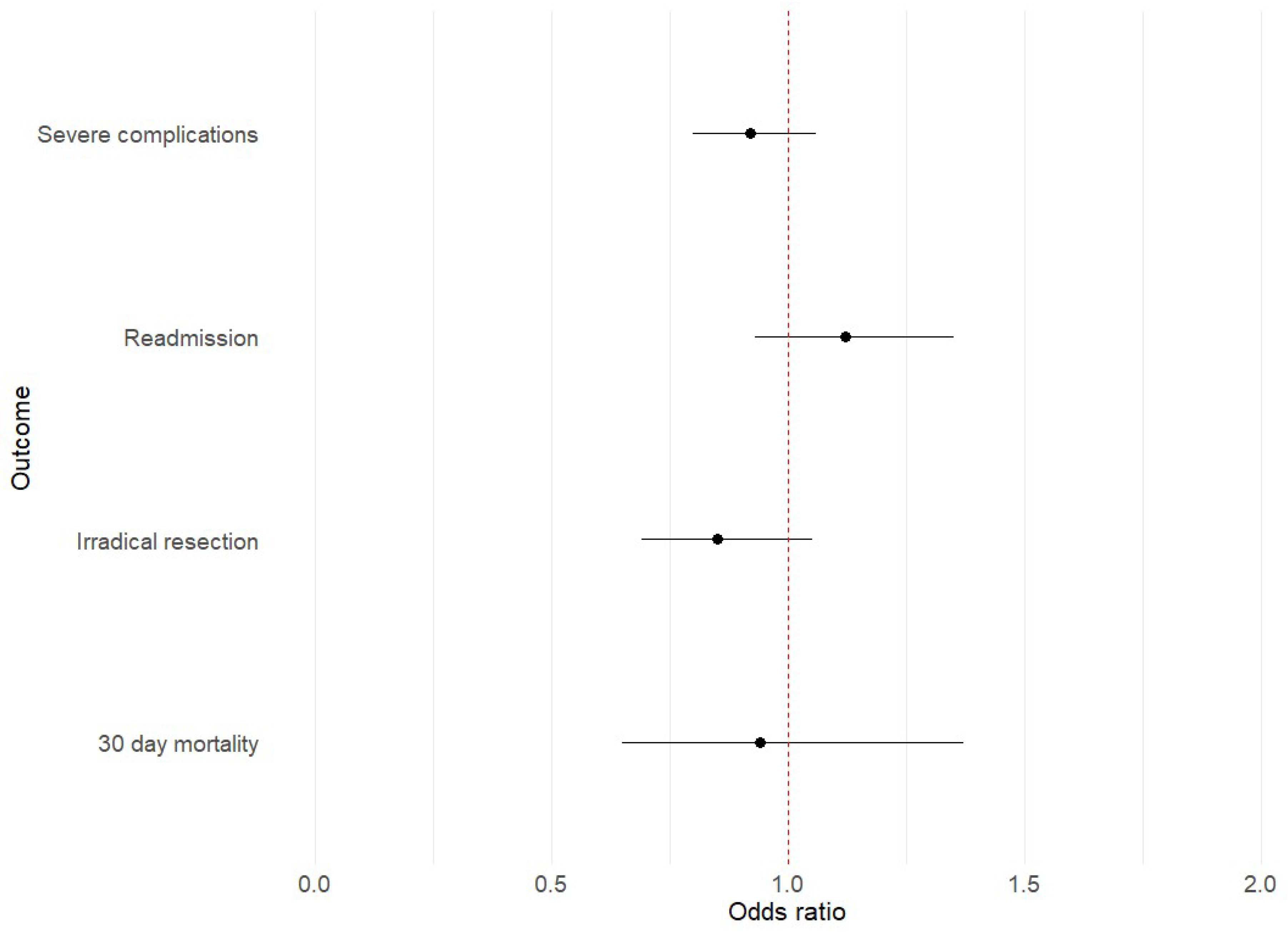
3.3. Postoperative Outcomes
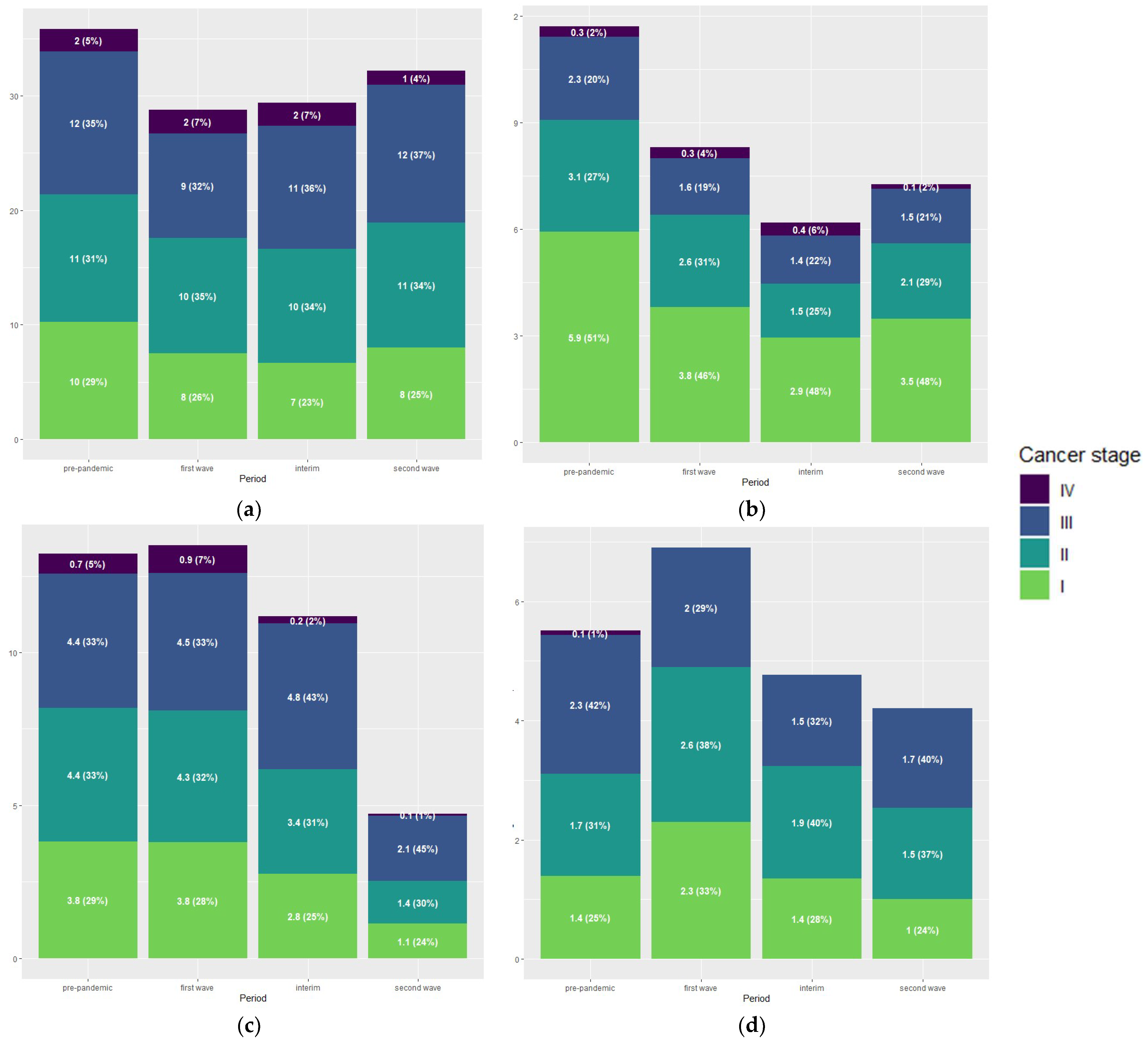
3.4. Tumour Stage Distribution
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lookhuyzen, L. Uitstel Kan, Maar Ook Dat Is Eindig—NRC. Available online: https://www.nrc.nl/nieuws/2020/03/26/andere-zorg-uitstellen-kan-maar-niet-oneindig-a3995039 (accessed on 8 June 2023).
- Stoffelen, A.; Huisman, C. Chemo Uitgesteld, Transplantatie Afgeblazen: In de Ziekenhuizen Voltrekt Zich Een Schaduwcrisis. Available online: https://www.volkskrant.nl/nieuws-achtergrond/chemo-uitgesteld-transplantatie-afgeblazen-in-de-ziekenhuizen-voltrekt-zich-een-schaduwcrisis~b9d4efb4/?referrer=https%3A%2F%2Fwww.google.com%2F (accessed on 8 June 2023).
- de Graaff, M.R.; Hogenbirk, R.N.M.; Janssen, Y.F.; Elfrink, A.K.E.; Liem, R.S.L.; Nienhuijs, S.W.; de Vries, J.-P.P.M.; Elshof, J.-W.; Verdaasdonk, E.; Melenhorst, J.; et al. Impact of the COVID-19 Pandemic on Surgical Care in The Netherlands. Br. J. Surg. 2022, 109, 1282–1292. [Google Scholar] [CrossRef]
- Federatie Medisch Specialisten. Normering Oncologische Zorg in N. SONCOS Normeringsrapport. 2020. Available online: https://demedischspecialist.nl/sites/default/files/SONCOS-normeringsrapport-versie-8-1.pdf (accessed on 21 June 2023).
- Busweiler, L.A.D.; Wijnhoven, B.P.L.; van Berge Henegouwen, M.I.; Henneman, D.; van Grieken, N.C.T.; Wouters, M.W.J.M.; van Hillegersberg, R.; van Sandick, J.W. Early Outcomes from the Dutch Upper Gastrointestinal Cancer Audit. Br. J. Surg. 2016, 103, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- van der Werf, L.R.; Kok, N.F.M.; Buis, C.I.; Grünhagen, D.J.; Hoogwater, F.J.H.; Swijnenburg, R.J.; den Dulk, M.; Dejong, K.C.H.C.; Klaase, J.M. Implementation and First Results of a Mandatory, Nationwide Audit on Liver Surgery. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2019, 21, 1400–1410. [Google Scholar] [CrossRef]
- van Rijssen, L.B.; Koerkamp, B.G.; Zwart, M.J.; Bonsing, B.A.; Bosscha, K.; van Dam, R.M.; van Eijck, C.H.; Gerhards, M.F.; van der Harst, E.; de Hingh, I.H.; et al. Nationwide Prospective Audit of Pancreatic Surgery: Design, Accuracy, and Outcomes of the Dutch Pancreatic Cancer Audit. HPB Off. J. Int. Hepato Pancreato Biliary Assoc. 2017, 19, 919–926. [Google Scholar] [CrossRef]
- Van Leersum, N.J.; Snijders, H.S.; Henneman, D.; Kolfschoten, N.E.; Gooiker, G.A.; ten Berge, M.G.; Eddes, E.H.; Wouters, M.W.J.M.; Tollenaar, R.A.E.M.; Bemelman, W.A.; et al. The Dutch Surgical Colorectal Audit. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2013, 39, 1063–1070. [Google Scholar] [CrossRef]
- Ten Berge, M.; Beck, N.; Heineman, D.J.; Damhuis, R.; Steup, W.H.; van Huijstee, P.J.; Eerenberg, J.P.; Veen, E.; Maat, A.; Versteegh, M.; et al. Dutch Lung Surgery Audit: A National Audit Comprising Lung and Thoracic Surgery Patients. Ann. Thorac. Surg. 2018, 106, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Number of Hospitals in The Netherlands. Available online: https://www.vzinfo.nl/ziekenhuiszorg/aanbod/instellingen (accessed on 25 August 2023).
- Inclusion & Exclusion Criteria DCRA—DICA. Available online: https://dica.nl/dcra/home (accessed on 8 June 2023).
- Inclusion & Exclusion Criteria DUCA—DICA. Available online: https://dica.nl/duca/home (accessed on 8 June 2023).
- Inclusion & Exclusion Criteria DHBA—DICA. Available online: https://dica.nl/dhba/home (accessed on 8 June 2023).
- Inclusion & Exclusion Criteria DPCA—DICA. Available online: https://dica.nl/dpca/home (accessed on 8 June 2023).
- Inclusion & Exclusion Criteria DLCA—DICA. Available online: https://dica.nl/dlca/home (accessed on 8 June 2023).
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A New Method of Classifying Prognostic Comorbidity in Longitudinal Studies: Development and Validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Clavien, P.A.; Barkun, J.; De Oliveira, M.L.; Vauthey, J.N.; Dindo, D.; Schulick, R.D.; De Santibañes, E.; Pekolj, J.; Slankamenac, K.; Bassi, C.; et al. The Clavien-Dindo Classification of Surgical Complications: Five-Year Experience. Ann. Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef] [PubMed]
- CBS Covid Waves. Available online: https://www.cbs.nl/nl-nl/nieuws/2021/27/in-tweede-golf-overleden-bijna-11-duizend-meer-mensen-dan-verwacht (accessed on 29 December 2023).
- Samenwerkende Kwaliteits Registraties, SKR Impact Report—Covid Waves. Available online: https://skr-zorg.nl/impact-report/opzet-onderzoek/ (accessed on 23 April 2024).
- Fu, R.; Sutradhar, R.; Li, Q.; Hanna, T.P.; Chan, K.K.W.; Irish, J.C.; Coburn, N.; Hallet, J.; Dare, A.; Singh, S.; et al. Timeliness and Modality of Treatment for New Cancer Diagnoses During the COVID-19 Pandemic in Canada. JAMA Netw. Open 2023, 6, e2250394. [Google Scholar] [CrossRef]
- Eijkelboom, A.H.; De Munck, L.; Willemien Menke-Van Der Houven Van Oordt, C.; Broeders, M.J.M.; Van Den Bongard, D.H.J.G.; Strobbe, L.J.A.; Mureau, M.A.M.; Lobbes, M.B.I.; Westenend, P.J.; Koppert, L.B.; et al. Changes in Breast Cancer Treatment during the COVID-19 Pandemic: A Dutch Population-Based Study. Breast Cancer Res. Treat. 2023, 197, 161–175. [Google Scholar] [CrossRef]
- Meijer, J.; Elferink, M.A.G.; van Hoeve, J.C.; Buijsen, J.; van Erning, F.; Nagtegaal, I.D.; Tanis, P.J.; Vink, G.R.; Wumkes, M.L.; de Hingh, I.H.J.T.; et al. Impact of the COVID-19 Pandemic on Colorectal Cancer Care in The Netherlands: A Population-Based Study. Clin. Colorectal Cancer 2022, 21, e171–e178. [Google Scholar] [CrossRef] [PubMed]
- Van Hoogstraten, L.M.C.; Kiemeney, L.A.; Meijer, R.P.; Van Leenders, G.J.L.H.; Vanneste, B.G.L.; Incrocci, L.; Smilde, T.J.; Siesling, S.; Witjes, J.A.; Aben, K.K.H. The Impact of the COVID-19 Pandemic on Bladder Cancer Care in The Netherlands. Bladder Cancer 2022, 8, 139–154. [Google Scholar] [CrossRef]
- Algera, M.D.; van Driel, W.J.; Slangen, B.F.M.; Kruitwagen, R.F.P.M.; Wouters, M.W.J.M.; Baalbergen, A.; Ten Cate, A.D.; Aalders, A.L.; van der Kolk, A.; Kruse, A.J.; et al. Impact of the COVID-19-Pandemic on Patients with Gynecological Malignancies Undergoing Surgery: A Dutch Population-Based Study Using Data from the ‘Dutch Gynecological Oncology Audit’. Gynecol. Oncol. 2022, 165, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Swift, B.E.; Mazuryk, J.; Yermakhanova, O.; Green, B.; Ferguson, S.R.; Kupets, R. Access to Surgery for Endometrial Cancer Patients During the COVID-19 Pandemic in Ontario, Canada: A Population-Based Study. J. Obstet. Gynaecol. Canada 2024, 46, 102226. [Google Scholar] [CrossRef]
- Graus, M.U.J.E.; de Hingh, I.H.J.T.; Besselink, M.G.; Bruno, M.J.; Wilmink, J.W.; de Meijer, V.E.; van Velthuysen, M.L.F.; Valkenburg-van Iersel, L.B.J.; van der Geest, L.G.M.; de Vos-Geelen, J.; et al. Population-Based Impact of COVID-19 on Incidence, Treatment, and Survival of Patients with Pancreatic Cancer. HPB 2023, 25, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Rasic, G.; Beaulieu-Jones, B.R.; Chung, S.H.; Romatoski, K.S.; Kenzik, K.; Ng, S.C.; Tseng, J.F.; Sachs, T.E. The Impact of the COVID-19 Pandemic on Hepatocellular Carcinoma Time to Treatment Initiation: A National Cancer Database Study. Ann. Surg. Oncol. 2023, 30, 4249–4259. [Google Scholar] [CrossRef] [PubMed]
- Schoonbeek, R.C.; de Jel, D.V.C.; van Dijk, B.A.C.; Willems, S.M.; Bloemena, E.; Hoebers, F.J.P.; van Meerten, E.; Verbist, B.M.; Smeele, L.E.; Halmos, G.B.; et al. Fewer Head and Neck Cancer Diagnoses and Faster Treatment Initiation during COVID-19 in 2020: A Nationwide Population-Based Analysis: Impact of COVID-19 on Head and Neck Cancer. Radiother. Oncol. 2022, 167, 42–48. [Google Scholar] [CrossRef]
- Eijkelboom, A.H.; de Munck, L.; Vrancken Peeters, M.J.T.F.D.; Broeders, M.J.M.; Strobbe, L.J.A.; Bos, M.E.M.M.; Schmidt, M.K.; Guerrero Paez, C.; Smidt, M.L.; Bessems, M.; et al. Impact of the COVID-19 Pandemic on Diagnosis, Stage, and Initial Treatment of Breast Cancer in The Netherlands: A Population-Based Study. J. Hematol. Oncol. 2021, 14, 64. [Google Scholar] [PubMed]
- Fligor, S.C.; Wang, S.; Allar, B.G.; Tsikis, S.T.; Ore, A.S.; Whitlock, A.E.; Calvillo-Ortiz, R.; Arndt, K.R.; Gangadharan, S.P.; Callery, M.P. Gastrointestinal Malignancies and the COVID-19 Pandemic: Evidence-Based Triage to Surgery. J. Gastrointest. Surg. 2020, 24, 2357–2373. [Google Scholar] [CrossRef]
- Fligor, S.C.; Tsikis, S.T.; Wang, S.; Ore, A.S.; Allar, B.G.; Whitlock, A.E.; Calvillo-Ortiz, R.; Arndt, K.; Callery, M.P.; Gangadharan, S.P. Time to Surgery in Thoracic Cancers and Prioritization during COVID-19: A Systematic Review. J. Thorac. Dis. 2020, 12, 6640–6654. [Google Scholar] [CrossRef]
- Ling, Q.; Huang, S.T.; Yu, T.H.; Liu, H.L.; Zhao, L.Y.; Chen, X.L.; Liu, K.; Chen, X.Z.; Yang, K.; Hu, J.K.; et al. Optimal Timing of Surgery for Gastric Cancer after Neoadjuvant Chemotherapy: A Systematic Review and Meta-Analysis. World J. Surg. Oncol. 2023, 21, 377. [Google Scholar] [CrossRef] [PubMed]
- Molenaar, C.J.L.; van Rooijen, S.J.; Fokkenrood, H.J.P.; Roumen, R.M.H.; Janssen, L.; Slooter, G.D. Prehabilitation versus No Prehabilitation to Improve Functional Capacity, Reduce Postoperative Complications and Improve Quality of Life in Colorectal Cancer Surgery. Cochrane Database Syst. Rev. 2022, 5, CD013259. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Endo, H.; Yamamoto, H.; Miyata, H.; Munekage, M.; Taketomi, A.; Kakeji, Y.; Seto, Y.; Yoshida, K.; Yamaue, H.; et al. Effects of the COVID-19 Pandemic on Gastroenterological Surgeries in 2020: A Study Using the National Clinical Database of Japan. Ann. Gastroenterol. Surg. 2022, 7, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Kong, J.C.; Singh, P.; Prabhakaran, S.; Warrier, S.K.; Bell, S. The Impact of the COVID-19 Pandemic on Colorectal Cancer Diagnosis and Management: A Binational Colorectal Cancer Audit Study. Anz J. Surg. 2021, 91, 2091–2096. [Google Scholar] [CrossRef] [PubMed]
- Toes-Zoutendijk, E.; Vink, G.; Nagtegaal, I.D.; Spaander, M.C.W.; Dekker, E.; van Leerdam, M.E.; Siesling, S.; Lansdorp-Vogelaar, I.; Elferink, M.A.G. Impact of COVID-19 and Suspension of Colorectal Cancer Screening on Incidence and Stage Distribution of Colorectal Cancers in The Netherlands. Eur. J. Cancer 2022, 161, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Yamamoto, H.; Ikeda, N.; Konishi, H.; Endo, S.; Okada, Y.; Kondo, H.; Shintani, Y.; Toyooka, S.; Nakamura, H.; et al. The Impact of COVID-19 on Thoracic Surgical Procedures in Japan: Analysis of Data from the National Clinical Database. Lung Cancer 2022, 172, 127–135. [Google Scholar] [CrossRef]
- Eklöv, K.; Nygren, J.; Bringman, S.; Löfgren, J.; Sjövall, A.; Nordenvall, C.; Everhov, Å.H. Trends in Treatment of Colorectal Cancer and Short-Term Outcomes During the First Wave of the COVID-19 Pandemic in Sweden. JAMA Netw. Open 2022, 5, e2211065. [Google Scholar] [CrossRef]

| Characteristic | Historic Cohort, N = 11,275 | Pandemic Cohort, N = 3292 | p-Value 1 |
|---|---|---|---|
| Age (years) Missing | 68 (10.6) | 68 (10.8) | 0.9 § |
| <0.1% | 0.2% | ||
| Female sex Missing | 4712 (42%) | 1403 (43%) | 0.4 |
| - | <0.1% | ||
| BMI > 30 Missing | 2024 (19%) | 577 (18%) | 0.3 |
| 4% | 2% | ||
| Pulmonary comorbidity Missing | 1360 (13%) | 373 (11%) | 0.003 |
| 10% | 0.5% | ||
| Charlson Comorbidity Index | 4.1 (2.3) | 4.5 (2.5) | <0.001 § |
| Missing | <0.1% | 0.2% | |
| ASA classification | 0.006 | ||
| 1 | 1064 (9.4%) | 273 (8.3%) | |
| 2 | 6325 (56.4%) | 1774 (54.5%) | |
| 3 | 3651 (32.5%) | 1160 (35.7%) | |
| 4 | 188 (1.7%) | 49 (1.5%) | |
| Missing | 0.4% | 1% | |
| Tumour type | <0.001 | ||
| CRC | 4369 (38.8%) | 1282 (39%) | |
| HPB | 3357 (29.8%) | 1169 (35.5%) | |
| Lung | 1556 (13.8%) | 347 (10.5%) | |
| Upper GI | 1993 (17.6%) | 494 (15%) | |
| Cancer stage 2 | <0.001 | ||
| I | 2459 (32.3%) | 587 (27.8%) | |
| II | 2343 (30.8%) | 703 (33%) | |
| III | 2467 (32.4%) | 734 (34.6%) | |
| IV | 339 (4.5%) | 98 (4.6%) | |
| Missing | 11% | 9% |
| Characteristic | Pre-Pandemic, N = 10,935 | First Wave, N = 920 | Interim, N = 1377 | Second Wave, N = 1335 | p-Value 1 |
|---|---|---|---|---|---|
| Emergency surgery 2 Missing | 619 (10%) | 77 (15%) | 99 (13%) | 67 (8.9%) | <0.001 |
| 43% | 44% | 44% | 44% | ||
| Neoadjuvant treatment Missing | 1581 (16%) | 195 (23%) | 250 (20%) | 224 (18%) | <0.001 |
| 8.0% | 7.4% | 8.9% | 8.4% | ||
| Surgical approach Open | 0.2 | ||||
| 2850 (27%) | 275 (30.7%) | 374 (28%) | 331 (25.4%) | ||
| Laparoscopic Conversion | 5965 (56.6%) | 474 (52.7%) | 726 (54.6%) | 752 (58%) | |
| 727 (6.9%) | 58 (6.5%) | 93 (7.0%) | 80 (6.2%) | ||
| Robot assisted | 308 (2.9%) | 29 (3.2%) | 52 (3.9%) | 46 (3.5%) | |
| Local/ablation | 691 (6.6%) | 62 (6.9%) | 86 (6.5%) | 90 (6.9%) | |
| Missing | 3.6% | 2.4% | 3.3% | 2.7% |
| Characteristic | Pre-Pandemic, N = 10,480 | First Wave, N = 789 | Interim, N = 1222 | Second Wave, N = 963 | p-Value |
|---|---|---|---|---|---|
| Time to treatment (days) | 27 (16, 43) | 27 (15, 43) | 25 (14, 39) | 26 (15, 39) | <0.001 1 |
| CRC | 19 (12, 29) | 15 (9, 31) | 16 (10, 28) | 18 (11, 28) | 0.01 |
| HPB | 33 (21, 50) | 31 (20, 47) | 29 (19, 46) | 30 (20, 42) | <0.001 |
| Upper GI | 22 (15, 34) | 26 (15, 36) | 21 (15, 33) | 26 (17, 40) | 0.2 |
| Lung | 50 (36, 69) | 48 (34, 63) | 46 (33, 63) | 42 (31, 60) | <0.001 |
| Treatment started < 6 w | 7636 (73%) | 573 (73%) | 945 (77%) | 757 (79%) | <0.001 2 |
| CRC (N = 4942) | 90% | 84% | 91% | 92% | 0.01 |
| HPB (N = 4387) | 66% | 68% | 71% | 73% | 0.009 |
| Upper GI (N = 2226) | 83% | 83% | 88% | 77% | 0.1 |
| Lung (N = 1899) | 35% | 41% | 42% | 47% | 0.02 |
| Cohort | Historic | Pandemic | ||||
|---|---|---|---|---|---|---|
| Outcome | <6 w, N = 7734 | >6 w, N = 2920 | p-Value 1 | <6 w, N = 2322 | >6 w, N = 729 | p-Value 1 |
| Length of hospital stay (days) | 7 (4, 11) | 7 (4, 11) | 0.7 | 6 (4, 10) | 7 (4, 10) | 0.4 |
| Missing | 0.4% | 0.6% | 0.8% | 1.0% | ||
| Readmission within 30 days | 745 (10%) | 254 (9.6%) | 0.2 | 220 (9.6%) | 75 (11%) | 0.5 |
| Missing | 8% | 10% | 2% | 2% | ||
| Severe complication | 1253 (16%) | 492 (17%) | 0.4 | 360 (16%) | 123 (17%) | 0.4 |
| Missing | 0.3% | 0.7% | 0.1% | 0 | ||
| 30-day mortality | 124 (1.6%) | 77 (2.6%) | <0.001 | 48 (2.1%) | 13 (1.8%) | 0.6 |
| Missing | 0.4% | 0.4% | 1.0% | 1.0% | ||
| Resection margins | 0.003 | 0.04 | ||||
| R0 | 6065 (93.7%) | 2000 (92.1%) | 1732 (91.5%) | 459 (88.3%) | ||
| R1 | 397 (6.1%) | 159 (7.3%) | 150 (7.9%) | 59 (11.3%) | ||
| R2 | 16 (0.2%) | 14 (0.6%) | 12 (0.6%) | 2 (0.4%) | ||
| Missing | 16% | 26% | 18% | 29% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Vuren, R.M.G.; Janssen, Y.F.; Hogenbirk, R.N.M.; de Graaff, M.R.; van den Hoek, R.; Kruijff, S.; Heineman, D.J.; van der Plas, W.Y.; Wouters, M.W.J.M. The Impact of the COVID-19 Pandemic on Time to Treatment in Surgical Oncology: A National Registry Study in The Netherlands. Cancers 2024, 16, 1738. https://doi.org/10.3390/cancers16091738
van Vuren RMG, Janssen YF, Hogenbirk RNM, de Graaff MR, van den Hoek R, Kruijff S, Heineman DJ, van der Plas WY, Wouters MWJM. The Impact of the COVID-19 Pandemic on Time to Treatment in Surgical Oncology: A National Registry Study in The Netherlands. Cancers. 2024; 16(9):1738. https://doi.org/10.3390/cancers16091738
Chicago/Turabian Stylevan Vuren, Roos M. G., Yester F. Janssen, Rianne N. M. Hogenbirk, Michelle R. de Graaff, Rinske van den Hoek, Schelto Kruijff, David J. Heineman, Willemijn Y. van der Plas, and Michel W. J. M. Wouters. 2024. "The Impact of the COVID-19 Pandemic on Time to Treatment in Surgical Oncology: A National Registry Study in The Netherlands" Cancers 16, no. 9: 1738. https://doi.org/10.3390/cancers16091738
APA Stylevan Vuren, R. M. G., Janssen, Y. F., Hogenbirk, R. N. M., de Graaff, M. R., van den Hoek, R., Kruijff, S., Heineman, D. J., van der Plas, W. Y., & Wouters, M. W. J. M. (2024). The Impact of the COVID-19 Pandemic on Time to Treatment in Surgical Oncology: A National Registry Study in The Netherlands. Cancers, 16(9), 1738. https://doi.org/10.3390/cancers16091738





