Hypoxia-Inducible Factor-Dependent and Independent Mechanisms Underlying Chemoresistance of Hypoxic Cancer Cells
Abstract
Simple Summary
Abstract
1. Brief Introduction
2. Hypoxic Microenvironment in Malignant Solid Tumors and Its Association with Therapy Resistance
3. HIF-Mediated Mechanisms behind Chemotherapy Resistance of Cancer Cells under Hypoxia
3.1. The Molecular Mechanisms behind the Regulation of HIFs’ Activity
3.2. HIF-Mediated Mechanisms behind Chemotherapy Resistance
3.3. HIF-Related Hypoxia-Responsive Non-Coding RNAs and Chemotherapy Resistance
3.4. HIF, EMT, and Chemotherapy Resistance
4. HIF-Independent Mechanisms behind Chemotherapy Resistance of Cancer Cells under Hypoxia
4.1. HIF-Independent Mechanisms behind Chemotherapy Resistance
4.2. Hypoxia-Associated Proteotoxicity, UPR, and Chemotherapy Resistance
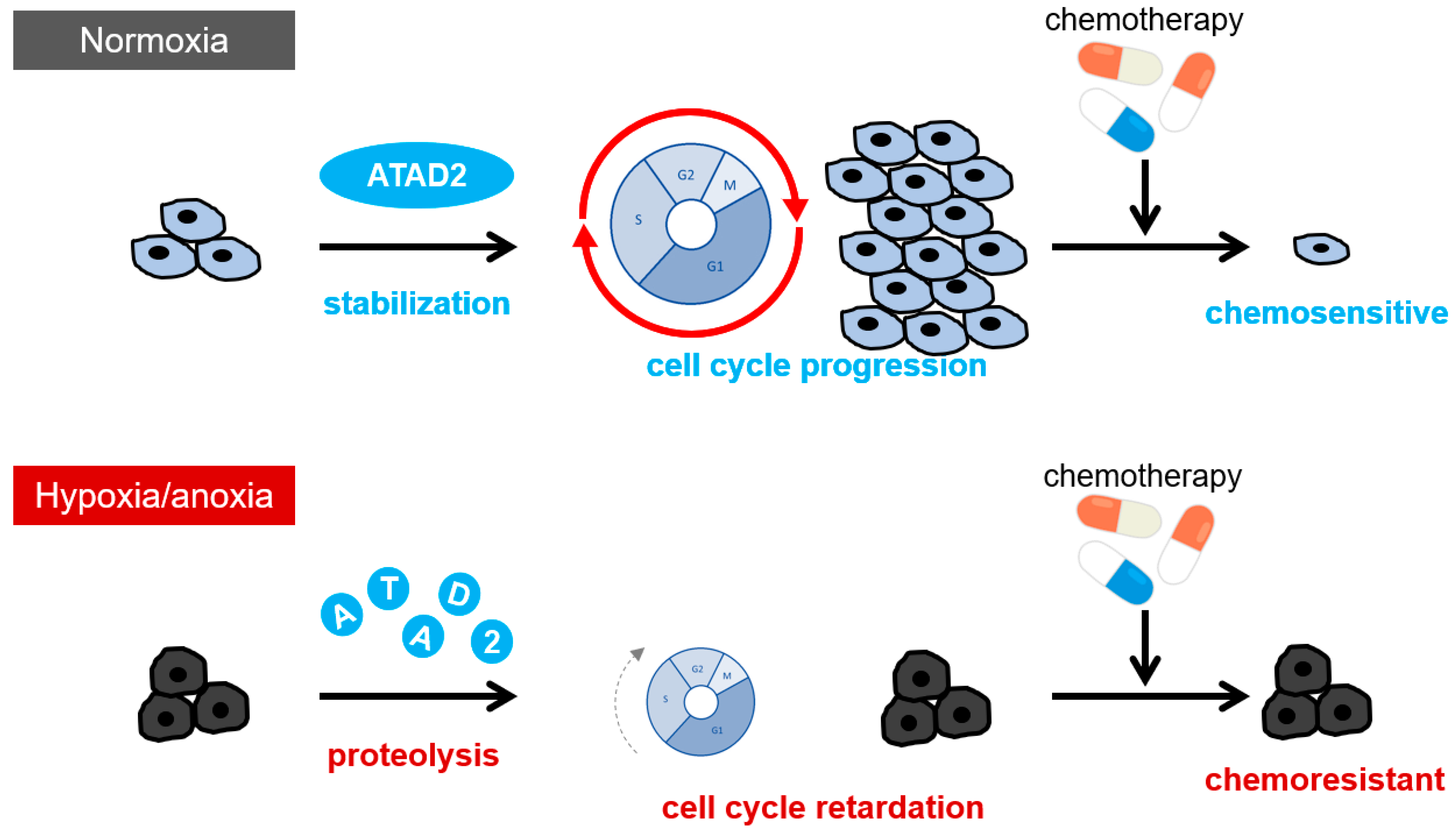
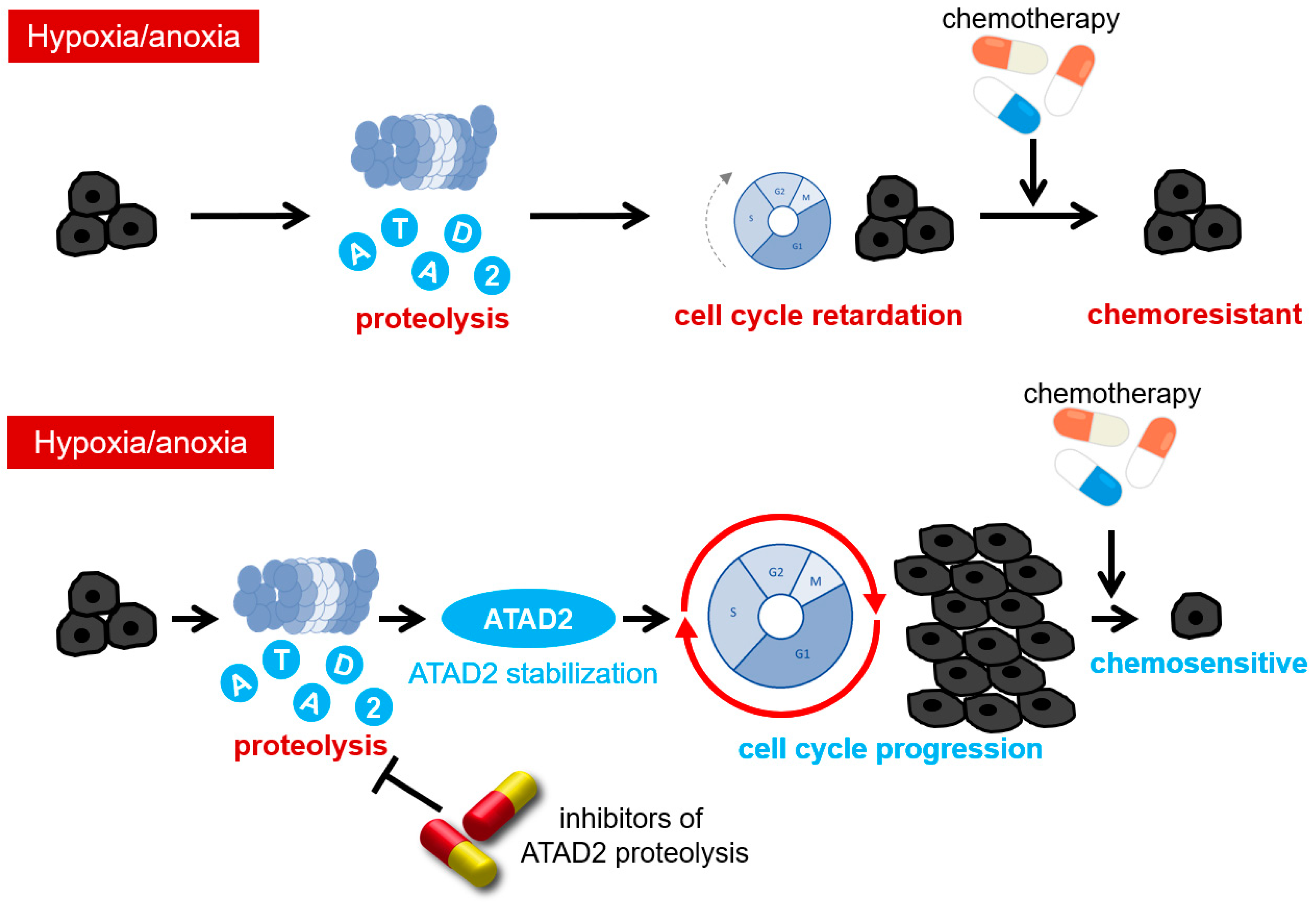
5. The Mechanisms of Therapy Resistance Acquisition through Hypoxia-Dependent Epigenetic Regulations: The Role of a Histone Acetyl Reader Protein, ATAD2
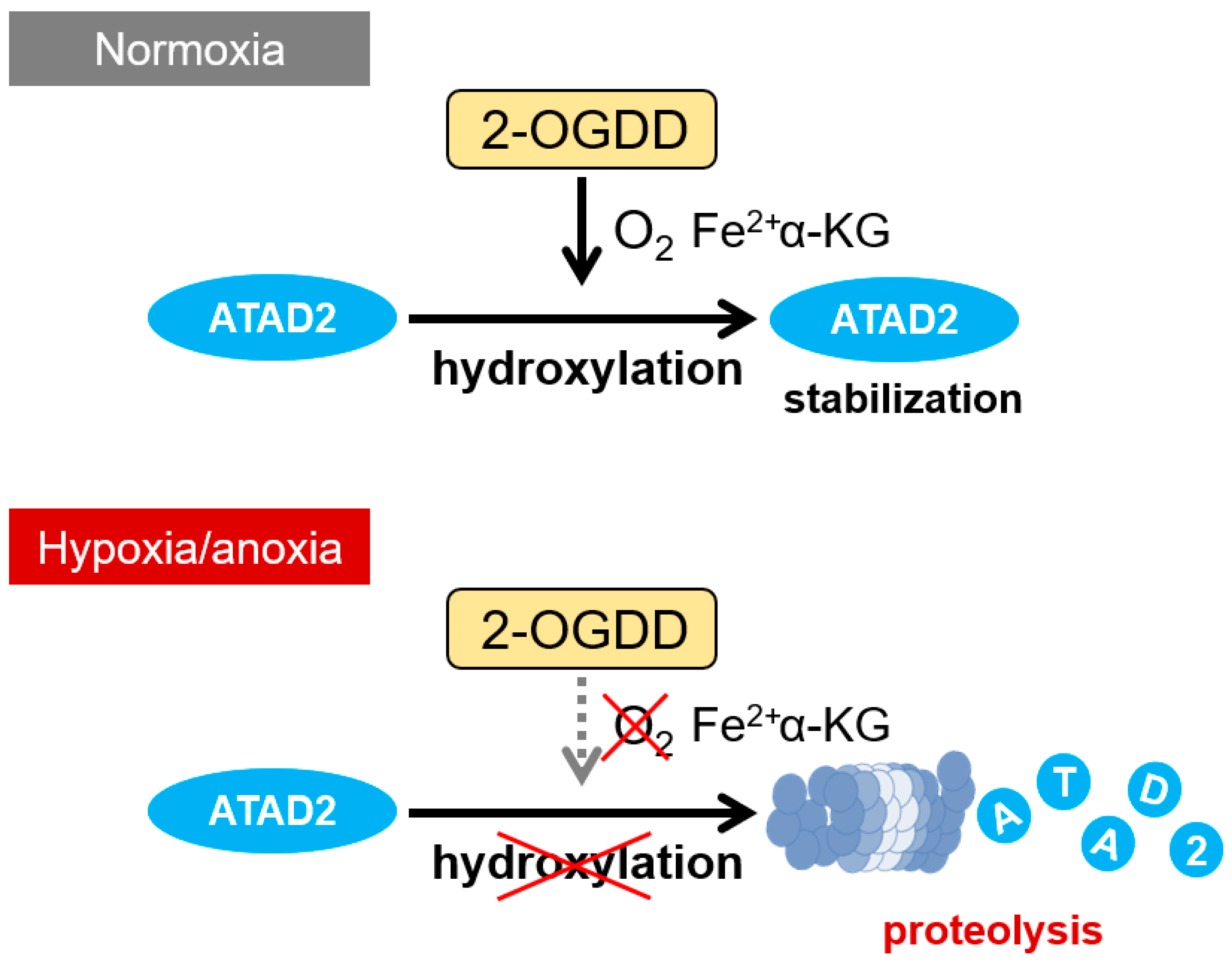
6. Oxygen-Dependent Regulatory Mechanism of ATAD2 Expression
7. Summary and Perspectives
| Name | Target/Effect | Resistance | Cancer Type, Model | Ref. | |
|---|---|---|---|---|---|
| HIF downstream | LINC03000-201 (lncMat2B, ENST00000486913.3) | ↓ ROS production ↓ DNA damage | Cisplatin | Breast cancer, in vitro | [223] |
| PVT1 | miR-140-3p/ATG5 ↓ autophagy | Cisplatin | Lung cancer, in vitro and in vivo | [60] | |
| lncRNA-CBSLR | YTHDF2/CBS ↓ ferroptosis | Cisplatin | Gastric cancer, in vivo | [224] | |
| ANRIL | miR-328/ABCG2, MDR1 | Cisplatin | Retinoblastoma, in vitro | [225] | |
| LUCAT1 | Interaction with PTBP1 ↓ DNA damage | 5-Fluorouracil, Camptothecin, Doxorubicin and Oxaliplatin | Colorectal cancer, in vitro, in vivo, and patient cohorts | [226] | |
| NLUCAT1 (HIF-2α-dependent) | ↓ ROS production | Cisplatin | Lung adenocarcinoma, in vitro | [227] | |
| HIF upstream | PVT1 | miR-194-5p/HIF1A ↑ proliferation | Cisplatin | Oral SCC, in vitro | [228] |
| HIF1A-AS1 | Interaction with YB1 ↑ HIF-1α (positive feedback) ↑ glycolysis | Gemcitabine | Pancreatic cancer, in vitro, in vivo, and patient cohorts | [229] | |
| HIF1A-AS2 | ↑ HIF-1α ↑ autophagy | Doxorubicin | Small cell lung cancer, in vitro | [230] | |
| NORAD | miR-495-3p/HIF-1α ↑ vasculogenic mimicry | 5-Fluorouracil | Colorectal cancer, in vitro | [231] | |
| Potentially HIF-dependent | HOTAIR | miR-1277-5p/ZEB1 ↑ EMT | Oxaliplatin | Colorectal cancer, in vitro and in vivo | [232] |
| lncRNA-EMS | miR-758-3p/WTAP | Cisplatin | Esophageal cancer, in vitro and in vivo | [233] |
Author Contributions
Funding
Conflicts of Interest
References
- Yeom, C.J.; Goto, Y.; Zhu, Y.; Hiraoka, M.; Harada, H. Microenvironments and cellular characteristics in the micro tumor cords of malignant solid tumors. Int. J. Mol. Sci. 2012, 13, 13949–13965. [Google Scholar] [CrossRef]
- Walsh, J.C.; Lebedev, A.; Aten, E.; Madsen, K.; Marciano, L.; Kolb, H.C. The clinical importance of assessing tumor hypoxia: Relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid. Redox Signal. 2014, 21, 1516–1554. [Google Scholar] [CrossRef]
- Osinsky, S.; Zavelevich, M.; Vaupel, P. Tumor hypoxia and malignant progression. Exp. Oncol. 2009, 31, 80–86. [Google Scholar]
- Haitani, T.; Kobayashi, M.; Koyasu, S.; Akamatsu, S.; Suwa, T.; Onodera, Y.; Nam, J.-M.; Nguyen, P.T.L.; Menju, T.; Date, H.; et al. Proteolysis of a histone acetyl reader, atad2, induces chemoresistance of cancer cells under severe hypoxia by inhibiting cell cycle progression in s phase. Cancer Lett. 2022, 528, 76–84. [Google Scholar] [CrossRef]
- Shirai, Y.; Chow, C.C.T.; Kambe, G.; Suwa, T.; Kobayashi, M.; Takahashi, I.; Harada, H.; Nam, J.-M. An overview of the recent development of anticancer agents targeting the hif-1 transcription factor. Cancers 2021, 13, 2813. [Google Scholar] [CrossRef]
- Yoshimura, M.; Itasaka, S.; Harada, H.; Hiraoka, M. Microenvironment and radiation therapy. Biomed. Res. Int. 2013, 2013, 685308. [Google Scholar] [CrossRef]
- Mellor, H.R.; Callaghan, R. Resistance to chemotherapy in cancer: A complex and integrated cellular response. Pharmacology 2008, 81, 275–300. [Google Scholar] [CrossRef]
- Yeldag, G.; Rice, A.; Del Río Hernández, A. Chemoresistance and the self-maintaining tumor microenvironment. Cancers 2018, 10, 471. [Google Scholar] [CrossRef]
- Weniger, M.; Honselmann, K.C.; Liss, A.S. The extracellular matrix and pancreatic cancer: A complex relationship. Cancers 2018, 10, 316. [Google Scholar] [CrossRef] [PubMed]
- Masamune, A.; Kikuta, K.; Watanabe, T.; Satoh, K.; Hirota, M.; Shimosegawa, T. Hypoxia stimulates pancreatic stellate cells to induce fibrosis and angiogenesis in pancreatic cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G709–G717. [Google Scholar] [CrossRef] [PubMed]
- Goda, N.; Ryan, H.E.; Khadivi, B.; McNulty, W.; Rickert, R.C.; Johnson, R.S. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol. Cell. Biol. 2003, 23, 359–369. [Google Scholar] [CrossRef]
- Harada, H. How can we overcome tumor hypoxia in radiation therapy? J. Radiat. Res. 2011, 52, 545–556. [Google Scholar] [CrossRef]
- Kizaka-Kondoh, S.; Inoue, M.; Harada, H.; Hiraoka, M. Tumor hypoxia: A target for selective cancer therapy. Cancer Sci. 2003, 94, 1021–1028. [Google Scholar] [CrossRef]
- Kizaka-Kondoh, S.; Tanaka, S.; Harada, H.; Hiraoka, M. The hif-1-active microenvironment: An environmental target for cancer therapy. Adv. Drug Deliv. Rev. 2009, 61, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, W.R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef]
- Begg, K.; Tavassoli, M. Inside the hypoxic tumour: Reprogramming of the ddr and radioresistance. Cell Death Discov. 2020, 6, 77. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Semenza, G.L. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 1995, 270, 1230–1237. [Google Scholar] [CrossRef]
- Hirota, K.; Semenza, G.L. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem. Biophys. Res. Commun. 2005, 338, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.C.; Gleadle, J.M.; McNeill, L.A.; Hewitson, K.S.; O’Rourke, J.; Mole, D.R.; Mukherji, M.; Metzen, E.; Wilson, M.I.; Dhanda, A.; et al. C. Elegans egl-9 and mammalian homologs define a family of dioxygenases that regulate hif by prolyl hydroxylation. Cell 2001, 107, 43–54. [Google Scholar] [CrossRef]
- Ivan, M.; Kondo, K.; Yang, H.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G., Jr. Hifalpha targeted for vhl-mediated destruction by proline hydroxylation: Implications for o2 sensing. Science 2001, 292, 464–468. [Google Scholar] [CrossRef]
- Jaakkola, P.; Mole, D.R.; Tian, Y.-M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; von Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of hif-alpha to the von hippel-lindau ubiquitylation complex by o2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef]
- Rosenberg, N.; Gervais, P. Evaluation of the sequelae of occupational asthma. Rev. Mal. Respir. 1989, 6, 35–38. [Google Scholar]
- Kaelin, W.G., Jr. The von hippel-lindau tumour suppressor protein: O2 sensing and cancer. Nat. Rev. Cancer 2008, 8, 865–873. [Google Scholar] [CrossRef]
- Tanimoto, K.; Makino, Y.; Pereira, T.; Poellinger, L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von hippel-lindau tumor suppressor protein. EMBO J. 2000, 19, 4298–4309. [Google Scholar] [CrossRef]
- Ohh, M.; Park, C.W.; Ivan, M.; Hoffman, M.A.; Kim, T.-Y.; Huang, L.E.; Pavletich, N.; Chau, V.; Kaelin, W.G. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von hippel-lindau protein. Nat. Cell Biol. 2000, 2, 423–427. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.-W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumour suppressor protein vhl targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Arany, Z.; Huang, L.E.; Eckner, R.; Bhattacharya, S.; Jiang, C.; Goldberg, M.A.; Bunn, H.F.; Livingston, D.M. An essential role for p300/cbp in the cellular response to hypoxia. Proc. Natl. Acad. Sci. USA 1996, 93, 12969–12973. [Google Scholar] [CrossRef]
- Lando, D.; Peet, D.J.; Whelan, D.A.; Gorman, J.J.; Whitelaw, M.L. Asparagine hydroxylation of the hif transactivation domain a hypoxic switch. Science 2002, 295, 858–861. [Google Scholar] [CrossRef]
- Mahon, P.C.; Hirota, K.; Semenza, G.L. Fih-1: A novel protein that interacts with hif-1alpha and vhl to mediate repression of hif-1 transcriptional activity. Genes Dev. 2001, 15, 2675–2686. [Google Scholar] [CrossRef]
- Koyasu, S.; Kobayashi, M.; Goto, Y.; Hiraoka, M.; Harada, H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: Two decades of knowledge. Cancer Sci. 2018, 109, 560–571. [Google Scholar] [CrossRef]
- Yeom, C.J.; Zeng, L.; Goto, Y.; Morinibu, A.; Zhu, Y.; Shinomiya, K.; Kobayashi, M.; Itasaka, S.; Yoshimura, M.; Hur, C.-G.; et al. Ly6e: A conductor of malignant tumor growth through modulation of the pten/pi3k/akt/hif-1 axis. Oncotarget 2016, 7, 65837–65848. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Morinibu, A.; Kobayashi, M.; Zhu, Y.; Wang, X.; Goto, Y.; Yeom, C.J.; Zhao, T.; Hirota, K.; Shinomiya, K.; et al. Aberrant idh3alpha expression promotes malignant tumor growth by inducing hif-1-mediated metabolic reprogramming and angiogenesis. Oncogene 2015, 34, 4758–4766. [Google Scholar] [CrossRef]
- Harada, H.; Kizaka-Kondoh, S.; Li, G.; Itasaka, S.; Shibuya, K.; Inoue, M.; Hiraoka, M. Significance of hif-1-active cells in angiogenesis and radioresistance. Oncogene 2007, 26, 7508–7516. [Google Scholar] [CrossRef]
- Liu, Y.; Cox, S.R.; Morita, T.; Kourembanas, S. Hypoxia regulates vascular endothelial growth factor gene expression in endothelial cells. Identification of a 5′ enhancer. Circ. Res. 1995, 77, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Zeng, L.; Yeom, C.J.; Zhu, Y.; Morinibu, A.; Shinomiya, K.; Kobayashi, M.; Hirota, K.; Itasaka, S.; Yoshimura, M.; et al. Uchl1 provides diagnostic and antimetastatic strategies due to its deubiquitinating effect on hif-1alpha. Nat. Commun. 2015, 6, 6153. [Google Scholar] [CrossRef]
- Semenza, G.L. Molecular mechanisms mediating metastasis of hypoxic breast cancer cells. Trends Mol. Med. 2012, 18, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; De Marzo, A.M.; Laughner, E.; Lim, M.; Hilton, D.A.; Zagzag, D.; Buechler, P.; Isaacs, W.B.; Semenza, G.L.; Simons, J.W. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999, 59, 5830–5835. [Google Scholar]
- Semenza, G.L. Mitochondrial autophagy: Life and breath of the cell. Autophagy 2008, 4, 534–536. [Google Scholar] [CrossRef] [PubMed]
- Comerford, K.M.; Wallace, T.J.; Karhausen, J.; Louis, N.A.; Montalto, M.C.; Colgan, S.P. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (mdr1) gene. Cancer Res. 2002, 62, 3387–3394. [Google Scholar]
- He, M.; Wu, H.; Jiang, Q.; Liu, Y.; Han, L.; Yan, Y.; Wei, B.; Liu, F.; Deng, X.; Chen, H.; et al. Hypoxia-inducible factor-2alpha directly promotes bcrp expression and mediates the resistance of ovarian cancer stem cells to adriamycin. Mol. Oncol. 2019, 13, 403–421. [Google Scholar] [CrossRef]
- Pinzón-Daza, M.L.; Cuellar-Saenz, Y.; Nualart, F.; Ondo-Mendez, A.; Del Riesgo, L.; Castillo-Rivera, F.; Garzón, R. Oxidative stress promotes doxorubicin-induced pgp and bcrp expression in colon cancer cells under hypoxic conditions. J. Cell. Biochem. 2017, 118, 1868–1878. [Google Scholar] [CrossRef]
- Wang, H.; Wu, X.; Hudkins, K.; Mikheev, A.; Zhang, H.; Gupta, A.; Unadkat, J.D.; Mao, Q. Expression of the breast cancer resistance protein (bcrp1/abcg2) in tissues from pregnant mice: Effects of pregnancy and correlations with nuclear receptors. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1295–E1304. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.-Y.; Chun, Y.-S.; Kim, T.-Y.; Kim, H.-L.; Kim, M.-S.; Park, J.-W. Hypoxia-inducible factor 1alpha-mediated resistance to phenolic anticancer. Chemotherapy 2004, 50, 119–126. [Google Scholar] [CrossRef]
- Chen, J.; Ding, Z.; Peng, Y.; Pan, F.; Li, J.; Zou, L.; Zhang, Y.; Liang, H. Hif-1alpha inhibition reverses multidrug resistance in colon cancer cells via downregulation of mdr1/p-glycoprotein. PLoS ONE 2014, 9, e98882. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-Y.; Fu, Z.-X.; Wang, X.-H. Effect of hypoxia-inducible factor 1-alpha on survivin in colorectal cancer. Mol. Med. Rep. 2010, 3, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Dong, X.; Lin, L.; Jiang, X.; Wei, Z.; Zhai, B.; Sun, B.; Zhang, Q.; Wang, X.; Jiang, H.; et al. Up-regulation of survivin by akt and hypoxia-inducible factor 1alpha contributes to cisplatin resistance in gastric cancer. FEBS J. 2014, 281, 115–128. [Google Scholar] [CrossRef]
- Li, H.; Sun, X.; Li, J.; Liu, W.; Pan, G.; Mao, A.; Liu, J.; Zhang, Q.; Rao, L.; Xie, X.; et al. Hypoxia induces docetaxel resistance in triple-negative breast cancer via the hif-1alpha/mir-494/survivin signaling pathway. Neoplasia 2022, 32, 100821. [Google Scholar] [CrossRef] [PubMed]
- Zichittella, C.; Barreca, M.M.; Cordaro, A.; Corrado, C.; Alessandro, R.; Conigliaro, A. Mir-675-5p supports hypoxia-induced drug resistance in colorectal cancer cells. BMC Cancer 2022, 22, 567. [Google Scholar] [CrossRef]
- Feng, L.; Shen, F.; Zhou, J.; Li, Y.; Jiang, R.; Chen, Y. Hypoxia-induced up-regulation of mir-27a promotes paclitaxel resistance in ovarian cancer. Biosci. Rep. 2020, 40, BSR20192457. [Google Scholar] [CrossRef]
- Xu, K.; Zhan, Y.; Yuan, Z.; Qiu, Y.; Wang, H.; Fan, G.; Wang, J.; Li, W.; Cao, Y.; Shen, X.; et al. Hypoxia induces drug resistance in colorectal cancer through the hif-1alpha/mir-338-5p/il-6 feedback loop. Mol. Ther. 2019, 27, 1810–1824. [Google Scholar] [CrossRef]
- Lu, H.; Samanta, D.; Xiang, L.; Zhang, H.; Hu, H.; Chen, I.; Bullen, J.W.; Semenza, G.L. Chemotherapy triggers hif-1-dependent glutathione synthesis and copper chelation that induces the breast cancer stem cell phenotype. Proc. Natl. Acad. Sci. USA 2015, 112, E4600–E4609. [Google Scholar] [CrossRef] [PubMed]
- Roncuzzi, L.; Pancotti, F.; Baldini, N. Involvement of hif-1alpha activation in the doxorubicin resistance of human osteosarcoma cells. Oncol. Rep. 2014, 32, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Ding, J.; Sun, W.; Wu, K.; Ning, B.; Gong, W.; He, G.; Huang, S.; Ding, X.; Yin, P.; et al. Suppression of cyclin d1 by hypoxia-inducible factor-1 via direct mechanism inhibits the proliferation and 5-fluorouracil-induced apoptosis of a549 cells. Cancer Res. 2010, 70, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Paré, G.C.; Frederiksen, L.J.; Semenza, G.L.; Graham, C.H. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor-1 activity. Mol. Cancer Ther. 2008, 7, 1961–1973. [Google Scholar] [CrossRef]
- Johnson, A.B.; Denko, N.; Barton, M.C. Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat. Res. 2008, 640, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Luo, D.; Li, X.M.; Li, Z.-Q.; Yu, X.; Zhu, H.-W. Pvt1 knockdown inhibits autophagy and improves gemcitabine sensitivity by regulating the mir-143/hif-1alpha/vmp1 axis in pancreatic cancer. Pancreas 2021, 50, 227–234. [Google Scholar] [CrossRef]
- Wu, H.-M.; Jiang, Z.-F.; Ding, P.-S.; Shao, L.-J.; Liu, R.-Y. Hypoxia-induced autophagy mediates cisplatin resistance in lung cancer cells. Sci. Rep. 2015, 5, 12291. [Google Scholar] [CrossRef]
- Liang, C.; Dong, Z.; Cai, X.; Shen, J.; Xu, Y.; Zhang, M.; Li, H.; Yu, W.; Chen, W. Hypoxia induces sorafenib resistance mediated by autophagy via activating foxo3a in hepatocellular carcinoma. Cell Death Dis. 2020, 11, 1017. [Google Scholar] [CrossRef]
- Yang, X.; Yin, H.; Zhang, Y.; Li, X.; Tong, H.; Zeng, Y.; Wang, Q.; He, W. Hypoxia-induced autophagy promotes gemcitabine resistance in human bladder cancer cells through hypoxia-inducible factor 1alpha activation. Int. J. Oncol. 2018, 53, 215–224. [Google Scholar] [CrossRef]
- Wang, J.; Dong, Z.; Sheng, Z.; Cai, Y. Hypoxia-induced pvt1 promotes lung cancer chemoresistance to cisplatin by autophagy via pvt1/mir-140-3p/atg5 axis. Cell Death Discov. 2022, 8, 104. [Google Scholar] [CrossRef]
- Huang, S.; Qi, P.; Zhang, T.; Li, F.; He, X. The hif-1alpha/mir-224-3p/atg5 axis affects cell mobility and chemosensitivity by regulating hypoxia-induced protective autophagy in glioblastoma and astrocytoma. Oncol. Rep. 2019, 41, 1759–1768. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Weng, L.; Jia, Y.; Liu, B.; Wu, S.; Xue, L.; Yin, X.; Mao, A.; Wang, Z.; Shang, M. Ptbp3 promotes malignancy and hypoxia-induced chemoresistance in pancreatic cancer cells by atg12 up-regulation. J. Cell. Mol. Med. 2020, 24, 2917–2930. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Dominguez, R.; Perez-Medina, M.; Lopez-Gonzalez, J.S.; Galicia-Velasco, M.; Aguilar-Cazares, D. The double-edge sword of autophagy in cancer: From tumor suppression to pro-tumor activity. Front. Oncol. 2020, 10, 578418. [Google Scholar] [CrossRef]
- Sannigrahi, M.; Singh, V.; Sharma, R.; Panda, N.; Khullar, M. Role of autophagy in head and neck cancer and therapeutic resistance. Oral Dis. 2015, 21, 283–291. [Google Scholar] [CrossRef]
- Sullivan, R.; Graham, C.H. Hypoxia prevents etoposide-induced DNA damage in cancer cells through a mechanism involving hypoxia-inducible factor 1. Mol. Cancer Ther. 2009, 8, 1702–1713. [Google Scholar] [CrossRef]
- Yan, Y.; He, M.; Zhao, L.; Wu, H.; Zhao, Y.; Han, L.; Wei, B.; Ye, D.; Lv, X.; Wang, Y.; et al. A novel hif-2alpha targeted inhibitor suppresses hypoxia-induced breast cancer stemness via sod2-mtros-pdi/gpr78-upr(er) axis. Cell Death Differ. 2022, 29, 1769–1789. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, F.; Han, L.; Zhao, L.; Chen, J.; I Olopade, O.; He, M.; Wei, M. Hif-2alpha promotes conversion to a stem cell phenotype and induces chemoresistance in breast cancer cells by activating wnt and notch pathways. J. Exp. Clin. Cancer Res. 2018, 37, 256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Tao, X.; Ji, B.; Gong, J. Hypoxia-driven m2-polarized macrophages facilitate cancer aggressiveness and temozolomide resistance in glioblastoma. Oxidative Med. Cell. Longev. 2022, 2022, 1614336. [Google Scholar] [CrossRef]
- Zheng, H.; Yu, S.; Zhu, C.; Guo, T.; Liu, F.; Xu, Y. Hif1alpha promotes tumor chemoresistance via recruiting gdf15-producing tams in colorectal cancer. Exp. Cell Res. 2021, 398, 112394. [Google Scholar] [CrossRef]
- Yu, S.; Li, Q.; Yu, Y.; Cui, Y.; Li, W.; Liu, T.; Liu, F. Activated hif1alpha of tumor cells promotes chemoresistance development via recruiting gdf15-producing tumor-associated macrophages in gastric cancer. Cancer Immunol. Immunother. 2020, 69, 1973–1987. [Google Scholar] [CrossRef]
- Eptaminitaki, G.C.; Stellas, D.; Bonavida, B.; Baritaki, S. Long non-coding rnas (lncrnas) signaling in cancer chemoresistance: From prediction to druggability. Drug Resist. Updat. 2022, 65, 100866. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Zhang, Q.; Guo, J.; Zhang, P.; Liu, H.; Tian, Z.-B.; Zhang, C.-P.; Li, X.-Y. The role of circular rnas in the drug resistance of cancers. Front. Oncol. 2021, 11, 790589. [Google Scholar] [CrossRef] [PubMed]
- Papatsirou, M.; I Artemaki, P.; Scorilas, A.; Kontos, C.K. The role of circular rnas in therapy resistance of patients with solid tumors. Pers. Med. 2020, 17, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Tan, H.-Y.; Chan, Y.-T.; Jiang, H.; Wang, N.; Wang, D. The functional role of long noncoding rna in resistance to anticancer treatment. Ther. Adv. Med. Oncol. 2020, 12, 1758835920927850. [Google Scholar] [CrossRef]
- Cui, C.; Yang, J.; Li, X.; Liu, D.; Fu, L.; Wang, X. Functions and mechanisms of circular rnas in cancer radiotherapy and chemotherapy resistance. Mol. Cancer 2020, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Pucci, P.; Rescigno, P.; Sumanasuriya, S.; de Bono, J.; Crea, F. Hypoxia and noncoding rnas in taxane resistance. Trends Pharmacol. Sci. 2018, 39, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Bakhshaei Shahrebabaki, P.; Fouladi, H.; Mansoori Derakhshan, S. The impact of micrornas on the resistance of breast cancer subtypes to chemotherapy. Pathol. Res. Pract. 2023, 249, 154702. [Google Scholar] [CrossRef] [PubMed]
- Funamizu, N.; Honjo, M.; Tamura, K.; Sakamoto, K.; Ogawa, K.; Takada, Y. Micrornas associated with gemcitabine resistance via emt, tme, and drug metabolism in pancreatic cancer. Cancers 2023, 15, 1230. [Google Scholar] [CrossRef]
- Doghish, A.S.; Ismail, A.; Elrebehy, M.A.; Elbadry, A.M.; Mahmoud, H.H.; Farouk, S.M.; Abu Serea, G.A.; Elghany, R.A.A.; El-Halwany, K.K.; Alsawah, A.O.; et al. A study of mirnas as cornerstone in lung cancer pathogenesis and therapeutic resistance: A focus on signaling pathways interplay. Pathol. Res. Pract. 2022, 237, 154053. [Google Scholar] [CrossRef]
- Karami Fath, M.; Azargoonjahromi, A.; Kiani, A.; Jalalifar, F.; Osati, P.; Akbari Oryani, M.; Shakeri, F.; Nasirzadeh, F.; Khalesi, B.; Nabi-Afjadi, M.; et al. The role of epigenetic modifications in drug resistance and treatment of breast cancer. Cell. Mol. Biol. Lett. 2022, 27, 52. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, Y.; Geng, X.; Zhang, Y.; Wen, X.; Yan, Q.; Wang, T.; Ling, C.; Xu, Y.; Duan, J.; et al. Ncrnas: Multi-angle participation in the regulation of glioma chemotherapy resistance (review). Int. J. Oncol. 2022, 60, 76. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Hashemi, F.; Moghadam, E.R.; Owrang, M.; Hashemi, F.; Makvandi, P.; Goharrizi, M.A.S.B.; Najafi, M.; et al. Lung cancer cells and their sensitivity/resistance to cisplatin chemotherapy: Role of micrornas and upstream mediators. Cell. Signal. 2021, 78, 109871. [Google Scholar] [CrossRef]
- Lin, Z.; Lu, S.; Xie, X.; Yi, X.; Huang, H. Noncoding rnas in drug-resistant pancreatic cancer: A review. Biomed. Pharmacother. 2020, 131, 110768. [Google Scholar] [CrossRef]
- Yete, S.; Saranath, D. Micrornas in oral cancer: Biomarkers with clinical potential. Oral Oncol. 2020, 110, 105002. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Hashemi, F.; Hashemi, F.; Samarghandian, S.; Najafi, M. Micrornas in cancer therapy: Their involvement in oxaliplatin sensitivity/resistance of cancer cells with a focus on colorectal cancer. Life Sci. 2020, 256, 117973. [Google Scholar] [CrossRef]
- Si, W.; Shen, J.; Zheng, H.; Fan, W. The role and mechanisms of action of micrornas in cancer drug resistance. Clin. Epigenetics 2019, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Cano, I.; Pattanayak, B.; Adam-Artigues, A.; Lameirinhas, A.; Torres-Ruiz, S.; Tormo, E.; Cervera, R.; Eroles, P. Micrornas as a clue to overcome breast cancer treatment resistance. Cancer Metastasis Rev. 2022, 41, 77–105. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Li, S.; Zhu, S.; Yi, M.; Luo, S.; Wu, K. Mirna-mediated emt and cscs in cancer chemoresistance. Exp. Hematol. Oncol. 2021, 10, 12. [Google Scholar] [CrossRef]
- Pan, G.; Liu, Y.; Shang, L.; Zhou, F.; Yang, S. Emt-associated micrornas and their roles in cancer stemness and drug resistance. Cancer Commun. 2021, 41, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Liang, W.; Liu, J.; Zhang, L.; Wei, J.; Yang, J.; Zhang, Y.; Huang, Z. Autophagy-mediating micrornas in cancer chemoresistance. Cell Biol. Toxicol. 2020, 36, 517–536. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, H.-Y.; Bai, S.-Y.; Pu, K.; Wang, Y.-P.; Zhou, Y.-N. The roles of micrornas in multidrug-resistance mechanisms in gastric cancer. Curr. Mol. Med. 2020, 20, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, C.; Zeng, J.; Zhao, Z.; Hu, Q. Microrna-210-3p is transcriptionally upregulated by hypoxia induction and thus promoting emt and chemoresistance in glioma cells. PLoS ONE 2021, 16, e0253522. [Google Scholar] [CrossRef] [PubMed]
- Jawad, S.F.; Altalbawy, F.M.A.; Hussein, R.M.; Fadhil, A.A.; Jawad, M.A.; Zabibah, R.S.; Taraki, T.Y.; Mohan, C.D.; Rangappa, K.S. The strict regulation of hif-1alpha by non-coding rnas: New insight towards proliferation, metastasis, and therapeutic resistance strategies. Cancer Metastasis Rev. 2024, 43, 5–27. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Sheng, L.; Qu, W.; Xue, X.; Chen, H.; Zheng, G.; Chen, W. Mir-194-5p enhances the sensitivity of nonsmall-cell lung cancer to doxorubicin through targeted inhibition of hypoxia-inducible factor-1. World J. Surg. Oncol. 2021, 19, 174. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Xia, X.; Wang, X.; Zhang, K.; Cao, J.; Jiang, T.; Zhao, Q.; Qiu, Z. Mir-301a plays a pivotal role in hypoxia-induced gemcitabine resistance in pancreatic cancer. Exp. Cell Res. 2018, 369, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Jasim, S.A.; Majeed, A.A.; Uinarni, H.; Alshuhri, M.; Alzahrani, A.A.; Ibrahim, A.A.; Alawadi, A.; Abed Al-Abadi, N.K.; Mustafa, Y.F.; Ahmed, B.A. Long non-coding rna (lncrna) pvt1 in drug resistance of cancers: Focus on pathological mechanisms. Pathol. Res. Pract. 2024, 254, 155119. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, M.; Deng, Y. Long noncoding rnas in taxane resistance of breast cancer. Int. J. Mol. Sci. 2023, 24, 12253. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, G.; Wang, X.; Wang, Y.; Wang, K. Functions and mechanisms of lncrna malat1 in cancer chemotherapy resistance. Biomark. Res. 2023, 11, 23. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, X.; Wang, Y.; Wang, K. Functions and underlying mechanisms of lncrna hotair in cancer chemotherapy resistance. Cell Death Discov. 2022, 8, 383. [Google Scholar] [CrossRef]
- Yao, W.; Li, S.; Liu, R.; Jiang, M.; Gao, L.; Lu, Y.; Liang, X.; Zhang, H. Long non-coding rna pvt1: A promising chemotherapy and radiotherapy sensitizer. Front. Oncol. 2022, 12, 959208. [Google Scholar] [CrossRef]
- Ashrafizaveh, S.; Ashrafizadeh, M.; Zarrabi, A.; Husmandi, K.; Zabolian, A.; Shahinozzaman; Aref, A.R.; Hamblin, M.R.; Nabavi, N.; Crea, F.; et al. Long non-coding rnas in the doxorubicin resistance of cancer cells. Cancer Lett. 2021, 508, 104–114. [Google Scholar] [CrossRef]
- Taheri, M.; Shoorei, H.; Anamag, F.T.; Ghafouri-Fard, S.; Dinger, M.E. Lncrnas and mirnas participate in determination of sensitivity of cancer cells to cisplatin. Exp. Mol. Pathol. 2021, 123, 104602. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guan, Z.; He, K.; Qian, J.; Cao, J.; Teng, L. Lncrna uca1 in anti-cancer drug resistance. Oncotarget 2017, 8, 64638–64650. [Google Scholar] [CrossRef]
- Jiao, B.; Liu, S.; Zhao, H.; Zhuang, Y.; Ma, S.; Lin, C.; Hu, J.; Liu, X. Hypoxia-responsive circrnas: A novel but important participant in non-coding rnas ushered toward tumor hypoxia. Cell Death Dis. 2022, 13, 666. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Cao, G.; Hua, J.; Shan, G.; Lin, W. Emerging roles of circular rnas in gastric cancer metastasis and drug resistance. J. Exp. Clin. Cancer Res. 2022, 41, 218. [Google Scholar] [CrossRef]
- Mu, Q.; Lv, Y.; Luo, C.; Liu, X.; Huang, C.; Xiu, Y.; Tang, L. Research progress on the functions and mechanism of circrna in cisplatin resistance in tumors. Front. Pharmacol. 2021, 12, 709324. [Google Scholar] [CrossRef]
- Ameli-Mojarad, M.; Ameli-Mojarad, M.; Hadizadeh, M.; Young, C.; Babini, H.; Nazemalhosseini-Mojarad, E.; Bonab, M.A. The effective function of circular rna in colorectal cancer. Cancer Cell Int. 2021, 21, 496. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Li, M.; Wu, J.; Qin, C.; Tao, Y.; He, H. Circular rna circnrip1 sponges microrna-138-5p to maintain hypoxia-induced resistance to 5-fluorouracil through hif-1alpha-dependent glucose metabolism in gastric carcinoma. Cancer Manag. Res. 2020, 12, 2789–2802. [Google Scholar] [CrossRef]
- Xu, M.; Liu, X.; Zhou, X.; Qin, Y.; Yang, L.; Wen, S.; Qiu, Y.; Chen, S.; Tang, R.; Guo, Y.; et al. Hypoxia-induced circstt3a enhances serine synthesis and promotes h3k4me3 modification to facilitate breast cancer stem cell formation. Pharmacol. Res. 2023, 197, 106964. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhao, Y.; Chen, Q.; Zhu, S.; Niu, Y.; Ye, Z.; Hu, P.; Chen, D.; Xu, P.; Chen, J.; et al. Hypoxic exosomal hif-1alpha-stabilizing circznf91 promotes chemoresistance of normoxic pancreatic cancer cells via enhancing glycolysis. Oncogene 2021, 40, 5505–5517. [Google Scholar] [CrossRef]
- Deng, K.; Zou, F.; Xu, J.; Xu, D.; Luo, Z. Cancer-associated fibroblasts promote stemness maintenance and gemcitabine resistance via hif-1alpha/mir-21 axis under hypoxic conditions in pancreatic cancer. Mol. Carcinog. 2024, 63, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Tam, S.Y.; Wu, V.W.C.; Law, H.K.W. Hypoxia-induced epithelial-mesenchymal transition in cancers: Hif-1alpha and beyond. Front. Oncol. 2020, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lou, Y.; Zhang, J.; Fu, Q.; Wei, T.; Sun, X.; Chen, Q.; Yang, J.; Bai, X.; Liang, T. Hypoxia-inducible factor-2alpha promotes tumor progression and has crosstalk with wnt/beta-catenin signaling in pancreatic cancer. Mol. Cancer 2017, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Kohnoh, T.; Hashimoto, N.; Ando, A.; Sakamoto, K.; Miyazaki, S.; Aoyama, D.; Kusunose, M.; Kimura, M.; Omote, N.; Imaizumi, K.; et al. Hypoxia-induced modulation of pten activity and emt phenotypes in lung cancers. Cancer Cell Int. 2016, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Tang, Y.-L.; Liang, X.-H. Emt: A new vision of hypoxia promoting cancer progression. Cancer Biol. Ther. 2011, 11, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, S.; Schuhwerk, H.; Brabletz, T.; Stemmler, M.P. Dynamic emt: A multi-tool for tumor progression. EMBO J. 2021, 40, e108647. [Google Scholar] [CrossRef] [PubMed]
- Gaponova, A.V.; Rodin, S.; Mazina, A.A.; Volchkov, P.V. Epithelial-mesenchymal transition: Role in cancer progression and the perspectives of antitumor treatment. Acta Naturae 2020, 12, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Tamma, R.; Annese, T. Epithelial-mesenchymal transition in cancer: A historical overview. Transl. Oncol. 2020, 13, 100773. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Arani, H.Z.; Orouei, S.; Fallah, S.; Ghorbani, A.; Khaledabadi, M.; Kakavand, A.; Tavakolpournegari, A.; Saebfar, H.; Heidari, H.; et al. Emt mechanism in breast cancer metastasis and drug resistance: Revisiting molecular interactions and biological functions. Biomed. Pharmacother. 2022, 155, 113774. [Google Scholar] [CrossRef]
- Palamaris, K.; Felekouras, E.; Sakellariou, S. Epithelial to mesenchymal transition: Key regulator of pancreatic ductal adenocarcinoma progression and chemoresistance. Cancers 2021, 13, 5532. [Google Scholar] [CrossRef]
- Sha, J.; Bai, Y.; Ngo, H.X.; Okui, T.; Kanno, T. Overview of evidence-based chemotherapy for oral cancer: Focus on drug resistance related to the epithelial-mesenchymal transition. Biomolecules 2021, 11, 893. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Mirzaei, S.; Hashemi, F.; Zarrabi, A.; Zabolian, A.; Saleki, H.; Sharifzadeh, S.O.; Soleymani, L.; Daneshi, S.; Hushmandi, K.; et al. New insight towards development of paclitaxel and docetaxel resistance in cancer cells: Emt as a novel molecular mechanism and therapeutic possibilities. Biomed. Pharmacother. 2021, 141, 111824. [Google Scholar] [CrossRef] [PubMed]
- De Las Rivas, J.; Brozovic, A.; Izraely, S.; Casas-Pais, A.; Witz, I.P.; Figueroa, A. Cancer drug resistance induced by emt: Novel therapeutic strategies. Arch. Toxicol. 2021, 95, 2279–2297. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hushmandi, K.; Kalantari, M.; Mohammadinejad, R.; Javaheri, T.; Sethi, G. Association of the epithelial-mesenchymal transition (emt) with cisplatin resistance. Int. J. Mol. Sci. 2020, 21, 4002. [Google Scholar] [CrossRef] [PubMed]
- Dudás, J.; Ladányi, A.; Ingruber, J.; Steinbichler, T.B.; Riechelmann, H. Epithelial to mesenchymal transition: A mechanism that fuels cancer radio/chemoresistance. Cells 2020, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Carstens, J.L.; Kim, J.; Scheible, M.; Kaye, J.; Sugimoto, H.; Wu, C.-C.; LeBleu, V.S.; Kalluri, R. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 2015, 527, 525–530. [Google Scholar] [CrossRef]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.C.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef]
- Debaugnies, M.; Rodríguez-Acebes, S.; Blondeau, J.; Parent, M.-A.; Zocco, M.; Song, Y.; de Maertelaer, V.; Moers, V.; Latil, M.; Dubois, C.; et al. Rhoj controls emt-associated resistance to chemotherapy. Nature 2023, 616, 168–175. [Google Scholar] [CrossRef]
- Petrella, G.; Corsi, F.; Ciufolini, G.; Germini, S.; Capradossi, F.; Pelliccia, A.; Torino, F.; Ghibelli, L.; Cicero, D.O. Metabolic reprogramming of castration-resistant prostate cancer cells as a response to chemotherapy. Metabolites 2022, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, Z.; Zhang, Z.; Zhao, C.; Li, J.; Jiang, J.; Huang, B.; Qin, Y. Puerarin inhibits emt induced by oxaliplatin via targeting carbonic anhydrase xii. Front. Pharmacol. 2022, 13, 969422. [Google Scholar] [CrossRef]
- Boulding, T.; McCuaig, R.D.; Tan, A.; Hardy, K.; Wu, F.; Dunn, J.; Kalimutho, M.; Sutton, C.R.; Forwood, J.K.; Bert, A.G.; et al. Lsd1 activation promotes inducible emt programs and modulates the tumour microenvironment in breast cancer. Sci. Rep. 2018, 8, 73. [Google Scholar] [CrossRef]
- Xie, S.; Fan, S.; Zhang, S.; Chen, W.; Li, Q.; Pan, G.; Zhang, H.; Wang, W.; Weng, B.; Zhang, Z.; et al. Sox8 regulates cancer stem-like properties and cisplatin-induced emt in tongue squamous cell carcinoma by acting on the wnt/beta-catenin pathway. Int. J. Cancer 2018, 142, 1252–1265. [Google Scholar] [CrossRef]
- Zheng, H.C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef]
- Li, P.; Zhang, X.; Wang, H.; Wang, L.; Liu, T.; Du, L.; Yang, Y.; Wang, C. Malat1 is associated with poor response to oxaliplatin-based chemotherapy in colorectal cancer patients and promotes chemoresistance through ezh2. Mol. Cancer Ther. 2017, 16, 739–751. [Google Scholar] [CrossRef]
- Li, Q.-Q.; Chen, Z.-Q.; Cao, X.-X.; Xu, J.-W.; Chen, Y.-Y.; Wang, W.-J.; Chen, Q.; Tang, F.; Liu, X.-P.; Xu, Z.-D. Involvement of nf-kappab/mir-448 regulatory feedback loop in chemotherapy-induced epithelial-mesenchymal transition of breast cancer cells. Cell Death Differ. 2011, 18, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, L.; Zhan, N.; Xu, P.; Yang, J.; Yuan, F.; Xu, Y.; Cai, Q.; Geng, R.; Chen, Q. Hypoxia induced ferritin light chain (ftl) promoted epithelia mesenchymal transition and chemoresistance of glioma. J. Exp. Clin. Cancer Res. 2020, 39, 137. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhou, S.; Yuan, W.; Cen, F.; Yan, Q. Mechanism of mir-210 involved in epithelial-mesenchymal transition of pancreatic cancer cells under hypoxia. J. Recept. Signal Transduct. Res. 2019, 39, 399–406. [Google Scholar] [CrossRef]
- Okumura, Y.; Noda, T.; Eguchi, H.; Sakamoto, T.; Iwagami, Y.; Yamada, D.; Asaoka, T.; Wada, H.; Kawamoto, K.; Gotoh, K.; et al. Hypoxia-induced plod2 is a key regulator in epithelial-mesenchymal transition and chemoresistance in biliary tract cancer. Ann. Surg. Oncol. 2018, 25, 3728–3737. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-E.; Zhuang, Y.-W.; Zhou, J.-Y.; Liu, S.-L.; Wang, R.-P.; Shu, P. Cinnamaldehyde enhances apoptotic effect of oxaliplatin and reverses epithelial-mesenchymal transition and stemnness in hypoxic colorectal cancer cells. Exp. Cell Res. 2019, 383, 111500. [Google Scholar] [CrossRef]
- Zhou, Q.-Y.; Tu, C.-Y.; Shao, C.-X.; Wang, W.-K.; Zhu, J.-D.; Cai, Y.; Mao, J.-Y.; Chen, W. Gc7 blocks epithelial-mesenchymal transition and reverses hypoxia-induced chemotherapy resistance in hepatocellular carcinoma cells. Am. J. Transl. Res. 2017, 9, 2608–2617. [Google Scholar]
- Adamski, J.; Price, A.; Dive, C.; Makin, G. Hypoxia-induced cytotoxic drug resistance in osteosarcoma is independent of hif-1alpha. PLoS ONE 2013, 8, e65304. [Google Scholar] [CrossRef] [PubMed]
- Cosse, J.-P.; Ronvaux, M.; Ninane, N.; Raes, M.J.; Michiels, C. Hypoxia-induced decrease in p53 protein level and increase in c-jun DNA binding activity results in cancer cell resistance to etoposide. Neoplasia 2009, 11, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Piret, J.-P.; Cosse, J.-P.; Ninane, N.; Raes, M.; Michiels, C. Hypoxia protects hepg2 cells against etoposide-induced apoptosis via a hif-1-independent pathway. Exp. Cell Res. 2006, 312, 2908–2920. [Google Scholar] [CrossRef] [PubMed]
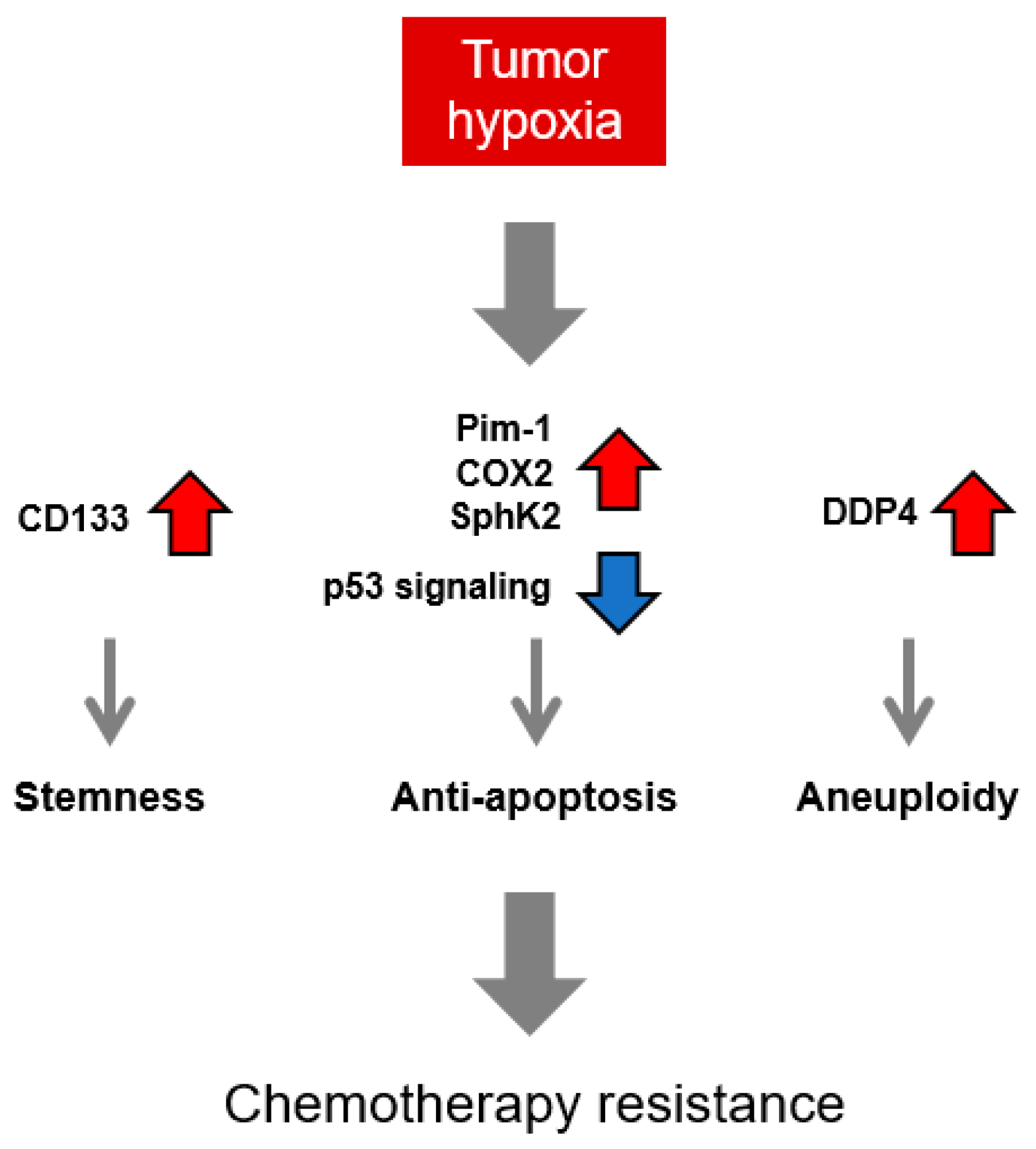
- Chen, J.; Kobayashi, M.; Darmanin, S.; Qiao, Y.; Gully, C.; Zhao, R.; Yeung, S.C.; Lee, M.H. Pim-1 plays a pivotal role in hypoxia-induced chemoresistance. Oncogene 2009, 28, 2581–2592. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, S.E.; Weigert, A.; Zhou, J.; Brüne, B. Hypoxia enhances sphingosine kinase 2 activity and provokes sphingosine-1-phosphate-mediated chemoresistance in a549 lung cancer cells. Mol. Cancer Res. 2009, 7, 393–401. [Google Scholar] [CrossRef]
- Schnitzer, S.E.; Schmid, T.; Zhou, J.; Brune, B. Hypoxia and hif-1alpha protect a549 cells from drug-induced apoptosis. Cell Death Differ. 2006, 13, 1611–1613. [Google Scholar] [CrossRef]
- Chen, X.; Liu, M.; Meng, F.; Sun, B.; Jin, X.; Jia, C. The long noncoding rna hif1a-as2 facilitates cisplatin resistance in bladder cancer. J. Cell Biochem. 2019, 120, 243–252. [Google Scholar] [CrossRef]
- Su, Y.; Yang, W.; Jiang, N.; Shi, J.; Chen, L.; Zhong, G.; Bi, J.; Dong, W.; Wang, Q.; Wang, C.; et al. Hypoxia-elevated circelp3 contributes to bladder cancer progression and cisplatin resistance. Int. J. Biol. Sci. 2019, 15, 441–452. [Google Scholar] [CrossRef]
- Huang, H.; Peng, J.; Yi, S.; Ding, C.; Ji, W.; Huang, Q.; Zeng, S. Circular rna circube2d2 functions as an oncogenic factor in hepatocellular carcinoma sorafenib resistance and glycolysis. Am. J. Transl. Res. 2021, 13, 6076–6086. [Google Scholar]
- Wang, H.; Min, J.; Xu, C.; Liu, Y.; Yu, Z.; Gong, A.; Xu, M. Hypoxia-elicited exosomes promote the chemoresistance of pancreatic cancer cells by transferring lncror via hippo signaling. J. Cancer 2023, 14, 1075–1087. [Google Scholar] [CrossRef]
- Tao, S.; Wang, J.; Li, F.; Shi, B.; Ren, Q.; Zhuang, Y.; Qian, X. Extracellular vesicles released by hypoxia-induced tumor-associated fibroblasts impart chemoresistance to breast cancer cells via long noncoding rna h19 delivery. FASEB J. 2024, 38, e23165. [Google Scholar] [CrossRef] [PubMed]
- Musah-Eroje, A.; Watson, S. A novel 3d in vitro model of glioblastoma reveals resistance to temozolomide which was potentiated by hypoxia. J. Neurooncol 2019, 142, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.M.; Bandopadhyay, G.; Coyle, B.; Grabowska, A. A hif-independent, cd133-mediated mechanism of cisplatin resistance in glioblastoma cells. Cell. Oncol. 2018, 41, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Mao, Q.; Zhang, Y.; Fu, X.; Xue, J.; Guo, W.; Meng, M.; Zhou, Z.; Mo, X.; Lu, Y. A tumor hypoxic niche protects human colon cancer stem cells from chemotherapy. J. Cancer Res. Clin. Oncol. 2013, 139, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, S.; Tanaka, K.; Toiyama, Y.; Yokoe, T.; Okugawa, Y.; Koike, Y.; Fujikawa, H.; Inoue, Y.; Miki, C.; Kusunoki, M. Clinical significance of cd133 and hypoxia inducible factor-1alpha gene expression in rectal cancer after preoperative chemoradiotherapy. Clin. Oncol. 2011, 23, 323–332. [Google Scholar] [CrossRef]
- Lu, C.; Mahajan, A.; Hong, S.-H.; Galli, S.; Zhu, S.; Tilan, J.U.; Abualsaud, N.; Adnani, M.; Chung, S.; Elmansy, N.; et al. Hypoxia-activated neuropeptide y/y5 receptor/rhoa pathway triggers chromosomal instability and bone metastasis in ewing sarcoma. Nat. Commun. 2022, 13, 2323. [Google Scholar] [CrossRef] [PubMed]
- Moffitt, L.R.; Bilandzic, M.; Wilson, A.L.; Chen, Y.; Gorrell, M.D.; Oehler, M.K.; Plebanski, M.; Stephens, A.N. Hypoxia regulates dpp4 expression, proteolytic inactivation, and shedding from ovarian cancer cells. Int. J. Mol. Sci. 2020, 21, 8110. [Google Scholar] [CrossRef] [PubMed]
- Tilan, J.U.; Lu, C.; Galli, S.; Izycka-Swieszewska, E.; Earnest, J.P.; Shabbir, A.; Everhart, L.M.; Wang, S.; Martin, S.; Horton, M.; et al. Hypoxia shifts activity of neuropeptide y in ewing sarcoma from growth-inhibitory to growth-promoting effects. Oncotarget 2013, 4, 2487–2501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.-Y.; Zhuang, L.-H.; Hu, Y.; Zhou, Y.-L.; Lin, W.-K.; Wang, D.-D.; Wan, Z.-Q.; Chang, L.-L.; Chen, Y.; Ying, M.-D.; et al. Inactivation of hypoxia-induced yap by statins overcomes hypoxic resistance tosorafenib in hepatocellular carcinoma cells. Sci. Rep. 2016, 6, 30483. [Google Scholar] [CrossRef]
- Lang, L. Fda approves sorafenib for patients with inoperable liver cancer. Gastroenterology 2008, 134, 379. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Q.; Ye, T.; Liu, Y.; Liu, D.; Song, S.; Zheng, C. Nrf2/abcb1-mediated efflux and parp1-mediated dampening of DNA damage contribute to doxorubicin resistance in chronic hypoxic hepg2 cells. Fundam. Clin. Pharmacol. 2020, 34, 41–50. [Google Scholar] [CrossRef]
- Syu, J.-P.; Chi, J.-T.; Kung, H.-N. Nrf2 is the key to chemotherapy resistance in mcf7 breast cancer cells under hypoxia. Oncotarget 2016, 7, 14659–14672. [Google Scholar] [CrossRef]
- Li, T.; Fu, X.; Wang, J.; Shang, W.; Wang, X.; Zhang, L.; Li, J. Mechanism of nurp1 in temozolomide resistance in hypoxia-treated glioma cells via the kdm3a/tfeb axis. Oncol. Res. 2023, 31, 345–359. [Google Scholar] [CrossRef]
- Koritzinsky, M.; Levitin, F.; van den Beucken, T.; Rumantir, R.A.; Harding, N.J.; Chu, K.C.; Boutros, P.C.; Braakman, I.; Wouters, B.G. Two phases of disulfide bond formation have differing requirements for oxygen. J. Cell Biol. 2013, 203, 615–627. [Google Scholar] [CrossRef]
- Bartoszewska, S.; Collawn, J.F.; Bartoszewski, R. The role of the hypoxia-related unfolded protein response (upr) in the tumor microenvironment. Cancers 2022, 14, 4870. [Google Scholar] [CrossRef]
- Chipurupalli, S.; Kannan, E.; Tergaonkar, V.; D’andrea, R.; Robinson, N. Hypoxia induced er stress response as an adaptive mechanism in cancer. Int. J. Mol. Sci. 2019, 20, 749. [Google Scholar] [CrossRef]
- Avril, T.; Vauléon, E.; Chevet, E. Endoplasmic reticulum stress signaling and chemotherapy resistance in solid cancers. Oncogenesis 2017, 6, e373. [Google Scholar] [CrossRef]
- Benedetti, R.; Romeo, M.A.; Arena, A.; Montani, M.S.G.; Di Renzo, L.; D’orazi, G.; Cirone, M. Atf6 prevents DNA damage and cell death in colon cancer cells undergoing er stress. Cell Death Discov. 2022, 8, 295. [Google Scholar] [CrossRef]
- Cho, J.; Min, H.-Y.; Pei, H.; Wei, X.; Sim, J.Y.; Park, S.-H.; Hwang, S.J.; Lee, H.-J.; Hong, S.; Shin, Y.K.; et al. The atf6-egf pathway mediates the awakening of slow-cycling chemoresistant cells and tumor recurrence by stimulating tumor angiogenesis. Cancers 2020, 12, 1772. [Google Scholar] [CrossRef] [PubMed]
- Akman, M.; Belisario, D.C.; Salaroglio, I.C.; Kopecka, J.; Donadelli, M.; De Smaele, E.; Riganti, C. Hypoxia, endoplasmic reticulum stress and chemoresistance: Dangerous liaisons. J. Exp. Clin. Cancer Res. 2021, 40, 28. [Google Scholar] [CrossRef] [PubMed]
- Pi, L.; Li, X.; Song, Q.; Shen, Y.; Lu, X.; DI, B. Knockdown of glucose-regulated protein 78 abrogates chemoresistance of hypopharyngeal carcinoma cells to cisplatin induced by unfolded protein in response to severe hypoxia. Oncol. Lett. 2014, 7, 685–692. [Google Scholar] [CrossRef]
- Lee, D.; Sun, S.; Ho, A.S.; Kiang, K.M.; Zhang, X.Q.; Xu, F.F.; Leung, G.K. Hyperoxia resensitizes chemoresistant glioblastoma cells to temozolomide through unfolded protein response. Anticancer. Res. 2014, 34, 2957–2966. [Google Scholar]
- Bouznad, N.; Rokavec, M.; Öner, M.G.; Hermeking, H. Mir-34a and ire1a/xbp-1(s) form a double-negative feedback loop to regulate hypoxia-induced emt, metastasis, chemo-resistance and autophagy. Cancers 2023, 15, 1143. [Google Scholar] [CrossRef]
- Moszyńska, A.; Collawn, J.F.; Bartoszewski, R. Ire1 endoribonuclease activity modulates hypoxic hif-1alpha signaling in human endothelial cells. Biomolecules 2020, 10, 895. [Google Scholar] [CrossRef]
- Lee, J.H.; Yoon, Y.M.; Lee, S.H. Hypoxic preconditioning promotes the bioactivities of mesenchymal stem cells via the hif-1alpha-grp78-akt axis. Int. J. Mol. Sci. 2017, 18, 1320. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Newton, I.P.; Zhang, L.; Ji, P.; Li, Z. Grp78 is implicated in the modulation of tumor aerobic glycolysis by promoting autophagic degradation of ikkbeta. Cell. Signal. 2015, 27, 1237–1245. [Google Scholar] [CrossRef]
- Chen, X.; Iliopoulos, D.; Zhang, Q.; Tang, Q.; Greenblatt, M.B.; Hatziapostolou, M.; Lim, E.; Tam, W.L.; Ni, M.; Chen, Y.; et al. Xbp1 promotes triple-negative breast cancer by controlling the hif1alpha pathway. Nature 2014, 508, 103–107. [Google Scholar] [CrossRef]
- Miharada, K.; Karlsson, G.; Rehn, M.; Rörby, E.; Siva, K.; Cammenga, J.; Karlsson, S. Hematopoietic stem cells are regulated by cripto, as an intermediary of hif-1alpha in the hypoxic bone marrow niche. Ann. N. Y. Acad. Sci. 2012, 1266, 55–62. [Google Scholar] [CrossRef]
- Kang, M.J.; Jung, S.M.; Kim, M.J.; Bae, J.H.; Kim, H.B.; Kim, J.Y.; Park, S.J.; Song, H.S.; Kim, D.W.; Kang, C.D.; et al. DNA-dependent protein kinase is involved in heat shock protein-mediated accumulation of hypoxia-inducible factor-1alpha in hypoxic preconditioned hepg2 cells. FEBS J. 2008, 275, 5969–5981. [Google Scholar] [CrossRef] [PubMed]
- Mivechi, N.F.; Koong, A.C.; Giaccia, A.J.; Hahn, G.M. Analysis of hsf-1 phosphorylation in a549 cells treated with a variety of stresses. Int. J. Hyperth. 1994, 10, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Cyran, A.M.; Zhitkovich, A. Heat shock proteins and hsf1 in cancer. Front. Oncol. 2022, 12, 860320. [Google Scholar] [CrossRef]
- Landriscina, M.; Maddalena, F.; Laudiero, G.; Esposito, F. Adaptation to oxidative stress, chemoresistance, and cell survival. Antioxid. Redox Signal 2009, 11, 2701–2716. [Google Scholar] [CrossRef]
- Vydra, N.; Toma, A.; Glowala-Kosinska, M.; Gogler-Piglowska, A.; Widlak, W. Overexpression of heat shock transcription factor 1 enhances the resistance of melanoma cells to doxorubicin and paclitaxel. BMC Cancer 2013, 13, 504. [Google Scholar] [CrossRef]
- Vilaboa, N.E.; Galán, A.; Troyano, A.; de Blas, E.; Aller, P. Regulation of multidrug resistance 1 (mdr1)/p-glycoprotein gene expression and activity by heat-shock transcription factor 1 (hsf1). J. Biol. Chem. 2000, 275, 24970–24976. [Google Scholar] [CrossRef]
- Yoo, H.J.; Im, C.-N.; Youn, D.-Y.; Yun, H.H.; Lee, J.-H. Bis is induced by oxidative stress via activation of hsf1. Korean J. Physiol. Pharmacol. 2014, 18, 403–409. [Google Scholar] [CrossRef]
- Shanker, M.; Willcutts, D.; Roth, J.A.; Ramesh, R. Drug resistance in lung cancer. Lung Cancer 2010, 1, 23–36. [Google Scholar]
- Banerjee Mustafi, S.; Chakraborty, P.K.; Dey, R.S.; Raha, S. Heat stress upregulates chaperone heat shock protein 70 and antioxidant manganese superoxide dismutase through reactive oxygen species (ros), p38mapk, and akt. Cell Stress Chaperones 2009, 14, 579–589. [Google Scholar] [CrossRef]
- Yoo, H.Y.; Chang, M.S.; Rho, H.M. The activation of the rat copper/zinc superoxide dismutase gene by hydrogen peroxide through the hydrogen peroxide-responsive element and by paraquat and heat shock through the same heat shock element. J. Biol. Chem. 1999, 274, 23887–23892. [Google Scholar] [CrossRef]
- Egger, G.; Liang, G.; Aparicio, A.; Jones, P.A. Epigenetics in human disease and prospects for epigenetic therapy. Nature 2004, 429, 457–463. [Google Scholar] [CrossRef]
- Marmorstein, R.; Zhou, M.-M. Writers and readers of histone acetylation: Structure, mechanism, and inhibition. Cold Spring Harb. Perspect. Biol. 2014, 6, a018762. [Google Scholar] [CrossRef]
- Kim, M.S.; Kwon, H.J.; Lee, Y.M.; Baek, J.H.; Jang, J.-E.; Lee, S.-W.; Moon, E.-J.; Kim, H.-S.; Lee, S.-K.; Chung, H.Y.; et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat. Med. 2001, 7, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Pluemsampant, S.; Safronova, O.S.; Nakahama, K.; Morita, I. Protein kinase ck2 is a key activator of histone deacetylase in hypoxia-associated tumors. Int. J. Cancer 2008, 122, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Kirmes, I.; Szczurek, A.; Prakash, K.; Charapitsa, I.; Heiser, C.; Musheev, M.; Schock, F.; Fornalczyk, K.; Ma, D.; Birk, U.; et al. A transient ischemic environment induces reversible compaction of chromatin. Genome Biol. 2015, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- Leszczynska, K.B.; Dzwigonska, M.; Estephan, H.; Moehlenbrink, J.; Bowler, E.; Giaccia, A.J.; Mieczkowski, J.; Kaminska, B.; Hammond, E.M. Hypoxia-mediated regulation of ddx5 through decreased chromatin accessibility and post-translational targeting restricts r-loop accumulation. Mol. Oncol. 2023, 17, 1173–1191. [Google Scholar] [CrossRef] [PubMed]
- Gujral, P.; Mahajan, V.; Lissaman, A.C.; Ponnampalam, A.P. Histone acetylation and the role of histone deacetylases in normal cyclic endometrium. Reprod. Biol. Endocrinol. 2020, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-Q.; Pan, B.-Z.; Huang, J.-Y.; Zhang, K.; Cui, S.-Y.; De, W.; Wang, R.; Chen, L.-B. Hdac 1/4-mediated silencing of microrna-200b promotes chemoresistance in human lung adenocarcinoma cells. Oncotarget 2014, 5, 3333–3349. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Langdon, S.P.; Tse, M.; Mullen, P.; Um, I.H.; Faratian, D.; Harrison, D.J. The role of hdac2 in chromatin remodelling and response to chemotherapy in ovarian cancer. Oncotarget 2016, 7, 4695–4711. [Google Scholar] [CrossRef] [PubMed]
- Taddei, A.; Roche, D.; Bickmore, W.A.; Almouzni, G. The effects of histone deacetylase inhibitors on heterochromatin: Implications for anticancer therapy? EMBO Rep. 2005, 6, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, S.A.; Sahu, K.K.; Sengupta, S.; Partap, S.; Karpoormath, R.; Kumar, B.; Kumar, D. Recent advancement of hdac inhibitors against breast cancer. Med. Oncol. 2023, 40, 201. [Google Scholar] [CrossRef]
- Falkenberg, K.J.; Johnstone, R.W. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat. Rev. Drug Discov. 2014, 13, 673–691. [Google Scholar] [CrossRef]
- Hu, Z.; Wei, F.; Su, Y.; Wang, Y.; Shen, Y.; Fang, Y.; Ding, J.; Chen, Y. Histone deacetylase inhibitors promote breast cancer metastasis by elevating nedd9 expression. Signal Transduct. Target. Ther. 2023, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Quinn, D.I.; Tsao-Wei, D.D.; Twardowski, P.; Aparicio, A.M.; Frankel, P.; Chatta, G.; Wright, J.J.; Groshen, S.G.; Khoo, S.; Lenz, H.-J.; et al. Phase ii study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid; saha) in recurrent or metastatic transitional cell carcinoma of the urothelium—An nci-ctep sponsored: California cancer consortium trial, nci 6879. Investig. New Drugs 2021, 39, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Filippakopoulos, P. Functions of bromodomain-containing proteins and their roles in homeostasis and cancer. Nat. Rev. Mol. Cell Biol. 2017, 18, 246–262. [Google Scholar] [CrossRef]
- Filippakopoulos, P.; Picaud, S.; Mangos, M.; Keates, T.; Lambert, J.-P.; Barsyte-Lovejoy, D.; Felletar, I.; Volkmer, R.; Müller, S.; Pawson, T.; et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell 2012, 149, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Zhao, Y.; Wang, X.; Xie, X.; Liu, M.; Zhang, K.; Wang, L.; Bai, D.; Foster, L.J.; Shu, R.; et al. Targeting bromodomain-containing proteins: Research advances of drug discovery. Mol. Biomed. 2023, 4, 13. [Google Scholar] [CrossRef]
- Zaware, N.; Zhou, M.-M. Bromodomain biology and drug discovery. Nat. Struct. Mol. Biol. 2019, 26, 870–879. [Google Scholar] [CrossRef]
- Muller, S.; Filippakopoulos, P.; Knapp, S. Bromodomains as therapeutic targets. Expert. Rev. Mol. Med. 2011, 13, e29. [Google Scholar] [CrossRef] [PubMed]
- Gajjela, B.K.; Zhou, M.-M. Bromodomain inhibitors and therapeutic applications. Curr. Opin. Chem. Biol. 2023, 75, 102323. [Google Scholar] [CrossRef] [PubMed]
- Lazarchuk, P.; Hernandez-Villanueva, J.; Pavlova, M.N.; Federation, A.; MacCoss, M.; Sidorova, J.M. Mutual balance of histone deacetylases 1 and 2 and the acetyl reader atad2 regulates the level of acetylation of histone h4 on nascent chromatin of human cells. Mol. Cell. Biol. 2020, 40, e00421-19. [Google Scholar] [CrossRef]
- Koo, S.J.; Fernández-Montalván, A.E.; Badock, V.; Ott, C.J.; Holton, S.J.; von Ahsen, O.; Toedling, J.; Vittori, S.; Bradner, J.E.; Gorjánácz, M. Atad2 is an epigenetic reader of newly synthesized histone marks during DNA replication. Oncotarget 2016, 7, 70323–70335. [Google Scholar] [CrossRef]
- Revenko, A.S.; Kalashnikova, E.V.; Gemo, A.T.; Zou, J.X.; Chen, H.-W. Chromatin loading of e2f-mll complex by cancer-associated coregulator ancca via reading a specific histone mark. Mol. Cell. Biol. 2010, 30, 5260–5272. [Google Scholar] [CrossRef]
- Ciró, M.; Prosperini, E.; Quarto, M.; Grazini, U.; Walfridsson, J.; McBlane, F.; Nucifero, P.; Pacchiana, G.; Capra, M.; Christensen, J.; et al. Atad2 is a novel cofactor for myc, overexpressed and amplified in aggressive tumors. Cancer Res. 2009, 69, 8491–8498. [Google Scholar] [CrossRef]
- Losman, J.-A.; Koivunen, P.; Kaelin, W.G., Jr. 2-oxoglutarate-dependent dioxygenases in cancer. Nat. Rev. Cancer 2020, 20, 710–726. [Google Scholar] [CrossRef]
- Kao, T.-W.; Bai, G.-H.; Wang, T.-L.; Shih, I.-M.; Chuang, C.-M.; Lo, C.-L.; Tsai, M.-C.; Chiu, L.-Y.; Lin, C.-C.; Shen, Y.-A. Novel cancer treatment paradigm targeting hypoxia-induced factor in conjunction with current therapies to overcome resistance. J. Exp. Clin. Cancer Res. 2023, 42, 171. [Google Scholar] [CrossRef]
- Meehan, R.; Kummar, S.; Do, K.; Coyne, G.O.; Juwara, L.; Zlott, J.; Rubinstein, L.; Doroshow, J.H.; Chen, A.P. A phase i study of ganetespib and ziv-aflibercept in patients with advanced carcinomas and sarcomas. Oncologist 2018, 23, 1269-e125. [Google Scholar] [CrossRef]
- Kim, A.; Lu, Y.; Okuno, S.H.; Reinke, D.; Maertens, O.; Perentesis, J.; Basu, M.; Wolters, P.L.; De Raedt, T.; Chawla, S.; et al. Targeting refractory sarcomas and malignant peripheral nerve sheath tumors in a phase i/ii study of sirolimus in combination with ganetespib (sarc023). Sarcoma 2020, 2020, 5784876. [Google Scholar] [CrossRef]
- Ramalingam, S.; Goss, G.; Rosell, R.; Schmid-Bindert, G.; Zaric, B.; Andric, Z.; Bondarenko, I.; Komov, D.; Ceric, T.; Khuri, F.; et al. A randomized phase ii study of ganetespib, a heat shock protein 90 inhibitor, in combination with docetaxel in second-line therapy of advanced non-small cell lung cancer (galaxy-1). Ann. Oncol. 2015, 26, 1741–1748. [Google Scholar] [CrossRef]
- Fallah, J.; Brave, M.H.; Weinstock, C.; Mehta, G.U.; Bradford, D.; Gittleman, H.; Bloomquist, E.W.; Charlab, R.; Hamed, S.S.; Miller, C.P.; et al. Fda approval summary: Belzutifan for von hippel-lindau disease-associated tumors. Clin. Cancer Res. 2022, 28, 4843–4848. [Google Scholar] [CrossRef]
- Strowd, R.; Ellingson, B.; Raymond, C.; Yao, J.; Wen, P.Y.; Ahluwalia, M.; Piotrowski, A.; Desai, A.; Clarke, J.L.; Lieberman, F.S.; et al. Activity of a first-in-class oral hif2-alpha inhibitor, pt2385, in patients with first recurrence of glioblastoma. J. Neuro-Oncol. 2023, 165, 101–112. [Google Scholar] [CrossRef]
- Xu, P.; Wu, Q.; Yu, J.; Rao, Y.; Kou, Z.; Fang, G.; Shi, X.; Liu, W.; Han, H. A systematic way to infer the regulation relations of mirnas on target genes and critical mirnas in cancers. Front. Genet. 2020, 11, 278. [Google Scholar] [CrossRef]
- He, Z.; Zhu, Q. Circular rnas: Emerging roles and new insights in human cancers. Biomed. Pharmacother. 2023, 165, 115217. [Google Scholar] [CrossRef]
- Brenner, A.J.; Floyd, J.; Fichtel, L.; Michalek, J.; Kanakia, K.P.; Huang, S.; Reardon, D.; Wen, P.Y.; Lee, E.Q. Phase 2 trial of hypoxia activated evofosfamide (th302) for treatment of recurrent bevacizumab-refractory glioblastoma. Sci. Rep. 2021, 11, 2306. [Google Scholar] [CrossRef]
- García-Venzor, A.; Mandujano-Tinoco, E.A.; Ruiz-Silvestre, A.; Sánchez, J.M.; Lizarraga, F.; Zampedri, C.; Melendez-Zajgla, J.; Maldonado, V. Lncmat2b regulated by severe hypoxia induces cisplatin resistance by increasing DNA damage repair and tumor-initiating population in breast cancer cells. Carcinogenesis 2020, 41, 1485–1497. [Google Scholar] [CrossRef]
- Yang, H.; Hu, Y.; Weng, M.; Liu, X.; Wan, P.; Hu, Y.; Ma, M.; Zhang, Y.; Xia, H.; Lv, K. Hypoxia inducible lncrna-cbslr modulates ferroptosis through m6a-ythdf2-dependent modulation of cbs in gastric cancer. J. Adv. Res. 2022, 37, 91–106. [Google Scholar] [CrossRef]
- Yin, X.; Liao, Y.; Xiong, W.; Zhang, Y.; Zhou, Y.; Yang, Y. Hypoxia-induced lncrna anril promotes cisplatin resistance in retinoblastoma cells through regulating abcg2 expression. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1049–1057. [Google Scholar] [CrossRef]
- Huan, L.; Guo, T.; Wu, Y.; Xu, L.; Huang, S.; Xu, Y.; Liang, L.; He, X. Hypoxia induced lucat1/ptbp1 axis modulates cancer cell viability and chemotherapy response. Mol. Cancer 2020, 19, 11. [Google Scholar] [CrossRef]
- Moreno Leon, L.; Gautier, M.; Allan, R.; Ilié, M.; Nottet, N.; Pons, N.; Paquet, A.; Lebrigand, K.; Truchi, M.; Fassy, J.; et al. The nuclear hypoxia-regulated nlucat1 long non-coding rna contributes to an aggressive phenotype in lung adenocarcinoma through regulation of oxidative stress. Oncogene 2019, 38, 7146–7165. [Google Scholar] [CrossRef]
- Wang, F.; Ji, X.; Wang, J.; Ma, X.; Yang, Y.; Zuo, J.; Cui, J. Lncrna pvt1 enhances proliferation and cisplatin resistance via regulating mir-194-5p/hif1a axis in oral squamous cell carcinoma. Onco Targets Ther. 2020, 13, 243–252. [Google Scholar] [CrossRef]
- Xu, F.; Huang, M.; Chen, Q.; Niu, Y.; Hu, Y.; Hu, P.; Chen, D.; He, C.; Huang, K.; Zeng, Z.; et al. Lncrna hif1a-as1 promotes gemcitabine resistance of pancreatic cancer by enhancing glycolysis through modulating the akt/yb1/hif1alpha pathway. Cancer Res. 2021, 81, 5678–5691. [Google Scholar] [CrossRef]
- Güçlü, E.; Güneş, C.E.; Kurar, E.; Vural, H. Knockdown of lncrna hif1a-as2 increases drug sensitivity of sclc cells in association with autophagy. Med. Oncol. 2021, 38, 113. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.; Zhang, Y.; Xiao, X.; Chu, F. Induction of lncrna norad accounts for hypoxia-induced chemoresistance and vasculogenic mimicry in colorectal cancer by sponging the mir-495-3p/ hypoxia-inducible factor-1alpha (hif-1alpha). Bioengineered 2022, 13, 950–962. [Google Scholar] [CrossRef]
- Weng, X.; Liu, H.; Ruan, J.; Du, M.; Wang, L.; Mao, J.; Cai, Y.; Lu, X.; Chen, W.; Huang, Y.; et al. Hotair/mir-1277-5p/zeb1 axis mediates hypoxia-induced oxaliplatin resistance via regulating epithelial-mesenchymal transition in colorectal cancer. Cell Death Discov. 2022, 8, 310. [Google Scholar] [CrossRef]
- Zhu, Z.-J.; Pang, Y.; Jin, G.; Zhang, H.-Y.; Wang, W.-H.; Liu, J.-W.; Tuo, G.-X.; Wu, P.; Yang, Y.; Wang, Z.-Q.; et al. Hypoxia induces chemoresistance of esophageal cancer cells to cisplatin through regulating the lncrna-ems/mir-758-3p/wtap axis. Aging 2021, 13, 17155–17176. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, P.W.T.; Koseki, L.R.; Haitani, T.; Harada, H.; Kobayashi, M. Hypoxia-Inducible Factor-Dependent and Independent Mechanisms Underlying Chemoresistance of Hypoxic Cancer Cells. Cancers 2024, 16, 1729. https://doi.org/10.3390/cancers16091729
Lee PWT, Koseki LR, Haitani T, Harada H, Kobayashi M. Hypoxia-Inducible Factor-Dependent and Independent Mechanisms Underlying Chemoresistance of Hypoxic Cancer Cells. Cancers. 2024; 16(9):1729. https://doi.org/10.3390/cancers16091729
Chicago/Turabian StyleLee, Peter Wai Tik, Lina Rochelle Koseki, Takao Haitani, Hiroshi Harada, and Minoru Kobayashi. 2024. "Hypoxia-Inducible Factor-Dependent and Independent Mechanisms Underlying Chemoresistance of Hypoxic Cancer Cells" Cancers 16, no. 9: 1729. https://doi.org/10.3390/cancers16091729
APA StyleLee, P. W. T., Koseki, L. R., Haitani, T., Harada, H., & Kobayashi, M. (2024). Hypoxia-Inducible Factor-Dependent and Independent Mechanisms Underlying Chemoresistance of Hypoxic Cancer Cells. Cancers, 16(9), 1729. https://doi.org/10.3390/cancers16091729






