The Association between Blood Test Trends and Undiagnosed Cancer: A Systematic Review and Critical Appraisal
Abstract
Simple Summary
Abstract
1. Background
2. Methods
2.1. Participants
2.2. Outcome
2.3. Search Strategy
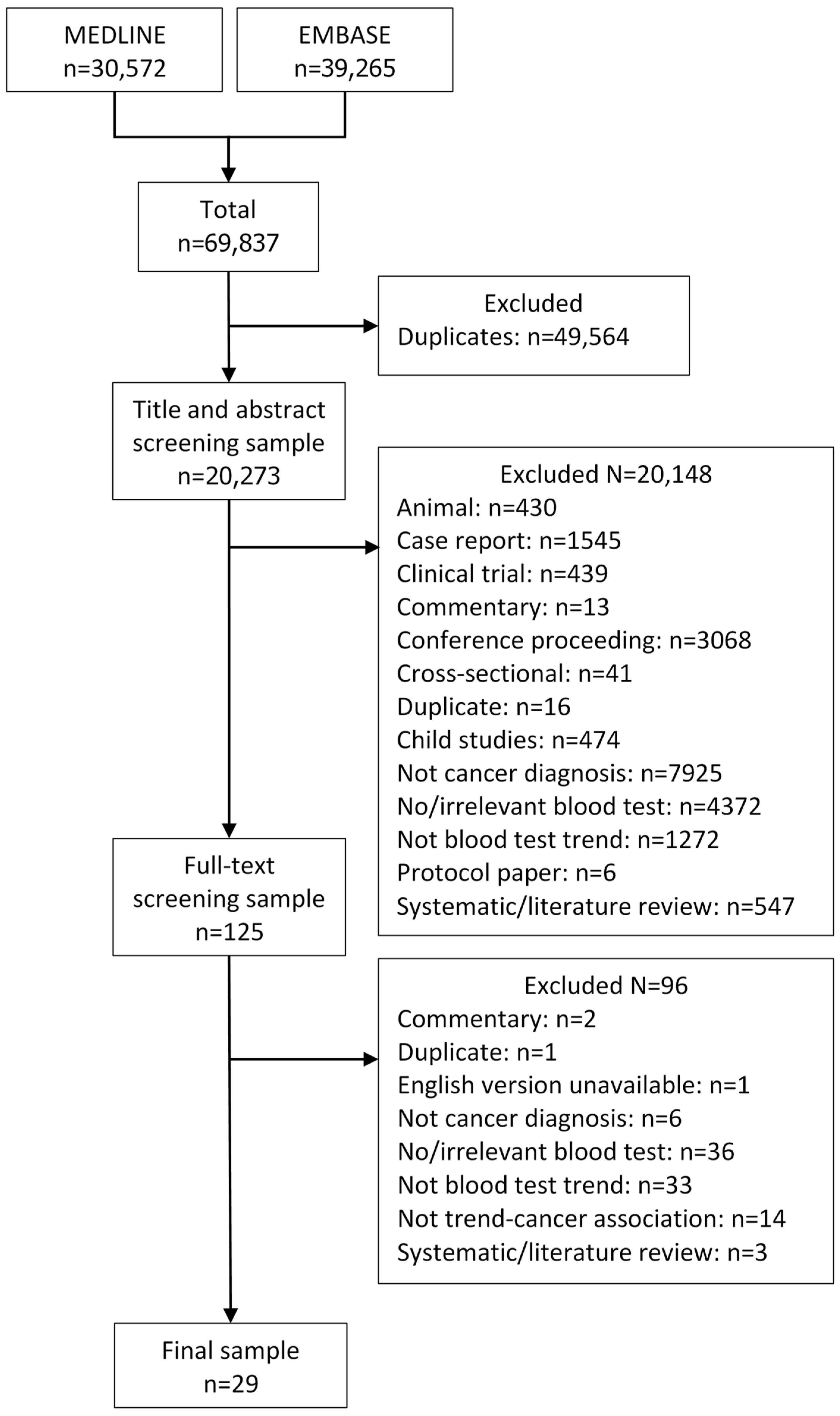
2.4. Study Selection
2.5. Data Extraction
2.6. Data Analysis and Synthesis
2.7. Risk of Bias Assessment
3. Results
3.1. Description of Studies
3.1.1. Study Design
3.1.2. Participants and Setting
3.2. Modelling Blood Test Trend
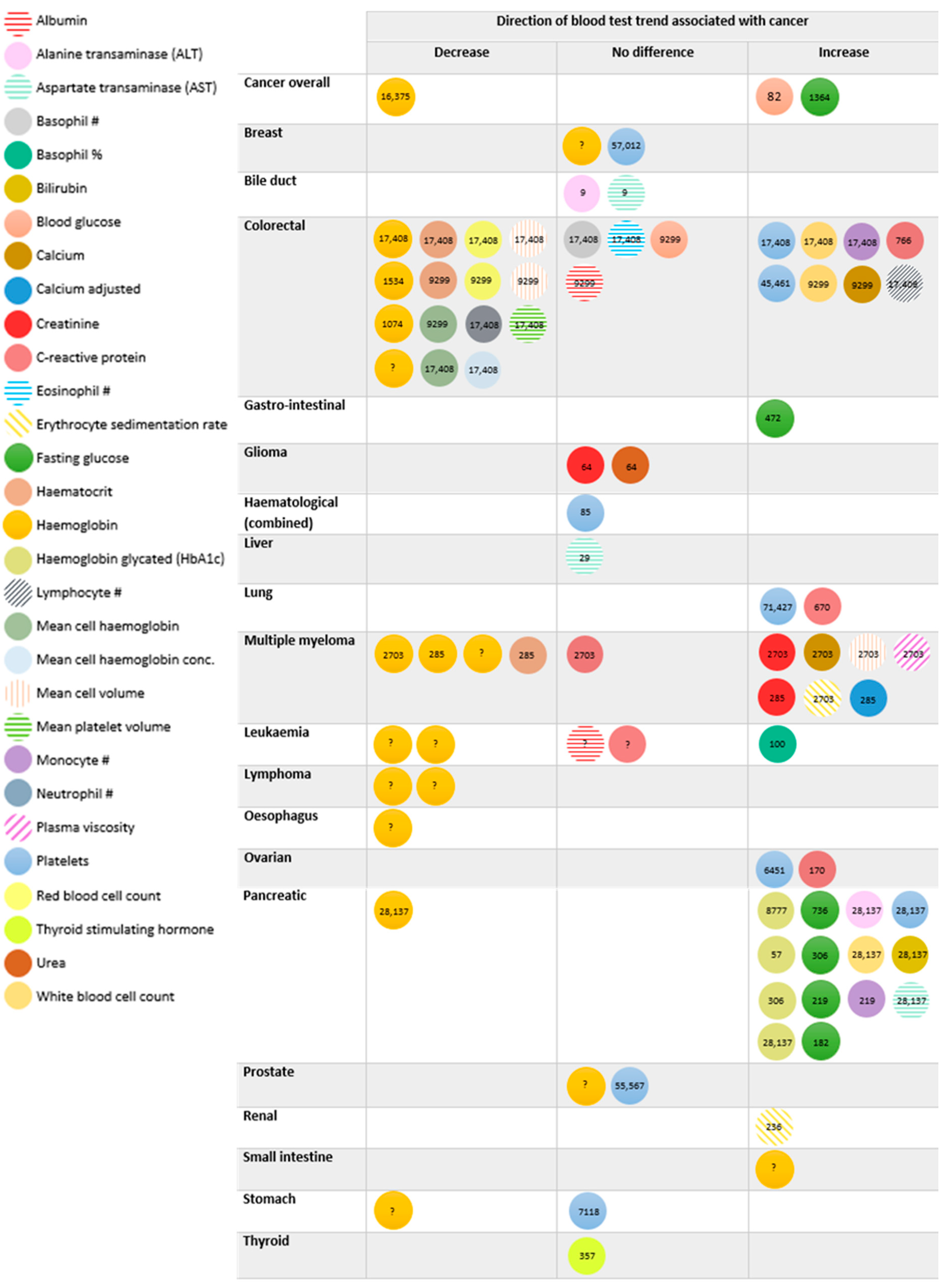
3.3. Cancers Associated with Blood Test Trends
3.4. Cancer Staging and Blood Test Trend
3.5. Blood Test Trend and Abnormality Thresholds
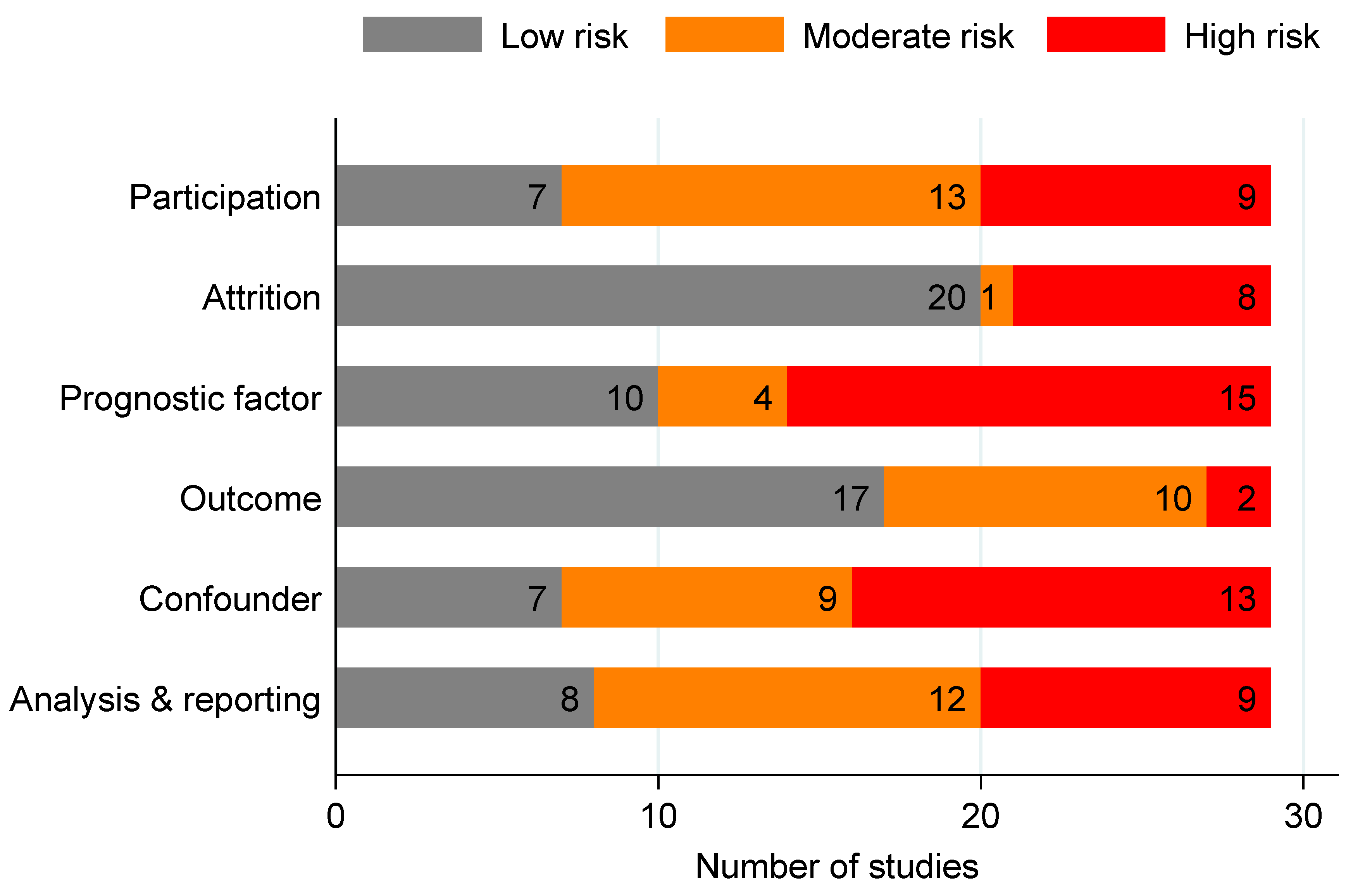
3.6. Risk of Bias
4. Discussion
4.1. Strengths and Limitations
4.2. Comparison with Existing Literature
4.3. Clinical and Research Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Research UK. Cancer Statistics for the UK-Cancer Risk. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk#heading-Three (accessed on 22 August 2023).
- Cancer Research UK. Cancer Statistics for the UK-Cancer Screening and Diagnosis. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk#heading-Four (accessed on 22 August 2023).
- Cancer Research UK. Survival for Lung Cancer. Available online: https://www.cancerresearchuk.org/about-cancer/lung-cancer/survival (accessed on 22 August 2023).
- Cancer Research UK. Survival for Bowel Cancer. Available online: https://www.cancerresearchuk.org/about-cancer/bowel-cancer/survival (accessed on 22 August 2023).
- Cancer Research UK. Survival for Breast Cancer. Available online: https://www.cancerresearchuk.org/about-cancer/breast-cancer/survival (accessed on 22 August 2023).
- Cancer Research UK. Survival of Prostate Cancer. Available online: https://www.cancerresearchuk.org/about-cancer/prostate-cancer/survival (accessed on 22 August 2023).
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. What Is Cancer Screening? 2022. Available online: https://www.cancerresearchuk.org/about-cancer/cancer-symptoms/spot-cancer-early/screening/what-is-cancer-screening#screening20 (accessed on 25 August 2023).
- Rubin, G.; Berendsen, A.; Crawford, S.M.; Dommett, R.; Earle, C.; Emery, J.; Fahey, T.; Grassi, L.; Grunfeld, E.; Gupta, S.; et al. The expanding role of primary care in cancer control. Lancet Oncol. 2015, 16, 1231–1272. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.D.; Aveyard, P.; Price, S.J.; Hobbs, F.R.; Koshiaris, C.; Hamilton, W. Prioritising primary care patients with unexpected weight loss for cancer investigation: Diagnostic accuracy study. BMJ 2020, 370, m2651. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.D.; Hamilton, W.; Koshiaris, C.; Oke, J.L.; Hobbs, F.D.R.; Aveyard, P. The association between unexpected weight loss and cancer diagnosis in primary care: A matched cohort analysis of 65,000 presentations. Br. J. Cancer 2020, 122, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.D.; Oke, J.L.; Aveyard, P.; Hamilton, W.T.; Hobbs, F.D.R. Individual inflammatory marker abnormalities or inflammatory marker scores to identify primary care patients with unexpected weight loss for cancer investigation? Br. J. Cancer 2021, 124, 1540–1542. [Google Scholar] [CrossRef] [PubMed]
- Rubin, G.P.; Saunders, C.L.; Abel, G.A.; McPhail, S.; Lyratzopoulos, G.; Neal, R.D. Impact of investigations in general practice on timeliness of referral for patients subsequently diagnosed with cancer: Analysis of national primary care audit data. Br. J. Cancer 2015, 112, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Mounce, L.; Bailey, S.E.; Cooper, S.L.; Hamilton, W. Blood markers for cancer. BMJ 2019, 367, l5774. [Google Scholar] [CrossRef] [PubMed]
- NICE. Suspected Cancer: Recognition and Referral (NG12). 2015. Available online: https://www.nice.org.uk/guidance/ng12 (accessed on 1 April 2023).
- Virdee, P.S.; Patnick, J.; Watkinson, P.; Holt, T.; Birks, J. Full Blood Count Trends for Colorectal Cancer Detection in Primary Care: Development and Validation of a Dynamic Prediction Model. Cancers 2022, 14, 4779. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- EndNote. EndNote 20. 2023. Available online: https://endnote.com/ (accessed on 22 April 2024).
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Cote, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Atkin, C.; Sapey, E.; Richter, A. Change in blood test results prior to diagnosis in multiple myeloma. Clin. Med. 2020, 20 (Suppl. S2), s99–s100. [Google Scholar] [CrossRef] [PubMed]
- Boursi, B.; Mamtani, R.; Hwang, W.T.; Haynes, K.; Yang, Y.X. A Risk Prediction Model for Sporadic CRC Based on Routine Lab Results. Dig. Dis. Sci. 2016, 61, 2076–2086. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Caporaso, N.E.; Katki, H.A.; Wong, H.L.; Chatterjee, N.; Pine, S.R.; Chanock, S.J.; Goedert, J.J.; Engels, E.A. C-reactive protein and risk of lung cancer. J. Clin. Oncol. 2010, 28, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, J.; Sharma, A.; Chari, S.; Majumder, S. Peripheral blood monocyte counts are elevated in the pre-diagnostic phase of pancreatic cancer: A population based study. Pancreatology 2019, 19, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Edgren, G.; Bagnardi, V.; Bellocco, R.; Hjalgrim, H.; Rostgaard, K.; Melbye, M.; Reilly, M.; Adami, H.O.; Hall, P.; Nyren, O. Pattern of declining hemoglobin concentration before cancer diagnosis. Int. J. Cancer 2010, 127, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, G.; Lyu, Z.; Chen, S.; Wei, L.; Li, X.; Wen, Y.; Chen, Y.; Xie, S.; Cui, H.; et al. The association between fasting blood glucose trajectory and cancer risk in Chinese population without diabetes. Int. J. Cancer 2020, 147, 958–966. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, R.; Tajima, H.; Nakata, K.; Kono, K.; Muro, T.; Sato, A.; Kawahara, K.; Ishii, N.; Kusumoto, Y.; Munehisa, T.; et al. Clinical significance of serum alpha-fetoprotein in patients with liver cirrhosis. Tumour Biol. 1984, 5, 327–338. [Google Scholar]
- Giannakeas, V. Trends in platelet count among cancer patients. Exp. Hematol. Oncol. 2022, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Goldshtein, I.; Neeman, U.; Chodick, G.; Shalev, V. Variations in hemoglobin before colorectal cancer diagnosis. Eur. J. Cancer Prev. 2010, 19, 342–344. [Google Scholar] [CrossRef] [PubMed]
- Gradel, K.O.; Povoa, P.; Garvik, O.S.; Vinholt, P.J.; Nielsen, S.L.; Jensen, T.G.; Chen, M.; Dessau, R.B.; Moller, J.K.; Coia, J.E.; et al. Longitudinal trajectory patterns of plasma albumin and C-reactive protein levels around diagnosis, relapse, bacteraemia, and death of acute myeloid leukaemia patients. BMC Cancer 2020, 20, 249. [Google Scholar] [CrossRef] [PubMed]
- Hauser, R.G.; Esserman, D.; Beste, L.A.; Ong, S.Y.; Colomb, D.G.; Bhargava, A.; Wadia, R.; Rose, M.G. A Machine Learning Model to Successfully Predict Future Diagnosis of Chronic Myelogenous Leukemia with Retrospective Electronic Health Records Data. Am. J. Clin. Pathol. 2021, 156, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, R.W.; Ravindran, A.; Hook, C.C.; Begna, K.H.; Ashrani, A.A.; Pruthi, R.K.; Marshall, A.L.; Hogan, W.; Litzow, M.; Hoyer, J.; et al. Etiologies of Extreme Thrombocytosis: A Contemporary Series. Mayo Clin. Proc. 2019, 94, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.Z.; Pandol, S.J.; Jeon, C.Y.; Chari, S.T.; Sugar, C.A.; Chao, C.R.; Zhang, Z.F.; Wu, B.U.; Setiawan, V.W. New-Onset Diabetes, Longitudinal Trends in Metabolic Markers, and Risk of Pancreatic Cancer in a Heterogeneous Population. Clin. Gastroenterol. Hepatol. 2020, 18, 1812. [Google Scholar] [CrossRef] [PubMed]
- Iversen, O.H.; Roger, M.; Solberg, H.E.; Wetteland, P. Rising erythrocyte sedimentation rate during several years before diagnosis can be a predictive factor in 70% of renal cell carcinoma patients. The benefit of knowing subject-based reference values. J. Intern. Med. 1996, 240, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.; Dahlqvist, P.; Johansson, M.; Svensson, J.; Billing, O.; Sund, M.; Franklin, O. Hyperglycemia as a risk factor in pancreatic cancer: A nested case-control study using prediagnostic blood glucose levels. Pancreatology 2021, 21, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, P.; Antti, H.; Spath, F.; Melin, B.; Bjorkblom, B. Identification of pre-diagnostic metabolic patterns for glioma using subset analysis of matched repeated time points. Cancers 2020, 12, 3349. [Google Scholar] [CrossRef] [PubMed]
- Koshiaris, C.; Van den Bruel, A.; Oke, J.L.; Nicholson, B.D.; Shephard, E.; Braddick, M.; Hamilton, W. Early detection of multiple myeloma in primary care using blood tests: A case-control study in primary care. Br. J. Gen. Pract. 2018, 68, e586–e593. [Google Scholar] [CrossRef] [PubMed]
- Kubo, S.; Takemura, S.; Tanaka, S.; Nishioka, T.; Kinoshita, M.; Hamano, G.; Ito, T.; Yamamoto, T.; Abue, M.; Aoki, M.; et al. Screening and surveillance for occupational cholangiocarcinoma in workers exposed to organic solvents. Surg. Today 2016, 46, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Lemanska, A.; Price, C.A.; Jeffreys, N.; Byford, R.; Dambha-Miller, H.; Fan, X.; Hinton, W.; Otter, S.; Rice, R.; Stunt, A.; et al. BMI and HbA1c are metabolic markers for pancreatic cancer: Matched case-control study using a UK primary care database. PLoS ONE 2022, 17, e0275369. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cao, Y.; Olsson, L. A population-based study on time trends of hemoglobin in primary care comparing prediagnostic colorectal cancer patients vs age- and sex-matched controls. Scand. J. Gastroenterol. 2021, 56, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Pannala, R.; Leibson, C.L.; Rabe, K.G.; Timmons, L.J.; Ransom, J.; de Andrade, M.; Petersen, G.M.; Chari, S.T. Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am. J. Gastroenterol. 2009, 104, 2318–2325. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Plummer, M.; Biessy, C.; Tsilidis, K.K.; Ostergaard, J.N.; Overvad, K.; Tjonneland, A.; Halkjaer, J.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; et al. Thyroid-stimulating hormone, thyroglobulin, and thyroid hormones and risk of differentiated thyroid carcinoma: The EPIC study. J. Natl. Cancer Inst. 2014, 106, dju097. [Google Scholar] [CrossRef] [PubMed]
- Sadr-Azodi, O.; Gudbjornsdottir, S.; Ljung, R. Pattern of increasing HbA1c levels in patients with diabetes mellitus before clinical detection of pancreatic cancer—A population-based nationwide case-control study. Acta Oncol. 2015, 54, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Smyrk, T.C.; Levy, M.J.; Topazian, M.A.; Chari, S.T. Fasting Blood Glucose Levels Provide Estimate of Duration and Progression of Pancreatic Cancer Before Diagnosis. Gastroenterology 2018, 155, 490–500.e2. [Google Scholar] [CrossRef] [PubMed]
- Stroud, A.M.; Dewey, E.N.; Husain, F.A.; Fischer, J.M.; Courcoulas, A.P.; Flum, D.R.; Mitchell, J.E.; Pories, W.J.; Purnell, J.Q.; Wolfe, B.M. Association between weight loss and serum biomarkers with risk of incident cancer in the Longitudinal Assessment of Bariatric Surgery cohort. Surg. Obes. Relat. Dis. 2020, 16, 1086–1094. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.S.; Garriga, C.; Clift, A.; Liao, W.; Patone, M.; Coupland, C.; Bashford-Rogers, R.; Sivakumar, S.; Hippisley-Cox, J. Temporality of body mass index, blood tests, comorbidities and medication use as early markers for pancreatic ductal adenocarcinoma (PDAC): A nested case-control study. Gut 2023, 72, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Toriola, A.T.; Cheng, T.Y.; Neuhouser, M.L.; Wener, M.H.; Zheng, Y.; Brown, E.; Miller, J.W.; Song, X.; Beresford, S.A.; Gunter, M.J.; et al. Biomarkers of inflammation are associated with colorectal cancer risk in women but are not suitable as early detection markers. Int. J. Cancer 2013, 132, 2648–2658. [Google Scholar] [CrossRef] [PubMed]
- Toriola, A.T.; Grankvist, K.; Agborsangaya, C.B.; Lukanova, A.; Lehtinen, M.; Surcel, H.M. Changes in pre-diagnostic serum C-reactive protein concentrations and ovarian cancer risk: A longitudinal study. Ann. Oncol. 2011, 22, 1916–1921. [Google Scholar] [CrossRef] [PubMed]
- Virdee, P.S.; Patnick, J.; Watkinson, P.; Birks, J.; Holt, T.A. Trends in the full blood count blood test and colorectal cancer detection: A longitudinal, case-control study of UK primary care patient data. NIHR Open Res. 2022, 2, 32. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Giustini, D.; Kramer, B.M. Comparing the coverage, recall, and precision of searches for 120 systematic reviews in Embase, MEDLINE, and Google Scholar: A prospective study. Syst. Rev. 2016, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Shamshirian, A.; Aref, A.R.; Yip, G.W.; Ebrahimi Warkiani, M.; Heydari, K.; Razavi Bazaz, S.; Hamzehgardeshi, Z.; Shamshirian, D.; Moosazadeh, M.; Alizadeh-Navaei, R. Diagnostic value of serum HER2 levels in breast cancer: A systematic review and meta-analysis. BMC Cancer 2020, 20, 1049. [Google Scholar] [CrossRef] [PubMed]
- Kane, L.E.; Mellotte, G.S.; Mylod, E.; O’Brien, R.M.; O’Connell, F.; Buckley, C.E.; Arlow, J.; Nguyen, K.; Mockler, D.; Meade, A.D.; et al. Diagnostic Accuracy of Blood-based Biomarkers for Pancreatic Cancer: A Systematic Review and Meta-analysis. Cancer Res. Commun. 2022, 2, 1229–1243. [Google Scholar] [CrossRef] [PubMed]
- Harlid, S.; Gunter, M.J.; Van Guelpen, B. Risk-Predictive and Diagnostic Biomarkers for Colorectal Cancer; a Systematic Review of Studies Using Pre-Diagnostic Blood Samples Collected in Prospective Cohorts and Screening Settings. Cancers 2021, 13, 4406. [Google Scholar] [CrossRef] [PubMed]
- Virdee, P.S.; Marian, I.R.; Mansouri, A.; Elhussein, L.; Kirtley, S.; Holt, T.; Birks, J. The Full Blood Count Blood Test for Colorectal Cancer Detection: A Systematic Review, Meta-Analysis, and Critical Appraisal. Cancers 2020, 12, 2348. [Google Scholar] [CrossRef] [PubMed]
- NICE. How Should I Interpret Platelet Count Results? 2021. Available online: https://cks.nice.org.uk/topics/platelets-abnormal-counts-cancer/diagnosis/interpreting-platelet-results/ (accessed on 11 April 2023).
- NICE. Anaemia-Iron Deficiency. 2021. Available online: https://cks.nice.org.uk/topics/anaemia-iron-deficiency/ (accessed on 11 April 2023).
- Virdee, P.S.; Fuller, A.; Jacobs, M.; Holt, T.; Birks, J. Assessing data quality from the Clinical Practice Research Datalink: A methodological approach applied to the full blood count blood test. J. Big Data 2020, 7, 96. [Google Scholar] [CrossRef]
- Bull, L.M.; Lunt, M.; Martin, G.P.; Hyrich, K.; Sergeant, J.C. Harnessing repeated measurements of predictor variables for clinical risk prediction: A review of existing methods. Diagn. Progn. Res. 2020, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Virdee, P.S.; Bankhead, C.; Koshiaris, C.; Drakesmith, C.W.; Oke, J.; Withrow, D.; Swain, S.; Collins, K.; Chammas, L.; Tamm, A.; et al. BLOod Test Trend for cancEr Detection (BLOTTED): Protocol for an observational and prediction model development study using English primary care electronic health record data. Diagn. Progn. Res. 2023, 7, 1. [Google Scholar] [CrossRef]
| Blood Test | Blood Level |
|---|---|
| Full Blood Count | red blood cell count, haemoglobin, haematocrit, mean cell volume, mean cell haemoglobin, mean cell haemoglobin concentration, red blood cell distribution width, platelet count, mean platelet volume, white blood cell count, basophil count, eosinophil count, lymphocyte count, monocyte count, neutrophil count, basophil %, eosinophil %, lymphocyte %, monocyte %, neutrophil % |
| Liver Function Tests | alanine aminotransaminase, albumin, alkaline phosphatase, aspartate transaminase, bilirubin |
| Renal Function | sodium, potassium, creatinine, urea |
| Inflammatory Markers | C-reactive protein, erythrocyte sedimentation rate, plasma viscosity |
| Other tests | amylase, HBA1c, calcium, calcium adjusted, total protein, blood glucose, fasting glucose, thyroid stimulating hormone |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Virdee, P.S.; Collins, K.K.; Friedemann Smith, C.; Yang, X.; Zhu, S.; Roberts, S.E.; Roberts, N.; Oke, J.L.; Bankhead, C.; Perera, R.; et al. The Association between Blood Test Trends and Undiagnosed Cancer: A Systematic Review and Critical Appraisal. Cancers 2024, 16, 1692. https://doi.org/10.3390/cancers16091692
Virdee PS, Collins KK, Friedemann Smith C, Yang X, Zhu S, Roberts SE, Roberts N, Oke JL, Bankhead C, Perera R, et al. The Association between Blood Test Trends and Undiagnosed Cancer: A Systematic Review and Critical Appraisal. Cancers. 2024; 16(9):1692. https://doi.org/10.3390/cancers16091692
Chicago/Turabian StyleVirdee, Pradeep S., Kiana K. Collins, Claire Friedemann Smith, Xin Yang, Sufen Zhu, Sophie E. Roberts, Nia Roberts, Jason L. Oke, Clare Bankhead, Rafael Perera, and et al. 2024. "The Association between Blood Test Trends and Undiagnosed Cancer: A Systematic Review and Critical Appraisal" Cancers 16, no. 9: 1692. https://doi.org/10.3390/cancers16091692
APA StyleVirdee, P. S., Collins, K. K., Friedemann Smith, C., Yang, X., Zhu, S., Roberts, S. E., Roberts, N., Oke, J. L., Bankhead, C., Perera, R., Hobbs, F. R., & Nicholson, B. D. (2024). The Association between Blood Test Trends and Undiagnosed Cancer: A Systematic Review and Critical Appraisal. Cancers, 16(9), 1692. https://doi.org/10.3390/cancers16091692







