COVID-19 and Cancer Detection in Russia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
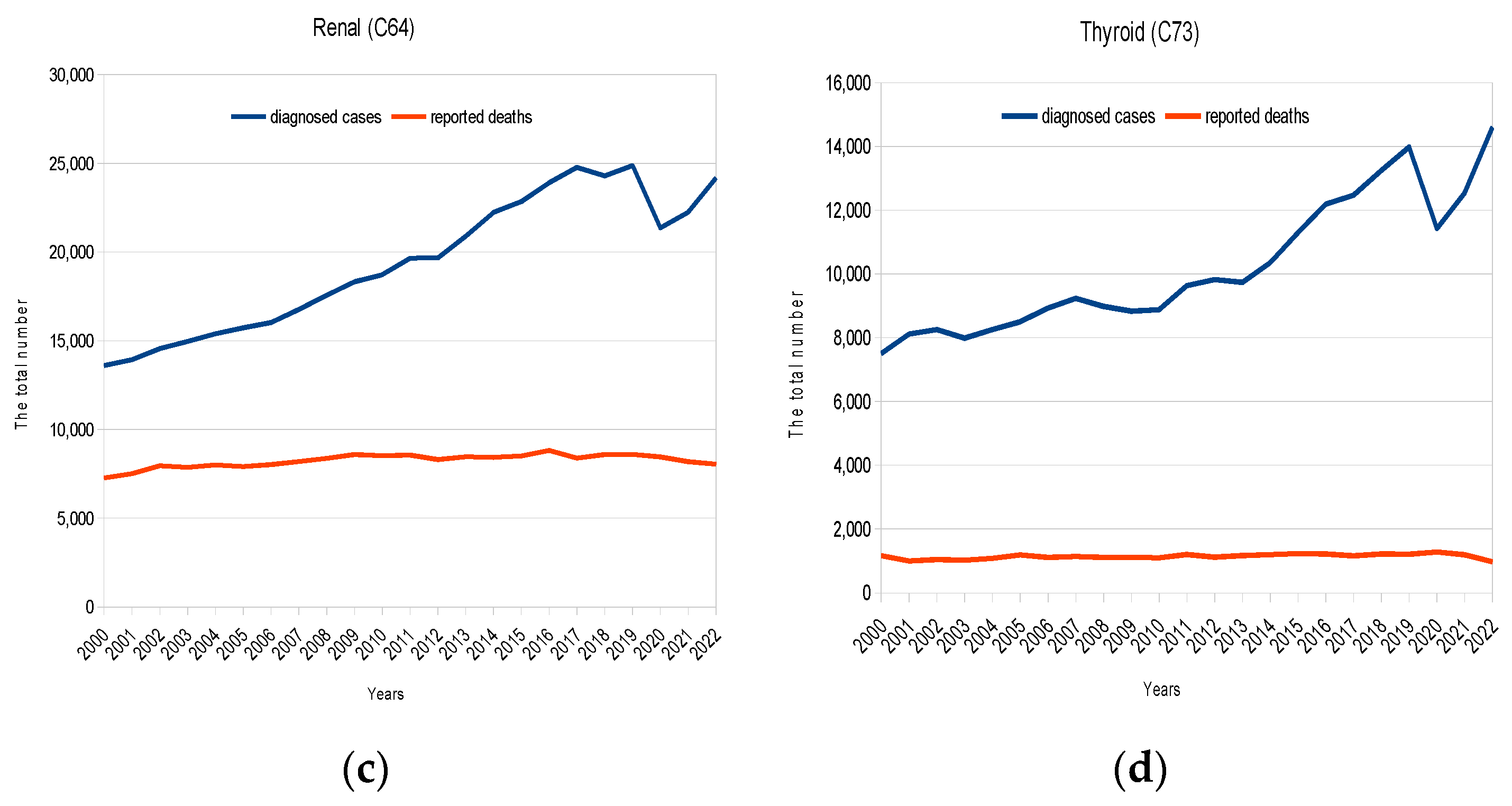
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crile, G. Common Sense in Cancer. Postgrad. Med. 1955, 17, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Crile, G. A plea against blind fear of cancer. Life 1955, 128–132. [Google Scholar]
- Timonen, S.; Nieminen, U.; Kauraniemi, T. Mass screening for cervical carcinoma in Finland. Organization and effect on morbidity and mortality. Ann. Chir. Gynaecol. Fenn. 1974, 63, 104–112. [Google Scholar] [PubMed]
- Papanicolaou, G.N.; Traut, H.F. The diagnostic value of vaginal smears in carcinoma of the uterus. Am. J. Obstet. Gynecol. 1941, 42, 193–206. [Google Scholar] [CrossRef]
- Harach, H.R.; Franssila, K.O.; Wasenius, V.M. Occult papillary carcinoma of the thyroid. A “normal” finding in Finland. A systematic autopsy study. Cancer 1985, 56, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Bastacky, S.; Chandran, U.; Becich, M.J.; Dhir, R. Prevalence of incidental prostate cancer in the general population: A study of healthy organ donors. J. Urol. 2008, 179, 892–895; discussion 895. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G.; Black, W.C. Using autopsy series to estimate the disease “reservoir” for ductal carcinoma in situ of the breast: How much more breast cancer can we find? Ann. Intern. Med. 1997, 127, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.K.; Wells, C.K.; McFarlane, M.J.; Feinstein, A.R. More lung cancer but better survival. Implications of secular trends in “necropsy surprise” rates. Chest 1989, 96, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, R.I.; Feinstein, A.R.; Horwitz, S.M.; Robboy, S.J. Necropsy diagnosis of endometrial cancer and detection-bias in case/control studies. Lancet 1981, 2, 66–68. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G.; Black, W.C. Overdiagnosis in Cancer. JNCI J. Natl. Cancer Inst. 2010, 102, 605–613. [Google Scholar] [CrossRef]
- Autier, P.; Boniol, M.; Gavin, A.; Vatten, L.J. Breast cancer mortality in neighbouring European countries with different levels of screening but similar access to treatment: Trend analysis of WHO mortality database. BMJ 2011, 343, d4411. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bleyer, A. US breast cancer mortality is consistent with European data. BMJ 2011, 343, d5630. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gøtzsche, P.C.; Jørgensen, K.J. Screening for breast cancer with mammography. Cochrane Database Syst. Rev. 2013, 2013, CD001877. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G. Screening mammography—A long run for a short slide? N. Engl. J. Med. 2010, 363, 1276–1278. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.S.; Kim, H.J.; Welch, H.G. Korea’s thyroid-cancer “epidemic”—Screening and overdiagnosis. N. Engl. J. Med. 2014, 371, 1765–1767. [Google Scholar] [CrossRef] [PubMed]
- Welch, H.G.; Skinner, J.S.; Schroeck, F.R.; Zhou, W.; Black, W.C. Regional Variation of Computed Tomographic Imaging in the United States and the Risk of Nephrectomy. JAMA Intern. Med. 2018, 178, 221–227. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Draisma, G.; Boer, R.; Otto, S.J.; van der Cruijsen, I.W.; Damhuis, R.A.; Schröder, F.H.; de Koning, H.J. Lead times and overdetection due to prostate-specific antigen screening: Estimates from the European Randomized Study of Screening for Prostate Cancer. J. Natl. Cancer Inst. 2003, 95, 868–878. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.H.; Hugosson, J.; Roobol, M.J.; Tammela, T.L.; Ciatto, S.; Nelen, V.; Kwiatkowski, M.; Lujan, M.; Lilja, H.; Zappa, M.; et al. Screening and prostate-cancer mortality in a randomized European study. N. Engl. J. Med. 2009, 360, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Shaukat, A.; Mongin, S.J.; Geisser, M.S.; Lederle, F.A.; Bond, J.H.; Mandel, J.S.; Church, T.R. Long-term mortality after screening for colorectal cancer. N. Engl. J. Med. 2013, 369, 1106–1114. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://oncology.ru/service/statistics/malignant_tumors/ (accessed on 21 March 2024).
- Kaprin, A.D.; Starinsky, V.V.; Shakhzadova, A.O.; Lisichnikova, I.V. (Eds.) Malignant Neoplasms in Russia in 2022: Morbidity and Mortality [Booklet in Russian]; Polygraphmaster Company LLC: Moscow, Russia, 2023; ISBN 978-5-85502-290-2. [Google Scholar]
- LibreOffice. Available online: https://www.libreoffice.org/ (accessed on 21 March 2024).
- SEER*Explorer: An Interactive Website for SEER Cancer Statistics [Internet]. Surveillance Research Program, National Cancer Institute. 19 April 2023. [Updated: 16 November 2023]; Data source(s): SEER Incidence Data, November 2022 Submission (1975–2020), SEER 22 Registries. U.S. Mortality Data (1969–2020), National Center for Health Statistics, CDC. Available online: https://seer.cancer.gov/statistics-network/explorer/ (accessed on 6 April 2024).
- Berlin, L. Overdiagnosed: Making People Sick in Pursuit of Health. JAMA 2011, 305, 1356. [Google Scholar] [CrossRef]
- Welch, H.G.; Schwartz, L.M.; Woloshin, S. Overdiagnosed: Making People Sick in the Pursuit of Health; Beacon Press: Boston, MA, USA, 2011; 228p, ISBN 978-0-807-02200-9. [Google Scholar]
- Lee, L.Y.W.; Cazier, J.B.; Starkey, T.; Briggs, S.E.W.; Arnold, R.; Bisht, V.; Booth, S.; Campton, N.A.; Cheng, V.W.T.; Collins, G.; et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: A prospective cohort study. Lancet Oncol. 2020, 21, 1309–1316, Erratum in Lancet Oncol. 2020, 21, 1309–1316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Figueroa, J.D.; Gray, E.; Pashayan, N.; Deandrea, S.; Karch, A.; Vale, D.B.; Elder, K.; Procopio, P.; van Ravesteyn, N.T.; Mutabi, M.; et al. The impact of the COVID-19 pandemic on breast cancer early detection and screening. Prev. Med. 2021, 151, 106585. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alagoz, O.; Lowry, K.P.; Kurian, A.W.; Mandelblatt, J.S.; Ergun, M.A.; Huang, H.; Lee, S.J.; Schechter, C.B.; Tosteson, A.N.A.; Miglioretti, D.L.; et al. Impact of the COVID-19 Pandemic on Breast Cancer Mortality in the US: Estimates from Collaborative Simulation Modeling. J. Natl. Cancer Inst. 2021, 113, 1484–1494. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Welch, H.G. Stumbling onto Cancer: Avoiding Overdiagnosis of Renal Cell Carcinoma. Am. Fam. Physician 2019, 99, 145–147. [Google Scholar] [PubMed]
- Talantov, P. 0.05. Evidence-Based Medicine from Magic to the Quest for Immortality; AST Publishing House: Corpus, Moscow, 2019; 560p, (Library of the Evolution Foundation); ISBN 978-5-17-114111-0. Available online: https://ast.ru/book/0-05-dokazatelnaya-meditsina-ot-magii-do-poiskov-bessmertiya-842384/ (accessed on 21 March 2024).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudarikov, A. COVID-19 and Cancer Detection in Russia. Cancers 2024, 16, 1673. https://doi.org/10.3390/cancers16091673
Sudarikov A. COVID-19 and Cancer Detection in Russia. Cancers. 2024; 16(9):1673. https://doi.org/10.3390/cancers16091673
Chicago/Turabian StyleSudarikov, Andrey. 2024. "COVID-19 and Cancer Detection in Russia" Cancers 16, no. 9: 1673. https://doi.org/10.3390/cancers16091673
APA StyleSudarikov, A. (2024). COVID-19 and Cancer Detection in Russia. Cancers, 16(9), 1673. https://doi.org/10.3390/cancers16091673





