Temporal Muscle Thickness Compared to Functional Scales as a Prognostic Parameter in Patients with Brain Metastases
Abstract
Simple Summary
Abstract
1. Introduction

2. Materials and Methods
3. Results
3.1. Cohort Description
3.2. TMT and Functional Scores
3.3. Interrelations of TMT
3.4. Overall Survival (OS)
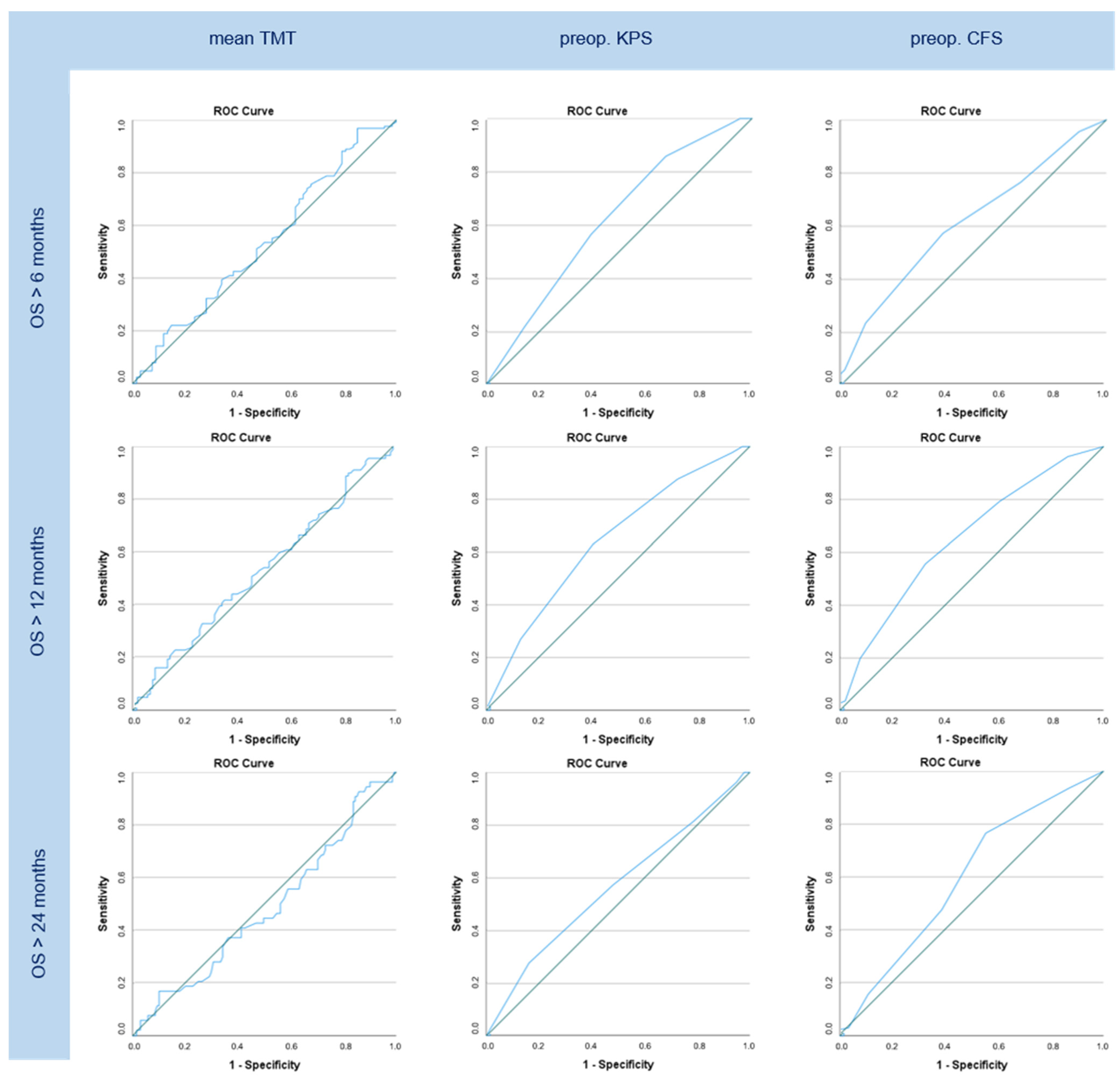
3.5. ROC/AUC Data
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.; Doehner, W.; Evans, J.; et al. Frailty Consensus: A Call to Action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Kerschbaumer, J.; Krigers, A.; Demetz, M.; Pinggera, D.; Klingenschmid, J.; Pichler, N.; Thomé, C.; Freyschlag, C.F. The Clinical Frailty Scale as useful tool in patients with brain metastases. J. Neuro-Oncol. 2022, 158, 51–57. [Google Scholar] [CrossRef]
- Kim, Y.I.; Shin, J.Y.; Yang, S.H.; Kim, H.H.; Shim, B.Y.; Ahn, S. Association between Temporal Muscle Thickness and Overall Survival in Non-Small Cell Lung Cancer Patients with Brain Metastasis. Curr. Oncol. 2022, 29, 6463–6471. [Google Scholar] [CrossRef] [PubMed]
- Furtner, J.; Berghoff, A.S.; Schöpf, V.; Reumann, R.; Pascher, B.; Woitek, R.; Asenbaum, U.; Pelster, S.; Leitner, J.; Widhalm, G.; et al. Temporal muscle thickness is an independent prognostic marker in melanoma patients with newly diagnosed brain metastases. J. Neuro-Oncol. 2018, 140, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Furtner, J.; Berghoff, A.S.; Albtoush, O.M.; Woitek, R.; Asenbaum, U.; Prayer, D.; Widhalm, G.; Gatterbauer, B.; Dieckmann, K.; Birner, P.; et al. Survival prediction using temporal muscle thickness measurements on cranial magnetic resonance images in patients with newly diagnosed brain metastases. Eur. Radiol. 2017, 27, 3167–3173. [Google Scholar] [CrossRef] [PubMed]
- Ilic, I.; Faron, A.; Heimann, M.; Potthoff, A.-L.; Schäfer, N.; Bode, C.; Borger, V.; Eichhorn, L.; Giordano, F.A.; Güresir, E.; et al. Combined Assessment of Preoperative Frailty and Sarcopenia Allows the Prediction of Overall Survival in Patients with Lung Cancer (NSCLC) and Surgically Treated Brain Metastasis. Cancers 2021, 13, 3353. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Beaudart, C.; McCloskey, E.; Bruyère, O.; Cesari, M.; Rolland, Y.; Rizzoli, R.; Araujo De Carvalho, I.; Amuthavalli Thiyagarajan, J.; Bautmans, I.; Bertière, M.-C.; et al. Sarcopenia in daily practice: Assessment and management. BMC Geriatr. 2016, 16, 170. [Google Scholar] [CrossRef] [PubMed]
- Leitner, J.; Pelster, S.; Schopf, V.; Berghoff, A.S.; Woitek, R.; Asenbaum, U.; Nenning, K.-H.; Widhalm, G.; Kiesel, B.; Gatterbauer, B.; et al. High correlation of temporal muscle thickness with lumbar skeletal muscle cross-sectional area in patients with brain metastases. PLoS ONE 2018, 13, e0207849. [Google Scholar] [CrossRef]
- Ranganathan, K.; Terjimanian, M.; Lisiecki, J.; Rinkinen, J.; Mukkamala, A.; Brownley, C.; Buchman, S.R.; Wang, S.C.; Levi, B. Temporalis muscle morphomics: The psoas of the craniofacial skeleton. J. Surg. Res. 2014, 186, 246–252. [Google Scholar] [CrossRef]
- Rockwood, K.; Theou, O. Using the Clinical Frailty Scale in Allocating Scarce Health Care Resources. Can. Geriatr. J. 2020, 23, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Mandel, R.; Fain, M.J. Frailty: An emerging geriatric syndrome. Am. J. Med. 2007, 120, 748–753. [Google Scholar] [CrossRef] [PubMed]
- Powell, C. Frailty: Help or hindrance? J. R. Soc. Med. 1997, 90, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Haas, L.E.M.; Boumendil, A.; Flaatten, H.; Guidet, B.; Ibarz, M.; Jung, C.; Moreno, R.; Morandi, A.; Andersen, F.H.; Zafeiridis, T.; et al. Frailty is associated with long-term outcome in patients with sepsis who are over 80 years old: Results from an observational study in 241 European ICUs. Age Ageing 2021, 50, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Congiusta, D.; Amer, K.; Suri, P.; Merchant, A.M.; Ahmed, I.H.; Vosbikian, M.M. A simplified preoperative risk assessment tool as a predictor of complications in the surgical management of forearm fractures. J. Clin. Orthop. Trauma 2021, 14, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Klingenschmid, J.; Krigers, A.; Pinggera, D.; Kerschbaumer, J.; Thomé, C.; Freyschlag, C.F. The Clinical Frailty Scale as predictor of overall survival after resection of high-grade glioma. J. Neuro-Oncol. 2022, 158, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Furtner, J.; Genbrugge, E.; Gorlia, T.; Bendszus, M.; Nowosielski, M.; Golfinopoulos, V.; Weller, M.; van den Bent, M.J.; Wick, W.; Preusser, M. Temporal muscle thickness is an independent prognostic marker in patients with progressive glioblastoma: Translational imaging analysis of the EORTC 26101 trial. Neuro Oncol. 2019, 21, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Huq, S.; Khalafallah, A.M.; Ruiz-Cardozo, M.A.; Botros, D.; Oliveira, L.A.; Dux, H.; White, T.; Jimenez, A.E.; Gujar, S.K.; Sair, H.I.; et al. A Novel Radiographic Marker of Sarcopenia with Prognostic Value in Glioblastoma. Clin. Neurol. Neurosurg. 2021, 207, 106782. [Google Scholar] [CrossRef]
- Muglia, R.; Simonelli, M.; Pessina, F.; Morenghi, E.; Navarria, P.; Persico, P.; Lorenzi, E.; Dipasquale, A.; Grimaldi, M.; Scorsetti, M.; et al. Prognostic relevance of temporal muscle thickness as a marker of sarcopenia in patients with glioblastoma at diagnosis. Eur. Radiol. 2021, 31, 4079–4086. [Google Scholar] [CrossRef]
- Wende, T.; Kasper, J.; Prasse, G.; Glass, A.; Kriesen, T.; Freiman, T.M.; Meixensberger, J.; Henker, C. Newly Diagnosed IDH-Wildtype Glioblastoma and Temporal Muscle Thickness: A Multicenter Analysis. Cancers 2021, 13, 5610. [Google Scholar] [CrossRef]
- Klingenschmid, J.; Krigers, A.; Schön, V.; Pinggera, D.; Kerschbaumer, J.; Grams, A.E.; Thomé, C.; Freyschlag, C.F. Temporal muscle thickness has no prognostic relevance in patients with high-grade glioma compared to functional scales. Front. Oncol. 2023, 13, 1237105. [Google Scholar] [CrossRef] [PubMed]
- Rockwood, K.; Song, X.; MacKnight, C.; Bergman, H.; Hogan, D.B.; McDowell, I.; Mitnitski, A. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005, 173, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Karvellas, C.J.; Baracos, V.; Williams, D.C.; Khadaroo, R.G. Sarcopenia is a predictor of outcomes in very elderly patients undergoing emergency surgery. Surgery 2014, 156, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Hennenberg, J.; Untersteiner, H.; Hirschmann, D.; Gatterbauer, B.; Zöchbauer-Müller, S.; Hochmair, M.J.; Preusser, M.; Rössler, K.; Dorfer, C.; et al. Influence of temporal muscle thickness on the outcome of radiosurgically treated patients with brain metastases from non–small cell lung cancer. J. Neurosurg. 2022, 137, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Steindl, A.; Leitner, J.; Schwarz, M.; Nenning, K.H.; Asenbaum, U.; Mayer, S.; Woitek, R.; Weber, M.; Schöpf, V.; Berghoff, A.S.; et al. Sarcopenia in Neurological Patients: Standard Values for Temporal Muscle Thickness and Muscle Strength Evaluation. J. Clin. Med. 2020, 9, 1272. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, M.; Narita, N.; Sasaki, K.; Sato, Y.; Suzuki, Y.; Mashiyama, S.; Tominaga, T. Standard values for temporal muscle thickness in the Japanese population who undergo brain check-up by magnetic resonance imaging. Surg. Neurol. Int. 2021, 12, 67. [Google Scholar] [CrossRef] [PubMed]
- Zapaishchykova, A.; Liu, K.X.; Saraf, A.; Ye, Z.; Catalano, P.J.; Benitez, V.; Ravipati, Y.; Jain, A.; Huang, J.; Hayat, H.; et al. Automated temporalis muscle quantification and growth charts for children through adulthood. Nat. Commun. 2023, 14, 6863. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.J.; Liu, H.; Garcia, J.M. Sex differences in muscle wasting. Adv. Exp. Med. Biol. 2017, 1043, 153–197. [Google Scholar] [PubMed]
- Moltoni, G.; D’arco, F.; Rossi-Espagnet, M.C.; James, G.; Hayward, R. Observations on the growth of temporalis muscle: A 3D CT imaging study. J. Anat. 2021, 238, 1218–1224. [Google Scholar] [CrossRef]
- Tuncel, U.; Gumus, M.; Kurt, A.; Güzel, N. An Extremely Rare Condition: Unilateral and Isolated Temporalis Muscle Hypertrophy. Plast. Reconstr. Surg.-Glob. Open 2017, 5, e1383. [Google Scholar] [CrossRef]
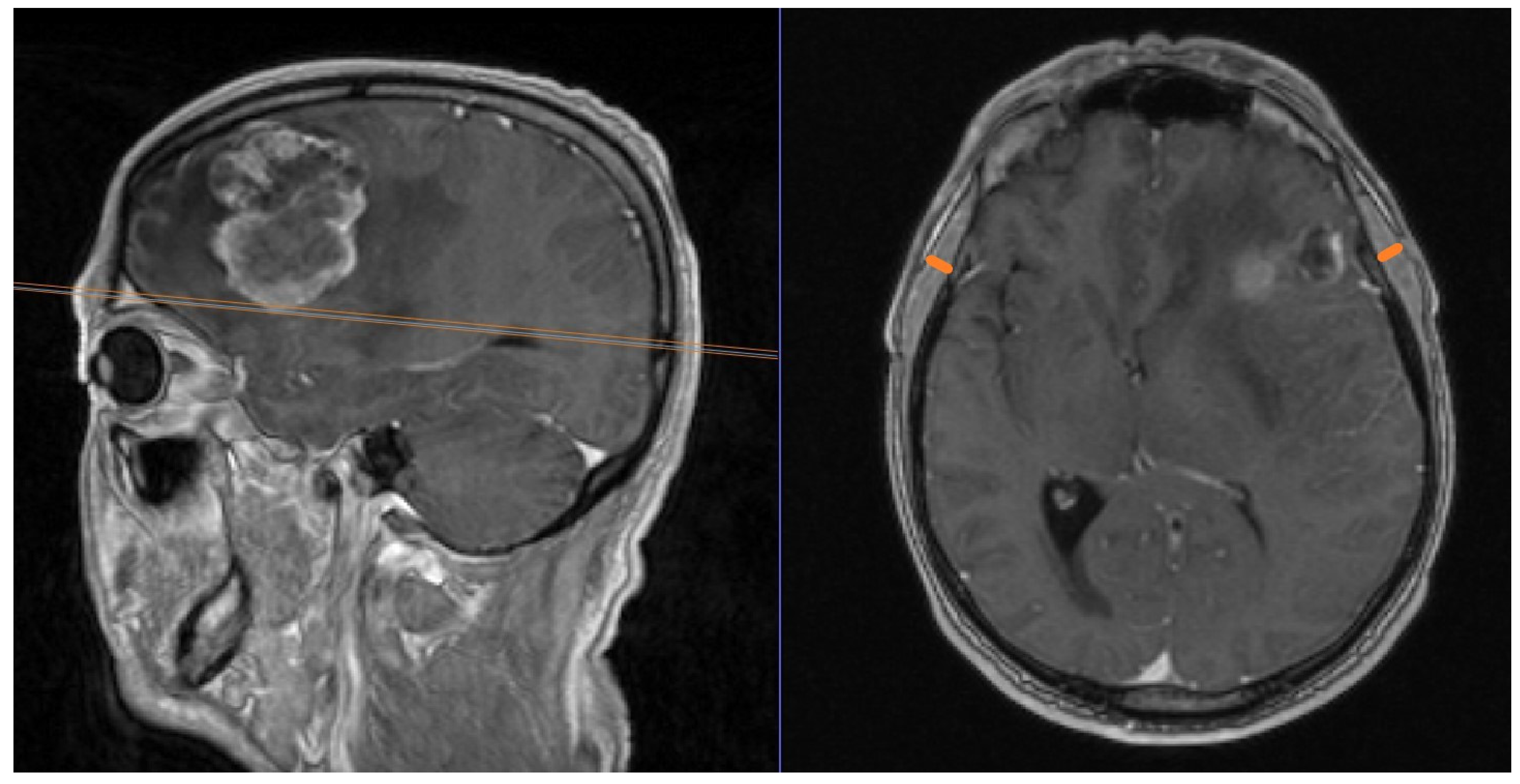
| Primary Tumor | n (%) |
|---|---|
| Lung | 89 (44.7%) |
| NSCLC | 75 (37.9%) |
| SCLC | 12 (6.1%) |
| Mamma | 23 (11.6%) |
| ER+ | 4 (2%) |
| HER2+ | 7 (3.5%) |
| ER+/HER2+ | 3 (1.5%) |
| Triple- | 8 (4%) |
| RCC | 5 (2.5%) |
| Colon | 8 (4%) |
| CUP | 6 (3%) |
| Other | 41 (20.6%) |
| KPS Preop. (n) | TMT (95CI) | KPS Postop. (n) | TMT (95CI) | KPS FU (n) | TMT (95CI) |
|---|---|---|---|---|---|
| 10 | 10 | 10 | |||
| 20 | 20 (1) | 20 | |||
| 30 | 30 | 30 (2) | 6.1 | ||
| 40 (1) | 40 (2) | 7.4 | 40 (2) | 8.0 | |
| 50 (2) | 5.6 | 50 (5) | 6.3 (5.2–7.4) | 50 (4) | 7.4 (3.6–11.3) |
| 60 (6) | 6.5 (5.2–7.9) | 60 (16) | 6.8 (6.1–7.6) | 60 (10) | 7.5 (6.2–8.8) |
| 70 (32) | 7.1 (6.7–7.6) | 70 (13) | 7.2 (6.3–8.1) | 70 (12) | 7.0 (6.0–8.1) |
| 80 (58) | 7.6 (7.1–8.1) | 80 (28) | 7.4 (6.8–8.0) | 80 (23) | 8.4 (7.5–9.2) |
| 90 (61) | 7.8 (7.4–8.2) | 90 (94) | 7.7 (7.4–8.0) | 90 (47) | 7.4 (7.0–7.9) |
| 100 (39) | 7.6 (7.1–8.1) | 100 (39) | 7.9 (7.3–8.5) | 100 (45) | 7.4 (7.0–7.9) |
| CFS Preop. (n) | TMT (95CI) | CFS Postop. (n) | TMT (95CI) | CFS FU (n) | TMT (95CI) |
|---|---|---|---|---|---|
| 1 (16) | 7.6 (7.0–8.2) | 1 (16) | 7.3 (6.5–8.2) | 1 (34) | 7.4 (6.8–7.9) |
| 2 (42) | 7.6 (7.0–8.1) | 2 (76) | 7.9 (7.5–8.3) | 2 (36) | 7.6 (7.1–8.2) |
| 3 (51) | 7.7 (7.3–8.1) | 3 (50) | 7.6 (7.1–8.1) | 3 (27) | 7.3 (6.8–7.8) |
| 4 (62) | 7.8 (7.3–8.2) | 4 (26) | 7.2 (6.6–7.8) | 4 (26) | 8.1 (7.3–8.9) |
| 5 (22) | 6.6 (5.9–7.2) | 5 (15) | 7.4 (6.7–8.2) | 5 (11) | 7.7 (6.5–8.9) |
| 6 (3) | 6.1 (4.9–7.3) | 6 (8) | 6.1 (5.4–6.8) | 6 (4) | 6.4 (4.0–8.9) |
| 7 (3) | 8.6 (4.1–13.2) | 7(5) | 7.6 (4.1–11.1) | 7 (1) | |
| 8 | 8 (3) | 6.7 (2.4–11.0) | 8 (6) | 7.6 (5.4–9.8) | |
| 9 | 9 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klingenschmid, J.; Krigers, A.; Pinggera, D.; Kerschbaumer, J.; Pichler, N.; Schoen, V.; Demetz, M.; Grams, A.E.; Thomé, C.; Freyschlag, C.F. Temporal Muscle Thickness Compared to Functional Scales as a Prognostic Parameter in Patients with Brain Metastases. Cancers 2024, 16, 1660. https://doi.org/10.3390/cancers16091660
Klingenschmid J, Krigers A, Pinggera D, Kerschbaumer J, Pichler N, Schoen V, Demetz M, Grams AE, Thomé C, Freyschlag CF. Temporal Muscle Thickness Compared to Functional Scales as a Prognostic Parameter in Patients with Brain Metastases. Cancers. 2024; 16(9):1660. https://doi.org/10.3390/cancers16091660
Chicago/Turabian StyleKlingenschmid, Julia, Aleksandrs Krigers, Daniel Pinggera, Johannes Kerschbaumer, Nadine Pichler, Victoria Schoen, Matthias Demetz, Astrid E. Grams, Claudius Thomé, and Christian F. Freyschlag. 2024. "Temporal Muscle Thickness Compared to Functional Scales as a Prognostic Parameter in Patients with Brain Metastases" Cancers 16, no. 9: 1660. https://doi.org/10.3390/cancers16091660
APA StyleKlingenschmid, J., Krigers, A., Pinggera, D., Kerschbaumer, J., Pichler, N., Schoen, V., Demetz, M., Grams, A. E., Thomé, C., & Freyschlag, C. F. (2024). Temporal Muscle Thickness Compared to Functional Scales as a Prognostic Parameter in Patients with Brain Metastases. Cancers, 16(9), 1660. https://doi.org/10.3390/cancers16091660







