Emotional Distress, Cognitive Complaints, and Care Needs among Advanced Cancer Survivors Treated with Immune Checkpoint Blockade: A Mixed-Method Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants and Procedures
2.1.1. Semi-Structured Interviews
2.1.2. Patient-Reported Outcome Measures
2.2. Statistical Analysis
3. Results
3.1. Study Population
3.2. Semi-Structured Interviews
3.2.1. Cancer as a Traumatic Experience
3.2.2. Changes in the Relationship with Spouse or Family
3.2.3. Most Burdensome Symptoms
3.2.4. Cognitive Complaints
3.2.5. Care Needs
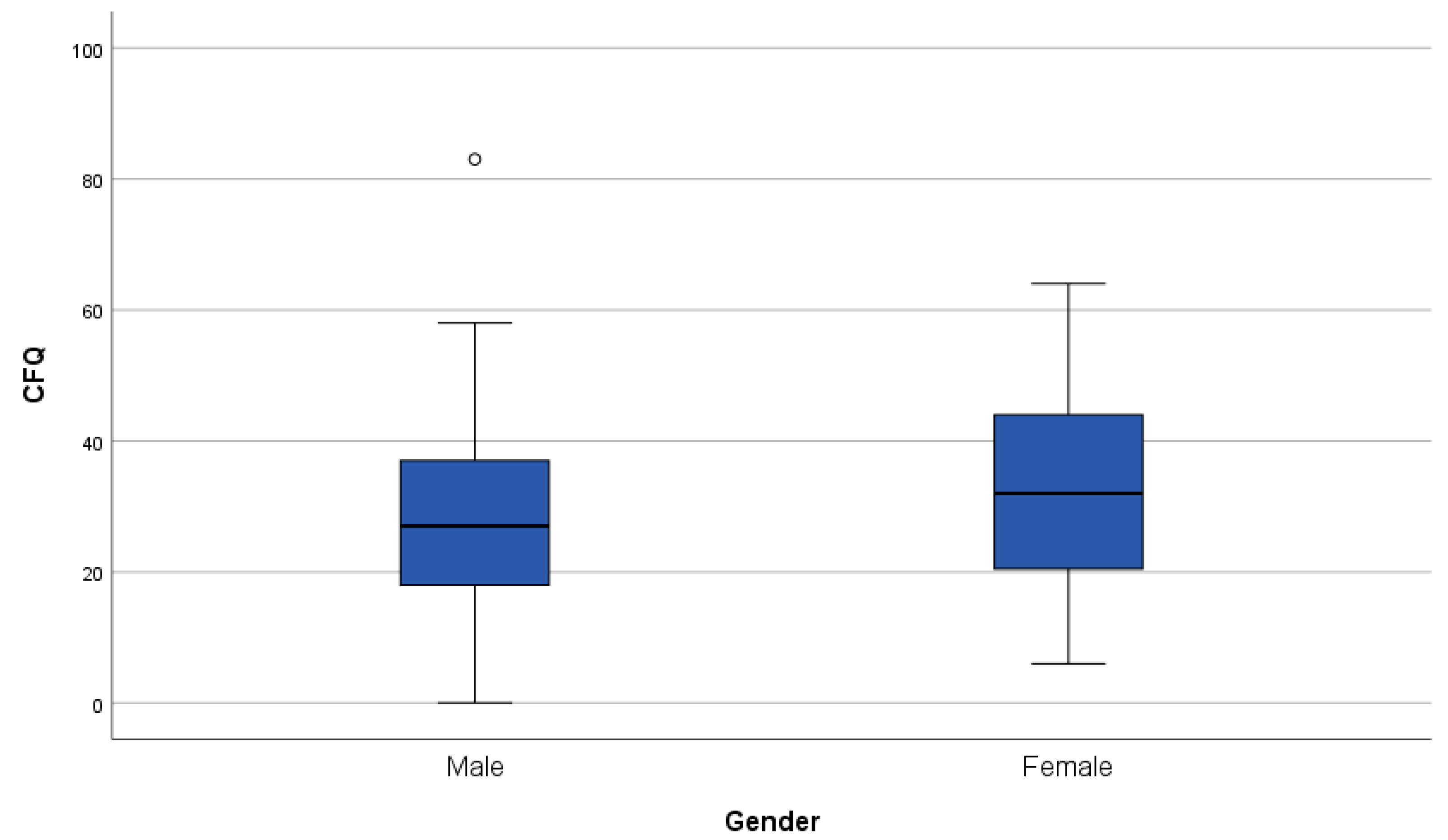
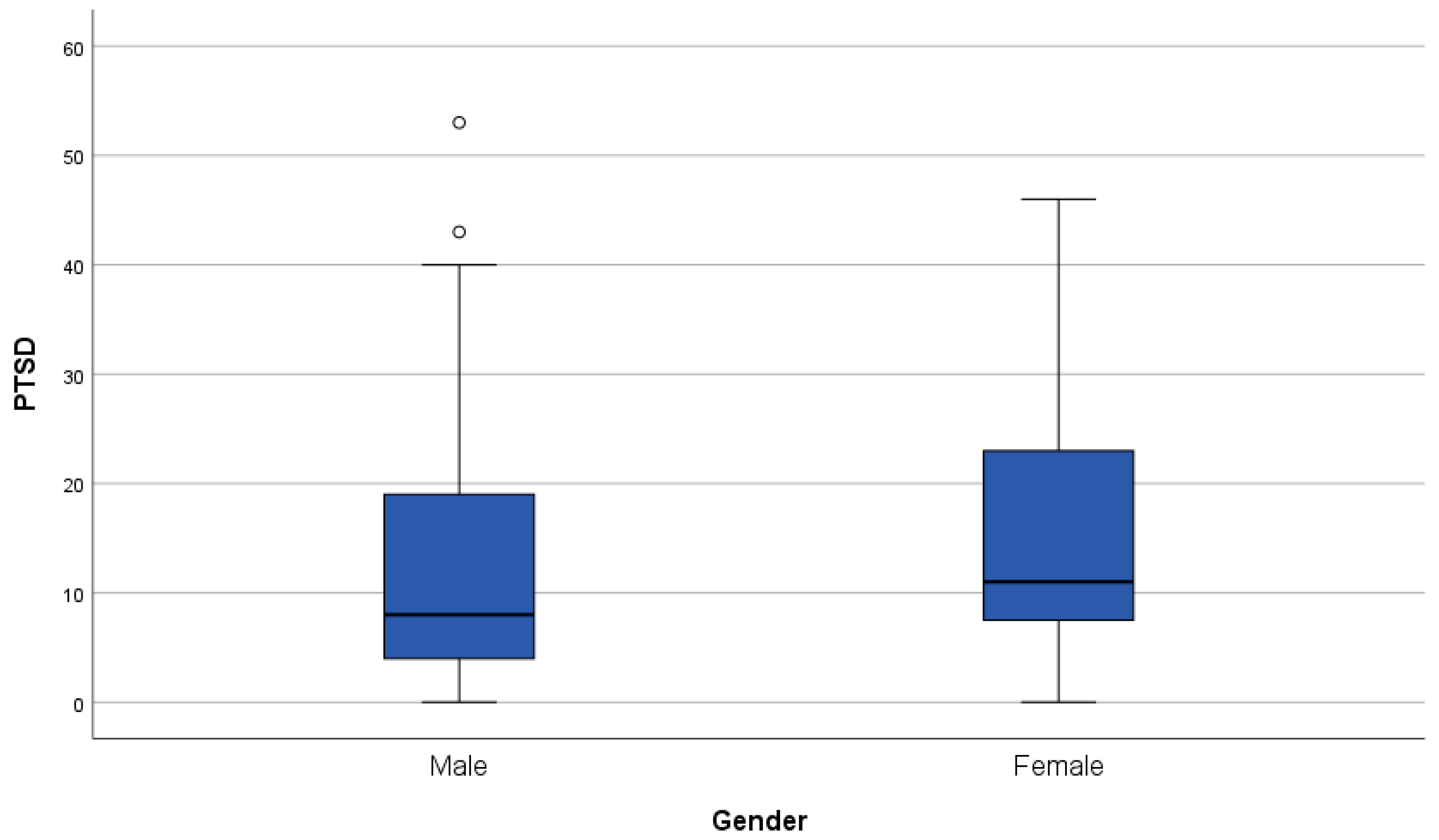
3.3. Patient-Reported Outcome Measures
3.3.1. Fear of Cancer Recurrence and Cancer-Related Post-Traumatic Stress
3.3.2. Subjective Cognitive Complaints
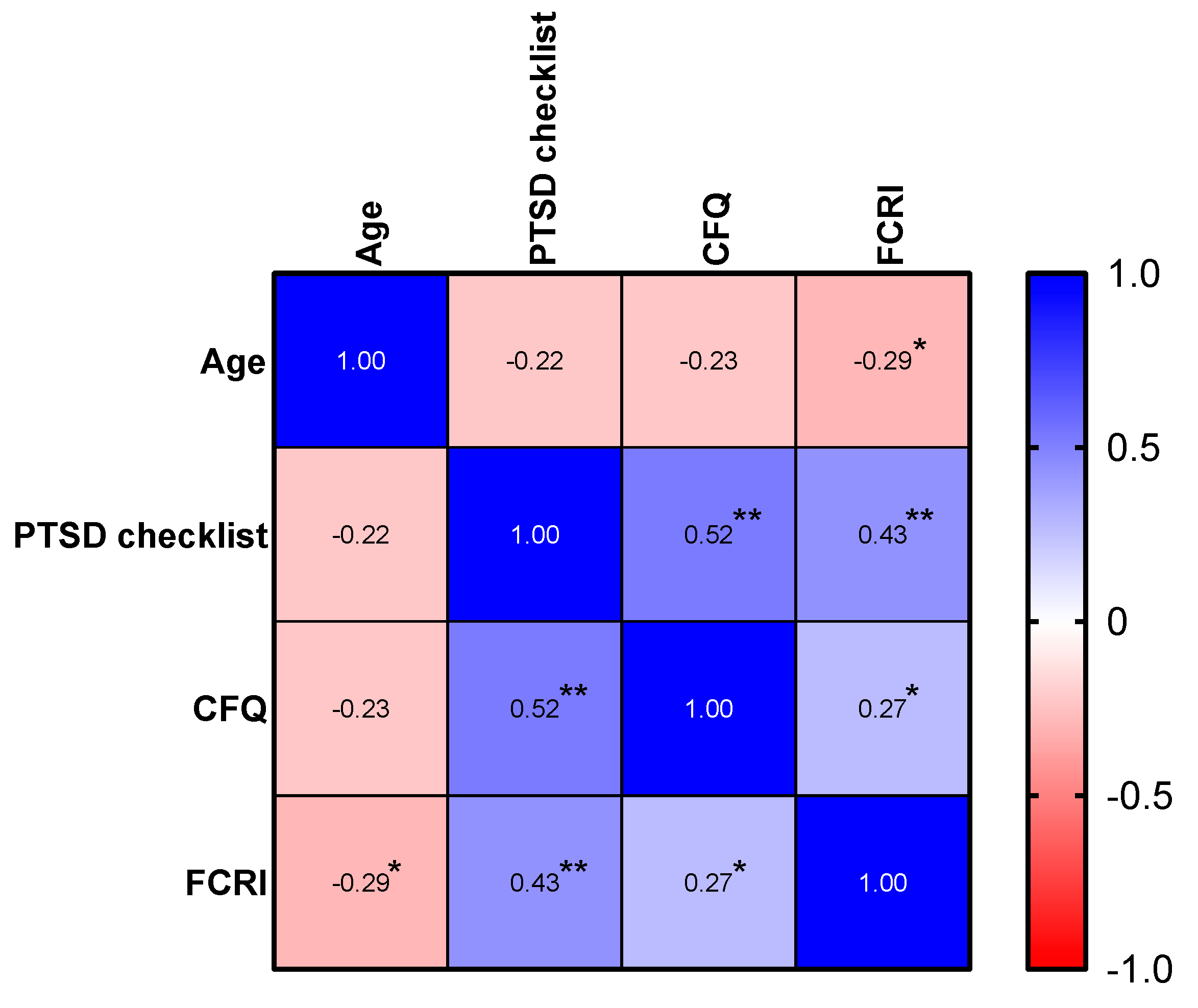
3.3.3. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Permission to Reproduce from Other Sources
References
- Mollica, M.A.; Smith, A.W.; Tonorezos, E.; Castro, K.; Filipski, K.K.; Guida, J.; Perna, F.; Green, P.; Jacobsen, P.B.; Mariotto, A.; et al. Survivorship for Individuals Living with Advanced and Metastatic Cancers: National Cancer Institute Meeting Report. J. Natl. Cancer Inst. 2022, 114, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Lai-Kwon, J.; Heynemann, S.; Hart, N.H.; Chan, R.J.; Smith, T.J.; Nekhlyudov, L.; Jefford, M. Evolving Landscape of Metastatic Cancer Survivorship: Reconsidering Clinical Care, Policy, and Research Priorities for the Modern Era. J. Clin. Oncol. 2023, 41, 3304–3310. [Google Scholar] [CrossRef] [PubMed]
- Patrinely, J.R., Jr.; Johnson, R.; Lawless, A.R.; Bhave, P.; Sawyers, A.; Dimitrova, M.; Yeoh, H.L.; Palmeri, M.; Ye, F.; Fan, R.; et al. Chronic Immune-Related Adverse Events Following Adjuvant Anti-PD-1 Therapy for High-risk Resected Melanoma. JAMA Oncol. 2021, 7, 744–748. [Google Scholar] [CrossRef]
- Lai-Kwon, J.; Khoo, C.; Lo, S.; Milne, D.; Mohamed, M.; Raleigh, J.; Smith, K.; Lisy, K.; Sandhu, S.; Jefford, M. The survivorship experience for patients with metastatic melanoma on immune checkpoint and BRAF-MEK inhibitors. J. Cancer Surviv. 2019, 13, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Emery, J.; Butow, P.; Lai-Kwon, J.; Nekhlyudov, L.; Rynderman, M.; Jefford, M. Management of common clinical problems experienced by survivors of cancer. Lancet 2022, 399, 1537–1550. [Google Scholar] [CrossRef]
- Langbaum, T.; Smith, T.J. Time to Study Metastatic-Cancer Survivorship. N. Engl. J. Med. 2019, 380, 1300–1302. [Google Scholar] [CrossRef]
- Rogiers, A.; Leys, C.; De Cremer, J.; Awada, G.; Schembri, A.; Theuns, P.; De Ridder, M.; Neyns, B. Health-related quality of life, emotional burden, and neurocognitive function in the first generation of metastatic melanoma survivors treated with pembrolizumab: A longitudinal pilot study. Support. Care Cancer 2020, 28, 3267–3278. [Google Scholar] [CrossRef]
- Rogiers, A.; Leys, C.; Lauwyck, J.; Schembri, A.; Awada, G.; Schwarze, J.K.; De Cremer, J.; Theuns, P.; Maruff, P.; De Ridder, M.; et al. Neurocognitive Function, Psychosocial Outcome, and Health-Related Quality of Life of the First-Generation Metastatic Melanoma Survivors Treated with Ipilimumab. J. Immunol. Res. 2020, 2020, 2192480. [Google Scholar] [CrossRef]
- Schagen, S.B.; Tsvetkov, A.S.; Compter, A.; Wefel, J.S. Cognitive adverse effects of chemotherapy and immunotherapy: Are interventions within reach? Nat. Rev. Neurol. 2022, 18, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Looman, E.L.; Cheng, P.F.; Lai-Kwon, J.; Morgan, L.; Wakkee, M.; Dummer, R.; Dimitriou, F. Health-related quality of life in survivors of advanced melanoma treated with anti-PD1-based immune checkpoint inhibitors. Cancer Med. 2023, 12, 12861–12873. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, A.; Hughes, P.; Mann, J.; Lai, Z.; Teh, J.J.; McLean, E.; Edmonds, K.; Lingard, K.; Chauhan, D.; Lynch, J.; et al. An immunotherapy survivor population: Health-related quality of life and toxicity in patients with metastatic melanoma treated with immune checkpoint inhibitors. Support. Care Cancer 2020, 28, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Lai-Kwon, J.; Inderjeeth, A.J.; Lisy, K.; Sandhu, S.; Rutherford, C.; Jefford, M. Impact of immune checkpoint inhibitors and targeted therapy on health-related quality of life of people with stage III and IV melanoma: A mixed-methods systematic review. Eur. J. Cancer 2023, 184, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Lee, J.S.; Ciuleanu, T.E.; Bernabe Caro, R.; Nishio, M.; Urban, L.; Audigier-Valette, C.; Lupinacci, L.; Sangha, R.; Pluzanski, A.; et al. Five-Year Survival Outcomes with Nivolumab Plus Ipilimumab Versus Chemotherapy as First-Line Treatment for Metastatic Non-Small-Cell Lung Cancer in CheckMate 227. J. Clin. Oncol. 2023, 41, 1200–1212. [Google Scholar] [CrossRef] [PubMed]
- Lai-Kwon, J.; Heynemann, S.; Flore, J.; Dhillon, H.; Duffy, M.; Burke, J.; Briggs, L.; Leigh, L.; Mileshkin, L.; Solomon, B.; et al. Living with and beyond metastatic non-small cell lung cancer: The survivorship experience for people treated with immunotherapy or targeted therapy. J. Cancer Surviv. 2021, 15, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Simard, S.; Savard, J. Fear of Cancer Recurrence Inventory: Development and initial validation of a multidimensional measure of fear of cancer recurrence. Support. Care Cancer 2009, 17, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Simard, S.; Savard, J. Screening and comorbidity of clinical levels of fear of cancer recurrence. J. Cancer Surviv. 2015, 9, 481–491. [Google Scholar] [CrossRef]
- Smith, A.B.; Costa, D.; Galica, J.; Lebel, S.; Tauber, N.; van Helmondt, S.J.; Zachariae, R. Spotlight on the Fear of Cancer Recurrence Inventory (FCRI). Psychol. Res. Behav. Manag. 2020, 13, 1257–1268. [Google Scholar] [CrossRef]
- Grassi, L.; Caruso, R.; Riba, M.B.; Lloyd-Williams, M.; Kissane, D.; Rodin, G.; McFarland, D.; Campos-Rodenas, R.; Zachariae, R.; Santini, D.; et al. Anxiety and depression in adult cancer patients: ESMO Clinical Practice Guideline. ESMO Open 2023, 8, 101155. [Google Scholar] [CrossRef]
- Weathers, F.W.; Litz, B.T.; Keane, T.M.; Palmieri, P.A.; Marx, B.P.; Schnurr, P.P. The PTSD Checklist for DSM-5 (PCL-5)—Standard [Measurement Instrument]. 2013. Available online: https://www.ptsd.va.gov/professional/assessment/documents/using-PCL5.pdf (accessed on 5 October 2023).
- Blevins, C.A.; Weathers, F.W.; Davis, M.T.; Witte, T.K.; Domino, J.L. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): Development and Initial Psychometric Evaluation. J. Trauma. Stress 2015, 28, 489–498. [Google Scholar] [CrossRef]
- Wortmann, J.H.; Jordan, A.H.; Weathers, F.W.; Resick, P.A.; Dondanville, K.A.; Hall-Clark, B.; Foa, E.B.; Young-McCaughan, S.; Yarvis, J.S.; Hembree, E.A.; et al. Psychometric analysis of the PTSD Checklist-5 (PCL-5) among treatment-seeking military service members. Psychol. Assess. 2016, 28, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Broadbent, D.E.; Cooper, P.F.; FitzGerald, P.; Parkes, K.R. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br. J. Clin. Psychol. 1982, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ponds, R.; Van Boxtel, M.; Jolles, J. De Cognitive Failure Questionnaire als maat voor subjectief cognitief functioneren. Tijdschr. Voor Neuropsychol. 2006, 2, 37–45. [Google Scholar]
- Carré, J.; Vom Hofe, A.; Boudoukha, A.-H. Psychopathologie de la vie quotidienne: Validation d’un nouveau questionnaire de défaillances cognitives. Psychol. Française 2014, 59, 167–182. [Google Scholar] [CrossRef]
- Elo, S.; Kyngas, H. The qualitative content analysis process. J. Adv. Nurs. 2008, 62, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Schotte, C.K.; Van Den Bossche, B.; De Doncker, D.; Claes, S.; Cosyns, P. A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depress. Anxiety 2006, 23, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Lynch, F.A.; Katona, L.; Jefford, M.; Smith, A.B.; Shaw, J.; Dhillon, H.M.; Ellen, S.; Phipps-Nelson, J.; Lai-Kwon, J.; Milne, D.; et al. Feasibility and Acceptability of Fear-Less: A Stepped-Care Program to Manage Fear of Cancer Recurrence in People with Metastatic Melanoma. J. Clin. Med. 2020, 9, 2969. [Google Scholar] [CrossRef] [PubMed]
- Andersen, B.L.; Lacchetti, C.; Ashing, K.; Berek, J.S.; Berman, B.S.; Bolte, S.; Dizon, D.S.; Given, B.; Nekhlyudov, L.; Pirl, W.; et al. Management of Anxiety and Depression in Adult Survivors of Cancer: ASCO Guideline Update. J. Clin. Oncol. 2023, 41, 3426–3453. [Google Scholar] [CrossRef]
- Deuning-Smit, E.; Custers, J.A.E.; Braam, C.I.W.; Hermens, R.; Prins, J.B. Toward implementation of an evidence-based intervention for fear of cancer recurrence: Feasibility in real-world psycho-oncology practice. Psychooncology 2024, 33, e6297. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, D.; Qin, N.; Liu, M.; Jiang, N.; Li, X. Factors Correlated With Fear of Cancer Recurrence in Cancer Survivors: A Meta-analysis. Cancer Nurs. 2022, 45, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Seiler, A.; Jenewein, J. Resilience in Cancer Patients. Front. Psychiatry 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Zwanenburg, L.C.; van der Lee, M.L.; Koldenhof, J.J.; Suijkerbuijk, K.P.M.; Schellekens, M.P.J. What patients with advanced cancer experience as helpful in navigating their life with a long-term response: A qualitative study. Support. Care Cancer 2024, 32, 222. [Google Scholar] [CrossRef]
- Bartels, F.; Strönisch, T.; Farmer, K.; Rentzsch, K.; Kiecker, F.; Finke, C. Neuronal autoantibodies associated with cognitive impairment in melanoma patients. Ann. Oncol. 2019, 30, 823–829. [Google Scholar] [CrossRef] [PubMed]

| Variables | n = 70 (100%) |
|---|---|
| Sex | |
| Male | 42 (60.0%) |
| Median age in years (range) | 65.0 (34–92) |
| Education | |
| Primary school | 3 (4.3%) |
| Lower secondary school | 8 (11.4%) |
| Higher secondary school | 21 (30.0%) |
| Vocational education | 7 (10.0%) |
| Higher education | 31 (44.3%) |
| Social status | |
| Professionally active | 29 (41.4%) |
| Sick leave | 6 (8.6%) |
| Retired | 35 (50.0%) |
| Civil status | |
| Married or cohabiting with partner | 59 (84.3%) |
| Divorced, single | 4 (5.7%) |
| Single, never married | 5 (7.1%) |
| Widowed | 2 (2.9%) |
| Children | |
| Young children at diagnosis (<18 y.o.) | 18 (25.7%) |
| Adult children at diagnosis (>18 y.o.) | 41 (58.6%) |
| No children | 11 (15.7%) |
| Psychiatric history | |
| Depression | 3 (4.3%) |
| Alcohol dependency | 1 (1.4%) |
| No psychiatric history | 66 (94.3%) |
| Psychotropic treatment | |
| Benzodiazepine | 5 (7.1%) |
| Antidepressant | 7 (10.6%) |
| Hypnotic Z-drug | 1 (1.5%) |
| No psychotropic treatment | 57 (81.4%) |
| Treatment and disease history | |
| ICB treatment | |
| Pembrolizumab | 36 (51.4%) |
| Ipilimumab + nivolumab (incl. nivolumab maintenance) | 13 (18.6%) |
| Nivolumab | 9 (12.9%) |
| Ipilimumab | 5 (7.1%) |
| Ipilimumab + dendritic cell therapy | 5 (7.1%) |
| Atezolizumab | 1 (1.4%) |
| Avelumab | 1 (1.4%) |
| Line of therapy | |
| 1st line | 30 (42.9%) |
| 2nd line | 23 (32.9%) |
| 3rd line | 14 (20.0%) |
| >3rd line | 3 (4.3%) |
| Previous systemic treatment before ICB | |
| Other ICB | 18 (25.7%) |
| BRAF/MEK inhibitor | 17 (24.3%) |
| Chemotherapy | 14 (20.0%) |
| Study drug (dendritic cell therapy or IFN) | 9 (12.9%) |
| Cancer type | |
| Melanoma | 57 (81.4%) |
| Non-small-cell lung carcinoma | 7 (10.0%) |
| Colorectal carcinoma | 2 (2.9%) |
| Renal-cell carcinoma | 2 (2.9%) |
| Transitional-cell carcinoma | 1 (1.4%) |
| Merkel-cell carcinoma | 1 (1.4%) |
| AJCC 8th edition cancer stage | |
| Melanoma | |
| IIIC | 4 (5.7%) |
| IV-M1a | 7 (10.0%) |
| IV-M1b | 14 (2.0%) |
| IV-M1c | 21 (30.0%) |
| IV-M1d | 11 (15.7%) |
| Non-small-cell lung carcinoma | |
| IIIA | 1 (1.4%) |
| IIIB | 1 (1.4%) |
| IVA | 3 (4.3%) |
| IVB | 3 (4.3%) |
| Colorectal carcinoma | |
| IV-M1c dMMR/MSI-high | 2 (2.9%) |
| Transitional-cell carcinoma | |
| IV-M1b | 1 (1.4%) |
| Renal-cell carcinoma | |
| IV | 2 (2.9%) |
| Merkel-cell carcinoma | |
| IIIB | 1 (1.4%) |
| Brain metastasis | 13 (18.6%) |
| Localized treatment for brain metastasis | |
| Stereotactical radiotherapy | 8 (11.4%) |
| Surgery | 2 (2.9%) |
| Surgery + post-surgical stereotactical radiotherapy | 1 (1.4%) |
| No localized treatment | 2 (2.9%) |
| Reason for stopping ICB treatment | |
| irAE | 27 (38.6%) |
| Elective | 40 (57.1%) |
| ICB ongoing | 3 (4.3%) |
| Years of survivorship since time of first ICB administration | |
| ≥5 years | 40 (57.1%) |
| 3–4 years | 18 (25.7%) |
| 1–2 years | 12 (17.1%) |
| Median years since starting ICB administration (range) | 5.1 (1.0–14.4) |
| Median years since the last ICB administration (range) | 3.3 (0.0–11.3) |
| Median years since complete metabolic remission (range) | 3.3 (0.0–13.3) |
| Median duration of ICB treatment in months (range) | 13.7 (0.6–68.7) |
| Theme | Category | Subcategory |
|---|---|---|
| Cancer as a traumatic experience | Contextual triggers of the certainty of dying | Diagnosis of metastasis Initial progression or recurrence of disease Oncologist communicating exhaustion of treatment options Grade 2 and 3 irAEs Pain and physical complaints due to bone metastasis |
| Coping strategies | Having confidence in the treatment and oncologist Undergoing the treatment in a detached way Mentally focusing on surviving | |
| Contextual triggers of the wish to die | Grade 2 and 3 irAEs Progression/recurrence of disease Difficulties in adjusting to “normal” life after achieving remission Co-existing life stressors Pain and physical complaints due to bone metastasis | |
| Contextual triggers of suicidal ideation | Difficulties in adjusting to “normal” life after achieving remission Co-existing life stressors Recurrence of disease | |
| Positive impact of cancer diagnosis | Mindful mindset Personal growth Changed perspective toward values of life More quality time with family and spouse | |
| Changes in relationship with spouse or family | Positive change | Becoming closer in relationship with partner or family Partner or family becoming more caring with patient |
| Both negative and positive impact | Not feeling emotional supported or not feeling understood Becoming closer while putting more burden on family | |
| Negative impact | Continued emotional and physical burden of the patient on the family Patient becoming more easily irritable and less tolerant Differences in values of life Not feeling emotional supported during the treatment | |
| Most burdensome symptoms | Physical symptoms | Chronic fatigue Lymphedema Cancer-related mobility problems Arthralgia Dyspnea |
| Emotional and cognitive complaints | Psychological distress Cognitive complaints | |
| Cognitive complaints | Diminished daily life activities | Difficulties with reading a book or newspaper Doing household tasks Doing job-related tasks in the workplace Doing hobbies Following a TV series or movie Driving |
| Care needs | Psychological care | Continued psychological care during and after treatment |
| Nutrition | General information on healthy nutrition Nutritional advice on losing weight, taking into account specific medication Nutritional advice to influence chronic fatigue |
| Diminished Daily Life Activities | Number of Patients Reporting Difficulties (n, %) | Examples from Patients |
|---|---|---|
| Household tasks | 12 (17.1%) |
|
| Following a TV series or movie | 6 (8.6%) |
|
| Professional context | 10 (14.3%) |
|
| Reading a book or newspaper | 17 (24.3%) |
|
| Doing hobbies | 7 (10.0%) |
|
| Driving | 3 (4.3%) |
|
| Cancer Survivors n = 70 | |
|---|---|
| Post-traumatic stress (PTSD checklist for DSM-5) | |
| Mean (sd) | 13.49 (12.1) |
| Median [range] | 10.0 [0–53] |
| Normal (n, %) | 65 (92.9%) |
| Indication of PTSD (n, %) | 5 (7.1%) |
| Fear of cancer recurrence (FCRI-SF) | |
| Mean (sd) | 14.43 (8.2) |
| Median [range] | 15.0 [0–34] |
| Normal | 32 (45.7%) |
| Clinical fear of cancer recurrence—sensitivity (≥13) | 38 (54.3%) |
| Clinical fear of cancer recurrence—specificity (≥16) | 32 (45.7%) |
| Pathological fear of cancer recurrence (≥22) | 15 (21.4%) |
| Cognitive complaints (CFQ) | |
| Mean (sd) | 29.9 (15.4) |
| Median [range] | 28.0 [0–83] |
| Normal (n, %) | 57 (81.4%) |
| Elevated (n, %) | 7 (10.0%) |
| Severe (n, %) | 6 (8.6%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanlaer, N.; Dirven, I.; Neyns, B.; Rogiers, A. Emotional Distress, Cognitive Complaints, and Care Needs among Advanced Cancer Survivors Treated with Immune Checkpoint Blockade: A Mixed-Method Study. Cancers 2024, 16, 1638. https://doi.org/10.3390/cancers16091638
Vanlaer N, Dirven I, Neyns B, Rogiers A. Emotional Distress, Cognitive Complaints, and Care Needs among Advanced Cancer Survivors Treated with Immune Checkpoint Blockade: A Mixed-Method Study. Cancers. 2024; 16(9):1638. https://doi.org/10.3390/cancers16091638
Chicago/Turabian StyleVanlaer, Nathalie, Iris Dirven, Bart Neyns, and Anne Rogiers. 2024. "Emotional Distress, Cognitive Complaints, and Care Needs among Advanced Cancer Survivors Treated with Immune Checkpoint Blockade: A Mixed-Method Study" Cancers 16, no. 9: 1638. https://doi.org/10.3390/cancers16091638
APA StyleVanlaer, N., Dirven, I., Neyns, B., & Rogiers, A. (2024). Emotional Distress, Cognitive Complaints, and Care Needs among Advanced Cancer Survivors Treated with Immune Checkpoint Blockade: A Mixed-Method Study. Cancers, 16(9), 1638. https://doi.org/10.3390/cancers16091638






