Outcomes of Modified Mayo Stage IIIa and IIIb Cardiac Light-Chain Amyloidosis: Real-World Experience in Clinical Characteristics and Treatment—67 Patients Multicenter Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Statistical Analysis
3. Results
3.1. Patient Information
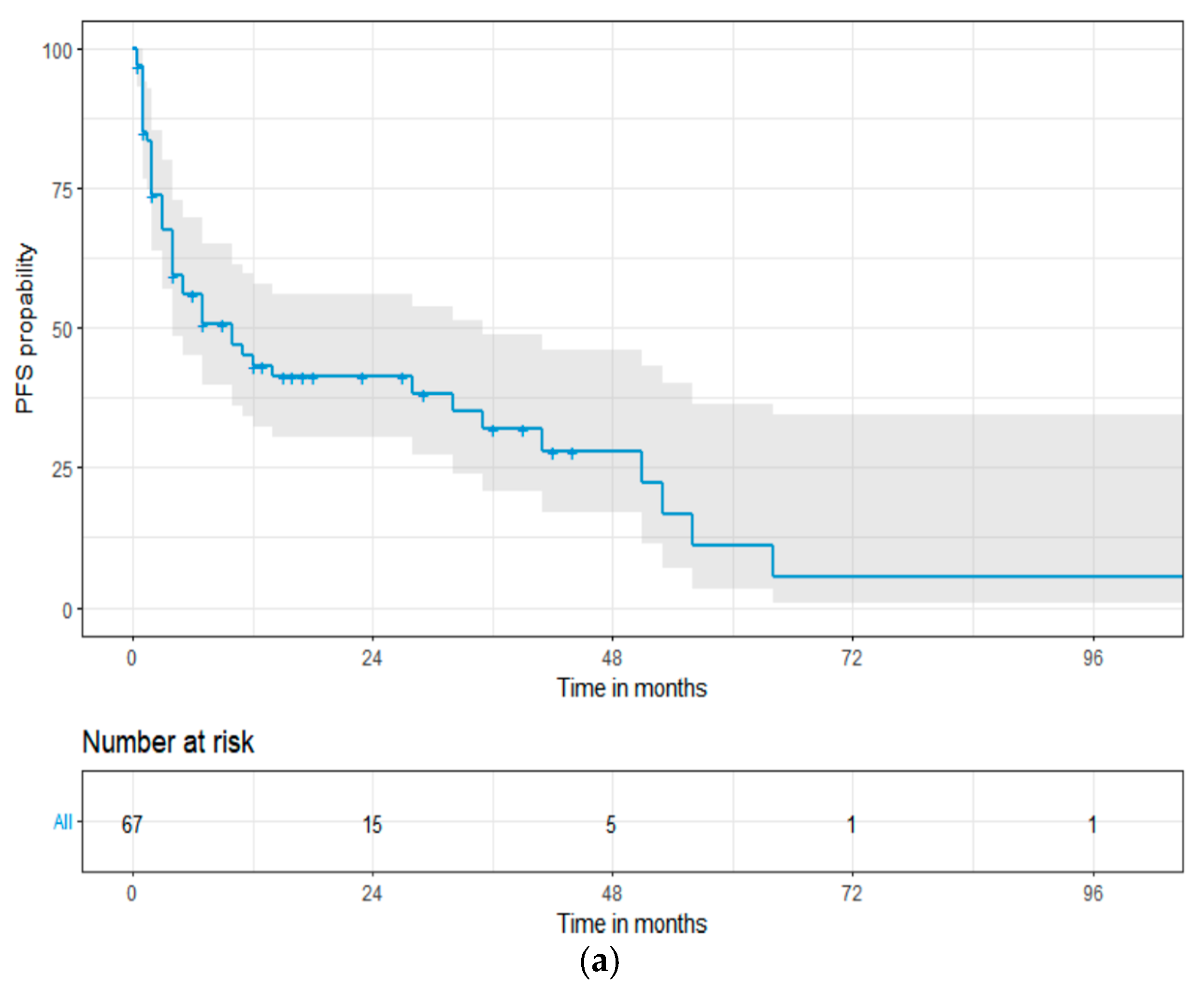
3.2. Methods and Effectiveness of First-Line Treatment
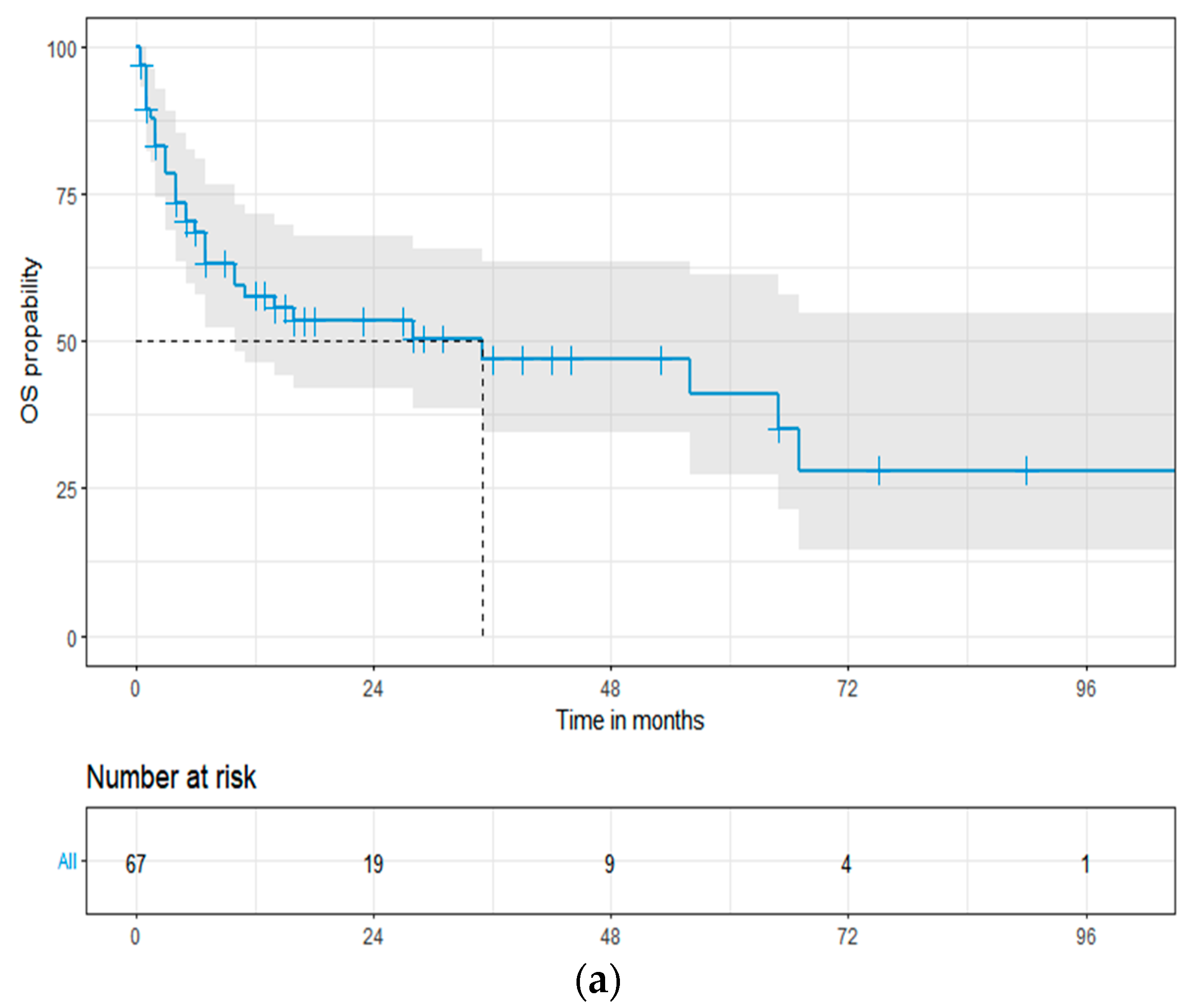
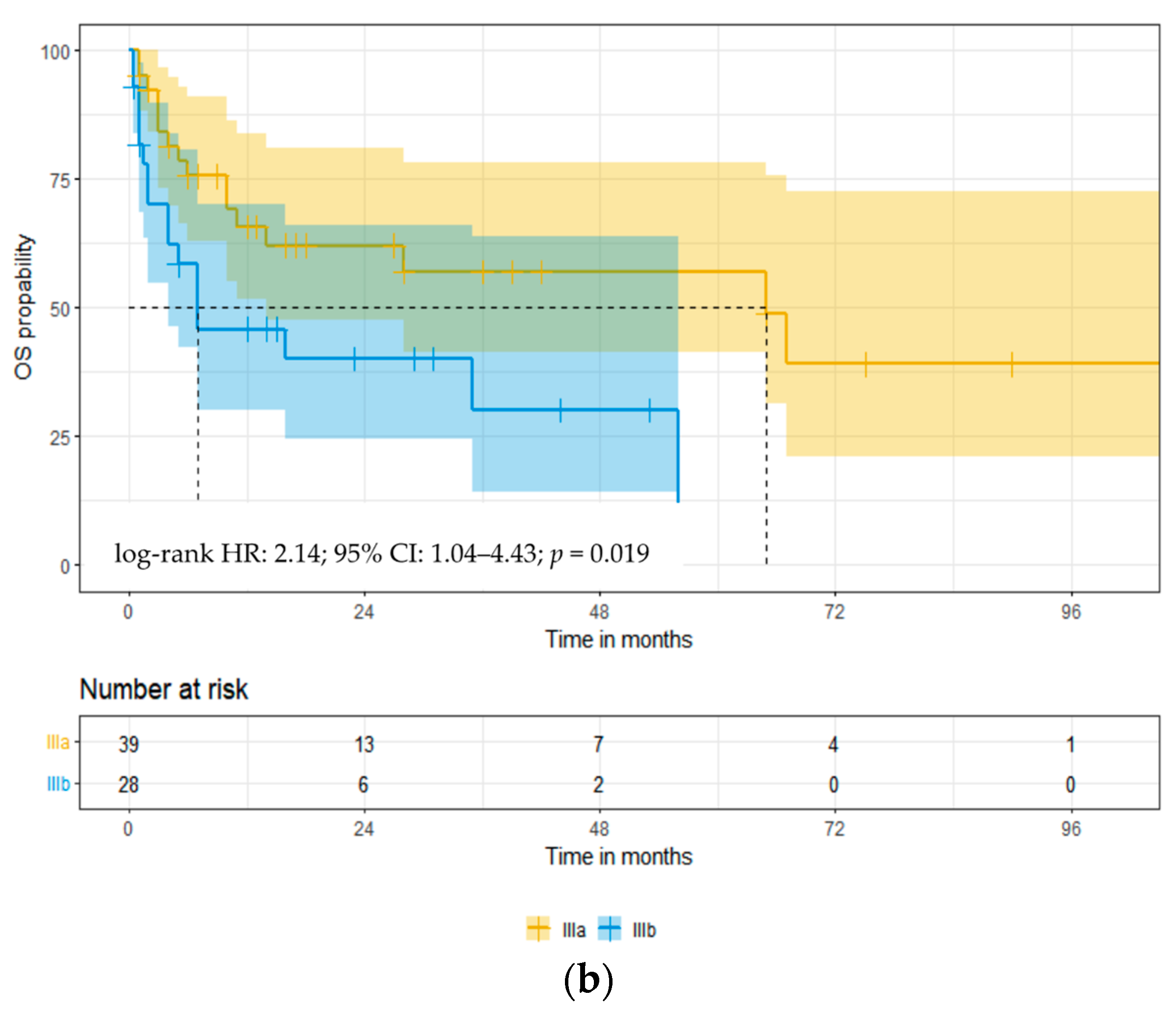
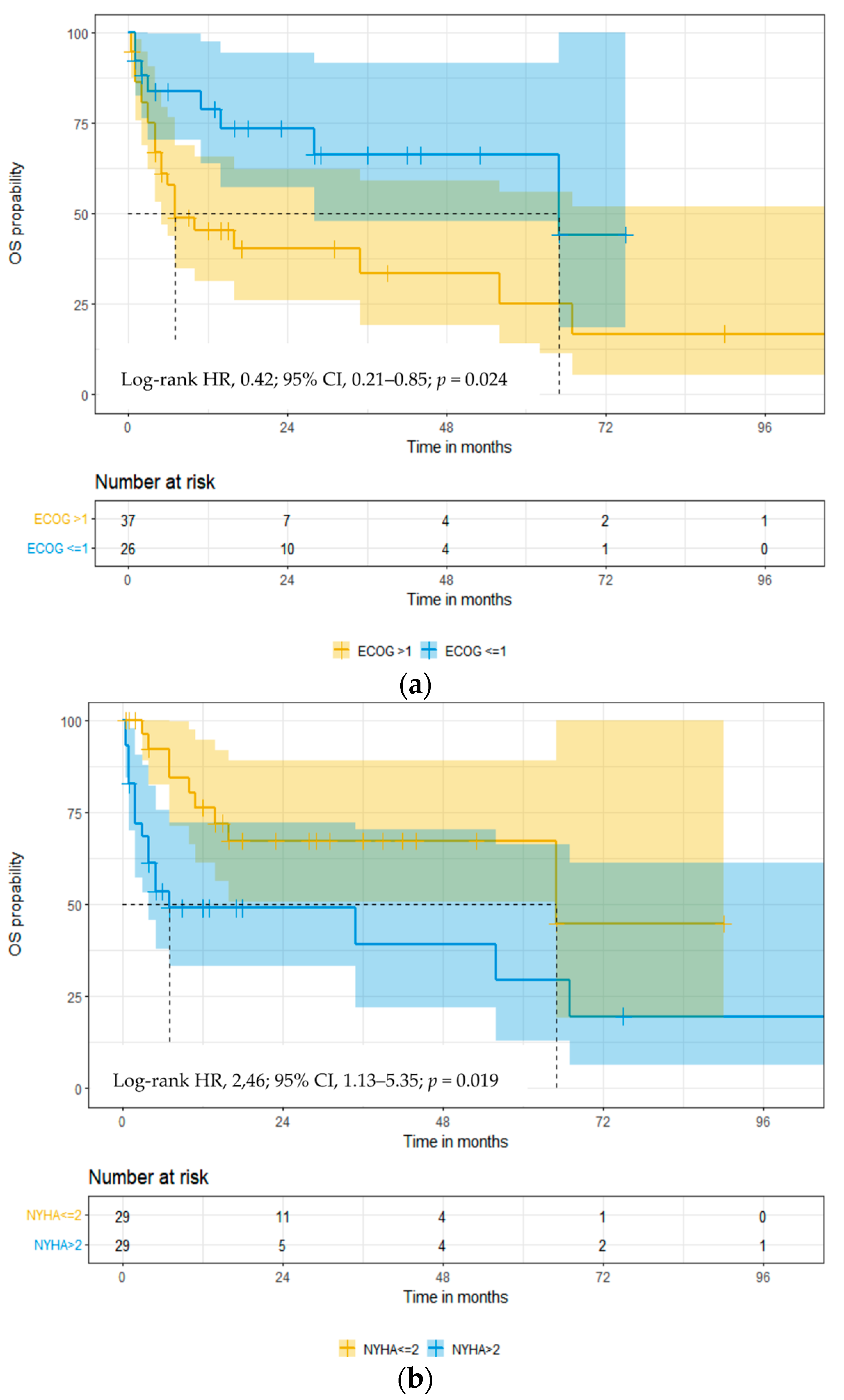
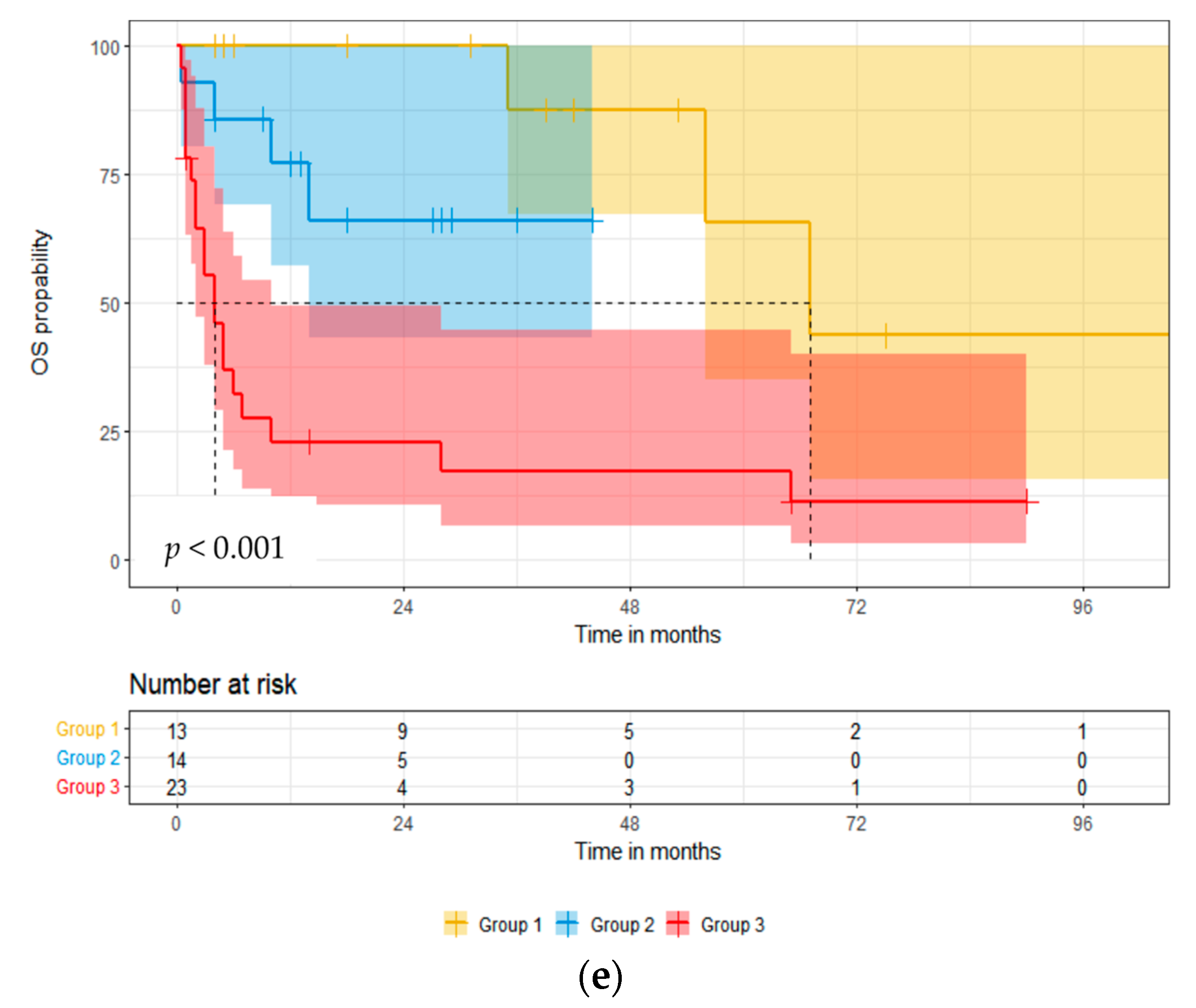
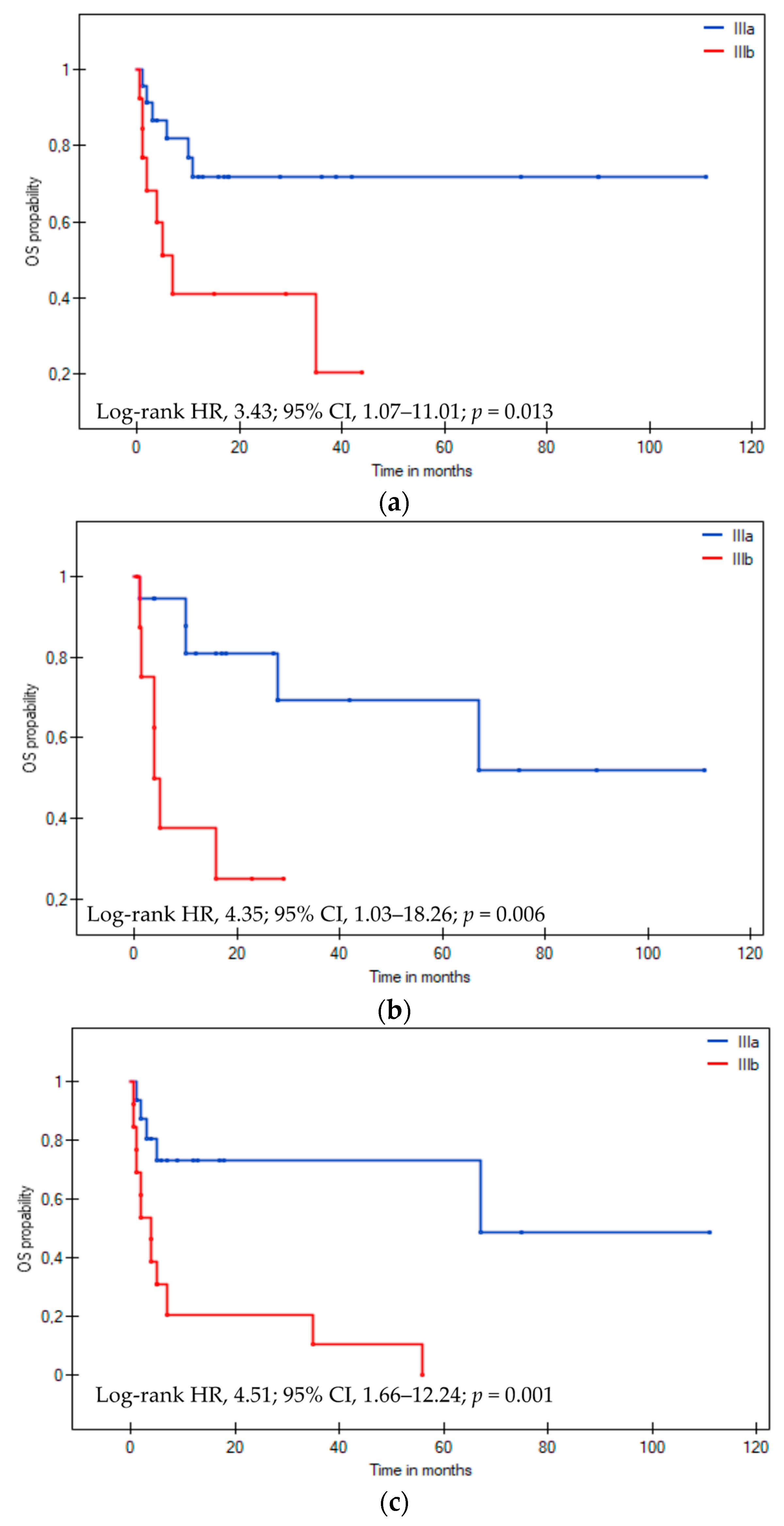
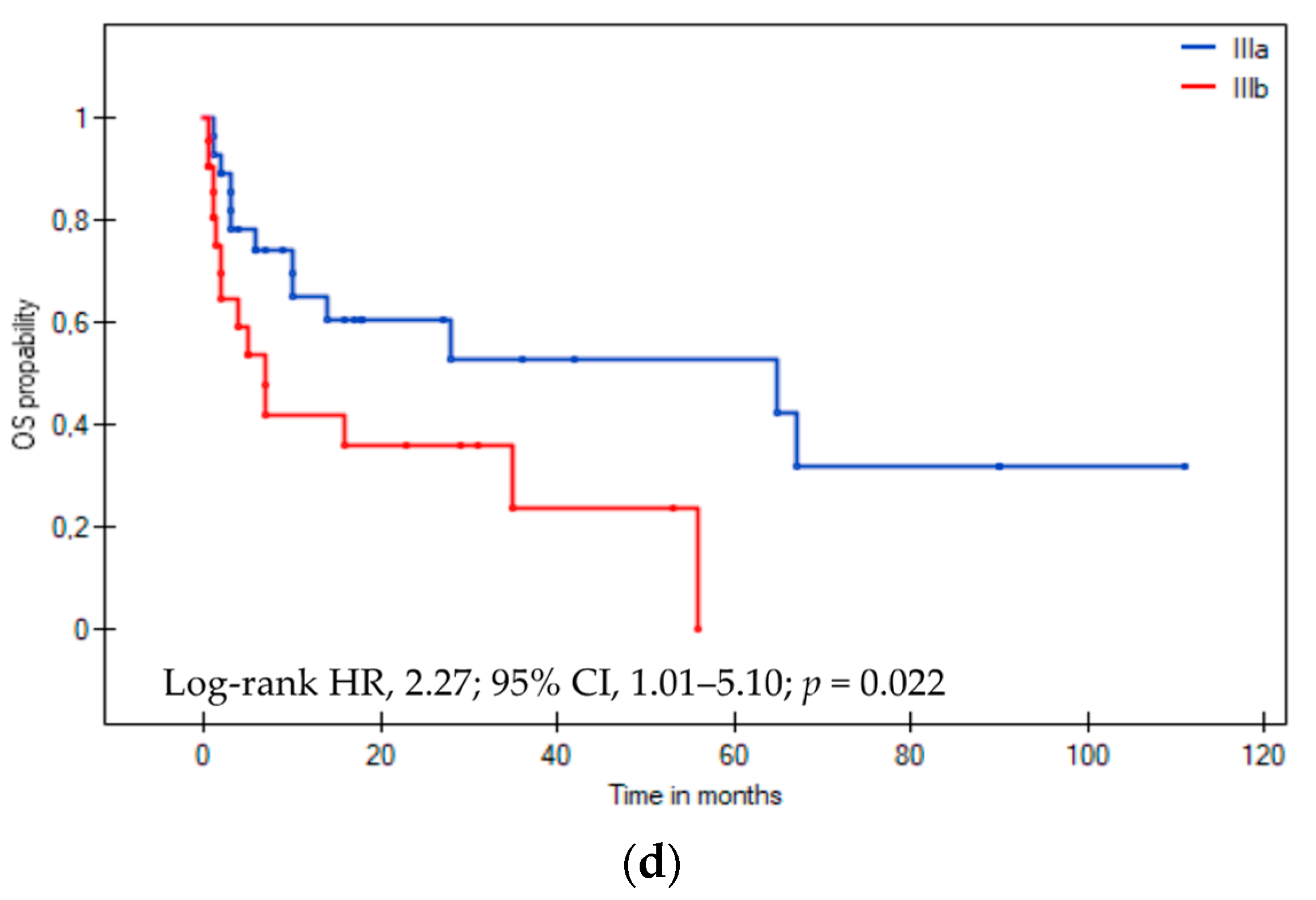
3.3. Overall Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Germain, P.; Vardazaryan, A.; Padoy, N.; Labani, A.; Roy, C.; Schindler, T.H.; El Ghannudi, S. Deep Learning Supplements Visual Analysis by Experienced Operators for the Diagnosis of Cardiac Amyloidosis by Cine-CMR. Diagnostics 2021, 12, 69. [Google Scholar] [CrossRef]
- Merlini, G.; Seldin, D.C.; Gertz, M.A. Amyloidosis: Pathogenesis and new therapeutic options. J. Clin. Oncol. 2011, 29, 1924–1933. [Google Scholar] [CrossRef]
- Blank, M.; Campbell, M.; Clarke, J.O.; Comenzo, R.; Dember, L.M.; Dispenzieri, A.; Dorbala, S.; Dunnmon, P.; Faller, D.V.; Rodney, H.F.; et al. The amyloidosis forum: A public private partnership to advance drug development in AL amyloidosis. Orphanet. J. Rare Dis. 2020, 15, 268. [Google Scholar]
- Desport, E.; Bridoux, F.; Sirac, C.; Delbes, S.; Bender, S.; Fermandez, B.; Quellard, N.; Lacombe, C.; Goujon, J.M.; Lavergne, D.; et al. AL amyloidosis. Orphanet. J. Rare Dis. 2012, 7, 54. [Google Scholar] [CrossRef]
- Quock, T.P.; Yan, T.; Chang, E.; Guthrie, S.; Broder, M.S. Epidemiology of AL amyloidosis: A real-world study using US claims data. Blood Adv. 2018, 2, 1046–1053. [Google Scholar] [CrossRef]
- Vaxman, I.; Gertz, M. Recent advances in the diagnosis, risk stratification, and management of systemic light-chain amyloidosis. Acta Haematol. 2019, 141, 93–106. [Google Scholar] [CrossRef]
- Gertz, M.A.; Dispenzieri, A. Systemic amyloidosis recognition, prognosis, and therapy: A systematic review. JAMA 2020, 324, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Gillmore, J.D.; Hawkins, P.N. Pathophysiology and treatment of systemic amyloidosis. Nat. Rev. Nephrol. 2013, 9, 574–586. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Schonland, S.; Merlini, G.; Milani, P.; Jaccard, A.; Bridoux, F.; Dimopoulos, M.A.; Ravichandran, S.; Hegenbart, U.; Roeloffzen, W.; et al. The management of light chain (AL) amyloidosis in Europe: Clinical characteristics, treatment patterns, and efficacy outcomes between 2004 and 2018. Blood Cancer J. 2023, 13, 19. [Google Scholar] [CrossRef]
- Kumar, S.K.; Gertz, M.A.; Lacy, M.Q.; Dingli, D.; Hayman, S.R.; Buadi, F.K.; Short-Detweiler, K.; Zeldenrust, S.R.; Leung, N.; Greipp, P.R.; et al. Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin. Proc. 2011, 86, 12–18. [Google Scholar] [CrossRef]
- Manwani, R.; Cohen, O.; Sharpley, F.; Mahmood, S.; Sachchithanantham, S.; Foard, D.; Lachmann, H.J.; Quarta, C.; Fontana, M.; Gillmore, J.D.; et al. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood 2019, 134, 2271–2280. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Dispenzieri, A.; Lacy, M.Q.; Hayman, S.R.; Buadi, F.K.; Colby, C.; Laumann, K.; Zeldenrust, S.R.; Leung, N.; Dingli, D.; et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J. Clin. Oncol. 2012, 30, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A.; Gertz, M.A.; Kyle, R.A.; Lacy, M.Q.; Burritt, M.F.; Therneau, T.M.; Greipp, P.R.; Witzing, T.E.; Lust, J.A.; Rajkumar, S.V.; et al. Serum cardiac troponins and N-terminal pro-brain natriuretic peptide: A staging system for primary systemic amyloidosis. J. Clin. Oncol. 2004, 22, 3751–3757. [Google Scholar] [CrossRef] [PubMed]
- Wechalekar, A.D.; Schonland, S.O.; Kastritis, E.; Gillmore, J.D.; Dimopoulos, M.A.; Lane, T.; Foli, A.; Foard, D.; Milani, P.; Rannigan, L.; et al. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood 2013, 121, 3420–3427. [Google Scholar] [CrossRef] [PubMed]
- Muchtar, E.; Gertz, M.A.; Kumar, S.K.; Lacy, M.Q.; Dingli, D.; Buadi, F.K.; Grogan, M.; Hayman, S.R.; Kapoor, P.; Leung, N.; et al. Improved outcomes for newly diagnosed AL amyloidosis between 2000 and 2014: Cracking the glass ceiling of early death. Blood 2017, 129, 2111–2119. [Google Scholar] [CrossRef] [PubMed]
- Wechalekar, A.D.; Gillmore, J.D.; Hawkins, P.N. Systemic amyloidosis. Lancet 2016, 387, 2641–2654. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Comenzo, R.; Falk, R.H.; Fermand, J.P.; Hazenberg, B.P.; Hawkins, P.N.; Merlini, G.; Moreau, P.; Ronco, P.; Sanchorawala, V.; et al. Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): A consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis, Tours, France, 18–22 April 2004. Am. J. Hematol. 2005, 79, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Palladini, G.; Dispenzieri, A.; Gertz, M.A.; Kumar, S.; Wechalekar, A.; Hawkins, P.N.; Schonland, S.; Hegenbart, U.; Comenzo, R.; Kastritis, E.; et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: Impact on survival outcomes. J. Clin. Oncol. 2012, 30, 4541–4549. [Google Scholar] [CrossRef] [PubMed]
- Muchtar, E.; Dispenzieri, A.; Wisniowski, B.; Palladini, G.; Milani, P.; Merlini, G.; Scholand, S.; Veelken, K.; Hegenbart, U.; Geyer, S.M.; et al. Graded cardiac response criteria for patients with systemic light chain amyloidosis. J. Clin. Oncol. 2023, 41, 1393–1403. [Google Scholar] [CrossRef]
- Kumar, S.K.; Mikhael, J.R.; Buadi, F.K.; Dingli, D.; Dispenzieri, A.; Fonseca, R.; Gertz, M.A.; Greipp, P.R.; Hayman, S.R.; Kyle, R.A.; et al. Management of Newly Diagnosed Symptomatic Multiple Myeloma: Updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) Consensus Guidelines. Mayo Clin. Proc. 2009, 84, 1095–1110. [Google Scholar] [CrossRef]
- Kaplan, E.; Meier, P. Non-parametric estimation from incomplete observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Kristen, A.V.; Brokbals, E.; Aus dem Siepen, F.; Bauer, R.; Hein, S.; Aurich, M.; Riffel, J.; Behrens, H.M.; Kruger, S.; Schirmacher, P.; et al. Cardiac amyloid load: A prognostic and predictive biomarker in patients with light-chain amyloidosis. J. Am. Coll. Cardiol. 2016, 68, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Lee, S.P.; Kim, Y.J.; Sohn, D.W. Incidence, diagnosis and prognosis of cardiac amyloidosis. Korean Circ. J. 2013, 43, 752–760. [Google Scholar] [CrossRef] [PubMed]
- Reece, D.E.; Hegenbart, U.; Sanchorawala, V.; Merlini, G.; Palladini, G.; Blade, J.; Fermand, J.P.; Hassoun, H.; Heffner, L.; Vescio, R.A.; et al. Efficacy and safety of once-weekly and twice- weekly bortezomib in patients with relapsed systemic AL amyloidosis: Results of a phase 1/2 study. Blood 2011, 118, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Wechalekar, A.D.; Dimopoulos, M.A.; Merlini, G.; Hawkins, P.N.; Perfetti, V.; Gillmore, J.D.; Palladini, G. Bortezomib with or without dexamethasone in primary systemic (light chain) amyloidosis. J. Clin. Oncol. 2010, 28, 1031–1037. [Google Scholar] [CrossRef]
- Venner, C.P.; Lane, T.; Foard, D.; Rannigan, L.; Gibbs, S.D.J.; Pinney, J.H.; Whelan, C.J.; Lachmann, H.J.; Gilmore, J.D.; Hawkins, P.N.; et al. Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood 2012, 119, 4387–4390. [Google Scholar] [CrossRef] [PubMed]
- Jaccard, A.; Comenzo, R.L.; Hari, P.; Hawkins, P.N.; Roussel, M.; Morel, P.; Macro, M.; Pellegrin, J.L.; Lazaro, E.; Mohty, D.; et al. Efficacy of bortezomib, cyclophosphamide and dexamethasone in treatment-naive patients with high-risk cardiac AL amyloidosis (Mayo Clinic stage III). Haematologica 2014, 99, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Sayago, I.; Krsnik, I.; Gomez-Bueno, M.; Garcia-Pavia, P.; Jaramillo, N.; Salas, C.; Mingo, S.; Oteo, J.F.; Alonso-Pulpon, L.; Segovia, J. Analysis of diagnostic and therapeutic strategies in advanced cardiac light-chain amyloidosis. J. Heart Lung Transplant. 2016, 35, 995–1002. [Google Scholar] [CrossRef]
- Palladini, G.; Kastritis, E.; Maurer, M.S.; Zonder, J.; Minnema, M.C.; Wechalekar, A.D.; Jaccard, A.; Lee, H.C.; Bumma, N.; Kaufman, J.L.; et al. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: Safety run-in results of ANDROMEDA. Blood 2020, 136, 71–80. [Google Scholar] [CrossRef]
- Charliński, G.; Tyczyńska, A.; Małecki, B.; Fornagiel, S.; Barchnicka, A.; Kołkowska, A.; Kopińska, A.; Usnarska-Zubkiewicz, L.; Robak, P.; Waszczuk-Gajda, A.; et al. Risk factors and causes of early mortality in patients with newly diagnosed multiple myeloma in a “real-world” study: Experiences of the Polish Myeloma Group. Pol. Arch. Intern. Med. 2021, 131, 527–534. [Google Scholar] [CrossRef]
- Gertz, M.A.; Landau, H.; Comenzo, R.L.; Seldin, D.; Weiss, B.; Zonder, J.; Merlini, G.; Schonland, S.; Walling, J.; Kinney, G.G.; et al. First-in-human phase I/II study of NEOD001 in patients with light chain amyloidosis and persistent organ dysfunction. J. Clin. Oncol. 2016, 34, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Gertz, M.A.; Cohen, A.; Comenzo, R.; Kastritis, E.; Landau, H.; Libby, H.; Liedtke, M.; Sanchorawala, V.; Schonland, S.; Wechalekar, A.; et al. Survival benefit of birtamimab in Mayo Stage IV AL. Amyloidosis in phase 3 VITAL study was consistent across all key baseline variables. HemaSphere 2023, 7 (Suppl. S2), 41–42. [Google Scholar] [CrossRef]
- Gertz, M.A.; Tripuraneni, R.; Kinney, G.G. Birtamimab in patients with Mayo stage IV AL. Amyloidosis: Rationale for confirmatory Affirm-AL phase 3 study design. Blood 2021, 138 (Suppl. S1), 2754–2756. [Google Scholar] [CrossRef]
- Nuvolone, M.; Nevone, A.; Merlini, G. Targeting amyloid fibrils by passive immunotherapy in systemic amyloidosis. BioDrugs 2022, 36, 591–608. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.V.; Gould, J.; Langer, A.L.; Mapara, M.; Radhakrishnan, J.; Maurer, M.S.; Raza, S.; Mears, J.G.; Wall, J.; Solomon, A.; et al. Analysis of the phase 1a/b study of chimeric fibril-reactive monoclonal antibody 11-1f4 in patients with AL amyloidosis. Blood 2016, 128, 643. [Google Scholar] [CrossRef]
- Edwards, C.V.; Gould, J.; Langer, A.L.; Mapara, M.; Radhakrishnan, J.; Maurer, M.S.; Raza, S.; Mears, J.G.; Wall, J.; Solomon, A.; et al. Interim analysis of the phase 1a/b study of chimeric fibril-reactive monoclonal antibody 11-1f4 in patients with AL amyloidosis. Amyloid 2017, 24, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.V.; Rao, N.; Bhutani, D.; Mapara, M.; Radhakrishnan, J.; Shames, S.; Maurer, M.S.; Leng, S.; Solomon, A.; Lentzsch, S.; et al. Phase 1a/b study of monoclonal antibody CAEL-101 (11-1f4) in patients with AL amyloidosis. Blood 2021, 138, 2632–2641. [Google Scholar] [CrossRef]
- Valent, J.; Liedtke, M.; Zonder, J.; Silowsky, J.; Kurman, M.; Daniel, E.; Jobes, J.; Harnet, M.; Bhattacharyya, A.; Quarta, C.C.; et al. Safety and tolerability of CAEL-101, an anti-amyloid monoclonal antibody, in combination with plasma cell dyscrasia therapy in patients with light-chain amyloidosis: Results from a phase 2 study. J. Am. Coll. Cardiol. 2022, 79 (Suppl. S9), 305. [Google Scholar] [CrossRef]


| Parameters | All Patients (n = 67) | Stage IIIa (n = 39; 58.2%) | Stage IIIb (n = 28, 41.8%) |
|---|---|---|---|
| n (%) or Median (Range) | n (%) or Median (Range) | n (%) or Median (Range) | |
| Male, n (%) | 36 (53.7) | 24 (61.5) | 12 (42.8) |
| Median age, years | 64 (41–83) | 63 (41–83) | 66 (41–83) |
| Distribution | |||
| <65 years | 36 (53.7) | 22 (56.4) | 14 (50.0) |
| ≥65 years | 31 (46.3) | 17 (43.6) | 14 (50.0) |
| ECOG PS, n = 63 | |||
| ≤1 | 26 (41.3) | 19 (52.8) | 7 (25.9) |
| >1 | 37 (58.7) | 17 (47.2) | 20 (74.1) |
| Comorbidities | |||
| 0/≥1 | 27 (40.3)/40 (59.7) | 18 (46.1)/21 (53.9) | 9 (32.1)/19 (67.9) |
| sFLC, n = 67 | |||
| lambda/kappa | 52 (77.6)/15 (22.4) | 32 (82.0)/7 (18.0) | 20 (71.4)/8 (28.6) |
| Heavy chain, n = 67 | |||
| IgG/IgA/IgM | 21 (31.3)/7 (10.4)/1 (1.5) | 14 (35.9)/5 (12.8)/0 (0.0) | 9 (32.1)/2 (7.1)/1 (3.6) |
| Other organ involvement | |||
| Kidneys, n = 66 | 42 (63.6) | 25 (64.1) | 17 (63.0) |
| Liver, n = 59 | 10 (16.9) | 5 (14.7) | 5 (20.0) |
| Peripheral neuropathy, n = 60 | 10 (16.7) | 6 (16.7) | 4 (16.7) |
| Autonomic neuropathy, n = 59 | 13 (22.0) | 6 (17.1) | 7 (29.2) |
| Gastrointestinal tract, n = 56 | 9 (16.1) | 5 (14.7) | 4 (18.2) |
| Cytogenetics | |||
| High-risk cytogenetics, n = 37 | 12 (32.4) | 8 (38.1) | 4 (25.0) |
| t(11;14) | 12 (32.4) | 7 (33.3) | 5 (31.2) |
| First-line chemotherapy | |||
| Bort-based | 64 (95.5) | 38 (97.4) | 26 (92.8) |
| Vd/VCd/Vd + IMiD | 28 (43.7)/25 (39.0)/11 (17.3) | 15 (39.5)/15 (39.5)/8 (21.0) | 13 (50.0)/10 (38.5)/3 (11.5) |
| IMiD-based (CTd, Rd) | 3 (4.5) | 1 (2.6) | 2 (7.2) |
| Daratumumab | 14 (20.9) | 8 (20.5) | 6 (21.4) |
| ASCT | 10 (14.9) | 9 (23.1) | 1 (3.6) |
| Laboratory parameters | |||
| CPBM > 10%, n = 67 | 20 (32.2) | 13 (33.3) | 7 (25.0) |
| Serum Hb (g/dL), n = 67 | 12.8 (7.7–17.5) | 12.7 (10.6–16.2) | 12.8 (7.7–17.5) |
| WBC count (×103/μL), n = 67 | 8.4 (3.8–17.1) | 8.2 (4.0–17.1) | 8.7 (3.8–16.4) |
| PLT count (×103/μL), n = 67 | 250.0 (114.0–624.0) | 260.0 (117.0–620.0) | 250.0 (114.0–624.0) |
| Serum albumin (mg/L), n = 67 | 3.2 (1.0–4.6) | 3.3 (1.0–4.6) | 3.2 (1.1–4.5) |
| Serum β2-microglobulin (mg/L), n = 49 | 4.0 (1.7–32.0) | 3.0 (1.7–18.0) | 5.5 (2.6–32.0) |
| sFLC lambda (mg/dL), n = 66 | 56.9 (0.7–7411.8) | 54.8 (0.7–1493.0) | 52.0 (0.9–7411.8) |
| sFLC kappa (mg/dL), n = 64 | 15.8 (0.2–3730.0) | 13.6 (0.5–588.0) | 16.0 (0.2–3730.0) |
| Serum LDH (IU/L), n = 49 | 267.0 (161.0–1021.0) | 250.0 (170.0–758.0) | 277.0 (161.0–1021.0) |
| Baseline echocardiography | 64 (95.5) | 39 (100.0) | 25 (89.3) |
| Baseline cardiac magnetic resonance | 31 (46.3) | 22 (56.4) | 9 (32.1) |
| Baseline endomyocardial biopsy | 15 (22.7) | 7 (17.9) | 8 (28.6) |
| Parameters | All Patients (n = 67) | Stage IIIa (n = 39; 58.2%) | Stage IIIb (n = 28; 41.8%) |
|---|---|---|---|
| n (%) or Median (Range) | n (%) or Median (Range) | n (%) or Median (Range) | |
| NYHA FC, grade, n = 58 | |||
| 1 | 3 (5.2) | 2 (6.3) | 1 (3.8) |
| 2 | 26 (44.8) | 14 (43.7) | 12 (46.2) |
| 3 | 24 (41.4) | 15 (46.9) | 9 (34.6) |
| 4 | 5 (8.6) | 1 (3.1) | 4 (15.4) |
| SBP (mmHg), n = 43 | 105 (32–152) | 105 (32–135) | 110 (70–152) |
| <100 mmHg | 16 (37.2) | 10 (37.0) | 6 (37.5) |
| LVEF (%), n = 60 | |||
| <50% | 14 (20.9) | 9 (25.0) | 5 (20.8) |
| ≥50% | 46 (68.7) | 27 (75.0) | 19 (79.2) |
| Cardiac Troponin T (μg/L), n = 24 | 54.5 (0.05–397.0) | 58.0 (0.07–242.0) | 97.8 (0.05–397.0) |
| ≥0.025 μg/L | 24 (100.0) | 15 (100.0) | 9 (100.0) |
| High sensitivity Troponin T (ng/L), n = 22 | 98.1 (45.0–566.0) | 68.5 (45.0–298.0) | 220.4 (57.0–566.0) |
| ≥40 ng/L | 22 (100.0) | 12 (100.0) | 10 (100.0) |
| Cardiac Troponin I (μg/L), n = 19 | 0.26 (0.02–4.7) | 0.23 (0.02–4.7) | 0.35 (0.04–0.36) |
| ≥0.1 μg/L | 15 (78.9) | 9 (75.0) | 6 (85.7) |
| NT-proBNP (ng/L), n = 67 | 6832.0 (655.0–70,000.0) | 4376.0 (655.0–8081.0) | 16,011.0 (8735.0–70,000.0) |
| ≥8500 μg/L | 28 (41.8) | 0 (0.0) | 28 (100.0) |
| Response | All Patients, n (%) | Stage IIIa, n (%) | Stage IIIb, n (%) |
|---|---|---|---|
| Hematological responses, n = 52 | |||
| Overall response rate | 37 (71.1) | 24 (75.0) | 13 (65.0) |
| Complete response | 15 (28.8) | 12 (37.6) | 3 (15.0) |
| Very good partial response | 9 (17.3) | 6 (18.7) | 3 (15.0) |
| Partial response | 13 (25.0) | 6 (18.7) | 7 (35.0) |
| No response | 15 (28.9) | 8 (25.0) | 7 (35.0) |
| Cardiac responses, n = 52 | |||
| Overall response rate | 20 (38.5) | 13 (40.6) | 7 (35.0) |
| Complete response | 3 (5.8) | 3 (9.4) | 0 (0.0) |
| Very good partial response | 2 (3.8) | 0 (0.0) | 2 (10.0) |
| Partial response | 15 (28.8) | 10 (31.2) | 5 (25.0) |
| No response | 32 (61.5) | 19 (59.4) | 13 (65.0) |
| Hematological and cardiac response, n = 50 | |||
| ≥hematological VGPR +≥cardiac PR | 13 (26.0) | 10 (33.3) | 3 (15.0) |
| Parameters | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Age ≥ 65 years | 1.76 (0.88–3.53) | 0.104 | ||
| Male | 1.06 (0.53–2.11 | 0.867 | ||
| Comorbidities ≥ 1 | 1.39 (0.81–2.38) | 0.226 | ||
| ECOG PS > 1 | 0.41 (0.18–0.92) | 0.032 | 0.23 (0.06–0.84) | 0.026 |
| NYHA FC > 2 | 2.54 (1.12–5.79) | 0.025 | 4.33 (1.09–17.21) | 0.037 |
| Stage IIIb at diagnosis | 2.25 (1.10–4.60) | 0.025 | 1.65 (0.56–4.84) | 0.36 |
| Clonal plasma cells in bone marrow ≥ 10% | 0.88 (0.41–1.86) | 0.739 | ||
| High-risk cytogenetic abnormalities | 2.62 (0.96–7.17) | 0.061 | ||
| ASCT treatment | 3.05 (1.12–8.25) | 0.028 | 0.35 (0.04–2.79) | 0.33 |
| Hematologic response ≥ VGPR | 0.21 (0.08–0.56) | 0.002 | 0.26 (0.08–0.83) | 0.023 |
| Cardiac response ≥ PR | 0.19 (0.06–0.58) | 0.003 | 0.06 (0.01–0.34) | 0.001 |
| Hematologic response ≥ VGPR + Cardiac response ≥ PR | 3.89 (1.79–8.41) | <0.001 | 4.30 (1.97–9.38) | <0.001 |
| Renal involvement | 0.97 (0.48–1.99) | 0.945 | ||
| Liver involvement | 1.81 (0.68–4.82) | 0.236 | ||
| GI tract involvement | 2.44 (1.03–5.80) | 0.042 | not included | |
| PN involvement | 1.45 (0.62–3.40) | 0.393 | ||
| Organs involved > 2 at diagnosis | 0.53 (0.18–1.52) | 0.238 | ||
| Hb < 12 g/dL | 1.38 (0.67–2.83) | 0.383 | ||
| SBP < 100 mmHg | 0.52 (0.18–1.45) | 0.209 | ||
| Serum albumin < 3.5 g/dL | 1.46 (0.65–3.29) | 0.352 | ||
| Serum β2-microglobulin ≥ 5.5 mg/L | 1.46 (0.66–3.25) | 0.346 | ||
| LDH > ULN | 2.47 (0.81–7.49) | 0.108 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charliński, G.; Steinhardt, M.; Rasche, L.; Gonzalez-Calle, V.; Peña, C.; Parmar, H.; Wiśniewska-Piąty, K.; Dávila Valls, J.; Olszewska-Szopa, M.; Usnarska-Zubkiewicz, L.; et al. Outcomes of Modified Mayo Stage IIIa and IIIb Cardiac Light-Chain Amyloidosis: Real-World Experience in Clinical Characteristics and Treatment—67 Patients Multicenter Analysis. Cancers 2024, 16, 1592. https://doi.org/10.3390/cancers16081592
Charliński G, Steinhardt M, Rasche L, Gonzalez-Calle V, Peña C, Parmar H, Wiśniewska-Piąty K, Dávila Valls J, Olszewska-Szopa M, Usnarska-Zubkiewicz L, et al. Outcomes of Modified Mayo Stage IIIa and IIIb Cardiac Light-Chain Amyloidosis: Real-World Experience in Clinical Characteristics and Treatment—67 Patients Multicenter Analysis. Cancers. 2024; 16(8):1592. https://doi.org/10.3390/cancers16081592
Chicago/Turabian StyleCharliński, Grzegorz, Maximilian Steinhardt, Leo Rasche, Veronica Gonzalez-Calle, Camila Peña, Harsh Parmar, Katarzyna Wiśniewska-Piąty, Julio Dávila Valls, Magdalena Olszewska-Szopa, Lidia Usnarska-Zubkiewicz, and et al. 2024. "Outcomes of Modified Mayo Stage IIIa and IIIb Cardiac Light-Chain Amyloidosis: Real-World Experience in Clinical Characteristics and Treatment—67 Patients Multicenter Analysis" Cancers 16, no. 8: 1592. https://doi.org/10.3390/cancers16081592
APA StyleCharliński, G., Steinhardt, M., Rasche, L., Gonzalez-Calle, V., Peña, C., Parmar, H., Wiśniewska-Piąty, K., Dávila Valls, J., Olszewska-Szopa, M., Usnarska-Zubkiewicz, L., Gozzetti, A., Ciofini, S., Gentile, M., Zamagni, E., Kurlapski, M., Legieć, W., Vesole, D. H., & Jurczyszyn, A. (2024). Outcomes of Modified Mayo Stage IIIa and IIIb Cardiac Light-Chain Amyloidosis: Real-World Experience in Clinical Characteristics and Treatment—67 Patients Multicenter Analysis. Cancers, 16(8), 1592. https://doi.org/10.3390/cancers16081592











