First-Line LV5FU2 with or without Aflibercept in Patients with Non-Resectable Metastatic Colorectal Cancer: A Randomized Phase II Trial (PRODIGE 25-FFCD-FOLFA)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Inclusion and Treatment
2.3. Study Objectives
2.4. Statistical Analyses
2.5. Ethics Approval
3. Results
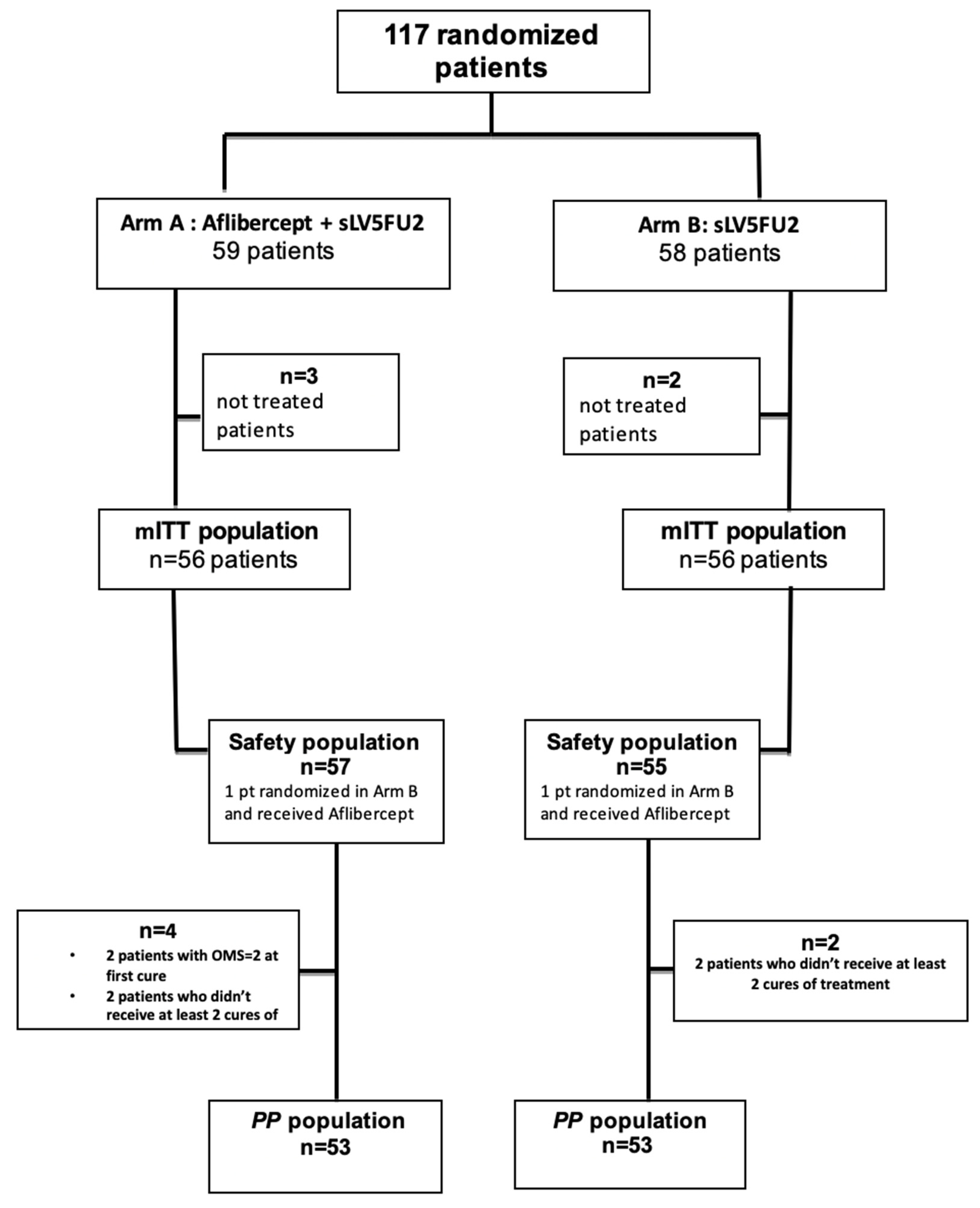
3.1. Population
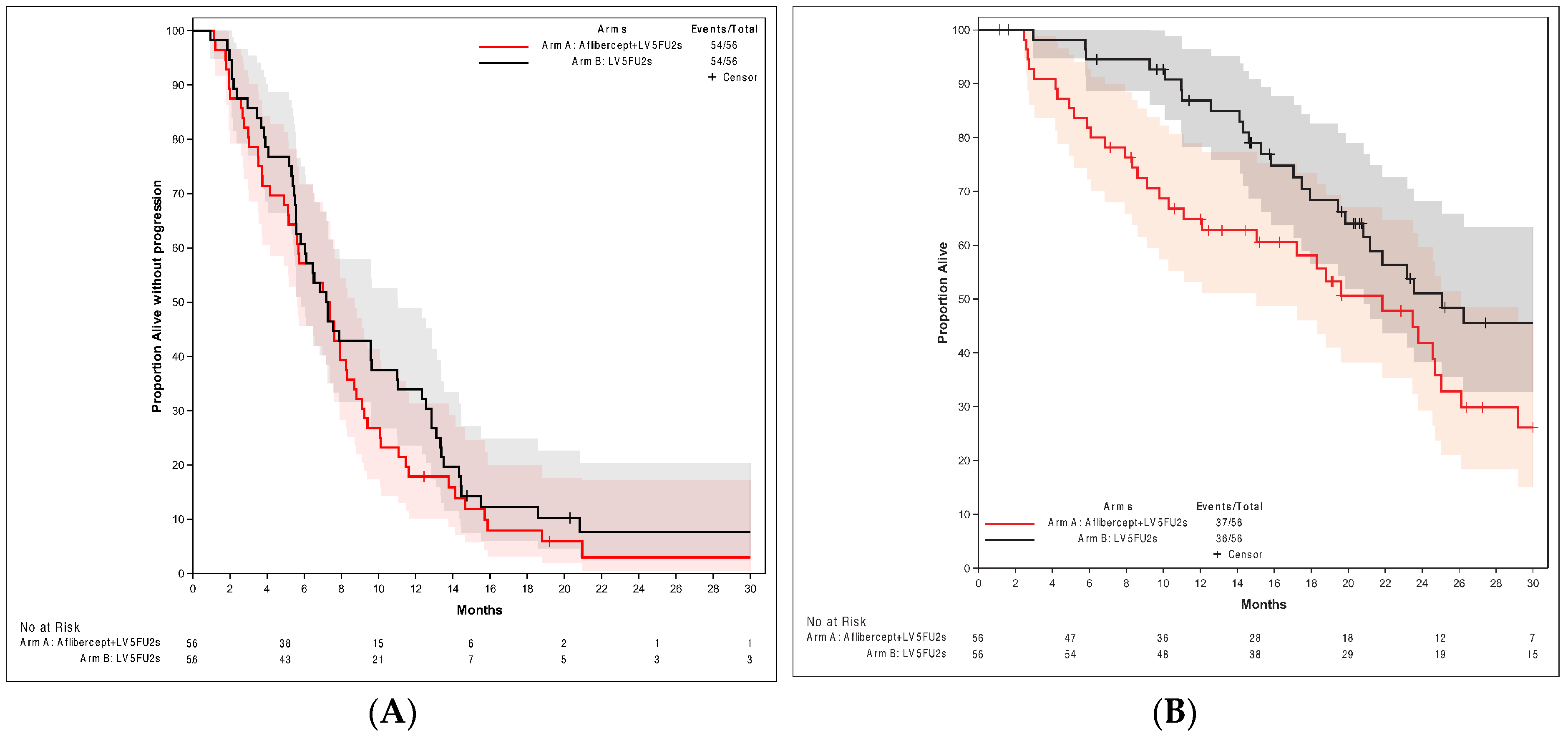
3.2. Treatment Efficacy
3.3. Toxicity
3.4. Follow-Up
4. Discussion
4.1. Choice of the Trial Design
4.2. PFS
4.3. OS
4.4. Toxicity
4.5. Limits of the Study
4.6. Place of Aflibercept in the Therapeutic Strategy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Jooste, V.; Remontet, L.; Colonna, M.; Belot, A.; Launoy, G.; Binder, F.; Faivre, J.; Bouvier, A.M. Trends in the incidence of digestive cancers in France between 1980 and 2005 and projections for the year 2010. Eur. J. Prev. 2011, 20, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, A.M.; Trétarre, B.; Delafosse, P.; Grosclaude, P.; Jéhannin-Ligier, K.; Marrer, E.; Molinié, F.; Woronoff, A.S.; Uhry, Z. Stade au diagnostic des cancers du sein, du côlon et du rectum. Etude réalisée à partir des registres des cancers du réseau Francim. Saint Maurice. Santé publique France. 2018, pp. 19–31. Available online: https://www.santepubliquefrance.fr/maladies-et-traumatismes/cancers/cancer-du-sein/documents/rapport-synthese/stade-au-diagnostic-des-cancers-du-sein-du-colon-et-du-rectum-etude-realisee-a-partir-des-registres-des-cancers-du-reseau-francim (accessed on 30 November 2023).
- Cottet, V.; Bouvier, V.; Rollot, F.; Jooste, V.; Bedenne, L.; Faivre, J.; Launoy, G.; Bouvier, A.M. Incidence and patterns of late recurrences in rectal cancer patients. Ann. Surg. Oncol. 2015, 22, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, A.M.; Launoy, G.; Bouvier, V.; Rollot, F.; Manfredi, S.; Faivre, J.; Cottet, V.; Jooste, V. Incidence and patterns of late recurrences in colon cancer patients. Int. J. Cancer 2015, 137, 2133–2138. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Lang, I.; Marcuello, E.; Lorusso, V.; Ocvirk, J.; Shin, D.B.; Jonker, D.; Stuart Osborne, S.; Andre, N.; Daniel Waterkamp, D.; et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): An open-label, randomised phase 3 trial. Lancet Oncol. 2013, 14, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Kabbinavar, F.F.; Schulz, J.; McCleod, M.; Patel, T.; Hamm, J.T.; Hecht, J.R.; Mass, R.; Brent Perrou, B.; Nelson, B.; Novotny, W.F. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: Results of a randomized phase II trial. J. Clin. Oncol. 2005, 23, 3697–3705. [Google Scholar] [CrossRef] [PubMed]
- Tebbutt, N.C.; Wilson, K.; Gebski, V.J.; Cummins, M.M.; Zannino, D.; van Hazel, G.A.; Robinson, B.; Broad, A.; Ganju, V.; Ackland, S.P.; et al. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: Results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J. Clin. Oncol. 2010, 28, 3191–3198. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Bouché, O.; Taieb, J.; Maillard, E.; Kirscher, S.; Etienne, P.L.; Faroux, F.; Khemissa Akouz, F.; El Hajbi, F.; Locher, C.; et al. Bevacizumab + chemotherapy versus chemotherapy alone in elderly patients with untreated metastatic colorectal cancer: A randomized phase II trial-PRODIGE 20 study results. Ann. Oncol. 2018, 29, 133–138, Erratum in Ann. Oncol. 2018, 29, 2270. https://doi.org/10.1093/annonc/mdx808. [Google Scholar] [CrossRef]
- Landre, T.; Maillard, E.; Taleb, C.; Ghebriou, D.; Des Guetz, G.; Laurent Zelek, L.; Aparicio, T. Impact of the addition of bevacizumab, oxaliplatin, or irinotecan to fluoropyrimidin in the first-line treatment of metastatic colorectal cancer in elderly patients. Int. J. Colorectal Dis. 2018, 33, 1125–1130. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Tabernero, J.; Lakomy, R.; Prenen, H.; Prausová, J.; Macarulla, T.; Ruff, P.; van Hazel, G.A.; Moiseyenko, V.; Ferry, D.; et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J. Clin. Oncol. 2012, 30, 3499–3506. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Khayat, D.; Verslype, C.; Billemont, B.; Tejpar, S.; Meric, J.B.; Soussan-Lazard, K.; Assadourian, S.; Cartot-Cotton, S.; Olivier Rixe, O. Phase I dose-escalation study of intravenous aflibercept administered in combination with irinotecan, 5-fluorouracil and leucovorin in patients with advanced solid tumours. Eur. J. Cancer 2013, 49, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ruff, P.; Van Cutsem, E.; Lakomy, R.; Prausova, J.; van Hazel, G.A.; Moiseyenko, V.M.; Soussan-Lazard, K.; Dochy, E.; Magherini, E.; Macarulla, T.; et al. Observed benefit and safety of aflibercept in elderly patients with metastatic colorectal cancer: An age-based analysis from the randomized placebo-controlled phase III VELOUR trial. J. Geriatr. Oncol. 2018, 9, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Folprecht, G.; Pericay, C.; Saunders, M.P.; Thomas, A.; Lopez Lopez, R.; Roh, J.K.; Chistyakov, V.; Höhler, T.; Kim, J.S.; Hofheinz, R.D.; et al. Oxaliplatin and 5-FU/folinic acid (modified FOLFOX6) with or without aflibercept in first-line treatment of patients with metastatic colorectal cancer: The AFFIRM study. Ann. Oncol. 2016, 27, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Seymour, M.T.; Thompson, L.C.; Wasan, H.S.; Middleton, G.; Brewster, A.E.; Shepherd, S.F.; Sinead O’Mahony, M.; Maughan, T.S.; Parmar, M.; Langley, R.E.; et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): An open label, randomised factorial trial. Lancet 2011, 377, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, A.; Adam, R.; Roselló, S.; Arnold, D.; Normanno, N.; Taïeb, J.; Seligmann, J.; De Baere, T.; Osterlund, P.; Yoshino, T.; et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2023, 34, 10–32. [Google Scholar] [CrossRef] [PubMed]
- Modest, D.P.; Fischer von Weikersthal, L.; Decker, T.; Vehling-Kaiser, U.; Uhlig, J.; Schenk, M.; Freiberg-Richter, J.; Peuser, B.; Denzlinger, C.; Peveling Genannt Reddemann, C.; et al. Sequential Versus Combination Therapy of Metastatic Colorectal Cancer Using Fluoropyrimidines, Irinotecan, and Bevacizumab: A Randomized, Controlled Study-XELAVIRI (AIO KRK0110). J. Clin. Oncol. 2019, 37, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Kurreck, A.; Heinemann, V.; Fischer von Weikersthal, L.; Decker, T.; Kaiser, F.; Uhlig, J.; Schenk, M.; Freiberg-Richter, J.; Peuser, B.; Denzlinger, C.; et al. Impact of age on efficacy and early mortality of initial sequential treatment versus upfront combination chemotherapy in patients with metastatic colorectal cancer: A subgroup analysis of a phase III trial (AIO KRK0110, XELAVIRI study). Eur. J. Cancer 2020, 137, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Jennings, B.A.; Kwok, C.S.; Willis, G.; Matthews, V.; Wawruch, P.; Loke, Y.K. Functional polymorphisms of folate metabolism and response to chemotherapy for colorectal cancer, a systematic review and meta-analysis. Pharmacogenet Genom. 2012, 22, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Boige, V.; Mendiboure, J.; Pignon, J.P.; Loriot, M.A.; Castaing, M.; Barrois, M.; Malka, D.; Trégouët, D.A.; Bouché, O.; Le Corre, D.; et al. Pharmacogenetic assessment of toxicity and outcome in patients with metastatic colorectal cancer treated with LV5FU2, FOLFOX, and FOLFIRI: FFCD 2000-05. J. Clin. Oncol. 2010, 28, 2556–2564. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). J. Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Aaronson, N.K.; Ahmedzai, S.; Bergman, B.; Bullinger, M.; Cull, A.; Duez, N.J.; Filiberti, A.; Flechtner, H.; Fleishman, S.B.; de Haes, J.C.; et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. J. Natl. Cancer Inst. 1993, 85, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Köhne, C.H.; Cunningham, D.; Di Costanzo, F.; Glimelius, B.; Blijham, G.; Aranda, E.; Scheithauer, W.; Rougier, P.; Palmer, M.; Wils, J.; et al. Clinical determinants of survival in patients with 5-fluorouracil based treatment for metastatic colorectal cancer: Results of a multivariate analysis of 3825 patients. Ann. Oncol. 2002, 13, 308–317. [Google Scholar] [CrossRef]
- Chionh, F.; Lau, D.; Yeung, Y.; Price, T.; Tebbutt, N. Oral versus intravenous fluoropyrimidines for colorectal cancer. Cochrane Database Syst. Rev. 2017, 7, CD008398. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Deng, Y. Capecitabine versus continuous infusion fluorouracil for the treatment of advanced or metastatic colorectal Cancer: A meta-analysis. Curr. Treat. Options Oncol. 2018, 19, 77. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Twelves, C.; Cassidy, J.; Allman, D.; Bajetta, E.; Boyer, M.; Bugat, R.; Findlay, M.; Frings, S.; Jahn, M.; et al. Oral capecitabine compared with intravenous fluorouracil plus leucovorin in patients with metastatic colorectal cancer: Results of a large phase III study. J. Clin. Oncol. 2001, 19, 4097–4106. [Google Scholar] [CrossRef]
- Hoff, P.M.; Ansari, R.; Batist, G.; Cox, J.; Kocha, W.; Kuperminc, M.; Maroun, J.; Walde, D.; Weaver, C.; Harrison, E.; et al. Comparison of oral capecitabine versus intravenous fluorouracil plus leucovorin as first-line treatment in 605 patients with metastatic colorectal cancer: Results of a randomized phase III study. J. Clin. Oncol. 2001, 19, 2282–2292. [Google Scholar] [CrossRef]
- Patel, A.; Spychalski, P.; Antoszewska, M.; Regula, J.; Kobiela, J. Proton pump inhibitors and colorectal cancer: A systematic review. World J. Gastroenterol. 2021, 27, 7716–7733. [Google Scholar] [CrossRef]
- Aparicio, T.; Lavau-Denes, S.; Phelip, J.M.; Maillard, E.; Jouve, J.L.; Gargot, D.; Gasmi, M.; Locher, C.; Adhoute, X.; Michel, M.; et al. Randomized phase III trial in elderly patients comparing LV5FU2 with or without irinotecan for first-line treatment of metastatic colorectal cancer (FFCD 2001-02). Ann. Oncol. 2016, 27, 121–127. [Google Scholar] [CrossRef]
- Seymour, M.T.; Maughan, T.S.; Ledermann, J.A.; Topham, C.; Roger James, R.; Gwyther, S.J.; Smith, D.B.; Shepherd, S.; Maraveyas, A.; Ferry, D.R.; et al. Different strategies of sequential and combination chemotherapy for patients with poor prognosis advanced colorectal cancer (MRC FOCUS): A randomised controlled trial. Lancet 2007, 370, 143–152. [Google Scholar] [CrossRef]
- Phelip, J.M.; Tougeron, D.; Léonard, D.; Benhaim, L.; Desolneux, G.; Dupré, A.; Michel, P.; Penna, C.; Tournigand, C.; Louvet, C.; et al. Metastatic colorectal cancer (mCRC): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, ACHBT, SFRO, SFR). Dig. Liver Dis. 2019, 51, 1357–1363, Update September 2023. Available online: https://www.snfge.org/tncd/cancer-colorectal-metastatique (accessed on 31 October 2023). [CrossRef]
- Bennouna, J.; Sastre, J.; Arnold, D.; Österlund, P.; Greil, R.; Van Cutsem, E.; von Moos, R.; Viéitez, J.M.; Bouché, O.; Borg, C.; et al. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Paccard, C.; Chiron, M.; Tabernero, J. Impact of prior bevacizumab treatment on VEGF-A and PlGF levels and outcome following second-line aflibercept treatment: Biomarker post hoc analysis of the VELOUR trial. Clin. Cancer Res. 2020, 26, 717–725. [Google Scholar] [CrossRef] [PubMed]
| Arm A: Aflibercept + sLV5FU2 a | Arm B: sLV5FU2 a | Total | ||
|---|---|---|---|---|
| (n = 56) | (n = 56) | (n = 112) | ||
| Age | n | 59 | 58 | 117 |
| Moy. (SD) | 80.27 (5.43) | 80.23 (5.40) | 80.25 (5.39) | |
| Médiane | 80.99 | 80.68 | 80.87 | |
| Q1; Q3 | 76.47; 83.68 | 78.07; 83.92 | 77.43; 83.76 | |
| Min; Max | 68.15; 90.49 | 67.05; 90.46 | 67.05; 90.49 | |
| ≤75 years | 13 (22.0%) | 9 (15.5%) | 22 (18.8%) | |
| >75 years | 46 (78.0%) | 49 (84.5%) | 95 (81.2%) | |
| Number of metastatic sites | n | 59 | 58 | 117 |
| 1 | 23 (39.0%) | 26 (44.8%) | 49 (41.9%) | |
| >1 | 36 (61.0%) | 32 (55.2%) | 68 (58.1%) | |
| TS-5UTR polymorphism | n | 59 | 58 | 117 |
| 2R2R genotype | 17 (28.8%) | 15 (25.9%) | 32(27.4%) | |
| 2R3R genotype | 28 (47.5%) | 29 (50.0%) | 57 (48.7%) | |
| 3R3R genotype | 14 (23.7%) | 14 (24.1%) | 28 (23.9%) | |
| BMI (Kg/m2) | n | 56 | 56 | 112 |
| Median | 24.84 | 26.12 | 25.56 | |
| Q1; Q3 | 21.85; 28.15 | 23.50; 28.04 | 22.94; 28.04 | |
| Min; Max | 18.36; 36.51 | 17.36; 37.10 | 17.36; 37.10 | |
| WHO performance status | n | 56 | 56 | 112 |
| 0 | 14 (25.0%) | 23 (41.1%) | 37 (33.0%) | |
| 1 | 42 (75.0%) | 33 (58.9%) | 75 (67.0%) | |
| Systolic blood pressure (mmHg) | n | 48 | 42 | 90 |
| Median | 139.00 | 134.50 | 136.50 | |
| Q1; Q3 | 124.00; 150.00 | 126.00; 145.00 | 125.00; 147.00 | |
| Min; Max | 103.00; 210.00 | 110.00; 188.00 | 103.00; 210.00 | |
| Diastolic blood pressure (mmHg) | n | 47 | 42 | 89 |
| Median | 70.00 | 74.50 | 73.00 | |
| Q1; Q3 | 68.00; 80.00 | 70.00; 80.00 | 69.00; 80.00 | |
| Min; Max | 59.00; 110.00 | 60.00; 91.00 | 59.00; 110.00 | |
| Köhne score | n | 56 | 56 | 112 |
| Low | 22 (39.3%) | 25 (44.6%) | 47 (42.0%) | |
| Middle | 27 (48.2%) | 30 (53.6%) | 57 (50.9%) | |
| High | 7 (12.5%) | 1 (1.8%) | 8 (7.1%) | |
| Creatinine clearance (mL/min) | n | 56 | 56 | 112 |
| Median | 65.0 | 67.5 | 66.5 | |
| Q1; Q3 | 57.5; 81.0 | 56.0; 84.5 | 57.0; 82.5 | |
| Min; Max | 42.00; 130.00 | 42.00; 126.00 | 42.00; 130.00 | |
| Albumin (g/L) | n | 53 | 53 | 106 |
| Median | 38.00 | 40.00 | 39.00 | |
| Q1; Q3 | 35.00; 41.00 | 38.00; 43.00 | 36.00; 41.00 | |
| Min; Max | 29.00; 45.00 | 22.00; 48.00 | 22.00; 48.00 | |
| Arm A: Aflibercept + sLV5FU2 a | Arm B: sLV5FU2 a | Total | p-Value | ||
|---|---|---|---|---|---|
| (n = 56) | (n = 56) | (n = 112) | |||
| Time from diagnosis to randomization (months) | n | 53 | 51 | 104 | |
| Median | 1.94 | 1.81 | 1.89 | ||
| Min; Max | 0.62; 57.82 | 0.33; 38.28 | 0.33; 57.82 | ||
| Location of the primary tumor | n | 55 | 55 | 110 | |
| Rectum | 10 (18.2%) | 13 (23.6%) | 23 (20.9%) | ||
| Right colon | 32 (58.2%) | 31 (56.4%) | 63 (57.3%) | ||
| Left colon | 13 (23.6%) | 11 (20.0%) | 24 (21.8%) | ||
| Resection of the primary tumor | n | 56 | 56 | 112 | |
| 31 (55.4%) | 39 (69.6%) | 70 (62.5%) | |||
| Synchronous or metachronous metastases | n | 50 | 51 | 101 | |
| Metachronous | 15 (30.0%) | 22 (43.1%) | 37 (36.6%) | ||
| Synchronous | 35 (70.0%) | 29 (56.9%) | 64 (63.4%) | ||
| Hepatic metastases | n | 56 | 56 | 112 | |
| 43 (76.8%) | 39 (69.6%) | 82 (73.2%) | |||
| Pulmonary metastases | n | 56 | 56 | 112 | |
| 30 (53.6%) | 33 (58.9%) | 63 (56.3%) | |||
| Peritoneal metastases | n | 56 | 56 | 112 | |
| 8 (14.3%) | 16 (28.6%) | 24 (21.4%) | |||
| Alkaline phosphatases (UI/L) | n | 56 | 55 | 111 | W: 0.02 |
| Median | 126.00 | 96.00 | 106.00 | ||
| Min; Max | 54.00; 978.00 | 31.00; 442.00 | 31.00; 978.00 | ||
| GGT (UI/L) | n | 56 | 54 | 110 | W: 0.04 |
| Median | 93.50 | 51.00 | 76.50 | ||
| Min; Max | 12.00; 1957.00 | 12.00; 868.00 | 12.00; 1957.00 | ||
| Lactate Dehydrogenase (UI/L) | n | 48 | 52 | 100 | |
| Median | 250.50 | 301.50 | 283.00 | ||
| Min; Max | 115.00; 2083.00 | 133.00; 1129.00 | 115.00; 2083.00 | ||
| RAS status | n | 56 | 56 | 112 | |
| Wild | 24 (42.9%) | 17 (30.4%) | 41 (36.6%) | ||
| Mutated | 24 (42.9%) | 34 (60.7%) | 58 (51.8%) | ||
| Not done | 8 (14.3%) | 5 (8.9%) | 13 (11.6%) | ||
| BRAF status | n | 56 | 56 | 112 | |
| Wild | 38 (67.9%) | 36 (64.3%) | 74 (66.1%) | ||
| Mutated | 6 (10.7%) | 3 (5.4%) | 9 (8.0%) | ||
| Not done | 12 (21.4%) | 17 (30.4%) | 29 (25.9%) |
| Arm A: Aflibercept + sLV5FU2 a | Arm B: sLV5FU2 a | |||
|---|---|---|---|---|
| Grade 1/2 | Grade 3/4/5 | Grade 1/2 | Grade 3/4/5 | |
| (n = 57) | (n = 57) | (n = 55) | (n = 55) | |
| At least one toxicity | 57 (100.0) | 47 (82.5) | 55 (100.0) | 32 (58.2) |
| Obstruction or sub-obstruction | 1 | 3 | 3 | |
| Digestive perforation | 1 (1.8) | |||
| Hemorrhage | 3 (5.3) | 2 (3.5) | 1 (1.8) | |
| Hematological toxicity | ||||
| Anemia | 35 (61.4) | 2 (3.5) | 41 (74.5) | 1 (1.8) |
| Neutropenia | 7 (12.3) | 2 (3.5) | 13 (23.6) | 3 (5.5) |
| Thrombopenia | 16 (28.1) | 15 (27.3) | ||
| Cardiovascular toxicity (all) | 22 (38.6) | 33 (57.8) | 20 (36.4) | 16 (29.1) |
| Rhythm disorders | 2 (3.6) | 2 (3.6) | ||
| Thoracic pain | 1 (1.8) | 1 (1.8) | ||
| Cardiac insufficiency | 1 (1.8) | |||
| Unspecified tromboembolic event | 1 (1.8) | 1 (1.8) | ||
| Arterial tromboembolic event | 2 (3.5) | 1 (1.8) | ||
| Venous tromboembolic event | 1 (1.8) | 4 (7.0) | 4 (7.3) | 2 (3.6) |
| Peripheral ischemia | 1 (1.8) | |||
| Stroke | 3 | 1 | 1 | |
| Hematoma | 1 (1.8) | |||
| Hypertension | 13 (22.8) | 24 (42.1) | 10 (18.2) | 10 (18.2) |
| Hypotension | 3 (5.3) | 1 (1.8) | ||
| Renal toxicity | ||||
| Proteinuria | 22 (38.6) | 7 (12.3) | 6 (10.9) | |
| Increased creatinine | 22 (38.6) | 1 (1.8) | 21 (38.2) | |
| Other toxicities | ||||
| Palmar-plantar erythrodysthesia syndrome | 15 (26.3) | 3 (5.3) | 12 (21.8) | 1 (1.8) |
| Oral mucositis | 24 (42.1) | 1 (1.8) | 23 (41.8) | 1 (1.8) |
| Nausea | 19 (33.3) | 22 (40.0) | ||
| Headache | 7 (12.3) | 1 (1.8) | 1 (1.8) | |
| Peripheral sensory neuropathy | 5 (8.8) | 1 (1.8) | ||
| Alteration of voice | 11 (19.3) | 1 (1.8) | ||
| Epistaxis | 14 (24.6) | 1 (1.8) | 9 (16.4) | |
| Cough | 8 (14.1) | 3 (5.5) | ||
| Weight loss | 9 (15.8) | 1 (1.8) | 4 (7.3) | 1 (1.8) |
| Anorexia | 29 (50.9) | 3 (5.3) | 15 (27.3) | 2 (3.6) |
| Fatigue | 41 (71.9) | 9 (15.8) | 37 (67.3) | 6 (10.9) |
| Hyperkalemia | 18 (31.6) | 5 (9.1) | ||
| A | ||||
|---|---|---|---|---|
| Arm A: Aflibercept + sLV5FU2 a | Arm B: sLV5FU2 a | p-Value | ||
| (n = 57) | (n = 55) | |||
| NCI-CTC classification: maximal grade per patient | n | 57 | 55 | p = 0.0356 |
| 1 | 2 (3.5%) | 2 (3.6%) | ||
| 2 | 8 (14.0%) | 21 (38.2%) | ||
| 3 | 39 (68.4%) | 26 (47.3%) | ||
| 4 | 4 (7.0%) | 5 (9.1%) | ||
| 5 | 4 (7.0%) | 1 (1.8%) | ||
| B | ||||
| Bras A: Aflibercept + sLV5FU2 a | Bras B: sLV5FU2 a | p-value | ||
| (n = 36) | (n = 32) | |||
| Grade Max | n | 36 | 32 | X2: 0.1819 |
| 1 | 1 (2.8%) | 1 (3.1%) | ||
| 2 | 6 (16.7%) | 14 (43.8%) | ||
| 3 | 23 (63.9%) | 13 (40.6%) | ||
| 4 | 4 (11.1%) | 3 (9.4%) | ||
| 5 | 2 (5.6%) | 1 (3.1%) | ||
| C | ||||
| Bras A: Aflibercept + sLV5FU2 b | Bras B: sLV5FU2 b | p-value | ||
| (n = 21) | (n = 23) | |||
| Grade Max | n | 21 | 23 | X2: 0.1353 |
| 1 | 1 (4.8%) | 1 (4.3%) | ||
| 2 | 2 (9.5%) | 7 (30.4%) | ||
| 3 | 16 (76.2%) | 13 (56.5%) | ||
| 4 | 0 (0.0) | 2 (8.7%) | ||
| 5 | 2 (9.5%) | 0 (0.0) | ||
| PFS | OS | |||||
|---|---|---|---|---|---|---|
| With Anti-Angiogenic | Without Anti-Angiogenic | p | With Anti-Angiogenic | Without Anti-Angiogenic | p | |
| FOLFA Aflibercept +/− LV5FU2 | 7.4 | 7.3 | ND | 21.8 | 25.1 | ND |
| MAX [8] Bevacizumab +/− capecitabine | 8.5 | 5.7 | p < 0.001 | 18.9 | 18.9 | ND |
| AVEX [6] Bevacizumab +/− capecitabine | 9.1 | 5.1 | p < 0.0001 | 20.7 | 16.8 | p = 0.18 |
| XELAVIRI [17] Bevacizumab + mixed | 8 | ND | - | 22 | ND | - |
| FFCD 2001-02 [27] LV5FU2 | ND | 5.2 | - | ND | 14.2 | - |
| FOCUS [28] LV5FU2 | ND | 6.3 | - | ND | 13.9 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Legoux, J.-L.; Faroux, R.; Barrière, N.; Le Malicot, K.; Tougeron, D.; Lorgis, V.; Guerin-Meyer, V.; Bourgeois, V.; Malka, D.; Aparicio, T.; et al. First-Line LV5FU2 with or without Aflibercept in Patients with Non-Resectable Metastatic Colorectal Cancer: A Randomized Phase II Trial (PRODIGE 25-FFCD-FOLFA). Cancers 2024, 16, 1515. https://doi.org/10.3390/cancers16081515
Legoux J-L, Faroux R, Barrière N, Le Malicot K, Tougeron D, Lorgis V, Guerin-Meyer V, Bourgeois V, Malka D, Aparicio T, et al. First-Line LV5FU2 with or without Aflibercept in Patients with Non-Resectable Metastatic Colorectal Cancer: A Randomized Phase II Trial (PRODIGE 25-FFCD-FOLFA). Cancers. 2024; 16(8):1515. https://doi.org/10.3390/cancers16081515
Chicago/Turabian StyleLegoux, Jean-Louis, Roger Faroux, Nicolas Barrière, Karine Le Malicot, David Tougeron, Véronique Lorgis, Véronique Guerin-Meyer, Vincent Bourgeois, David Malka, Thomas Aparicio, and et al. 2024. "First-Line LV5FU2 with or without Aflibercept in Patients with Non-Resectable Metastatic Colorectal Cancer: A Randomized Phase II Trial (PRODIGE 25-FFCD-FOLFA)" Cancers 16, no. 8: 1515. https://doi.org/10.3390/cancers16081515
APA StyleLegoux, J.-L., Faroux, R., Barrière, N., Le Malicot, K., Tougeron, D., Lorgis, V., Guerin-Meyer, V., Bourgeois, V., Malka, D., Aparicio, T., Baconnier, M., Lebrun-Ly, V., Egreteau, J., Khemissa Akouz, F., Terme, M., Lepage, C., & Boige, V. (2024). First-Line LV5FU2 with or without Aflibercept in Patients with Non-Resectable Metastatic Colorectal Cancer: A Randomized Phase II Trial (PRODIGE 25-FFCD-FOLFA). Cancers, 16(8), 1515. https://doi.org/10.3390/cancers16081515





