Simple Summary
The clinical and diagnostic utility of comprehensive genomic profiling (CGP) in Japan has not been thoroughly investigated. To address this gap, this large-scale study aimed to determine the usefulness of CGP in diagnosing digestive cancer. A total of 547 cases of digestive cancers were analyzed using an original scoring system. Through this approach, the characteristic genomic profiles of each digestive cancer type were identified, with the presence or absence of APC, KRAS, and CDKN2A alterations being characteristic of each organ. Based on the patterns of genomic alterations characteristic of each digestive cancer type, we suggested a classification flowchart specifically designed for digestive adenocarcinomas. Our findings highlight not only the clinical utility of CGP but also its diagnostic utility for digestive cancers.
Abstract
The usefulness of comprehensive genomic profiling (CGP) in the Japanese healthcare insurance system remains underexplored. Therefore, this large-scale study aimed to determine the usefulness of CGP in diagnosing digestive cancers. Patients with various cancer types recruited between March 2020 and October 2022 underwent the FoundationOne® CDx assay at the Keio PleSSision Group (19 hospitals in Japan). A scoring system was developed to identify potentially actionable genomic alterations of biological significance and actionable genomic alterations. The detection rates for potentially actionable genomic alterations, actionable genomic alterations, and alterations equivalent to companion diagnosis (CDx), as well as the signaling pathways associated with these alterations in each digestive cancer, were analyzed. Among the 1587 patients, 547 had digestive cancer. The detection rates of potentially actionable genomic alterations, actionable genomic alterations, and alterations equivalent to CDx were 99.5%, 62.5%, and 11.5%, respectively. APC, KRAS, and CDKN2A alterations were frequently observed in colorectal, pancreatic, and biliary cancers, respectively. Most digestive cancers, except esophageal cancer, were adenocarcinomas. Thus, the classification flowchart for digestive adenocarcinomas proposed in this study may facilitate precise diagnosis. CGP has clinical and diagnostic utility in digestive cancers.
1. Introduction
The advent of next-generation sequencing (NGS) has enabled the comprehensive and rapid analysis of genomic information at low costs. Comprehensive cancer gene analysis, defined as the simultaneous analysis of a large number of cancer-related genes in a single test, has gained popularity in recent years. Among the estimated 20,000 genes present in the human genome, approximately 400 are cancer-related genes [1]. Cancer-related genes have been analyzed using NGS-based cancer gene panels to detect genomic alterations. The process of testing cancer-related genes using a cancer gene panel is known as comprehensive genomic profiling (CGP), as it yields a comprehensive genomic profile of the cancer. CGP has become an indispensable test in the domain of cancer genome medicine [2,3,4]. Tumors have been assessed using NGS-based CGP to detect genomic alterations, such as base substitutions, insertions and deletions (indels), and fusions/rearrangements. Copy number alterations (CNAs) [5], a part of precision cancer medicine, have been used to identify effective targeted therapies that consider individual genomic variability and susceptibility. CGP testing of tissue samples using the FoundationOne® CDx assay (Foundation Medicine, Cambridge, MA, USA) was approved for insurance coverage in Japan in June 2019 [6].
FoundationOne® CDx, a CGP platform that exclusively examines tumor tissue, focuses on a targeted sequence capable of analyzing the hotspots of 324 genes, including 36 fusion genes [7]. FoundationOne® CDx also possesses companion diagnostic (CDx) functions in multiple molecular-targeted therapeutics for specific genes [8]. Its use has enabled the application of NGS to in vitro diagnostics via a hybrid capture-based target enrichment approach and the construction of a whole-genome shotgun library for the identification of substitutions, indels, CNAs, and select rearrangements, the four classes of somatic genomic alterations [9]. Formalin-fixed paraffin-embedded (FFPE) specimens that are 5 × 5 mm in size, have a tumor content of at least 20%, and have been collected within the last 3 years are recommended. FoundationOne CDx, the representative CGP in Japan, accounts for 74% of the CGP performed by our group up to October 2022 [based on unpublished data from the authors’ facility].
Previous studies have examined the clinical utility of CGP tests covered by the Japanese public health insurance system mainly from the clinical perspective, including genomic profiling in light of pharmacotherapy and other treatments [10,11,12,13]. However, the correlation of genomic profiles with pathological features and the signaling pathways involved in digestive cancers remain underexplored. The genomic profiles contain many kinds of diagnostically significant information that describes the nature of the tumor, and CGP is a necessary test for qualitative diagnosis of tumors. In particular, the WHO classification gives diagnoses to most tumors according to their genomic alterations for hematological [14], brain [15], and bone and soft tissue tumors [16]. Therefore, genomic analysis is essential when making a diagnosis.
This study aimed to characterize the pathological genomic features of different types of digestive cancers in clinical practice using a CGP with insurance coverage and establish an original scoring system. The information obtained using CGP will aid in the regularization of its use in routine clinical practice across Japan and facilitate the creation of a database for optimizing treatment strategies for cancer.
2. Materials and Methods
This study was approved by the Ethics Committee of the Keio University School of Medicine (approval number: 2021-1159). Between March 2020 and October 2022, 1587 patients with cancer were selected for this study. These patients underwent the FoundationOne CDx [7] assay at one of the hospitals affiliated with the Keio PleSSison Group, the Keio University Hospital (a core hospital for cancer genome medicine recognized by the Japanese Ministry of Health, Labour and Welfare) and its 18 partner hospitals.
CGP was performed under the coverage of the Japanese insurance system. The requirements for insurance reimbursement are as follows: (1) patients, excluding those with hematologic tumors, who have completed or were expected to complete standard medical therapy, and (2) patients with an unknown primary cancer or rare cancer with no established treatment protocol.
Figure S1 presents the clinical workflow of the cancer genome testing in the Keio PleSSision Group. Consent was obtained via the opt-out method at the outpatient clinic of each hospital. The tumor specimens were sequenced at Foundation Medicine, Inc. (FMI) (Cambridge, MA, USA) and curated in bulk by the Molecular Tumor Board at the Genomics unit, Keio University, based on reports analyzed by the Center for Cancer Genomics [17] and Mitsubishi Electric Software Corporation (Amagasaki-shi, Hyogo, Japan). The Clinical Tumor Board of each hospital reviewed the treatment recommended based on the sequencing results. The results were explained to the patients in the outpatient clinic at each hospital after a web-based consultation [18].
2.1. Sequencing and Identification of Genomic Alterations
DNA extraction and data acquisition via sequencing were performed in accordance with predefined protocols followed at the facilities designated by the FMI. A total of 324 genes were sequenced via NGS using FoundationOne CDx. The presence of genomic alterations, such as base substitution, indels, fusions/rearrangements, and CNAs, were evaluated. CGP was performed as described in a previous study [8]. Although corrected to some extent by tumor content, a variant allele frequency of 10% was established as the cut-off value.
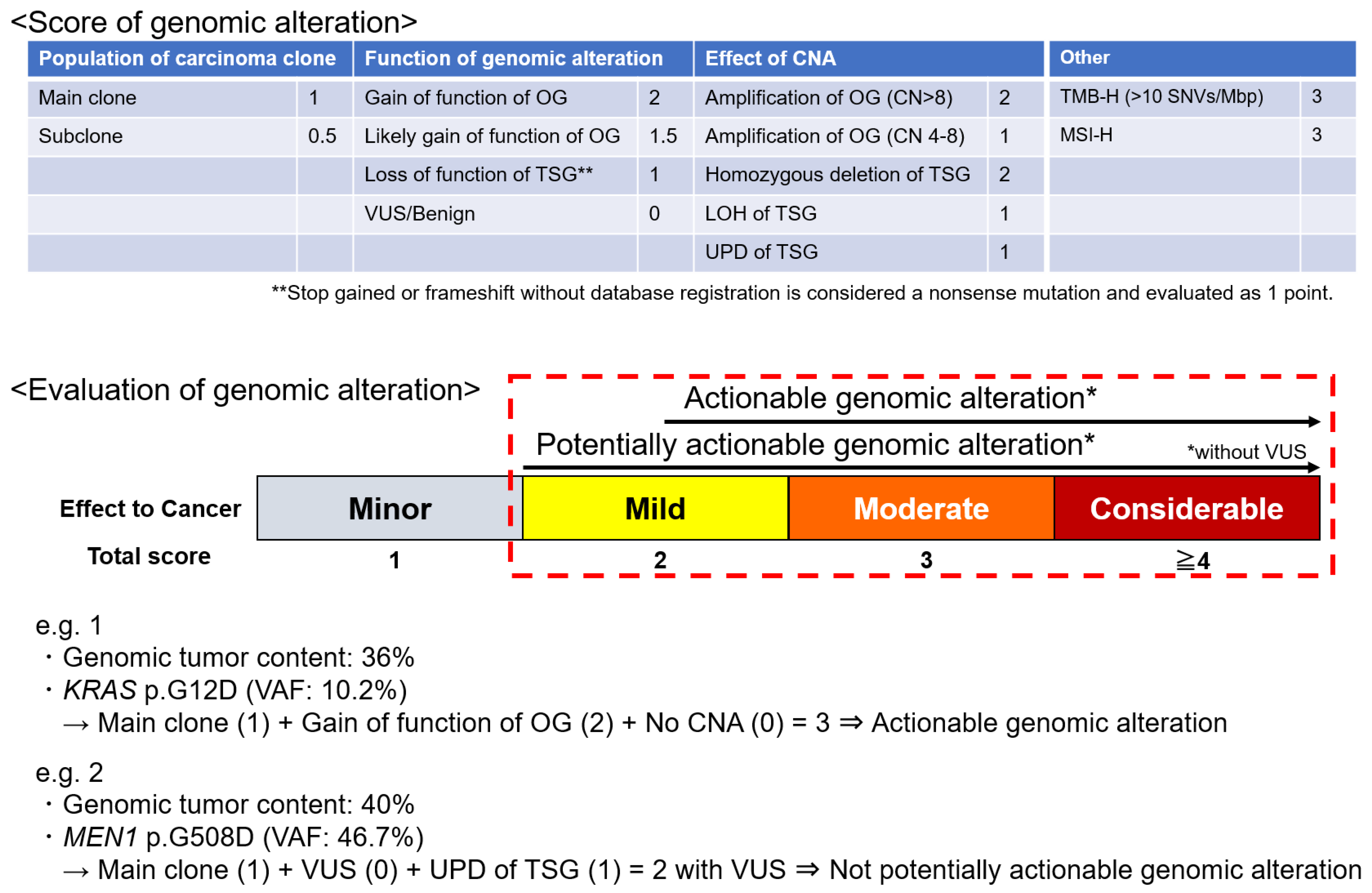
An original scoring system was developed based on the following factors to evaluate and score the genomic alterations: population of carcinoma clones, the function of gene alteration (using reference databases such as COSMIC, ClinVar, OncoKB, CIViC, and JAX CKB), and the effect of CNAs (Figure 1). The total score of these three categories was defined as the alteration score. Tumor mutation burden (TMB) and microsatellite instability (MSI) status were also evaluated [5,19]; high TMB (TMB-H) was defined as the presence of ≥10 single-nucleotide variants/Mbp. A scoring system for genomic alterations was developed based on these parameters.
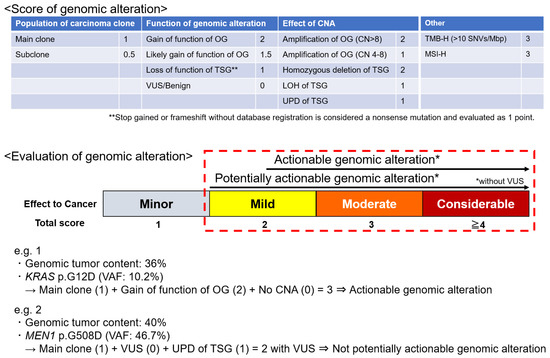
Figure 1.
Scoring system of genomic alteration. A score is assigned to each item based on its impact on carcinogenesis. The sum of the scores of the population of carcinoma clones, the function of genomic alteration, and the effect of CNA is the score for that alteration. TMB and MSI are scored as high, in addition to individual genomic alterations. Potentially actionable genomic alteration with biological significance is defined by a score of ≥2 points without VUSs. An actionable genomic alteration candidate for drugs that may be useful is defined by a score of ≥2.5 points without VUSs. CN, copy number; CAN, copy number alteration; LOH, loss of heterozygosity; MSI-H, high microsatellite instability; OG, oncogene; TMB-H, high tumor mutation burden; TSG, tumor suppressor gene; UPD, uniparental disomy; VUS, variant of uncertain significance.
“Potentially actionable genomic alterations” were defined as genomic alterations with a biological significance of ≥2 but no variants of unknown significance (VUSs). “Actionable genomic alterations” were defined as genomic alterations eligible for drug development with a potential usefulness of ≥2.5; an evidence level of D or higher according to the guidance provided by the Japanese Society of Medical Oncology, Japanese Society of Clinical Oncology, and Japanese Cancer Association; and without VUSs (Figure 1) [20].
“Genomic alterations equivalent to CDx” were defined as genomic alterations with which the physician could use the specific drugs under insurance coverage in Japan (Table S1). The detection rate of potentially actionable genomic alterations, actionable genomic alterations, and genomic alterations equivalent to CDx, as well as the signaling pathways activated or inactivated by genomic alterations, were evaluated for each type of digestive cancer. The Japanese version of the Cancer Genome Atlas (JCGA) was used as the reference database [1]. Table S2 presents the signaling pathways associated with genomic alterations.
2.2. Statistical Analysis
The detection rate, sensitivity, specificity, and positive likelihood ratio were calculated using Microsoft Excel 2019 (Microsoft, Redmond, WA, USA). All statistical analyses were performed using IBM SPSS Statistics ver. 25 (International Business Machines Co., Armonk, NY, USA). The rate of characteristic genomic alterations was evaluated using the χ-square test or Fisher’s exact test, and p-values < 0.05 were considered statistically significant.
3. Results
Among the 1587 patients that underwent the FoundationOne CDx, 547 had digestive cancer. There were 333 male and 214 female patients and the median age at diagnosis was 62 years (range, 15–85) (Table S3). Figure S2 presents the alteration plots for each case. The primary sites of cancer were as follows: esophageal cancer (n = 27), gastric cancer (n = 43), duodenal cancer (n = 4), small intestine cancer (n = 6), colorectal cancer (n = 217), pancreatic cancer (n = 127), and biliary tract cancer (n = 123) (Table S3).
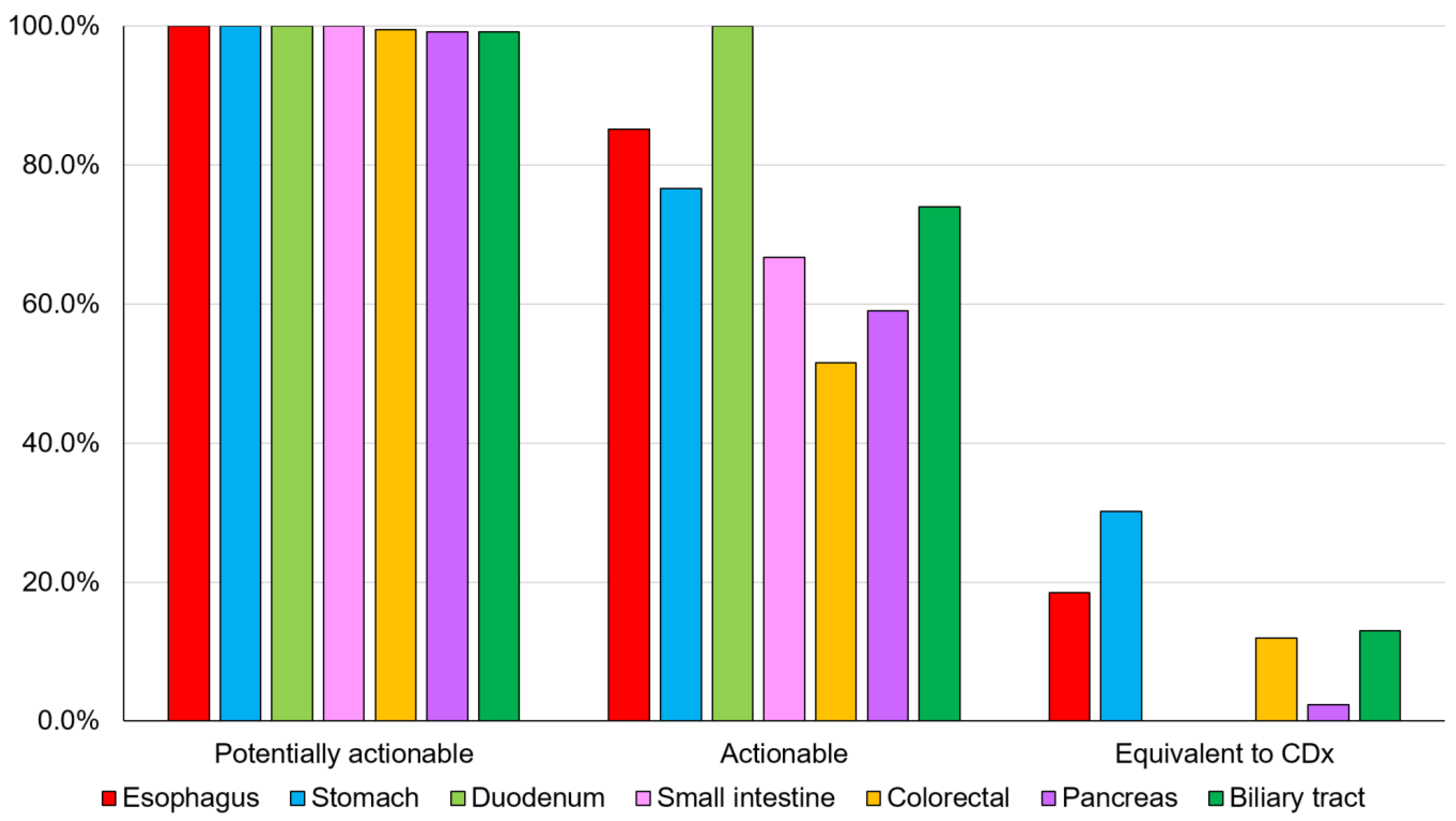
The overall detection rates for potentially actionable genomic alterations, actionable genomic alterations, and genomic alterations equivalent to CDx for digestive cancers were 99.5% (544/547), 62.5% (342/547), and 11.5% (63/547), respectively (Figure 2 and Table S3).
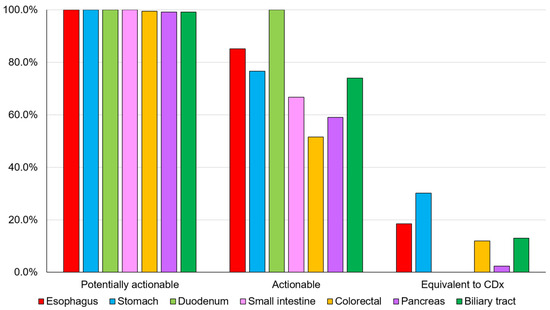
Figure 2.
Detection rate of genomic alterations. The detection rates for potentially actionable genomic alterations, actionable genomic alterations, and CDx-equivalent genomic alterations for each digestive cancer (Table S3). CDx, companion diagnostic.
3.1. Detection Rate of Genomic Alterations in Digestive Cancers
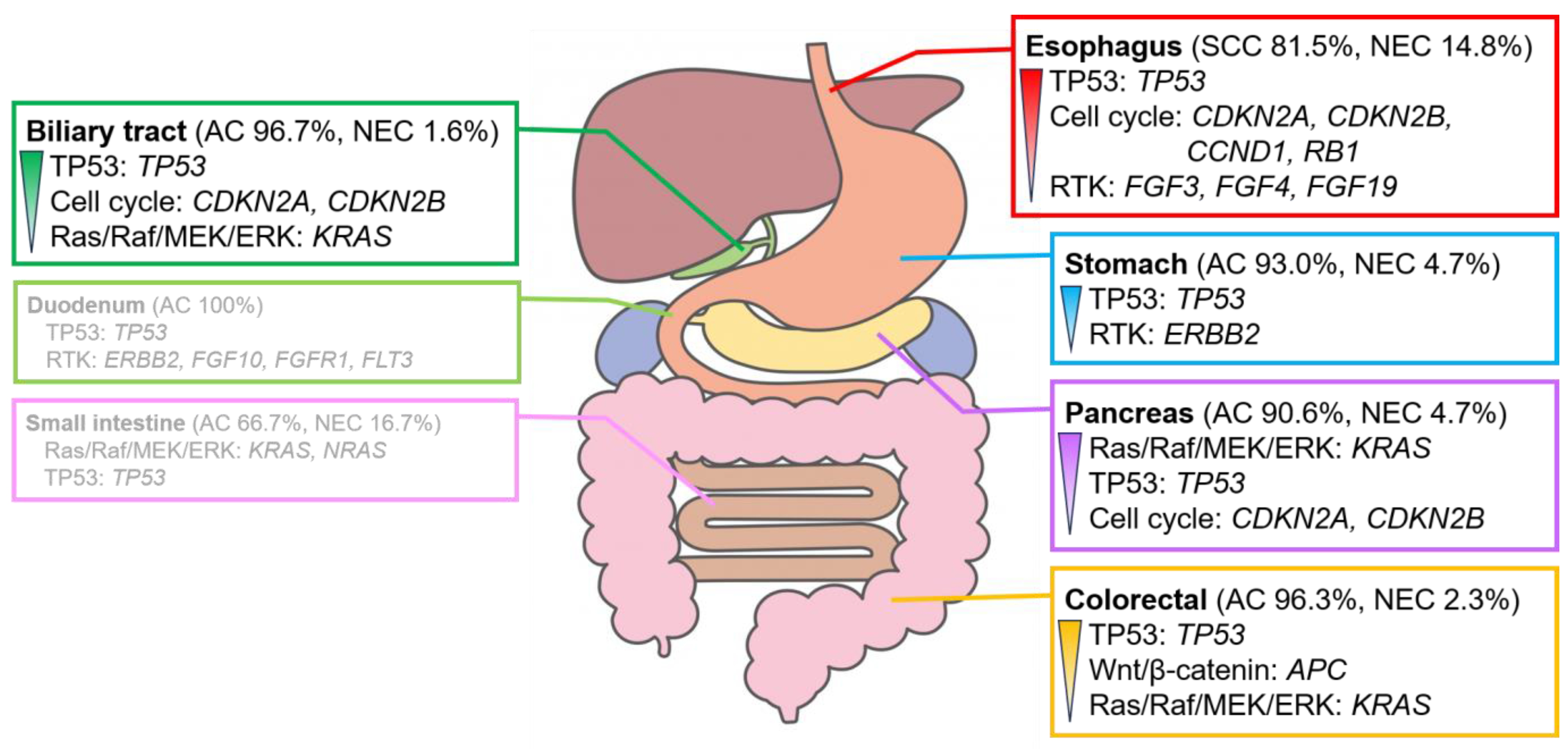
Several genomic alterations were observed in the genes involved in the TP53 signaling pathway, mainly in TP53, in patients with digestive cancer. However, the genomic alterations and signaling pathways with the highest number of genomic alterations differed for each organ (Figure 3).
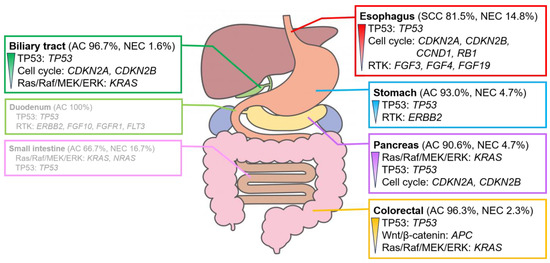
Figure 3.
Characteristic signaling pathways with genomic alterations in digestive cancers. Signaling pathways with genomic alterations of >30% in each digestive cancer. The pathways are listed in the order of their prevalence. Genomic alterations are observed in >20%. The duodenum and small intestine show no clear trend owing to the small number of cases analyzed. For further details, please refer to Figures S3 and S4. AC, adenocarcinoma; NEC, neuroendocrine carcinoma; SCC, squamous cell carcinoma.
3.1.1. Esophagus/Stomach Cancer
Histologically, 81.5% (22/27) and 14.8% (4/27) of esophageal cancers were classified as squamous cell carcinoma (SCC) and neuroendocrine carcinoma (NEC), respectively. The detection rates for potentially actionable genomic alterations, actionable genomic alterations, and genomic alterations equivalent to CDx were 100.0% (27/27), 85.2% (23/27), and 18.5% (5/27), respectively (Table S2). MSI-H (0/27) was not observed in any of the cases; in contrast, TMB-H was observed in 14.8% (4/27) of cases (Figure S2). Several alterations were observed in the genes involved in the TP53 pathway, primarily in TP53 (85.2%), as well as the cell cycle pathway, mainly in CDKN2A (59.3%) and CDKN2B (40.7%) (Figures S3 and S4).
Adenocarcinoma, accounting for 93.0% (40/43) of cases, was the most common histological type of gastric cancer. The detection rates for potentially actionable genomic alterations, actionable genomic alterations, and genomic alterations equivalent to CDx were 100.0% (43/43), 76.7% (33/43), and 30.2% (13/43), respectively (Table S3). MSI-H was observed in 9.3% (4/43) of cases, whereas TMB-H was observed in 2.3% (1/43) of cases (Figure S2). Several alterations were observed in the genes in the TP53 pathway, mainly in TP53 (69.8%), as well as the RTK pathway, mainly in ERBB2 (27.9%) (Figures S3 and S4).
3.1.2. Bowel Cancer
Duodenal, small intestinal, and colorectal cancers were grouped as bowel cancers. Adenocarcinomas accounted for all cases of duodenal cancers (4/4). The detection rates for potentially actionable genomic alterations, actionable genomic alterations, and genomic alterations equivalent to CDx were 100.0% (4/4), 100.0% (4/4), and 0.0% (0/4), respectively (Table S3). MSI-H or TMB-H (0/4) was not detected in any of the cases (Figure S2). Several alterations were observed in genes involved in the TP53 pathway, mainly in TP53 (75%) (Figures S3 and S4).
Adenocarcinoma, accounting for 66.7% (4/6) of cases, was the most common histological type of small intestine cancer. The detection rates for potentially actionable genomic alterations, actionable genomic alterations, and genomic alterations equivalent to CDx were 100% (6/6), 66.7% (4/6), and 0.0% (0/6), respectively (Table S3). MSI-H or TMB-H (0/6) was not observed in any of the cases (Figure S2). Several alterations were observed in the genes involved in the Ras/Raf/MEK/ERK pathway, mainly in KRAS (33.3%) and NRAS (33.3%) (Figures S3 and S4).
Adenocarcinoma, accounting for 96.3% (209/217) of cases, was the most common histological type of colorectal cancer. The detection rates for potentially actionable genomic alterations, actionable genomic alterations, and genomic alterations equivalent to CDx were 99.5% (216/217), 51.6% (112/217), and 12.0% (26/217), respectively (Table S3). MSI-H was observed in 0.9% (2/217) of cases, whereas TMB-H was observed in 5.1% (11/217) of cases (Figure S2). Several alterations were observed in the genes involved in the TP53 pathway, mainly in TP53 (81.1%), as well as the Wnt/β-catenin pathway, mainly in APC (78.8%) (Figures S3 and S4).
3.1.3. Pancreatic Cancer
Adenocarcinoma, accounting for 90.6% (115/127) of cases, was the most common histological type of pancreatic cancer. The detection rates for potentially actionable genomic alterations, actionable genomic alterations, and genomic alterations equivalent to CDx were 99.2% (126/127), 59.1% (75/127), and 2.4% (3/127), respectively (Table S3). MSI-H was not observed in any of the cases (0/127); in contrast, TMB-H was observed in 0.8% (1/127) of cases (Figure S2). Several alterations were observed in the genes involved in the TP53 pathway, mainly in TP53 (69.3%), as well as the Ras/Raf/MEK/ERK pathway, mainly in KRAS (87.4%) (Figures S3 and S4).
3.1.4. Biliary Tract Cancer
Adenocarcinoma, accounting for 96.7% (119/123) of cases, was the most common histological type of biliary tract cancer. The detection rates for potentially actionable genomic alterations, actionable genomic alterations, and genomic alterations equivalent to CDx were 99.2% (122/123), 74.0% (91/123), and 13.0% (16/123), respectively (Table S3). MSI-H was observed in 1.6% (2/123) of cases, whereas TMB-H was observed in 7.3% (9/123) of cases (Figure S2). Several alterations were observed in the genes involved in the TP53 pathway, mainly in TP53 (48.8%), as well as the cell cycle pathway, mainly in CDKN2A (31.7%) and CDKN2B (21.1%) (Figures S3 and S4).
3.2. Genomic Alteration of Digestive Adenocarcinomas
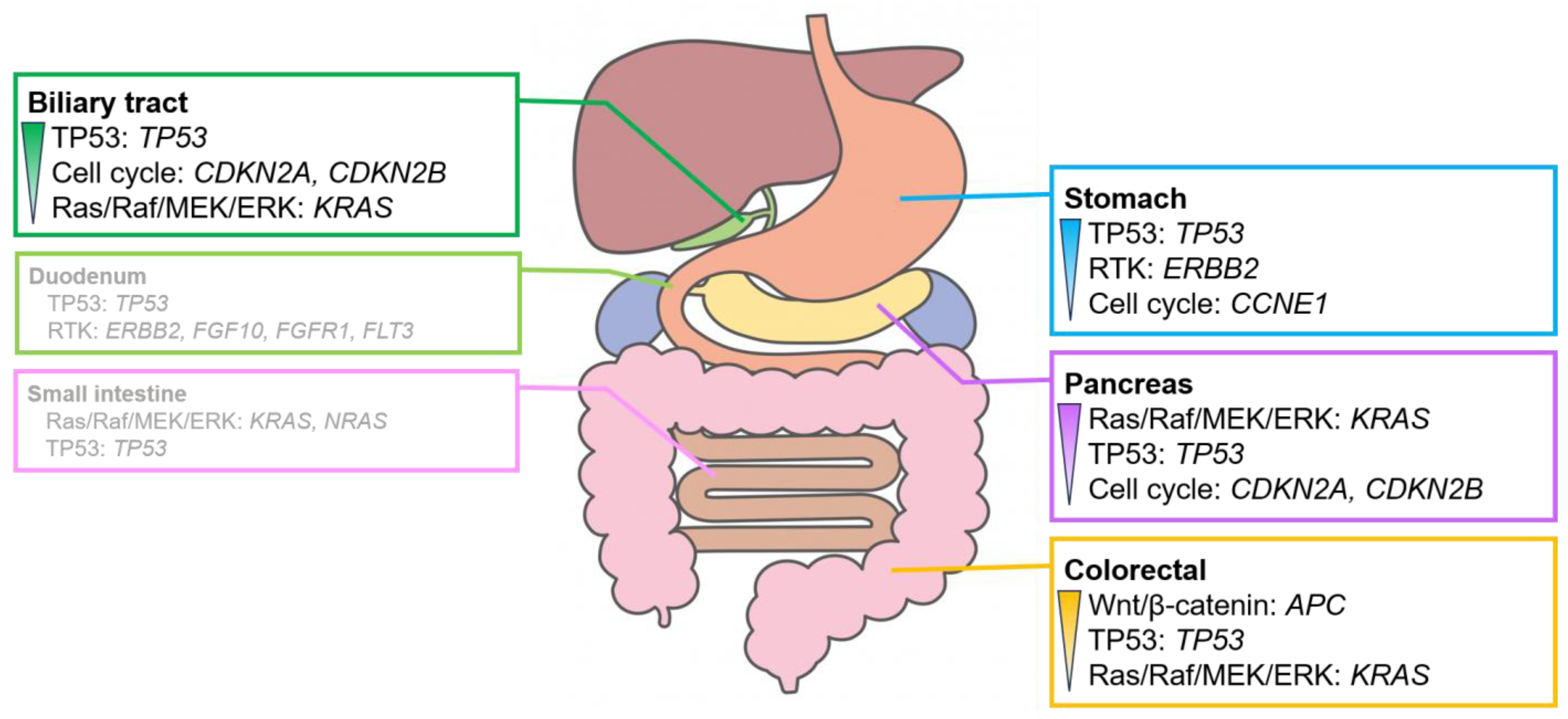
Adenocarcinoma was the most common histologic type of digestive cancer, except in esophageal cancer. The detection rates for signaling pathways with genomic alterations (Figure S5) and overall genomic alterations (Figure S6) were analyzed for each organ. However, no significant differences were observed between the detection rates for adenocarcinoma and the other histological types owing to the high proportion of adenocarcinomas in each organ (Figure 3 and Figure 4).
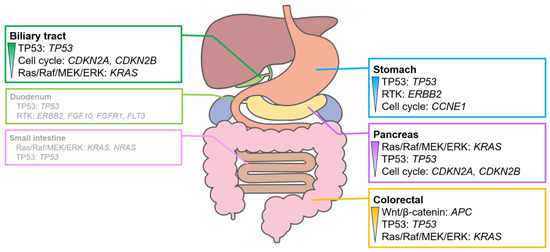
Figure 4.
Characteristic signaling pathways with genomic alterations in digestive adenocarcinomas. Signaling pathways with genomic alterations exceeded 30% in digestive adenocarcinomas. The pathways are listed in order of their prevalence. Genomic alterations are observed in over 20% of cases. The duodenum and the small intestine exhibit no clear trend owing to the small number of cases analyzed. For further details, please refer to Figures S5 and S6.
Alterations in the genes involved in the RTK system, such as ERBB2 amplification, were often observed in gastric adenocarcinoma. Alterations in the genes involved in the Wnt/β-catenin pathway, mainly APC, were observed in colorectal adenocarcinoma. Alterations in KRAS, which plays a role in the Ras/Raf/MEK/ERK pathway, were observed in pancreatic adenocarcinoma. Alterations in the genes involved in the cell cycle pathway, mainly in CDKN2A and CDKN2B, were frequently observed in biliary tract adenocarcinoma. Adenocarcinomas of the duodenum and small intestine showed no clear trend owing to the small number of cases analyzed.
The genes with numerous alterations in digestive adenocarcinomas, excluding duodenal and small intestinal adenocarcinomas, were cross-organized and evaluated across different types of digestive organs. The frequency of APC alterations in colorectal adenocarcinoma (80.9%) was significantly higher than that in stomach (2.5%), pancreatic (1.7%), and biliary tract (1.7%) adenocarcinomas (p < 0.01). The frequency of KRAS alterations in pancreatic (92.2%) and colorectal (45.5%) adenocarcinomas was significantly higher than that in the stomach (20.0%) and biliary tract (21.2%) adenocarcinomas (p < 0.01). The frequency of CDKN2A alterations in pancreatic (47.8%) and biliary tract (31.1%) adenocarcinomas was significantly higher than that in stomach (17.5%) and colorectal (1.4%) adenocarcinomas (p < 0.01) (Table S4).
3.3. Diagnostic Flowchart of Digestive Adenocarcinomas
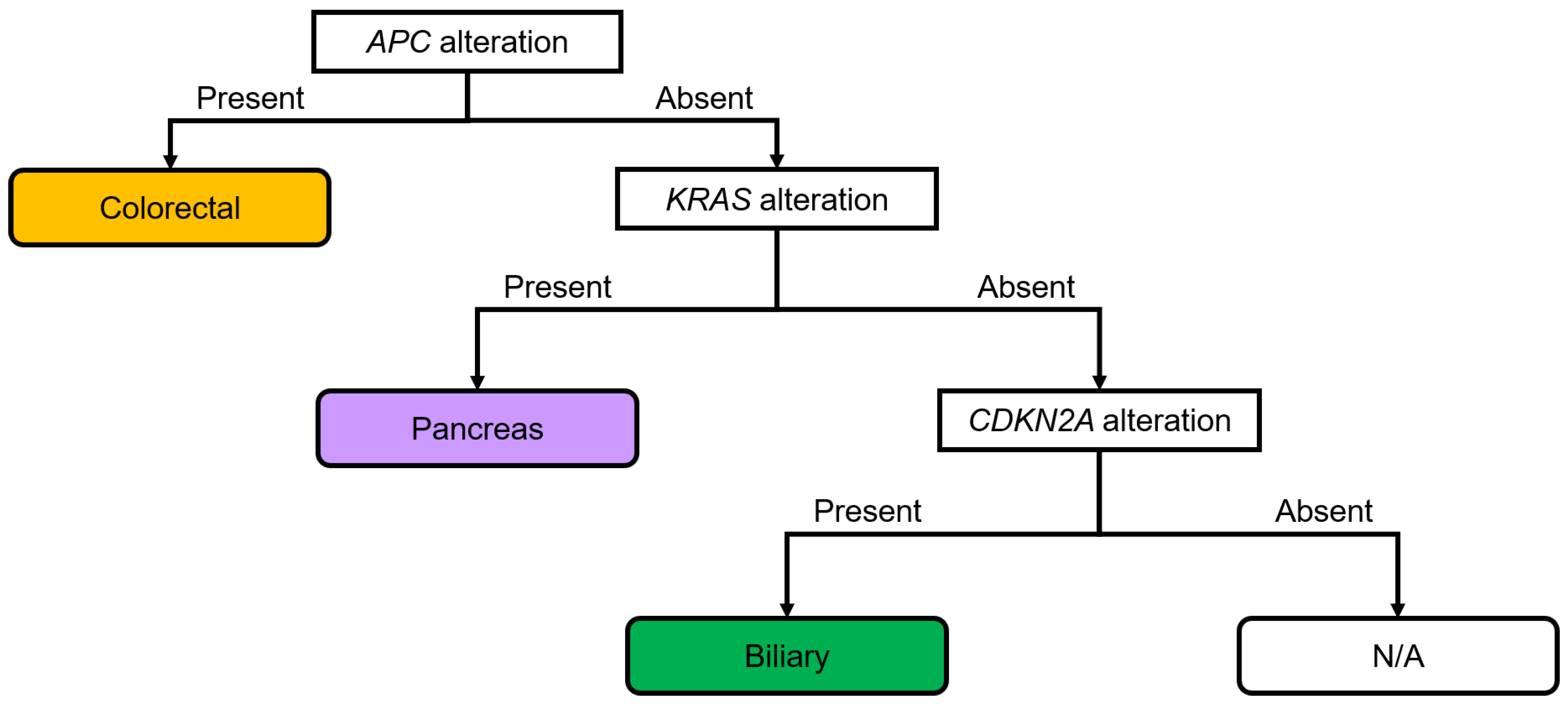
A diagnostic flowchart of genomic alterations in digestive adenocarcinomas was created based on these results (Figure 5). APC, KRAS, and CDKN2A were focused on in the present study, and cases with adenocarcinomas were classified based on the presence or absence of each alteration. Among the adenocarcinomas with APC alterations, 97.1% were colorectal adenocarcinomas (Figure S7a). Colorectal (40.6%) and pancreatic (45.3%) adenocarcinomas were the most common digestive adenocarcinomas with KRAS alterations (Figure S7b). Pancreatic (53.9%) and biliary tract (36.3%) adenocarcinomas were the most common digestive adenocarcinomas with CDKN2A alterations (Figure S7c). Pancreatic adenocarcinoma (67.5%) was the most prevalent digestive adenocarcinoma without APC alteration but with KRAS alteration (Figure S7d). Biliary tract adenocarcinoma (75.0%) was the most prevalent digestive adenocarcinoma without APC and KRAS alteration but with CDKN2A alteration (Figure S7e). The rates of digestive adenocarcinoma without APC, KRAS, and CDKN2A alterations in patients with biliary tract, gastric, colorectal, and pancreatic adenocarcinomas were 54.8%, 21.0%, 18.5%, and 5.6%, respectively (Figure S7f). A diagnostic flowchart was created and the sensitivity, specificity, and positive likelihood ratio were calculated to validate the usefulness of the diagnostic flow. The sensitivity, specificity, and positive likelihood ratio were calculated for gastric (65.0%, 77.8%, and 2.9, respectively), colorectal (80.9%, 98.2%, and 44.3, respectively), pancreatic (90.4%, 86.4%, and 6.6, respectively), and biliary tract cancers (20.2%, 97.8%, and 9.2, respectively) using this flowchart (Figure S8). Thus, APC alterations were highly associated with colorectal cancer; KRAS alterations and normal APC were highly associated with pancreatic cancer; and CDKN2A alterations and normal APC and KRAS were highly associated with biliary tract cancer.
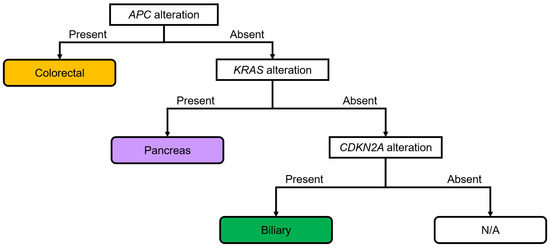
Figure 5.
Diagnostic flowchart based on the genomic alterations in digestive adenocarcinomas. APC alterations show strong associations with colorectal cancer. KRAS alterations and lack of alterations in APC show strong associations with pancreatic cancer. A lack of alterations in APC and KRAS, but the presence of alterations in CDKN2A, shows strong associations with biliary tract cancer. The sensitivity, specificity, and positive likelihood ratios were calculated for each cancer type (Figure S8). N/A, not applicable.
4. Discussion
We established a characteristic genomic profile for each organ by separating actionable genomic alterations from those with minimal impact on carcinogenesis and VUSs using the scoring system developed in the present study. Notably, this profile was comparable with those reported in previous studies [21,22,23,24]. Evaluating CGP results using this scoring system may elucidate the genomic profile of each case. Subsequent comparison with the genomic profile of each organ established in the present study could help identify the primary site of unknown primary cancers.
In esophageal cancers, CDKN2A, CDKN2B, and CCND1 alterations in the cell cycle pathway were frequently observed (Figure S4). A previous study reported CCND1 amplification in the cell cycle pathway, and also TP63/SOX2 amplification and KDM6A deletion in transcriptional regulation [25]. In gastric cancers, ERBB2 alterations in the RTK pathway were observed. In a previous study, gastric cancers were classified into four categories—EBV (Epstein–Barr virus), MSI (microsatellite instability), CIN (chromosomal instability), and GS (genomically stable). Of these, CIN was characterized by TP53 mutation, ERBB2 amplification, VEGFA amplification, and RTK-RAS activation [26]. In colorectal cancers, APC alterations in the Wnt/β-catenin pathway were mainly observed. In a previous study, colorectal cancers were classified into four categories—CMS1 (MSI immune), CMS2 (canonical), CMS3 (metabolic), and CMS4 (mesenchymal). CMS2 was characterized by WNT and MYC activation and high SCNAs (somatic copy number alteration) [27]. In the present study, CMS2 was probably the most common. In pancreatic cancers, KRAS alterations in the Ras/Raf/MEK/ERK pathway were mainly observed. Molecular genomic analyses revealed that pancreatic ductal adenocarcinoma (PDAC) was composed of KRAS, TP53, SMAD4, and CDKN2A. In particular, KRAS alterations were found in over 90% of PDACs [28]. In biliary tract cancers, CDKN2A and CDKN2B alterations in the cell cycle pathway were observed; however, these are not effective therapeutic target genes. In a previous study, biliary tract cancers were classified according to druggable genes—FGFR (FGFR pathway alterations or FGFR2 fusion/rearrangement), HER2 (ERBB2 amplification and mutations), IDH1 (IDH1 alterations), BRAF (BRAF alterations), MSI (MSI-H or MSI-deficient mismatch repair), NTRK (NTRK fusion/rearrangement), and others [29]. Although trastuzumab is expected to be an effective molecular target drug for ERBB2 alterations, which were observed in gastric cancers, there are no effective drugs currently available for KRAS and APC alterations, which are frequently observed in other organs, or for TP53 alterations, which are frequently observed in digestive cancers. In the future, more drugs would need to be developed.
Nevertheless, more than before, the treatment landscape for cancer is rapidly evolving owing to the increase in the number of approved drugs targeting specific genomic modifications [30,31]. In the present study, actionable genomic alterations were observed in 62.5% of all digestive cancers. Moreover, genomic alterations equivalent to CDx were observed in 11.5% of patients, demonstrating the clinical utility of genomic panel testing. A previous study, which analyzed advanced solid tumors using FoundationOne CDx or FoundationOne Heme, revealed one or more alterations in 94.6% of cases, as well as actionable alterations with candidates for therapeutic agents in 87.7% of cases [12]. Several clinical trials on CGP in patients with advanced or metastatic solid tumors have reported that the prevalence of actionable genomic alterations per patient ranges from 40% to 94% [32,33]. Furthermore, compared with previous reports, the percentage of patients with treatment recommendations ranged from 11% to 39% [13,34,35,36,37]. However, the rate of genotype-matched therapy was 9.4% in Japan (between June 2019 and June 2022) [38]. This is due to the Japanese healthcare insurance system, which permits the use of some molecular-targeted drugs and immune checkpoint inhibitors only if CDx-positive. In our study, the alterations observed in several patients with esophageal cancer (actionable, 85.2%; CDx-equivalent, 18.5%), gastric cancer (actionable, 76.7%; CDx-equivalent, 30.2%), and biliary tract cancer (actionable, 74.0%; CDx-equivalent, 13.0%) were associated with therapeutic agents. This finding suggests that this subset of patients would benefit from the active use of CGP. CGP is available only after the completion or expected completion of standard therapy in most cases in Japan [34]. However, the adoption of CGP during first-line chemotherapy can aid in therapeutic decision making. Furthermore, obtaining a genomic profile at the time of pre-treatment, especially when making a pathological diagnosis, would allow molecular classification, which would provide information on the nature of the cancer and prognostic prediction.
A diagnostic flowchart of genomic alterations in digestive adenocarcinomas was created based on our results (Figure 5). APC, KRAS, and CDKN2A were focused on in the present study, and cases with adenocarcinomas were classified based on the presence or absence of each alteration. The sensitivity, specificity, and positive likelihood ratio were high for colorectal adenocarcinoma. Similarly, the specificity was high and the positive likelihood ratio was relatively high for biliary tract adenocarcinoma. Thus, the established diagnostic flowchart was considered useful for the diagnosis of colorectal and biliary tract adenocarcinomas.
Nevertheless, the present study has some limitations. First, the definition of actionable alterations remains controversial. Second, the progress of the disease was not followed up. Third, although insurance requirements stipulate that the test should be performed after or at the time of completion or expected completion of standard treatment, in reality, the test may be performed at any time point at the discretion of clinicians [39]. Fourth, various types of tissue, such as endoscopic ultrasound–fine needle aspiration (EUS-FNA) or operative tissue, were used in the present study. Therefore, it is possible that not all genomic alterations were detected in cases with a low tumor content. Fifth, the tissue specimens were subjected to treatments that may have altered the true genomic profiles of each digestive cancer. As the specimens were obtained at various time points before and after chemotherapy and belonged to both primary tumors and metastases, it is difficult to discern whether they were from a pure genomic profile of digestive cancer. Lastly, the flowchart developed in the present study has only been validated for digestive adenocarcinomas, but not adenocarcinomas of other organs. Thus, the usefulness of the flowchart must be validated using the CGP results of metastatic tissue with known primary sites. Furthermore, it is expected that the flowchart will become more practical by evaluating the results of liquid biopsy. In the future, a flowchart including all organ adenocarcinomas can be used to identify the primary site of an unknown primary cancer. Despite these limitations, the findings of the present study, along with the proposed diagnostic flowchart based on the genome analysis of real-world data, add unique value to clinical practice owing to their versatility, practicality, and suitability.
5. Conclusions
This large-scale study assessed the utility of CGP in diagnosing digestive cancers and proposed a diagnostic flowchart based on gene alterations. The detection rates of actionable genomic alterations were high across various digestive cancer types. We believe that a comprehensive diagnosis based on histopathological images and CGP will aid in the precise diagnosis and treatment of cancer. Further prospective clinical trials assessing the overall survival and quality of CGP-based targeted therapies must be conducted to aid in decision making in the era of personalized treatment.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cancers16081504/s1: Figure S1: Workflow of the genomic profiling test performed by the Keio PleSSision Group; Figure S2: Genomic alteration plots of 547 digestive cancers; Figure S3: Detection rate for the signaling pathways with genomic alterations in digestive cancers; Figure S4: Detection rate of genomic alterations in digestive cancers; Figure S5: Detection rate of signaling pathways with genomic alterations in digestive adenocarcinomas; Figure S6: Detection rate of genomic alterations in digestive adenocarcinomas; Figure S7: Breakdown of digestive adenocarcinoma cases based on the alterations in different genes; Figure S8: Process of diagnostic flowchart of digestive adenocarcinoma; Table S1: List of genes for CDx of digestive cancers in Japan; Table S2: List of signaling pathways affected by genomic alterations; Table S3: Characteristics of 547 digestive cancers; Table S4: Rate of characteristic genomic alterations in each digestive adenocarcinoma.
Author Contributions
Conceptualization, M.I.; methodology, M.I.; formal analysis, M.I.; resources, K.N. (Kohei Nakamura), R.K., H.H., T.I. (Tatsuru Ikeda), M.S., Y.N., J.S., H.O., S.I., M.Y., H.S., T.I. (Takeshi Isobe), Y.Y., A.Y., H.K., S.Y., T.A., K.T., S.U., J.N. and Y.S.; data curation, M.I., S.N., C.O., K.N. (Ko Nishimiya) and S.T.; writing—original draft preparation, M.I.; writing—review and editing, M.I.; visualization, M.I.; supervision, M.I.; project administration, M.I.; funding acquisition, M.I. and H.N. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a Research Grant from the Princess Takamatsu Cancer Research Fund, the Japan Agency for Medical Research and Development (AMED), under grant number JP21ck0106448, and JSPS KAKENHI Grant Number JP22K15977.
Institutional Review Board Statement
This study was approved by the Ethics Committee of the Keio University School of Medicine (2021-1159, approved on 5 April 2022).
Informed Consent Statement
Consent was obtained on an opt-out basis at the outpatient clinic of each hospital.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and the Supplementary Materials.
Conflicts of Interest
Marin Ishikawa has received research grants from funding agencies, JSPS KAKENHI (grant number JP22K15977). Hiroshi Nishihara has received research grants from funding agencies, the Japan Agency for Medical Research and Development (AMED) (grant number JP21ck0106448), and a Research Grant from the Princess Takamatsu Cancer Research Fund. Sachio Nohara, Chihiro Okada, Ko Nishimiya, and Shigeki Tanishima are employees of a company. The other authors declare no conflicts of interest.
References
- Serizawa, M.; Mizuguchi, M.; Urakami, K.; Nagashima, T.; Ohshima, K.; Hatakeyama, K.; Ohnami, S.; Ohnami, S.; Maruyama, K.; Ashizawa, T.; et al. JCGA: The: Japanese version of the Cancer Genome Atlas and its contribution to the interpretation of gene alterations detected in clinical cancer genome sequencing. Hum. Genome Var. 2021, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.H.; Hsu, G.; Siden, E.G.; Thorlund, K.; Mills, E.J. An overview of precision oncology basket and umbrella trials for clinicians. CA Cancer J. Clin. 2020, 70, 12–137. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T. Implementation of “clinical sequencing” in cancer genome medicine in Japan. Cancer Sci. 2018, 109, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Kou, T.; Kanai, M.; Yamamoto, Y.; Kamada, M.; Nakatsui, M.; Sakuma, T.; Mochizuki, H.; Hiroshima, A.; Sugiyama, A.; Nakamura, E.; et al. Clinical sequencing using a next-generation sequencing-based multiplex gene assay in patients with advanced solid tumors. Cancer Sci. 2017, 108, 1440–1446. [Google Scholar] [CrossRef]
- Frampton, G.M.; Fichtenholtz, A.; Otto, G.A.; Wang, K.; Downing, S.R.; He, J.; Schnall-Levin, M.; White, J.; Sanford, E.M.; An, P.; et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat. Biotechnol. 2013, 31, 1023–1031. [Google Scholar] [CrossRef]
- Ebi, H.; Bando, H. Precision oncology and the universal health coverage system in Japan. JCO Precis. Oncol. 2019, 3, PO.19.00291. [Google Scholar] [CrossRef] [PubMed]
- As Our Understanding of Cancer Evolves, So Do Our Tests: FoundationOne®, CDx Gene List. FoundationOne®, CDx. Available online: https://www.foundationmedicine.in/content/dam/rfm/in_v2-en_in/CDx/FoundationOne%20CDx%20Gene%20list.pdf (accessed on 10 March 2024).
- Milbury, C.A.; Creeden, J.; Yip, W.K.; Smith, D.L.; Pattani, V.; Maxwell, K.; Sawchyn, B.; Gjoerup, O.; Meng, W.; Skoletsky, J.; et al. Clinical and analytical validation of FoundationOne®CDx, a comprehensive genomic profiling assay for solid tumors. PLoS ONE 2022, 17, e0264138. [Google Scholar] [CrossRef] [PubMed]
- Ida, H.; Koyama, T.; Mizuno, T.; Sunami, K.; Kubo, T.; Sudo, K.; Tao, K.; Hirata, M.; Yonemori, K.; Kato, K.; et al. Clinical utility of comprehensive genomic profiling tests for advanced or metastatic solid tumor in clinical practice. Cancer Sci. 2022, 113, 4300–4310. [Google Scholar] [CrossRef] [PubMed]
- Koguchi, D.; Tsumura, H.; Tabata, K.; Shimura, S.; Satoh, T.; Ikeda, M.; Watanabe, A.; Yoshida, T.; Sasaki, J.; Matsumoto, K.; et al. Real-world data on the comprehensive genetic profiling test for Japanese patients with metastatic castration-resistant prostate cancer. Jpn. J. Clin. Oncol. 2024, hyae003. [Google Scholar] [CrossRef]
- Higashigawa, S.; Matsubayashi, H.; Kiyozumi, Y.; Kado, N.; Nishimura, S.; Oishi, T.; Sugino, T.; Fushiki, K.; Shirasu, H.; Yasui, H.; et al. Present status of germline findings in precision medicine for Japanese cancer patients: Issues in the current system. Jpn. J. Clin. Oncol. 2022, 52, 599–608. [Google Scholar] [CrossRef]
- Aoyagi, Y.; Kano, Y.; Tohyama, K.; Matsudera, S.; Kumaki, Y.; Takahashi, K.; Mitsumura, T.; Harada, Y.; Sato, A.; Nakamura, H.; et al. Clinical utility of comprehensive genomic profiling in Japan: Result of PROFILE-F study. PLoS ONE 2022, 17, e0266112. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, J.; Ohhara, Y.; Takada, K.; Tanabe, H.; Hatanaka, K.; Amano, T.; C Hatanaka, K.; Hatanaka, Y.; Mitamura, T.; Kato, M.; et al. Clinical significance of comprehensive genomic profiling tests covered by public insurance in patients with advanced solid cancers in Hokkaido, Japan. Jpn. J. Clin. Oncol. 2021, 51, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Sbaraglia, M.; Bellan, E.; Tos, A.P.D. The 2020 WHO Classification of Soft Tissue Tumours: News and perspectives. Pathologica 2021, 113, 70–84. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Kato, M.; Kohsaka, S.; Sudo, T.; Tamai, I.; Shiraishi, Y.; Okuma, Y.; Ogasawara, D.; Suzuki, T.; Yoshida, T.; et al. C-CAT: The National Datacenter for Cancer Genomic Medicine in Japan. Cancer Discov. 2022, 12, 2509–2515. [Google Scholar] [CrossRef] [PubMed]
- Sunami, K.; Naito, Y.; Aimono, E.; Amano, T.; Ennishi, D.; Kage, H.; Kanai, M.; Komine, K.; Koyama, Y.; Maeda, T.; et al. The initial assessment of expert panel performance in core hospitals for cancer genomic medicine in Japan. Int. J. Clin. Oncol. 2021, 26, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 human cancer genomes reveals the landscape of tumor mutational burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Aburatani, H.; Amano, T.; Baba, E.; Furukawa, T.; Hayashida, T.; Hiyama, E.; Ikeda, S.; Kanai, M.; Kato, M.; et al. Clinical Practice Guidance for Next-Generation Sequencing in Cancer Diagnosis and Treatment (edition 2.1). Int. J. Clin. Oncol. 2021, 26, 233–283. [Google Scholar] [CrossRef]
- González-González, M.; Gutiérrez, M.L.; Sayagués, J.M.; Muñoz-Bellvís, L.; Orfao, A. Genomic profiling of sporadic liver metastatic colorectal cancer. Semin. Cancer Biol. 2021, 71, 98–108. [Google Scholar] [CrossRef]
- Yamai, T.; Ikezawa, K.; Sugimoto, N.; Urabe, M.; Kai, Y.; Takada, R.; Nakabori, T.; Uehara, H.; Kawamura, T.; Kunimasa, K.; et al. Utility of comprehensive genomic profiling tests for patients with incurable pancreatic cancer in clinical practice. Cancers 2023, 15, 970. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Bridgewater, J.; Normanno, N. Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann. Oncol. 2021, 32, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Bonelli, P.; Borrelli, A.; Tuccillo, F.M.; Silvestro, L.; Palaia, R.; Buonaguro, F.M. Precision medicine in gastric cancer. World J. Gastrointest. Oncol. 2019, 11, 804–829. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic characterization of oesophageal carcinoma. Nature 2017, 541, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Guinney, J.; Dienstmann, R.; Wang, X.; Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated Genomic Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell 2017, 32, 185–203.e13. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Kelley, R.K.; Nervi, B.; Oh, D.Y.; Zhu, A.X. Biliary tract cancer. Lancet 2021, 397, 428–444. [Google Scholar] [CrossRef]
- Shirota, H.; Komine, K.; Takahashi, M.; Takahashi, S.; Miyauchi, E.; Niizuma, H.; Tada, H.; Shimada, M.; Niihori, T.; Aoki, Y.; et al. Clinical decisions by the molecular tumor board on comprehensive genomic profiling tests in Japan: A retrospective observational study. Cancer Med. 2023, 12, 6170–6181. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Abate, R.E.; Morabito, A.; Milella, M.; Tabbò, F.; Curigliano, G.; Masini, C.; Marchetti, P.; Pruneri, G.; et al. Current practice of genomic profiling of patients with advanced solid tumours in Italy: The Italian Register of Actionable Mutations (RATIONAL) study. Eur. J. Cancer 2023, 187, 174–184. [Google Scholar] [CrossRef]
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Sunami, K.; Ichikawa, H.; Kubo, T.; Kato, M.; Fujiwara, Y.; Shimomura, A.; Koyama, T.; Kakishima, H.; Kitami, M.; Matsushita, H.; et al. Feasibility and utility of a panel testing for 114 cancer-associated genes in a clinical setting: A hospital-based study. Cancer Sci. 2019, 110, 1480–1490. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Matsubara, J.; Quy, P.N.; Fukuyama, K.; Nomura, M.; Funakoshi, T.; Doi, K.; Sakamori, Y.; Yoshioka, M.; Yokoyama, A.; et al. Comprehensive genomic profiling for patients with chemotherapy-naïve advanced cancer. Cancer Sci. 2021, 112, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.L.; Huang, B.; Weiss, H.L.; Hull, P.; Westgate, P.M.; Miller, R.W.; Arnold, S.M.; Kolesar, J.M. Clinical outcomes of molecular tumor boards: A systematic review. JCO Precis. Oncol. 2021, 5, PO.20.00495. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Takahama, T.; Sakai, K.; Shimizu, S.; Watanabe, S.; Kawakami, H.; Tanaka, K.; Sato, C.; Hayashi, H.; Nonagase, Y.; et al. Clinical application of the FoundationOne CDx assay to therapeutic decision-making for patients with advanced solid tumors. Oncologist 2021, 26, e588–e596. [Google Scholar] [CrossRef]
- Seet, A.O.L.; Tan, A.C.; Tan, T.J.; Ng, M.C.H.; Tai, D.W.M.; Lam, J.Y.C.; Tan, G.S.; Gogna, A.; Too, C.W.; Tan, B.S.; et al. Individualized molecular profiling for allocation to clinical trials Singapore study—An Asian tertiary cancer center experience. JCO Precis. Oncol. 2021, 5, PO.20.00261. [Google Scholar] [CrossRef]
- C-CAT Registration Status. Available online: https://for-patients.c-cat.ncc.go.jp/registration_status/ (accessed on 5 April 2024).
- Mukai, Y.; Ueno, H. Establishment and implementation of cancer genomic medicine in Japan. Cancer Sci. 2021, 112, 970–977. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).