Precision Immuno-Oncology in NSCLC through Gender Equity Lenses
Abstract
Simple Summary
Abstract
1. Introduction
2. Sex Differences in Response to Immunotherapy
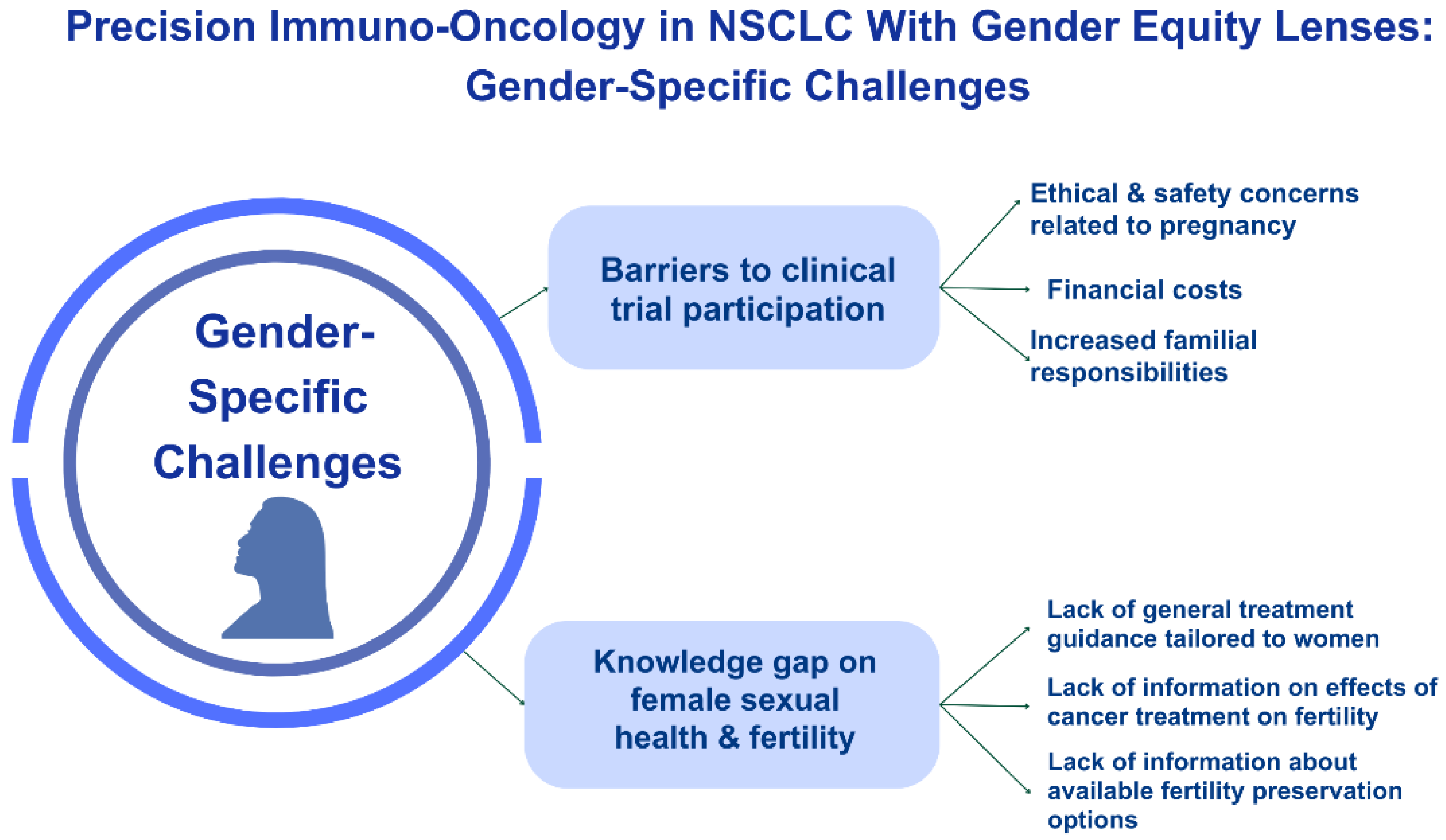
3. Lack of Inclusion in Clinical Trials
4. Future Directions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Research Council. Committee on A Framework for Developing a New Taxonomy of Disease. The National Academies Collection: Reports funded by National Institutes of Health. Toward Precision Medicine: Building a Knowledge Network for Biomedical Research and a New Taxonomy of Disease. 2011. Available online: https://pubmed.ncbi.nlm.nih.gov/22536618/ (accessed on 2 February 2024).
- Kumar-Sinha, C.; Chinnaiyan, A.M. Precision oncology in the age of integrative genomics. Nat. Biotechnol. 2018, 36, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Boca, S.M.; Panagiotou, O.A.; Rao, S.; McGarvey, P.B.; Madhavan, S. Future of evidence synthesis in precision oncology: Between systematic reviews and biocuration. JCO Precis. Oncol. 2018, 2. [Google Scholar] [CrossRef] [PubMed]
- Paggi, M.G.; Vona, R.; Abbruzzese, C.; Malorni, W. Gender-related disparities in non-small cell lung cancer. Cancer Lett. 2010, 298, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Ando, K.; Shinmyo, T.; Morita, K.; Mochizuki, A.; Kurimoto, N.; Tatsunami, S. Female gender is an independent prognostic factor in non-small-cell lung cancer: A meta-analysis. Ann. Thorac. Cardiovasc. Surg. 2011, 17, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Baiu, I.; Titan, A.L.; Martin, L.W.; Wolf, A.; Backhus, L. The role of gender in non-small cell lung cancer: A narrative review. J. Thorac. Dis. 2021, 13, 3816. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Murayama, T.; Sato, Y.; Suzuki, Y.; Saito, H.; Nomura, Y. Gender Differences in Long-Term Survival after Surgery for Non-Small Cell Lung Cancer. Thorac. Cardiovasc. Surg. 2016, 64, 507–514. [Google Scholar] [CrossRef]
- Donington, J.S.; Colson, Y.L. Sex and gender differences in non-small cell lung cancer. Semin. Thorac. Cardiovasc. Surg. 2011, 23, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Florez, N.; Kiel, L.; Riano, I.; Patel, S.; DeCarli, K.; Dhawan, N.; Franco, I.; Odai-Afotey, A.; Meza, K.; Swami, N.; et al. Lung Cancer in Women: The Past, Present, and Future. Clin. Lung Cancer 2023, 25, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, F.; Yoneda, K.; Takenaka, M.; Kuroda, K. Treatment strategy of EGFR-mutated non-small cell lung cancer. J. Thorac. Dis. 2022, 14, 602. [Google Scholar] [CrossRef]
- Wetterstrand, K. The Cost of Sequencing a Human Genome. Genome. 2016. Available online: https://www.genome.gov/about-genomics/fact-sheets/Sequencing-Human-Genome-cost (accessed on 3 February 2024).
- Chen, H.Z.; Bonneville, R.; Roychowdhury, S. Implementing precision cancer medicine in the genomic era. Semin. Cancer Biol. 2019, 55, 16–27. [Google Scholar] [CrossRef]
- Huang, L.; Guo, Z.; Wang, F.; Fu, L. KRAS mutation: From undruggable to druggable in cancer. Signal Transduct. Target. Ther. 2021, 6, 386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, J.; Gao, Y.; Li, Y.; Li, Y. Strategies for targeting undruggable targets. Expert Opin. Drug Discov. 2022, 17, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Murciano-Goroff, Y.R.; Suehnholz, S.P.; Drilon, A.; Chakravarty, D. Precision Oncology: 2023 in Review. Cancer Discov. 2023, 13, 2525–2531. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Mermel, C.H.; Robinson, J.T.; Garraway, L.A.; Golub, T.R.; Meyerson, M.; Gabriel, S.B.; Lander, E.S.; Getz, G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 2014, 505, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Chehrazi-Raffle, A.; Reddi, S.; Salgia, R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J. Immunother. Cancer 2018, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Prasad, V. Estimation of the percentage of US patients with cancer who are eligible for and respond to checkpoint inhibitor immunotherapy drugs. JAMA Netw. Open 2019, 2, e192535. [Google Scholar] [CrossRef] [PubMed]
- Fridman, W.H.; Pagès, F.; Sautès-Fridman, C.; Galon, J. The immune contexture in human tumours: Impact on clinical outcome. Nat. Rev. Cancer 2012, 12, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.S.; Mellman, I. Elements of cancer immunity and the cancer–immune set point. Nature 2017, 541, 321–330. [Google Scholar] [CrossRef]
- White, K.; Connor, K.; Meylan, M.; Bougoüin, A.; Salvucci, M.; Bielle, F.; O’farrell, A.C.; Sweeney, K.; Weng, L.; Bergers, G.; et al. Identification, validation and biological characterisation of novel glioblastoma tumour microenvironment subtypes: Implications for precision immunotherapy. Ann. Oncol. 2023, 34, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, M.; Pandozzi, C.; Sciarra, F.; Campolo, F.; Gelibter, A.J.; Sirgiovanni, G.; Cortesi, E.; Lenzi, A.; Isidori, A.M.; Sbardella, E.; et al. Circulating natural killer cells as prognostic value for non-small-cell lung cancer patients treated with immune checkpoint inhibitors: Correlation with sarcopenia. Cancers 2023, 15, 3592. [Google Scholar] [CrossRef] [PubMed]
- Tenuta, M.; Gelibter, A.; Pandozzi, C.; Sirgiovanni, G.; Campolo, F.; Venneri, M.A.; Caponnetto, S.; Cortesi, E.; Marchetti, P.; Isidori, A.M.; et al. Impact of sarcopenia and inflammation on patients with advanced non-small cell lung cancer (NCSCL) treated with immune checkpoint inhibitors (ICIs): A prospective study. Cancers 2021, 13, 6355. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.S.; Kopciuk, K.; Dean, M.L.; D’Silva, A.; Otsuka, S.; Klimowicz, A.; Hao, D.; Morris, D.; Bebb, D.G. CXCR4 expression in lung carcinogenesis: Evaluating gender-specific differences in survival outcomes based on CXCR4 expression in early stage non-small cell lung cancer patients. PLoS ONE 2021, 16, e0241240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Guo, S.; Liu, M.; Burow, M.E.; Wang, G. Targeting CXCL12/CXCR4 axis in tumor immunotherapy. Curr. Med. Chem. 2019, 26, 3026–3041. [Google Scholar] [CrossRef] [PubMed]
- Anagnostou, V.; Landon, B.V.; Medina, J.E.; Forde, P.; Velculescu, V.E. Translating the evolving molecular landscape of tumors to biomarkers of response for cancer immunotherapy. Sci. Transl. Med. 2022, 14, eabo3958. [Google Scholar] [CrossRef] [PubMed]
- Wheler, J.; Lee, J.J.; Kurzrock, R. Unique molecular landscapes in cancer: Implications for individualized, curated drug combinations. Cancer Res. 2014, 74, 7181–7184. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Zhao, Y.; Shen, M.; Wu, L.; Yang, H.; Yao, Y.; Yang, Q.; Du, J.; Liu, L.; Li, Y.; Bai, Y. Stromal cells in the tumor microenvironment: Accomplices of tumor progression? Cell Death Dis. 2023, 14, 587. [Google Scholar] [CrossRef]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef] [PubMed]
- Turley, S.J.; Cremasco, V.; Astarita, J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015, 15, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, W.; Lin, S.; Liu, B.; Wu, P.; Li, L. Fibroblast diversity and plasticity in the tumor microenvironment: Roles in immunity and relevant therapies. Cell Commun. Signal. 2023, 21, 234. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Valkenburg, K.C.; De Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, A.R.; Alkhatib, N.; Martin, J.; Babiker, H.M.; Garland, L.L.; McBride, A.; Abraham, I. Comparative efficacy and safety of immunotherapies targeting the PD-1/PD-L1 pathway for previously treated advanced non-small cell lung cancer: A Bayesian network meta-analysis. Crit. Rev. Oncol. Hematol. 2019, 142, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Jin, L.; Chen, P.; Li, D.; Gao, W.; Dong, G. Colorectal cancer immunotherapy-Recent progress and future directions. Cancer Lett. 2022, 545, 215816. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Verma, V.; Gay, C.M.; Chen, Y.; Liang, F.; Lin, Q.; Wang, J.; Zhang, W.; Hui, Z.; Zhao, M.; et al. Neoadjuvant immunotherapy for advanced, resectable non-small cell lung cancer: A systematic review and meta-analysis. Cancer 2023, 129, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Jácome, A.A.; Castro, A.C.G.; Vasconcelos, J.P.S.; Silva, M.H.C.; Lessa, M.A.O.; Moraes, E.D.; Andrade, A.C.; Lima, F.M.; Farias, J.P.F.; Gil, R.A.; et al. Efficacy and safety associated with immune checkpoint inhibitors in unresectable hepatocellular carcinoma: A meta-analysis. JAMA Netw. Open 2021, 4, e2136128. [Google Scholar] [CrossRef]
- Duma, N.; Vera Aguilera, J.; Paludo, J.; Haddox, C.L.; Gonzalez Velez, M.; Wang, Y.; Leventakos, K.; Hubbard, J.M.; Mansfield, A.S.; Go, R.S.; et al. Representation of minorities and women in oncology clinical trials: Review of the past 14 years. J. Oncol. Pract. 2018, 14, e1–e10. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.H.; Krumholz, H.M.; Gross, C.P. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA 2004, 291, 2720–2726. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Vom Steeg, L.G.; Klein, S.L. SeXX matters in infectious disease pathogenesis. PLoS Pathog. 2016, 12, e1005374. [Google Scholar] [CrossRef]
- Quintero, O.L.; Amador-Patarroyo, M.J.; Montoya-Ortiz, G.; Rojas-Villarraga, A.; Anaya, J.M. Autoimmune disease and gender: Plausible mechanisms for the female predominance of autoimmunity. J. Autoimmun. 2012, 38, J109–J119. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Jedlicka, A.; Pekosz, A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010, 10, 338–349. [Google Scholar] [CrossRef]
- Cook, M.B.; McGlynn, K.A.; Devesa, S.S.; Freedman, N.D.; Anderson, W.F. Sex disparities in cancer mortality and survival. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Pala, L.; De Pas, T.; Catania, C.; Giaccone, G.; Mantovani, A.; Minucci, S.; Viale, G.; Gelber, R.D.; Conforti, F. Sex and cancer immunotherapy: Current understanding and challenges. Cancer Cell 2022, 40, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Jin, J.; Yang, Y.; Sun, H.; Wu, L.; Shen, M.; Hong, X.; Li, W.; Lu, L.; Cao, D.; et al. Androgen receptor-mediated CD8+ T cell stemness programs drive sex differences in antitumor immunity. Immunity 2022, 55, 1268–1283.e9. [Google Scholar] [CrossRef] [PubMed]
- Polanczyk, M.J.; Hopke, C.; Vandenbark, A.A.; Offner, H. Estrogen-mediated immunomodulation involves reduced activation of effector T cells, potentiation of Treg cells, and enhanced expression of the PD-1 costimulatory pathway. J. Neurosci. Res. 2006, 84, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Haupt, S.; Caramia, F.; Klein, S.L.; Rubin, J.B.; Haupt, Y. Sex disparities matter in cancer development and therapy. Nat. Rev. Cancer 2021, 21, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Mertowska, P.; Mertowski, S.; Podgajna, M.; Grywalska, E. The Importance of the Transcription Factor Foxp3 in the Development of Primary Immunodeficiencies. J. Clin. Med. 2022, 11, 947. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Bagnardi, V.; De Pas, T.; Martinetti, M.; Viale, G.; Gelber, R.D.; Goldhirsch, A. Cancer immunotherapy efficacy and patients’ sex: A systematic review and meta-analysis. Lancet Oncol. 2018, 19, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Pagan, E.; Bagnardi, V.; De Pas, T.; Queirolo, P.; Pennacchioli, E.; Catania, C.; Cocorocchio, E.; Ferrucci, P.F.; et al. Sex-Based Dimorphism of Anticancer Immune Response and Molecular Mechanisms of Immune Evasion. Clin. Cancer Res. 2021, 27, 4311–4324. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, L.; Zhai, D.; Song, M.; Li, W.; Xu, S.; Jiang, S.; Meng, H.; Liang, J.; Wang, Y.; et al. The role of the sex hormone-gut microbiome axis in tumor immunotherapy. Gut Microbes 2023, 15, 2185035. [Google Scholar] [CrossRef]
- Santos-Marcos, J.A.; Rangel-Zuñiga, O.A.; Jimenez-Lucena, R.; Quintana-Navarro, G.M.; Garcia-Carpintero, S.; Malagon, M.M.; Landa, B.B.; Tena-Sempere, M.; Perez-Martinez, P.; Lopez-Miranda, J.; et al. Influence of gender and menopausal status on gut microbiota. Maturitas 2018, 116, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Wong, M.; Battisti, N.M.L.; Kordbacheh, T.; Kiderlen, M.; Greystoke, A.; Luciani, A. Immunotherapy in older patients with non-small cell lung cancer: Young International Society of Geriatric Oncology position paper. Br. J. Cancer 2020, 123, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Conforti, F.; Pala, L.; Bagnardi, V.; Viale, G.; De Pas, T.; Pagan, E.; Pennacchioli, E.; Cocorocchio, E.; Ferrucci, P.F.; De Marinis, F.; et al. Sex-Based Heterogeneity in Response to Lung Cancer Immunotherapy: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2019, 111, 772–781. [Google Scholar] [CrossRef]
- Liang, J.; Hong, J.; Tang, X.; Qiu, X.; Zhu, K.; Zhou, L.; Guo, D. Sex difference in response to non-small cell lung cancer immunotherapy: An updated meta-analysis. Ann. Med. 2022, 54, 2605–2615. [Google Scholar] [CrossRef]
- Wu, Y.; Ju, Q.; Jia, K.; Yu, J.; Shi, H.; Wu, H.; Jiang, M. Correlation between sex and efficacy of immune checkpoint inhibitors (PD-1 and CTLA-4 inhibitors). Int. J. Cancer 2018, 143, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Baas, P.; Kim, D.W.; Felip, E.; Pérez-Gracia, J.L.; Han, J.Y.; Molina, J.; Kim, J.H.; Arvis, C.D.; Ahn, M.J.; et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 2016, 387, 1540–1550. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer with PD-L1 Tumor Proportion Score ≥ 50. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-Year Outcomes from the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar] [CrossRef]
- Pinto, J.A.; Vallejos, C.S.; Raez, L.E.; Mas, L.A.; Ruiz, R.; Torres-Roman, J.S.; Morante, Z.; Araujo, J.M.; Gómez, H.L.; Aguilar, A.; et al. Gender and outcomes in non-small cell lung cancer: An old prognostic variable comes back for targeted therapy and immunotherapy? ESMO Open 2018, 3, e000344. [Google Scholar] [CrossRef] [PubMed]
- Wallis, C.J.; Butaney, M.; Satkunasivam, R.; Freedland, S.J.; Patel, S.P.; Hamid, O.; Pal, S.K.; Klaassen, Z. Association of Patient Sex with Efficacy of Immune Checkpoint Inhibitors and Overall Survival in Advanced Cancers: A Systematic Review and Meta-analysis. JAMA Oncol. 2019, 5, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Onesti, C.E.; Zizzari, I.; Cerbelli, B.; Sciattella, P.; Occhipinti, M.; Roberto, M.; Di Pietro, F.; Bonifacino, A.; Ghidini, M.; et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget 2017, 8, 99336. [Google Scholar] [CrossRef]
- Grassadonia, A.; Sperduti, I.; Vici, P.; Iezzi, L.; Brocco, D.; Gamucci, T.; Pizzuti, L.; Maugeri-Saccà, M.; Marchetti, P.; Cognetti, G.; et al. Effect of gender on the outcome of patients receiving immune checkpoint inhibitors for advanced cancer: A systematic review and meta-analysis of phase III randomized clinical trials. J. Clin. Med. 2018, 7, 542. [Google Scholar] [CrossRef]
- Wang, C.; Qiao, W.; Jiang, Y.; Zhu, M.; Shao, J.; Ren, P.; Liu, D.; Li, W. Effect of sex on the efficacy of patients receiving immune checkpoint inhibitors in advanced non-small cell lung cancer. Cancer Med. 2019, 8, 4023–4031. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Wen, P. Gender and sex disparity in cancer trials. ESMO Open 2020, 5, e000773. [Google Scholar] [CrossRef]
- Perera, N.D.; Bellomo, T.R.; Schmidt, W.M.; Litt, H.K.; Shyu, M.; Stavins, M.A.; Wang, M.M.; Bell, A.; Saleki, M.; Wolf, K.I.; et al. Analysis of Female Participant Representation in Registered Oncology Clinical Trials in the United States from 2008 to 2020. Oncologist 2023, 28, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Heymach, J.V.; Harpole, D.; Mitsudomi, T.; Taube, J.M.; Galffy, G.; Hochmair, M.; Winder, T.; Zukov, R.; Garbaos, G.; Gao, S.; et al. Abstract CT005: AEGEAN: A phase 3 trial of neoadjuvant durvalumab+ chemotherapy followed by adjuvant durvalumab in patients with resectable NSCLC. Cancer Res. 2023, 83, CT005. [Google Scholar] [CrossRef]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.H.; Gao, S.; Chen, K.N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. Perioperative Pembrolizumab for Early-Stage Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.W.; Brannigan, B.W.; Matsuo, K.; Finkelstein, D.M.; Sordella, R.; Settleman, J.; Mitsudomi, T.; Haber, D.A. Increased prevalence of EGFR-mutant lung cancer in women and in East Asian populations: Analysis of estrogen-related polymorphisms. Clin. Cancer Res. 2008, 14, 4079–4084. [Google Scholar] [CrossRef] [PubMed]
- Durant, R.W.; Wenzel, J.A.; Scarinci, I.C.; Paterniti, D.A.; Fouad, M.N.; Hurd, T.C.; Martin, M.Y. Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: Enhancing minority participation in clinical trials (EMPaCT). Cancer 2014, 120, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Morgan, R. The impact of sex and gender on immunotherapy outcomes. Biol. Sex Differ. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Unger, J.M.; Vaidya, R.; Hershman, D.L.; Minasian, L.M.; Fleury, M.E. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J. Natl. Cancer Inst. 2019, 111, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Kothadia, S.M.; Azam, T.U.; Yadav, S.; Paludo, J.; Vera Aguilera, J.; Gonzalez Velez, M.; Halfdanarson, T.R.; Molina, J.R.; Hubbard, J.M.; et al. Characterization of comorbidities limiting the recruitment of patients in early phase clinical trials. Oncologist 2019, 24, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Idossa, D.; Velazquez Manana, A.I.; Horiguchi, M.; Suero-Abreu, G.A.; Franco, I.I.; Borrero, M.; Patel, S.R.; Dhawan, N.; Florez, N. What is your preferred language? Evaluating equal access to oncology clinical studies for non-English-speaking participants. Am. Soc. Clin. Oncol. 2023, 41, 16. [Google Scholar] [CrossRef]
- Smith, A.; Agar, M.; Delaney, G. Lower trial participation by culturally and linguistically diverse (CALD) cancer patients is largely due to language barriers. Asia-Pac. J. Clin. Oncol. 2018, 14, 52–60. [Google Scholar] [CrossRef]
- Guo, X.M.; Neuman, M.K.; Vallejo, A.; Matsuo, K.; Roman, L.D. An absence of translated consent forms limits oncologic clinical trial enrollment for limited English proficiency participants. Gynecol. Oncol. 2024, 180, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.M.; Nolan, T.S.; Gregory, J.; Joseph, J.J. Diversity in clinical trials: An opportunity and imperative for community engagement. Lancet Gastroenterol. Hepatol. 2021, 6, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Boulware, L.E.; Cooper, L.A.; Ratner, L.E.; LaVeist, T.A.; Powe, N.R. Race and trust in the health care system. Public Health Rep. 2003, 118. [Google Scholar] [CrossRef]
- Doescher, M.P.; Saver, B.G.; Franks, P.; Fiscella, K. Racial and Ethnic Disparities in Perceptions of Physician Style and Trust. 2000. Available online: https://repository.escholarship.umassmed.edu/handle/20.500.14038/37165 (accessed on 6 February 2024).
- Huey, R.W.; George, G.C.; Phillips, P.; White, R.; Fu, S.; Janku, F.; Karp, D.D.; Naing, A.; Piha-Paul, S.; Subbiah, V.; et al. Patient-reported out-of-pocket costs and financial toxicity during early-phase oncology clinical trials. Oncologist 2021, 26, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Chino, F.; Zafar, S.Y. Financial toxicity and equitable access to clinical trials. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Kahn, J.M.; Gray, D.M., 2nd; Oliveri, J.M.; Washington, C.M.; DeGraffinreid, C.R.; Paskett, E.D. Strategies to improve diversity, equity, and inclusion in clinical trials. Cancer 2022, 128, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Hamel, L.M.; Penner, L.A.; Albrecht, T.L.; Heath, E.; Gwede, C.K.; Eggly, S. Barriers to clinical trial enrollment in racial and ethnic minority patients with cancer. Cancer Control 2016, 23, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Weisman, C.S.; Cassard, S.D.; Mastroianni, A.C.; Faden, R.; Federman, D. Health consequences of exclusion or underrepresentation of women in clinical studies (I). Women Health Res. 1994, 2, 35–40. [Google Scholar]
- Unger, J.M.; Vaidya, R.; Albain, K.S.; LeBlanc, M.; Minasian, L.M.; Gotay, C.C.; Henry, N.L.; Fisch, M.J.; Lee, S.M.; Blanke, C.D.; et al. Sex differences in risk of severe adverse events in patients receiving immunotherapy, targeted therapy, or chemotherapy in cancer clinical trials. J. Clin. Oncol. 2022, 40, 1474. [Google Scholar] [CrossRef] [PubMed]
- Monestime, S.; Page, R.; Jordan, W.M.; Aryal, S. Prevalence and predictors of patients reporting adverse drug reactions to health care providers during oral targeted cancer treatment. J. Am. Pharm. Assoc. 2021, 61, 53–59. [Google Scholar] [CrossRef]
- Dogan, S.; Shen, R.; Ang, D.C.; Johnson, M.L.; D’Angelo, S.P.; Paik, P.K.; Brzostowski, E.B.; Riely, G.J.; Kris, M.G.; Zakowski, M.F.; et al. Molecular Epidemiology of EGFR and KRAS Mutations in 3026 Lung Adenocarcinomas: Higher Susceptibility of Women to Smoking-Related KRAS-Mutant Cancers. Clin. Cancer Res. 2012, 18, 6169–6177. [Google Scholar] [CrossRef] [PubMed]
- Del Pup, L.; Villa, P.; Amar, I.D.; Bottoni, C.; Scambia, G. Approach to sexual dysfunction in women with cancer. Int. J. Gynecol. Cancer 2019, 29, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Duma, N.; Acharya, R.; Wei, Z.; Seaborne, L.; Heisler, C.; Fidler, M.J.; Elkins, I.; Feldman, J.; Moore, A.; King, J.; et al. MA14. 04 Sexual Health Assessment in Women with Lung Cancer (SHAWL) Study. J. Thorac. Oncol. 2022, 17, S93–S94. [Google Scholar] [CrossRef]
- Reese, J.B.; Sorice, K.; Beach, M.C.; Porter, L.S.; Tulsky, J.A.; Daly, M.B.; Lepore, S.J. Patient-provider communication about sexual concerns in cancer: A systematic review. J. Cancer Surviv. 2017, 11, 175–188. [Google Scholar] [CrossRef]
- Walter, J.R.; Xu, S.; Paller, A.S.; Choi, J.N.; Woodruff, T.K. Oncofertility considerations in adolescents and young adults given a diagnosis of melanoma: Fertility risk of Food and Drug Administration–approved systemic therapies. J. Am. Acad. Dermatol. 2016, 75, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.A.; Clatot, F.; Demeestere, I.; Lambertini, M.; Morgan, A.; Nelson, S.M.; Peccatori, F.; Cameron, D. Cancer survivorship: Reproductive health outcomes should be included in standard toxicity assessments. Eur. J. Cancer 2021, 144, 310–316. [Google Scholar] [CrossRef]
- Esposito, S.; Tenconi, R.; Preti, V.; Groppali, E.; Principi, N. Chemotherapy against cancer during pregnancy: A systematic review on neonatal outcomes. Medicine 2016, 95, e4899. [Google Scholar] [CrossRef]
- Angarita, A.M.; Johnson, C.A.; Fader, A.N.; Christianson, M.S. Fertility preservation: A key survivorship issue for young women with cancer. Front. Oncol. 2016, 6, 102. [Google Scholar] [CrossRef]
- Duma, N.; Lambertini, M. It is time to talk about fertility and immunotherapy. Oncologist 2020, 25, 277–278. [Google Scholar] [CrossRef]
- Rambhatla, A.; Strug, M.R.; De Paredes, J.G.; Cordoba Munoz, M.I.; Thakur, M. Fertility considerations in targeted biologic therapy with tyrosine kinase inhibitors: A review. J. Assist. Reprod. Genet. 2021, 38, 1897–1908. [Google Scholar] [CrossRef]
- Khan, S.Z.; Arecco, L.; Villarreal-Garza, C.; Sirohi, B.; Ponde, N.F.; Habeeb, B.; Brandão, M.; Azim, H.A., Jr.; Chowdhury, A.R.; Bozovic-Spasojevic, I.; et al. Knowledge, practice, and attitudes of physicians in low-and middle-income countries on fertility and pregnancy-related issues in young women with breast cancer. JCO Glob. Oncol. 2022, 8, e2100153. [Google Scholar] [CrossRef] [PubMed]
- Alshamsan, B.; Kushnir, I.; Al-Saadi, S.; Srikanthan, A. Perceptions and attitudes of medical oncologists regarding fertility preservation and pregnancy in high-risk cancer patients: A survey among Canadian medical oncologists. Cancer Med. 2023, 12, 1912–1921. [Google Scholar] [CrossRef]
- Adams, E.; Hill, E.; Watson, E. Fertility preservation in cancer survivors: A national survey of oncologists’ current knowledge, practice and attitudes. Br. J. Cancer 2013, 108, 1602–1615. [Google Scholar] [CrossRef] [PubMed]
- Peddie, V.L.; Porter, M.A.; Barbour, R.; Culligan, D.; MacDonald, G.; King, D.; Horn, J.; Bhattacharya, S. Factors affecting decision making about fertility preservation after cancer diagnosis: A qualitative study. BJOG Int. J. Obstet. Gynaecol. 2012, 119, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Cantero, M.T.R.; Pardo, M.A. European Medicines Agency policies for clinical trials leave women unprotected. J. Epidemiol. Community Health 2006, 60, 911–913. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Parekh, A.; Fadiran, E.O.; Uhl, K.; Throckmorton, D.C. Adverse effects in women: Implications for drug development and regulatory policies. Expert Rev. Clin. Pharmacol. 2011, 4, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Cortellini, A.; Tucci, M.; Adamo, V.; Stucci, L.S.; Russo, A.; Tanda, E.T.; Spagnolo, F.; Rastelli, F.; Bisonni, R.; Santini, D.; et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J. Immunother. Cancer 2020, 8, e001361. [Google Scholar] [CrossRef] [PubMed]
- Subramaniapillai, S.; Galea, L.A.; Einstein, G.; de Lange, A.-M.G. Sex and gender in health research: Intersectionality matters. Front. Neuroendocrinol. 2023, 72, 101104. [Google Scholar] [CrossRef]
- Liu, K.A.; Dipietro Mager, N.A. Women’s involvement in clinical trials: Historical perspective and future implications. Pharm. Pract. 2016, 14, 708. [Google Scholar] [CrossRef] [PubMed]
- Peters, U.; Spahn, J.; Patel, S. Carrots or Sticks: An Industry Perspective on the Significance of Regulatory Guidance in Promoting Participant Diversity in Clinical Trials. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 115–117. [Google Scholar] [CrossRef]

| Study | Type of Study | Sample Size | Cancers Included | Treatment Regimen | Outcome | Sex Differences in OS | Hazard Ratios (If Available) |
|---|---|---|---|---|---|---|---|
| [66] | Meta-Analysis | 11 RCTs | Solid tumors | ICI vs. chemotherapy | OS, PFS | No significant difference in males and females noted for OS or PFS | Anti-PD1: OS (males vs. females: HR 0.72, 95% CI 0.64–0.83 vs. HR 0.81, 95% CI 0.70–0.94, p = 0.285) Anti PD-1 PFS: (males vs. females: HR 0.66, 95% CI 0.52–0.82 vs. HR 0.85, 95% CI 0.66–1.09, p =0.158). |
| [67] | Systematic Review & Meta-Analysis | 21 RCTs, 26,598 patients | Solid tumors | ICI alone or with chemotherapy vs. chemotherapy | OS, PFS | Similar OS in males and females for anti-PD-1/PDL-1. Anti-CTLA-4 use was associated with longer OS in men only | OS: Females (HR, 0.77; 95% CI 0.67–0.89, p < 0.001) Males (HR, 0.73; 95% CI 0.66–0.80, p < 0.001) |
| [53] | Meta-Analysis | 20 RCTs, 11,351 patients | Solid tumors | CTLA-4 or PD-1 inhibitors vs. chemotherapy | OS | Men experienced longer OS when compared to females | OS: Women (HR, 0.86; 95% CI 0.79–0.93), Male (HR, 0.72; 95% CI 0.65–0.79) |
| [58] | Meta-Analysis | 8 RCTs, 574 NSCLC patients | NSCLC | PD-L1 or PD-L1 alone or with chemotherapy vs. chemotherapy | OS, PFS | Women had better OS with PD-1 and chemotherapy combination when compared to Males. Males had a better OS in the immunotherapy alone arm | OS PD-1/PD-L1 alone: Females (HR, 0.97; 95% CI = 0.79 to 1.19), Male (HR, 0.78 (95% CI = 0.60 to 1.00) OS combination: Females (HR, 0.44 95% CI = 0.25 to 0.76), Male (HR, 0.76; 95% CI = 0.64 to 0.91) |
| [65] | Meta-Analysis | 23 RCTs, 13,271 patients | Solid Tumors | ICI vs. standard therapies | OS | Benefit noted in both men and women with no statistical difference noted between the sexes | OS: Females (HR, 0.77; 95% CI, 0.67–0.88; p = 0.002), Men (HR, 0.75; 95% CI, 0.69–0.81; p < 0.001) |
| [60] | Meta-Analysis | 11 RCTs, 6096 patients | Solid tumors, (4 lung cancer RCTs) | CTLA-4 or PD-1 inhibitors vs. chemotherapy | OS, PFS | Better PFS and OS seen in males vs. females treated with ICI. However, this was not noted in the NSCLC cohort. | OS: Females (HR = 0.74; 95% CI, 0.65–0.84; p < 0.001) Males (HR = 0.62; 95% CI, 0.53–0.71, p < 0.001) |
| [59] | Meta-Analysis | 16 RCTs, 10,155 patients | NSCLC | ICI alone or with chemotherapy vs. chemotherapy alone | OS | Those who received ICIs (with or without chemotherapy) had longer OS than those who did not receive ICIs and was comparable between both genders | Overall: Females (HR: 0.74, 95%Cl 0.63–0.87), Males (HR: 0.76, 95%Cl 0.71–0.81) ICI + Chemo: females (HR: 0.63, 95%Cl 0.42–0.92), males (HR: 0.79, 95%Cl 0.70–0.89) ICI alone: Females (HR: 0.83, 95%Cl 0.73–0.95), Males (HR: 0.74, 95%Cl 0.67–0.81) |
| [68] | Meta-Analysis | 15 RCTs, 9583 patients | Lung cancer | ICI alone or with chemotherapy vs. chemotherapy alone | OS, PFS | Both females and males benefited from anti-PD-1 therapies and benefit was seen only for males with anti-PD-L1 therapies. | Anti-PD-1: Females (HR = 0.69, 95% CI, 0.52–0.93) Males (HR = 0.73, 95% CI, 0.67–0.80) Anti-PD-L1: Females (HR = 0.69, 95% CI, 0.44–1.07), Males: (HR = 0.80, 95% CI, 0.69–0.92) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marks, J.; Sridhar, A.; Ai, A.; Kiel, L.; Kaufman, R.; Abioye, O.; Mantz, C.; Florez, N. Precision Immuno-Oncology in NSCLC through Gender Equity Lenses. Cancers 2024, 16, 1413. https://doi.org/10.3390/cancers16071413
Marks J, Sridhar A, Ai A, Kiel L, Kaufman R, Abioye O, Mantz C, Florez N. Precision Immuno-Oncology in NSCLC through Gender Equity Lenses. Cancers. 2024; 16(7):1413. https://doi.org/10.3390/cancers16071413
Chicago/Turabian StyleMarks, Jennifer, Arthi Sridhar, Angela Ai, Lauren Kiel, Rebekah Kaufman, Oyepeju Abioye, Courtney Mantz, and Narjust Florez. 2024. "Precision Immuno-Oncology in NSCLC through Gender Equity Lenses" Cancers 16, no. 7: 1413. https://doi.org/10.3390/cancers16071413
APA StyleMarks, J., Sridhar, A., Ai, A., Kiel, L., Kaufman, R., Abioye, O., Mantz, C., & Florez, N. (2024). Precision Immuno-Oncology in NSCLC through Gender Equity Lenses. Cancers, 16(7), 1413. https://doi.org/10.3390/cancers16071413






