Low-Volume Metastases in Apparent Early-Stage Endometrial Cancer: Prevalence, Clinical Significance, and Future Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
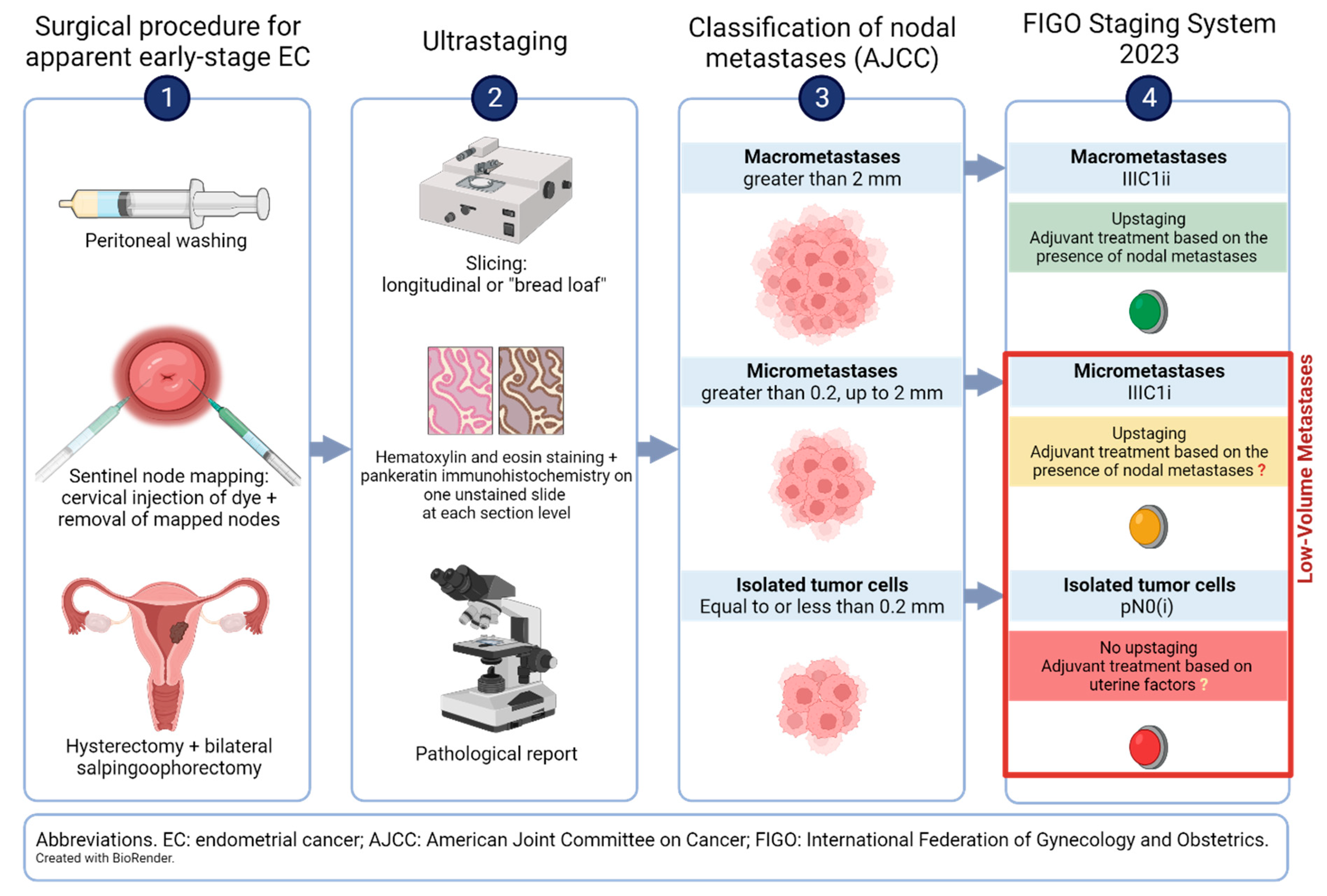
1.1. Sentinel Node Mapping Technique: Site of Injection, Dye, and Sentinel Node Algorithm
1.2. Ultrastaging of the Sentinel Nodes
2. Prevalence of Low-Volume Metastases
| First Author | Study Period | Country | Number of Patients | Patients with LVM (%) | Patients with MM (%) | Patients with ITCs (%) |
|---|---|---|---|---|---|---|
| Studies reporting data on prevalence of LVM, MM, and ITCs | ||||||
| Ballester [31] | 2007–2009 | France | 111 | 7 (6.3) | 6 (5.4) | 1 (0.9) |
| Holloway [37] | 2006–2013 | USA | 119 | 22 (18.5) | 10 (8.4) | 12 (10.1) |
| Clinton [62] | 2012–2015 | USA | 185 | 12 (6.5) | 3 (1.6) | 9 (4.9) |
| Euscher [51] | 2007–2014 | USA | 178 | 22 (12.4) | 14 (7.9) | 8 (4.5) |
| Plante [63] | 2010–2015 | Canada | 519 | 42 (8) | 11 (2) | 31 (6) |
| Rossi [25] | 2012–2015 | USA | 293 | 19 (6.5) | 9 (3.1) | 10 (3.4) |
| Backes [64] | 2013–2016 | USA | 184 | 11 (6) | 2 (1.1) | 9 (4.9) |
| Kennard [65] | 2011–2016 | USA | 414 | 57 (13.7) | 21 (5) | 36 (8.7) |
| Martinelli [66] | 2005–2019 | Italy | 221 | 14 (6.3) | 6 (2.7) | 8 (3.6) |
| Garcia Pineda [67] | 2007–2016 | Spain | 230 | 14 (6.1) | 8 (3.5) | 6 (2.6) |
| Mueller [60] | 2005–2018 | USA | 1044 | 106 (10.2) | 45 (4.4) | 61 (5.8) |
| Lavecchia [59] | 2015–2019 | Canada, Korea | 1012 | 19 (1.9) | 7 (0.7) | 12 (1.2) |
| Buda [68] | 2012–2020 | Europe | 1428 | 76 (5.3) | 50 (3.5) | 26 (1.8) |
| Total | 5938 | 421 (7.1) | 192 (3.2) | 229 (3.9) | ||
| Studies reporting data on prevalence of LVM only | ||||||
| Desai [69] | 2011–2013 | USA | 103 | 5 (4.9) | NR | NR |
| Studies reporting data on prevalence of ITCs only | ||||||
| Goebel [70] | 2012–2016 | USA | 155 | NR | NR | 21 (13.5) |
| Matsuo [71] | 2018 | USA | 6472 | NR | NR | 111 (1.7) |
| Mumford [72] | 2017–2020 | USA | 848 | NR | NR | 33 (3.9) |
| First Author | Study Period | Definition of Risk | Country | Number of Patients | Patients with LVM (%) | Patients with MM (%) | Patients with ITCs (%) |
|---|---|---|---|---|---|---|---|
| Raimond [73] | 2000–2012 | Apparent stage I, ESMO low- § and intermediate-risk ¥ EC | France | 136 | 15 (11) | NR | NR |
| Todo [74] | 1997–2004 | Intermediate-risk EC: early-stage with any of the following: >50% MI, grade 3 disease, non-endometrioid, with cervical involvement, LVSI, PPC | Japan | 61 | 9 (14.8) | 3 (5) | 6 (9.8) |
| Zahl-Eriksson [75] | 2004–2013 | Low-risk EC: MI < 50%, endometrioid, any grade | USA | 642 | 22 (3.4) | 2 (0.3) | 20 (3.1) |
| Ducie [76] | 2004–2013 | Intermediate-risk EC: apparent early-stage with >50% MI, any grade, endometrioid; high-risk EC: serous, clear-cell | USA | 202 | 20 (9.9) | 8 (4) | 12 (5.9) |
| Persson [33] | 2014-2018 | High-risk EC: apparent early stage with any of the following: endometrioid grade 3, non-endometrioid, >50% MI, CSI, non-diploid cytometry | Sweden | 257 | 19 (7.4) | 9 (3.5) | 10 (3.9) |
| Bjornholt [77] | 2017–2022 | Apparent early-stage endometrioid EC, low-grade, any MI | Denmark | 591 | 36 (5.7) | 20 (3.1) | 16 (2.6) |
| Burg [78] | 2016–2021 | Apparent early-stage, ESGO low- £ and intermediate-risk ¤ EC | Netherlands | 152 | NR | NR | 3 (2) |
2.1. Low-Volume Metastases in Apparent Early-Stage Endometrial Cancer
2.2. Prevalence of Low-Volume Metastases after Stratification of Patients in Low- and Intermediate-Risk Groups
2.3. Prevalence of Low-Volume Metastases after Stratification of Patients in Intermediate- and High-Risk Groups
2.4. Prevalence of Low-Volume Metastases after Stratification of Patients in Low-, Intermediate-, and High-Risk Groups
2.5. Considerations on the Prevalence of Low-Volume Metastases across Studies
3. Clinical Significance of Low-Volume Metastases
4. Present and Future Challenges
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Creasman, W. Revised FIGO staging for carcinoma of the endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 109. [Google Scholar] [CrossRef]
- Lewin, S.N.; Herzog, T.J.; Barrena Medel, N.I.; Deutsch, I.; Burke, W.M.; Sun, X.; Wright, J.D. Comparative performance of the 2009 international Federation of gynecology and obstetrics’ staging system for uterine corpus cancer. Obstet. Gynecol. 2010, 116, 1141–1149. [Google Scholar] [CrossRef]
- Barlin, J.N.; Soslow, R.A.; Lutz, M.; Zhou, Q.C.; St Clair, C.M.; Leitao, M.M., Jr.; Iasonos, A.; Hensley, M.L.; Barakat, R.R.; Matias-Guiu, X.; et al. Redefining stage I endometrial cancer: Incorporating histology, a binary grading system, myometrial invasion, and lymph node assessment. Int. J. Gynecol. Cancer 2013, 23, 1620–1628. [Google Scholar] [CrossRef]
- Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; Benz, C.C.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N. FIGO staging of endometrial cancer: 2023. Int. J. Gynaecol. Obstet. 2023, 162, 383–394. [Google Scholar] [CrossRef]
- Wilson, T.O.; Podratz, K.C.; Gaffey, T.A.; Malkasian, G.D., Jr.; O’Brien, P.C.; Naessens, J.M. Evaluation of unfavorable histologic subtypes in endometrial adenocarcinoma. Am. J. Obstet. Gynecol. 1990, 162, 418–423; discussion 423–426. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, J.H. Revised FIGO staging for gynaecological cancer. Br. J. Obstet. Gynaecol. 1989, 96, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Maggino, T.; Romagnolo, C.; Landoni, F.; Sartori, E.; Zola, P.; Gadducci, A. An analysis of approaches to the management of endometrial cancer in North America: A CTF study. Gynecol. Oncol. 1998, 68, 274–279. [Google Scholar] [CrossRef]
- Maggino, T.; Romagnolo, C.; Zola, P.; Sartori, E.; Landoni, F.; Gadducci, A. An analysis of approaches to the treatment of endometrial cancer in western Europe: A CTF study. Eur. J. Cancer 1995, 31, 1993–1997. [Google Scholar] [CrossRef] [PubMed]
- Konno, R.; Sato, S.; Yajima, A. A questionnaire survey on current surgical procedures for endometrial cancer in Japan. Tohoku J. Exp. Med. 2000, 190, 193–203. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Soliman, P.T.; Frumovitz, M.; Spannuth, W.; Greer, M.J.; Sharma, S.; Schmeler, K.M.; Ramirez, P.T.; Levenback, C.F.; Ramondetta, L.M. Lymphadenectomy during endometrial cancer staging: Practice patterns among gynecologic oncologists. Gynecol. Oncol. 2010, 119, 291–294. [Google Scholar] [CrossRef]
- Kitchener, H.; Swart, A.M.; Qian, Q.; Amos, C.; Parmar, M.K. Efficacy of systematic pelvic lymphadenectomy in endometrial cancer (MRC ASTEC trial): A randomised study. Lancet 2009, 373, 125–136. [Google Scholar] [CrossRef]
- Benedetti Panici, P.; Basile, S.; Maneschi, F.; Alberto Lissoni, A.; Signorelli, M.; Scambia, G.; Angioli, R.; Tateo, S.; Mangili, G.; Katsaros, D.; et al. Systematic pelvic lymphadenectomy vs. no lymphadenectomy in early-stage endometrial carcinoma: Randomized clinical trial. J. Natl. Cancer Inst. 2008, 100, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Emons, G.; Kim, J.W.; Weide, K.; de Gregorio, N.; Wimberger, P.; Trillsch, F.; Gabriel, B.; Denschlag, D.; Kommoss, S.; Aydogdu, M.; et al. Endometrial Cancer Lymphadenectomy Trial (ECLAT) (pelvic and para-aortic lymphadenectomy in patients with stage I or II endometrial cancer with high risk of recurrence; AGO-OP.6). Int. J. Gynecol. Cancer 2021, 31, 1075–1079. [Google Scholar] [CrossRef]
- Burke, T.W.; Levenback, C.; Tornos, C.; Morris, M.; Wharton, J.T.; Gershenson, D.M. Intraabdominal lymphatic mapping to direct selective pelvic and paraaortic lymphadenectomy in women with high-risk endometrial cancer: Results of a pilot study. Gynecol. Oncol. 1996, 62, 169–173. [Google Scholar] [CrossRef]
- Holub, Z.; Jabor, A.; Kliment, L. Comparison of two procedures for sentinel lymph node detection in patients with endometrial cancer: A pilot study. Eur. J. Gynaecol. Oncol. 2002, 23, 53–57. [Google Scholar] [PubMed]
- Echt, M.L.; Finan, M.A.; Hoffman, M.S.; Kline, R.C.; Roberts, W.S.; Fiorica, J.V. Detection of sentinel lymph nodes with lymphazurin in cervical, uterine, and vulvar malignancies. South. Med. J. 1999, 92, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Frumovitz, M.; Bodurka, D.C.; Broaddus, R.R.; Coleman, R.L.; Sood, A.K.; Gershenson, D.M.; Burke, T.W.; Levenback, C.F. Lymphatic mapping and sentinel node biopsy in women with high-risk endometrial cancer. Gynecol. Oncol. 2007, 104, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Gien, L.T.; Kwon, J.S.; Carey, M.S. Sentinel node mapping with isosulfan blue dye in endometrial cancer. J. Obstet. Gynaecol. Can. 2005, 27, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): A multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Togami, S.; Kawamura, T.; Fukuda, M.; Yanazume, S.; Kamio, M.; Kobayashi, H. Prospective study of sentinel lymph node mapping for endometrial cancer. Int. J. Gynaecol. Obstet. 2018, 143, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Rajanbabu, A.; Agarwal, R. A prospective evaluation of the sentinel node mapping algorithm in endometrial cancer and correlation of its performance against endometrial cancer risk subtypes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 224, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Farzaneh, F.; Moridi, A.; Azizmohammadi, Z.; Ansari, J.M.; Hosseini, M.S.; Arab, M.; Ashrafganjoei, T.; Mazaheri, M. Value of Sentinel Lymph Node (SLN) Mapping and Biopsy using Combined Intracervical Radiotracers and Blue Dye Injections for Endometrial Cancer. Asian Pac. J. Cancer Prev. 2017, 18, 431–435. [Google Scholar] [CrossRef]
- Holloway, R.W.; Ahmad, S.; Kendrick, J.E.; Bigsby, G.E.; Brudie, L.A.; Ghurani, G.B.; Stavitzski, N.M.; Gise, J.L.; Ingersoll, S.B.; Pepe, J.W. A Prospective Cohort Study Comparing Colorimetric and Fluorescent Imaging for Sentinel Lymph Node Mapping in Endometrial Cancer. Ann. Surg. Oncol. 2017, 24, 1972–1979. [Google Scholar] [CrossRef]
- Soliman, P.T.; Westin, S.N.; Dioun, S.; Sun, C.C.; Euscher, E.; Munsell, M.F.; Fleming, N.D.; Levenback, C.; Frumovitz, M.; Ramirez, P.T.; et al. A prospective validation study of sentinel lymph node mapping for high-risk endometrial cancer. Gynecol. Oncol. 2017, 146, 234–239. [Google Scholar] [CrossRef]
- Ballester, M.; Dubernard, G.; Lécuru, F.; Heitz, D.; Mathevet, P.; Marret, H.; Querleu, D.; Golfier, F.; Leblanc, E.; Rouzier, R.; et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early stage endometrial cancer: A prospective multicentre study (SENTI-ENDO). Lancet Oncol. 2011, 12, 469–476. [Google Scholar] [CrossRef]
- Hagen, B.; Valla, M.; Aune, G.; Ravlo, M.; Abusland, A.B.; Araya, E.; Sundset, M.; Tingulstad, S. Indocyanine green fluorescence imaging of lymph nodes during robotic-assisted laparoscopic operation for endometrial cancer. A prospective validation study using a sentinel lymph node surgical algorithm. Gynecol. Oncol. 2016, 143, 479–483. [Google Scholar] [CrossRef]
- Persson, J.; Salehi, S.; Bollino, M.; Lönnerfors, C.; Falconer, H.; Geppert, B. Pelvic Sentinel lymph node detection in High-Risk Endometrial Cancer (SHREC-trial)-the final step towards a paradigm shift in surgical staging. Eur. J. Cancer 2019, 116, 77–85. [Google Scholar] [CrossRef]
- Cusimano, M.C.; Vicus, D.; Pulman, K.; Maganti, M.; Bernardini, M.Q.; Bouchard-Fortier, G.; Laframboise, S.; May, T.; Hogen, L.F.; Covens, A.L.; et al. Assessment of Sentinel Lymph Node Biopsy vs Lymphadenectomy for Intermediate- and High-Grade Endometrial Cancer Staging. JAMA Surg. 2021, 156, 157–164. [Google Scholar] [CrossRef]
- Uterine Neoplasms (Version 1.2023); National Comprehensive Cancer Network: Plymouth Meeting, PA, USA, 2023.
- Khoury-Collado, F.; Murray, M.P.; Hensley, M.L.; Sonoda, Y.; Alektiar, K.M.; Levine, D.A.; Leitao, M.M.; Chi, D.S.; Barakat, R.R.; Abu-Rustum, N.R. Sentinel lymph node mapping for endometrial cancer improves the detection of metastatic disease to regional lymph nodes. Gynecol. Oncol. 2011, 122, 251–254. [Google Scholar] [CrossRef]
- Holloway, R.W.; Gupta, S.; Stavitzski, N.M.; Zhu, X.; Takimoto, E.L.; Gubbi, A.; Bigsby, G.E.; Brudie, L.A.; Kendrick, J.E.; Ahmad, S. Sentinel lymph node mapping with staging lymphadenectomy for patients with endometrial cancer increases the detection of metastasis. Gynecol. Oncol. 2016, 141, 206–210. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Khoury-Collado, F.; Pandit-Taskar, N.; Soslow, R.A.; Dao, F.; Sonoda, Y.; Levine, D.A.; Brown, C.L.; Chi, D.S.; Barakat, R.R.; et al. Sentinel lymph node mapping for grade 1 endometrial cancer: Is it the answer to the surgical staging dilemma? Gynecol. Oncol. 2009, 113, 163–169. [Google Scholar] [CrossRef]
- Rossi, E.C.; Jackson, A.; Ivanova, A.; Boggess, J.F. Detection of sentinel nodes for endometrial cancer with robotic assisted fluorescence imaging: Cervical versus hysteroscopic injection. Int. J. Gynecol. Cancer 2013, 23, 1704–1711. [Google Scholar] [CrossRef]
- Holloway, R.W.; Abu-Rustum, N.R.; Backes, F.J.; Boggess, J.F.; Gotlieb, W.H.; Jeffrey Lowery, W.; Rossi, E.C.; Tanner, E.J.; Wolsky, R.J. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol. Oncol. 2017, 146, 405–415. [Google Scholar] [CrossRef]
- Burg, L.C.; Verheijen, S.; Bekkers, R.L.M.; IntHout, J.; Holloway, R.W.; Taskin, S.; Ferguson, S.E.; Xue, Y.; Ditto, A.; Baiocchi, G.; et al. The added value of SLN mapping with indocyanine green in low- and intermediate-risk endometrial cancer management: A systematic review and meta-analysis. J. Gynecol. Oncol. 2022, 33, e66. [Google Scholar] [CrossRef]
- Frumovitz, M.; Plante, M.; Lee, P.S.; Sandadi, S.; Lilja, J.F.; Escobar, P.F.; Gien, L.T.; Urbauer, D.L.; Abu-Rustum, N.R. Near-infrared fluorescence for detection of sentinel lymph nodes in women with cervical and uterine cancers (FILM): A randomised, phase 3, multicentre, non-inferiority trial. Lancet Oncol. 2018, 19, 1394–1403. [Google Scholar] [CrossRef]
- Barlin, J.N.; Khoury-Collado, F.; Kim, C.H.; Leitao, M.M., Jr.; Chi, D.S.; Sonoda, Y.; Alektiar, K.; DeLair, D.F.; Barakat, R.R.; Abu-Rustum, N.R. The importance of applying a sentinel lymph node mapping algorithm in endometrial cancer staging: Beyond removal of blue nodes. Gynecol. Oncol. 2012, 125, 531–535. [Google Scholar] [CrossRef]
- Leitao, M.M., Jr.; Khoury-Collado, F.; Gardner, G.; Sonoda, Y.; Brown, C.L.; Alektiar, K.M.; Hensley, M.L.; Soslow, R.A.; Barakat, R.R.; Abu-Rustum, N.R. Impact of incorporating an algorithm that utilizes sentinel lymph node mapping during minimally invasive procedures on the detection of stage IIIC endometrial cancer. Gynecol. Oncol. 2013, 129, 38–41. [Google Scholar] [CrossRef]
- Burg, L.C.; Hengeveld, E.M.; In’t Hout, J.; Bulten, J.; Bult, P.; Zusterzeel, P.L.M. Ultrastaging methods of sentinel lymph nodes in endometrial cancer—A systematic review. Int. J. Gynecol. Cancer 2021, 31, 744–753. [Google Scholar] [CrossRef]
- Cote, R.J.; Peterson, H.F.; Chaiwun, B.; Gelber, R.D.; Goldhirsch, A.; Castiglione-Gertsch, M.; Gusterson, B.; Neville, A.M. Role of immunohistochemical detection of lymph-node metastases in management of breast cancer. International Breast Cancer Study Group. Lancet 1999, 354, 896–900. [Google Scholar] [CrossRef]
- Joseph, E.; Messina, J.; Glass, F.L.; Cruse, C.W.; Rapaport, D.P.; Berman, C.; Reintgen, D.S. Radioguided surgery for the ultrastaging of the patient with melanoma. Cancer J. Sci. Am. 1997, 3, 341–345. [Google Scholar]
- Yabushita, H.; Shimazu, M.; Yamada, H.; Sawaguchi, K.; Noguchi, M.; Nakanishi, M.; Kawai, M. Occult lymph node metastases detected by cytokeratin immunohistochemistry predict recurrence in node-negative endometrial cancer. Gynecol. Oncol. 2001, 80, 139–144. [Google Scholar] [CrossRef]
- Kim, C.H.; Soslow, R.A.; Park, K.J.; Barber, E.L.; Khoury-Collado, F.; Barlin, J.N.; Sonoda, Y.; Hensley, M.L.; Barakat, R.R.; Abu-Rustum, N.R. Pathologic ultrastaging improves micrometastasis detection in sentinel lymph nodes during endometrial cancer staging. Int. J. Gynecol. Cancer 2013, 23, 964–970. [Google Scholar] [CrossRef]
- Kim, C.H.; Khoury-Collado, F.; Barber, E.L.; Soslow, R.A.; Makker, V.; Leitao, M.M., Jr.; Sonoda, Y.; Alektiar, K.M.; Barakat, R.R.; Abu-Rustum, N.R. Sentinel lymph node mapping with pathologic ultrastaging: A valuable tool for assessing nodal metastasis in low-grade endometrial cancer with superficial myoinvasion. Gynecol. Oncol. 2013, 131, 714–719. [Google Scholar] [CrossRef]
- Euscher, E.; Sui, D.; Soliman, P.; Westin, S.; Ramalingam, P.; Bassett, R.; Malpica, A. Ultrastaging of Sentinel Lymph Nodes in Endometrial Carcinoma According to Use of 2 Different Methods. Int. J. Gynecol. Pathol. 2018, 37, 242–251. [Google Scholar] [CrossRef]
- Casarin, J.; Multinu, F.; Pasupathy, K.; Weaver, A.; McGree, M.; Tortorella, L.; Torres, D.; Kumar, A.; Langstraat, C.; Huang, Y.; et al. Frozen Section for Detection of Lymph Nodes After Cervical Injection with Indocyanine Green (ICG) for Sentinel Lymph Node Technique in Endometrial Cancer Staging. Ann. Surg. Oncol. 2018, 25, 3692–3698. [Google Scholar] [CrossRef]
- Bellaminutti, S.; Bonollo, M.; Gasparri, M.L.; Clivio, L.; Migliora, P.; Mazzucchelli, L.; Papadia, A. Sentinel lymph node intraoperative analysis in endometrial cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 3199–3205. [Google Scholar] [CrossRef]
- Bodurtha Smith, A.J.; Fader, A.N.; Tanner, E.J. Sentinel lymph node assessment in endometrial cancer: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2017, 216, 459–476.e10. [Google Scholar] [CrossRef]
- Nagai, T.; Niikura, H.; Okamoto, S.; Nakabayashi, K.; Matoda, M.; Utsunomiya, H.; Nagase, S.; Watanabe, M.; Takeshima, N.; Yaegashi, N. A new diagnostic method for rapid detection of lymph node metastases using a one-step nucleic acid amplification (OSNA) assay in endometrial cancer. Ann. Surg. Oncol. 2015, 22, 980–986. [Google Scholar] [CrossRef]
- Kosťun, J.; Pešta, M.; Sláma, J.; Slunéčko, R.; Vlasák, P.; Bouda, J.; Novotný, Z.; Topolčan, O.; Kučera, R.; Kulda, V.; et al. One-step nucleic acid amplification vs ultrastaging in the detection of sentinel lymph node metastasis in endometrial cancer patients. J. Surg. Oncol. 2019, 119, 361–369. [Google Scholar] [CrossRef]
- Togami, S.; Tanimoto, A.; Yanazume, S.; Tokunaga, H.; Nagai, T.; Watanabe, M.; Yahata, H.; Asanoma, K.; Yamamoto, H.; Tanaka, T.; et al. Evaluation of the one-step nucleic acid amplification assay for detecting lymph node metastasis in patients with cervical and endometrial cancer: A multicenter prospective study. Gynecol. Oncol. 2023, 170, 70–76. [Google Scholar] [CrossRef]
- Olawaiye, A.B.; Mutch, D.G. Lymphnode staging update in the American Joint Committee on Cancer 8th Edition cancer staging manual. Gynecol. Oncol. 2018, 150, 7–8. [Google Scholar] [CrossRef]
- Lavecchia, M.; Jang, J.H.; Lee, H.J.; Pin, S.; Steed, H.; Lee, J.Y.; Ghosh, S.; Kwon, J.S. Sentinel lymph node biopsy in endometrial cancer: The new norm—A multicentre, international experience. Surg. Oncol. 2023, 48, 101922. [Google Scholar] [CrossRef]
- Mueller, J.J.; Pedra Nobre, S.; Braxton, K.; Alektiar, K.M.; Leitao, M.M., Jr.; Aghajanian, C.; Ellenson, L.H.; Abu-Rustum, N.R. Incidence of pelvic lymph node metastasis using modern FIGO staging and sentinel lymph node mapping with ultrastaging in surgically staged patients with endometrioid and serous endometrial carcinoma. Gynecol. Oncol. 2020, 157, 619–623. [Google Scholar] [CrossRef]
- Amezcua, C.A.; MacDonald, H.R.; Lum, C.A.; Yi, W.; Muderspach, L.I.; Roman, L.D.; Felix, J.C. Endometrial cancer patients have a significant risk of harboring isolated tumor cells in histologically negative lymph nodes. Int. J. Gynecol. Cancer 2006, 16, 1336–1341. [Google Scholar] [CrossRef]
- Clinton, L.K.; Kondo, J.; Carney, M.E.; Tauchi-Nishi, P.; Terada, K.; Shimizu, D. Low-Volume Lymph Node Metastases in Endometrial Carcinoma. Int. J. Gynecol. Cancer 2017, 27, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Plante, M.; Stanleigh, J.; Renaud, M.C.; Sebastianelli, A.; Grondin, K.; Grégoire, J. Isolated tumor cells identified by sentinel lymph node mapping in endometrial cancer: Does adjuvant treatment matter? Gynecol. Oncol. 2017, 146, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Backes, F.J.; Cohen, D.; Salani, R.; Cohn, D.E.; O’Malley, D.M.; Fanning, E.; Suarez, A.A.; Fowler, J.M. Prospective clinical trial of robotic sentinel lymph node assessment with isosulfane blue (ISB) and indocyanine green (ICG) in endometrial cancer and the impact of ultrastaging (NCT01818739). Gynecol. Oncol. 2019, 153, 496–499. [Google Scholar] [CrossRef]
- Kennard, J.A.; Stephens, A.J.; Ahmad, S.; Zhu, X.; Singh, C.; McKenzie, N.D.; Kendrick, J.E.; Holloway, R.W. Sentinel lymph nodes (SLN) in endometrial cancer: The relationship between primary tumor histology, SLN metastasis size, and non-sentinel node metastasis. Gynecol. Oncol. 2019, 154, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, F.; Ditto, A.; Bogani, G.; Leone Roberti Maggiore, U.; Signorelli, M.; Chiappa, V.; Raspagliesi, F. Sentinel lymph node mapping in endometrial cancer: Performance of hysteroscopic injection of tracers. Int. J. Gynecol. Cancer 2020, 30, 332–338. [Google Scholar] [CrossRef] [PubMed]
- García Pineda, V.; Hernández Gutiérrez, A.; Gracia Segovia, M.; Siegrist Ridruejo, J.; Diestro Tejeda, M.D.; Zapardiel, I. Low-Volume Nodal Metastasis in Endometrial Cancer: Risk Factors and Prognostic Significance. J. Clin. Med. 2020, 9, 1999. [Google Scholar] [CrossRef]
- Buda, A.; Paniga, C.; Taskin, S.; Mueller, M.; Zapardiel, I.; Fanfani, F.; Puppo, A.; Casarin, J.; Papadia, A.; De Ponti, E.; et al. The Risk of Recurrence in Endometrial Cancer Patients with Low-Volume Metastasis in the Sentinel Lymph Nodes: A Retrospective Multi-Institutional Study. Cancers 2023, 15, 2052. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.H.; Hughes, P.; Tobias, D.H.; Tchabo, N.; Heller, P.B.; Dise, C.; Slomovitz, B.M. Accuracy of robotic sentinel lymph node detection (RSLND) for patients with endometrial cancer (EC). Gynecol. Oncol. 2014, 135, 196–200. [Google Scholar] [CrossRef]
- Goebel, E.A.; St Laurent, J.D.; Nucci, M.R.; Feltmate, C.M. Retrospective detection of isolated tumor cells by immunohistochemistry in sentinel lymph node biopsy performed for endometrial carcinoma: Is there clinical significance? Int. J. Gynecol. Cancer 2020, 30, 291–298. [Google Scholar] [CrossRef]
- Matsuo, K.; Khetan, V.U.; Brunette, L.L.; Jooya, N.D.; Klar, M.; Wright, J.D.; Roman, L.D. Characterizing isolated tumor cells in regional lymph nodes of early endometrial cancer. Gynecol. Oncol. 2022, 165, 264–269. [Google Scholar] [CrossRef]
- Mumford, B.S.; Garrett, A.A.; Lesnock, J.L. The role of secondary full lymphadenectomy for patients with early-stage endometrial cancer and isolated tumor cells detected on sentinel lymph node biopsy. Gynecol. Oncol. Rep. 2022, 44, 101074. [Google Scholar] [CrossRef]
- Raimond, E.; Ballester, M.; Hudry, D.; Bendifallah, S.; Daraï, E.; Graesslin, O.; Coutant, C. Impact of sentinel lymph node biopsy on the therapeutic management of early-stage endometrial cancer: Results of a retrospective multicenter study. Gynecol. Oncol. 2014, 133, 506–511. [Google Scholar] [CrossRef]
- Todo, Y.; Kato, H.; Okamoto, K.; Minobe, S.; Yamashiro, K.; Sakuragi, N. Isolated tumor cells and micrometastases in regional lymph nodes in stage I to II endometrial cancer. J. Gynecol. Oncol. 2016, 27, e1. [Google Scholar] [CrossRef]
- Zahl Eriksson, A.G.; Ducie, J.; Ali, N.; McGree, M.E.; Weaver, A.L.; Bogani, G.; Cliby, W.A.; Dowdy, S.C.; Bakkum-Gamez, J.N.; Abu-Rustum, N.R.; et al. Comparison of a sentinel lymph node and a selective lymphadenectomy algorithm in patients with endometrioid endometrial carcinoma and limited myometrial invasion. Gynecol. Oncol. 2016, 140, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Ducie, J.A.; Eriksson, A.G.Z.; Ali, N.; McGree, M.E.; Weaver, A.L.; Bogani, G.; Cliby, W.A.; Dowdy, S.C.; Bakkum-Gamez, J.N.; Soslow, R.A.; et al. Comparison of a sentinel lymph node mapping algorithm and comprehensive lymphadenectomy in the detection of stage IIIC endometrial carcinoma at higher risk for nodal disease. Gynecol. Oncol. 2017, 147, 541–548. [Google Scholar] [CrossRef]
- Bjørnholt, S.M.; Sponholtz, S.E.; Mogensen, O.; Bouchelouche, K.; Parner, E.T.; Neumann, G.; Jochumsen, K.M.; Hamid, B.H.; Davidsen, M.B.; Bjørn, S.F.; et al. The SENTIREC-endo study—Risks and benefits of a national adoption of sentinel node mapping in low and intermediate risk endometrial cancer. Gynecol. Oncol. 2023, 171, 121–128. [Google Scholar] [CrossRef]
- Burg, L.C.; Kruitwagen, R.; de Jong, A.; Bulten, J.; Bonestroo, T.J.J.; Kraayenbrink, A.A.; Boll, D.; Lambrechts, S.; Smedts, H.P.M.; Bouman, A.; et al. Sentinel Lymph Node Mapping in Presumed Low- and Intermediate-Risk Endometrial Cancer Management (SLIM): A Multicenter, Prospective Cohort Study in The Netherlands. Cancers 2022, 15, 271. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Mariani, A.; Paolini, B.; Ditto, A.; Raspagliesi, F. Low-volume disease in endometrial cancer: The role of micrometastasis and isolated tumor cells. Gynecol. Oncol. 2019, 153, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Mariani, A.; Webb, M.J.; Rao, S.K.; Lesnick, T.G.; Podratz, K.C. Significance of pathologic patterns of pelvic lymph node metastases in endometrial cancer. Gynecol. Oncol. 2001, 80, 113–120. [Google Scholar] [CrossRef]
- Zanfagnin, V.; Huang, Y.; Mc Gree, M.E.; Weaver, A.L.; Casarin, J.; Multinu, F.; Cappuccio, S.; Ferrero, A.; Mariani, A.; Glaser, G.E. Predictors of extensive lymphatic dissemination and recurrences in node-positive endometrial cancer. Gynecol. Oncol. 2019, 154, 480–486. [Google Scholar] [CrossRef]
- St Clair, C.M.; Eriksson, A.G.; Ducie, J.A.; Jewell, E.L.; Alektiar, K.M.; Hensley, M.L.; Soslow, R.A.; Abu-Rustum, N.R.; Leitao, M.M., Jr. Low-Volume Lymph Node Metastasis Discovered During Sentinel Lymph Node Mapping for Endometrial Carcinoma. Ann. Surg. Oncol. 2016, 23, 1653–1659. [Google Scholar] [CrossRef]
- Ignatov, A.; Lebius, C.; Ignatov, T.; Ivros, S.; Knueppel, R.; Papathemelis, T.; Ortmann, O.; Eggemann, H. Lymph node micrometastases and outcome of endometrial cancer. Gynecol. Oncol. 2019, 154, 475–479. [Google Scholar] [CrossRef]
- Piedimonte, S.; Richer, L.; Souhami, L.; Arseneau, J.; Fu, L.; Gilbert, L.; Alfieri, J.; Jardon, K.; Zeng, X.Z. Clinical significance of isolated tumor cells and micrometastasis in low-grade, stage I endometrial cancer. J. Surg. Oncol. 2018, 118, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Backes, F.J.; Felix, A.S.; Plante, M.; Grégoire, J.; Sullivan, S.A.; Rossi, E.C.; Tanner, E.J., 3rd; Stewart, K.I.; Soliman, P.T.; Holloway, R.W.; et al. Sentinel lymph node (SLN) isolated tumor cells (ITCs) in otherwise stage I/II endometrioid endometrial cancer: To treat or not to treat? Gynecol. Oncol. 2021, 161, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Ghoniem, K.; Larish, A.M.; Dinoi, G.; Zhou, X.C.; Alhilli, M.; Wallace, S.; Wohlmuth, C.; Baiocchi, G.; Tokgozoglu, N.; Raspagliesi, F.; et al. Oncologic outcomes of endometrial cancer in patients with low-volume metastasis in the sentinel lymph nodes: An international multi-institutional study. Gynecol. Oncol. 2021, 162, 590–598. [Google Scholar] [CrossRef]
- Cucinella, G.; Schivardi, G.; Zhou, X.C.; AlHilli, M.; Wallace, S.; Wohlmuth, C.; Baiocchi, G.; Tokgozoglu, N.; Raspagliesi, F.; Buda, A.; et al. Prognostic value of isolated tumor cells in sentinel lymph nodes in low risk endometrial cancer: Results from an international multi-institutional study. Int. J. Gynecol. Cancer 2024, 34, 179–187. [Google Scholar] [CrossRef]
- Gómez-Hidalgo, N.R.; Ramirez, P.T.; Ngo, B.; Pérez-Hoyos, S.; Coreas, N.; Sanchez-Iglesias, J.L.; Cabrera, S.; Franco, S.; Benavente, A.P.; Gil-Moreno, A. Oncologic impact of micrometastases or isolated tumor cells in sentinel lymph nodes of patients with endometrial cancer: A meta-analysis. Clin. Transl. Oncol. 2020, 22, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Narasimhulu, D.M.; Yang, J.; Swanson, A.A.; Schoolmeester, K.J.; Mariani, A. Low-volume lymphatic metastasis (isolated tumor cells) in endometrial cancer: Management and prognosis. Int. J. Gynecol. Cancer 2021, 31, 1080–1084. [Google Scholar] [CrossRef]
- Schivardi, G.; Cucinella, G.; Mariani, A.; Shahi, M.; C., L.; Weaver, A.L.; Mc Gree, M.E.; Multinu, F.; Zanagnolo, V.; Betella, I.; et al. 2022-RA-987-ESGO Molecular characterization of endometrial cancer with low volume metastasis in the sentinel lymph node: A multicentric international study. Int. J. Gynecol. Cancer 2022, 32, A124. [Google Scholar] [CrossRef]
| First Author | Number of Patients | Recurrences in Patients with Negative Nodes (%) | Recurrences in Patients with MM (%) | Recurrences in Patients with ITCs (%) | Non-Vaginal Recurrences in Patients with ITCs (%) | Recurrences in Patients with ITCs and Otherwise Low-Risk EC | Follow-Up (Months) |
|---|---|---|---|---|---|---|---|
| Kim [49] | 508 | NR | NR | 2 (10.5) | 2 (10.5) | 0 | NR |
| Raimond [73] | 136 | 11 (9.7) | 1 (6.7) | NR | NR | NR | NR |
| Todo [74] | 61 | 8 (15) | 1 (33.3) | 3 (50) | 3 (50) | 0 | 107 |
| St Clair [82] | 844 | 47 (6) | 2 (9.5) | 2 (8.7) | 1 (4.3) | NR | 26 |
| Plante [63] | 519 | NR | NR | 1 (3.2) | 1 (3.2) | 0 | 29 |
| Backes [64] | 184 | NR | 1 (50) | 0 | 0 | 0 | 31 |
| Garcia Pineda [67] | 230 | NR | 2 (25) | 3 (33.3) | 0 | 1 | 60 |
| Lavecchia [59] | 1012 | 44 (4.6) | 1 (14.3) | 0 | 0 | 0 | 27 |
| Buda [68] | 1428 | 75 (5.9) | 6 (12) | 5 (19.2) | NR | NR | 33.3 |
| Backes [85] | 175 | 16 (3.5) | NR | 9 (5.1) | 8 (4.6) | 1 | 31 |
| Ghoniem [86] | 247 | NR | 21 (18.3) | 17 (12.9) | NR | 1 (of 18 with low-risk EC) | 29.6 |
| Cucinella [87] | 494 | 16 (3.5) | NR | 5 (11.9) | 4 (9.5) | Only low-risk patients included | 28 in ITCs and 31 in node-negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fumagalli, D.; De Vitis, L.A.; Caruso, G.; Occhiali, T.; Palmieri, E.; Guillot, B.E.; Pappalettera, G.; Langstraat, C.L.; Glaser, G.E.; Reynolds, E.A.; et al. Low-Volume Metastases in Apparent Early-Stage Endometrial Cancer: Prevalence, Clinical Significance, and Future Perspectives. Cancers 2024, 16, 1338. https://doi.org/10.3390/cancers16071338
Fumagalli D, De Vitis LA, Caruso G, Occhiali T, Palmieri E, Guillot BE, Pappalettera G, Langstraat CL, Glaser GE, Reynolds EA, et al. Low-Volume Metastases in Apparent Early-Stage Endometrial Cancer: Prevalence, Clinical Significance, and Future Perspectives. Cancers. 2024; 16(7):1338. https://doi.org/10.3390/cancers16071338
Chicago/Turabian StyleFumagalli, Diletta, Luigi A. De Vitis, Giuseppe Caruso, Tommaso Occhiali, Emilia Palmieri, Benedetto E. Guillot, Giulia Pappalettera, Carrie L. Langstraat, Gretchen E. Glaser, Evelyn A. Reynolds, and et al. 2024. "Low-Volume Metastases in Apparent Early-Stage Endometrial Cancer: Prevalence, Clinical Significance, and Future Perspectives" Cancers 16, no. 7: 1338. https://doi.org/10.3390/cancers16071338
APA StyleFumagalli, D., De Vitis, L. A., Caruso, G., Occhiali, T., Palmieri, E., Guillot, B. E., Pappalettera, G., Langstraat, C. L., Glaser, G. E., Reynolds, E. A., Fruscio, R., Landoni, F., Mariani, A., & Grassi, T. (2024). Low-Volume Metastases in Apparent Early-Stage Endometrial Cancer: Prevalence, Clinical Significance, and Future Perspectives. Cancers, 16(7), 1338. https://doi.org/10.3390/cancers16071338






