1. Introduction
Despite advances in immunotherapy and surgical treatments of tumors, drug treatment remains a principal strategy against cancer [
1]. The advantages of anti-cancer drug treatments lie in better and wider access to target cancer cells throughout the body with higher sensitivity, recovery rates, and relative cost-effectiveness [
2]. Recent developments of anti-cancer drugs that specifically target mutated oncogenes or signaling molecules of a regulatory network in cancer cells not only compensates for causes of tumorigenesis but also complies with the principles of precision medicine [
3]. Nevertheless, the recurring challenge is that cancer still cannot be treated with drug treatments, since it eventually acquires resistance through repetitive drug treatments [
4].
Drug resistance is classified into three types: primary, acquired, and adaptive [
5]. Primary resistance occurs through the impact of pre-existing mutations on the regulatory network that initiate tumorigenesis and block the effects of anti-cancer drugs. Acquired resistance emerges when cancer cells accumulate additional mutations, causing them to become unresponsive to treatment. Adaptive resistance, perhaps the most complex, arises from undesirably activated regulatory motifs such as feedback or crosstalk during the cancer cell state transition, which reduces drug efficacy [
6,
7]. Therefore, unlike primary and acquired resistance, it is necessary to address the dynamic mechanisms of cell state transitions after drug treatment beyond static information such as mutation profiles to overcome adaptive resistance [
8,
9].
With recent developments in systems biology and control engineering, understanding of dynamics of state transitions within various cellular networks have been greatly improved [
10,
11]. In particular, a Boolean modeling scheme recapitulates the switch-like behaviors of molecules within a regulatory network by representing a functional activity of each node in the network with only ON (1) or OFF (0) states [
12]. The scheme can successfully implement the complex state transition dynamics of biological networks by using parameter-free logical equations [
13]. The activity of marker molecules can define cellular phenotypes, often indicating cell viabilities and thus representing drug responses [
14]. In a nominal state without any external disturbance, the cellular network state naturally transits towards a more stable state through a series of state transitions, which is known as a state transition path. As a result, all the states eventually converge to attractors, those of a few states with higher stabilities. These attractors can be represented as local valleys among an attractor landscape, which is the plane surface of possible network states [
15]. The stability of each state on the landscape can be measured by the size of its basin of attraction, which defines the relative size of neighboring states that converge to an attractor [
16]. With this, various studies on state control algorithms with Boolean networks have suggested probable molecular targets called “control targets” to regulate cellular phenotypes by pinning or permanently fixing the functional activity of each target node to ON (1, activation) or OFF (0, inhibition) [
17]. However, these strategies utilize only the initial and final states of a network, which cannot recapitulate the drug response dynamics that trigger adaptive resistance due to dynamic stochasticity after drug treatments.
After anti-cancer drug administration of a target molecule in the network, its state changes gradually influence the state of other molecules in the network. The sequence of which molecules are randomly affected depends on dynamic stochasticity, such as expression levels, molecular activity, and probability of physiochemical interactions [
18]. Despite a uniform regulatory mechanism of the molecular network, this results in drug influences that activate different signaling cascades in cancer cells and ultimately form their own stably activated regulatory motifs [
19]. Molecular regulatory networks within a cell can be subdivided into interconnected strongly connected components (SCCs) called multi-stable motifs that have multiple stable states in their respective attractors [
20]. However, as some of these stable states are biologically undesirable, drug-treated cancer cells consist of undesirably activated multi-stable motifs that then can inhibit desirably activated regulatory motifs that increase drug efficacy. Thus, a population of single-drug-treated cancer cells can heterogeneously be stabilized, and some of them may enter a drug-resistant state. These diverse responses cause adaptive resistance to the anti-cancer drug, which requires additional drug treatments in combination to compensate for the heterogeneous responses. Hence, the question arises as to whether we can induce desirable homogeneous responses in cellular systems by suggesting an optimal combination target for the given anti-cancer drug treatment.
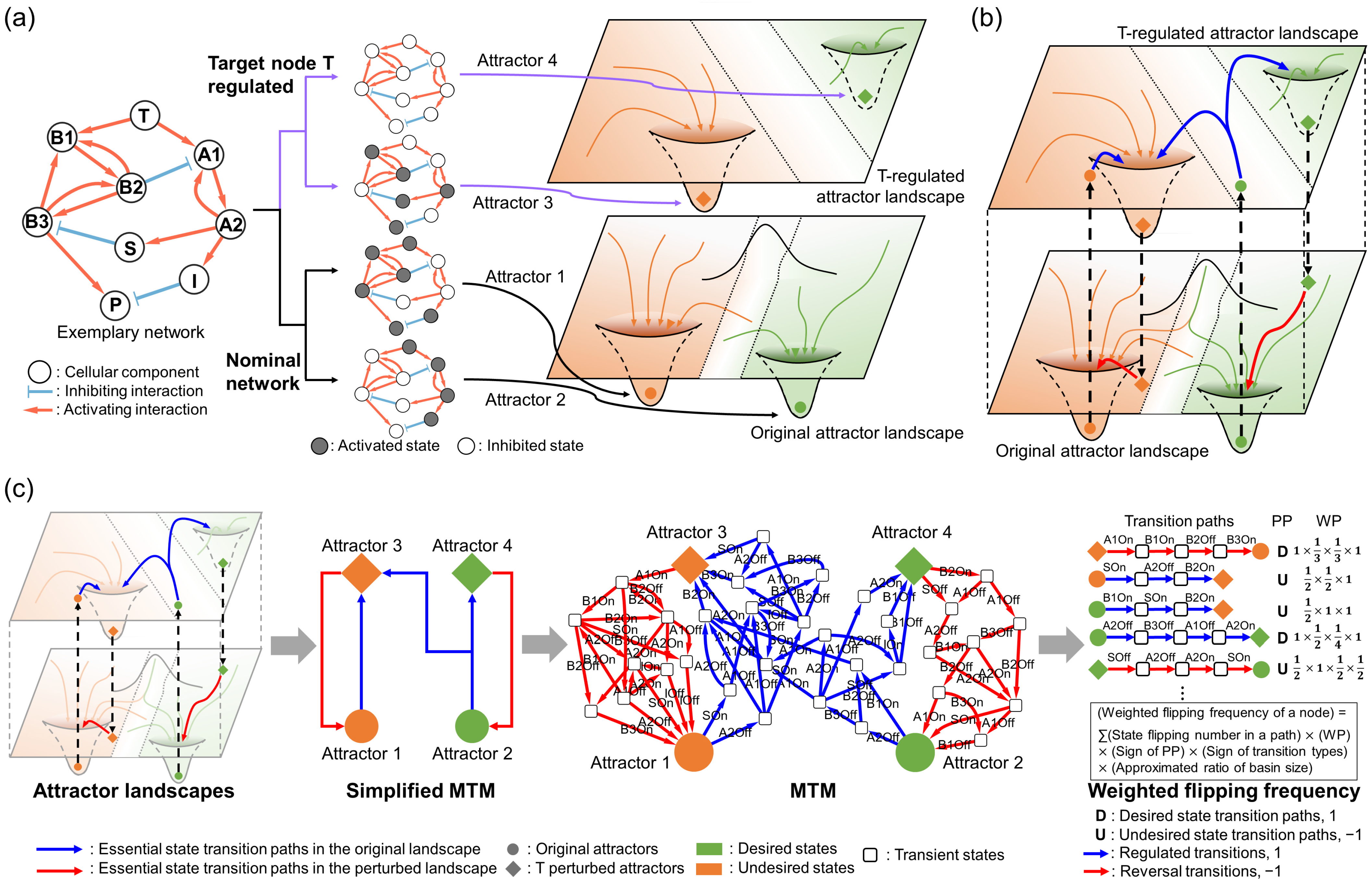
To answer this question, we have developed a new combinatorial target search algorithm called “merged transition map” (MTM). The MTM considers essential network dynamics for identifying combinatorial control targets that reduce heterogeneous responses of a given anti-cancer drug by extracting transition paths between initial and desired network states. We applied the MTM to a gastric cancer model [
21] to validate and test the performance of our strategy. By extracting essential state transition dynamics induced by target node perturbations, we revealed that competitive stabilization between multi-stable motifs caused by the dynamic stochasticity of drug effects spreads throughout the network and ultimately increases the heterogeneity of drug responses. Moreover, we found that reducing dynamic stochasticity of the molecules with frequent activity changes during the drug-induced state transitions can reduce competitive stabilization of multi-stable motifs. In addition, this can also synergistically increase the efficacy of the given targeted drug. We also discovered that conflicts between multi-stable motifs can steer the network into undesired states that can cause adaptive resistance. Thus, the suggested MTM combinatorial target can overcome resistance by regulating the nodes interconnecting these motifs. The MTM also identifies the nodes that frequently flip the most after a specific drug perturbation, positioning them as optimal combination targets for the given drug. Our findings underscore the possibility of the MTM identifying synergistic targets to counteract adaptive resistance by diminishing heterogeneous drug responses. Altogether, our study provides new insights into enhancement of a biological systems approach that can open a new paradigm in identifying combinatorial targets to compensate adaptive drug resistance in cancers.
3. Discussion
It has long been suggested that evolutionary gains in network complexity in a cell are not merely incidental but the results from adaptive capacities that reinforce information processing [
27]. Our results indicate that the conflicts between multi-stable motifs cause heterogeneous responses upon a perturbation, which may not be beneficial to cells. Such varied responses may be detrimental to the cells, which may ultimately become diminished through natural selection, since uniform responses might be more efficient in optimizing their responses to external stimuli. Yet intriguingly, these conflicting structures between multi-stable motifs are more prevalent in biological networks than in random networks (see
Supplementary Figure S7 for further details). These structures commonly appear across various gene sets, suggesting that the conflicting dynamics induced by positive feedback loops are a prevalent feature of cellular networks selected over evolutionary time [
28]. Cancers, being evolutionarily flexible, often exhibit heterogeneous responses to single drug treatments due to their possession of diverse cancer hallmarks, providing survival advantages [
29]. This suggests that conflicting dynamics between multi-stable motifs in fluctuating environments may confer survival benefits, but also complicate achieving desired cell states with single-drug treatment [
30]. Thus, understanding how these multi-stable motif structures are mutationally enriched in cancers and dynamically affect each other is crucial for overcoming adaptive drug resistance in cancers. The MTM suggests combinatorial targets based on essential network dynamics independently of tissue contexts and holds promise for identifying synergistic drugs across cancer types.
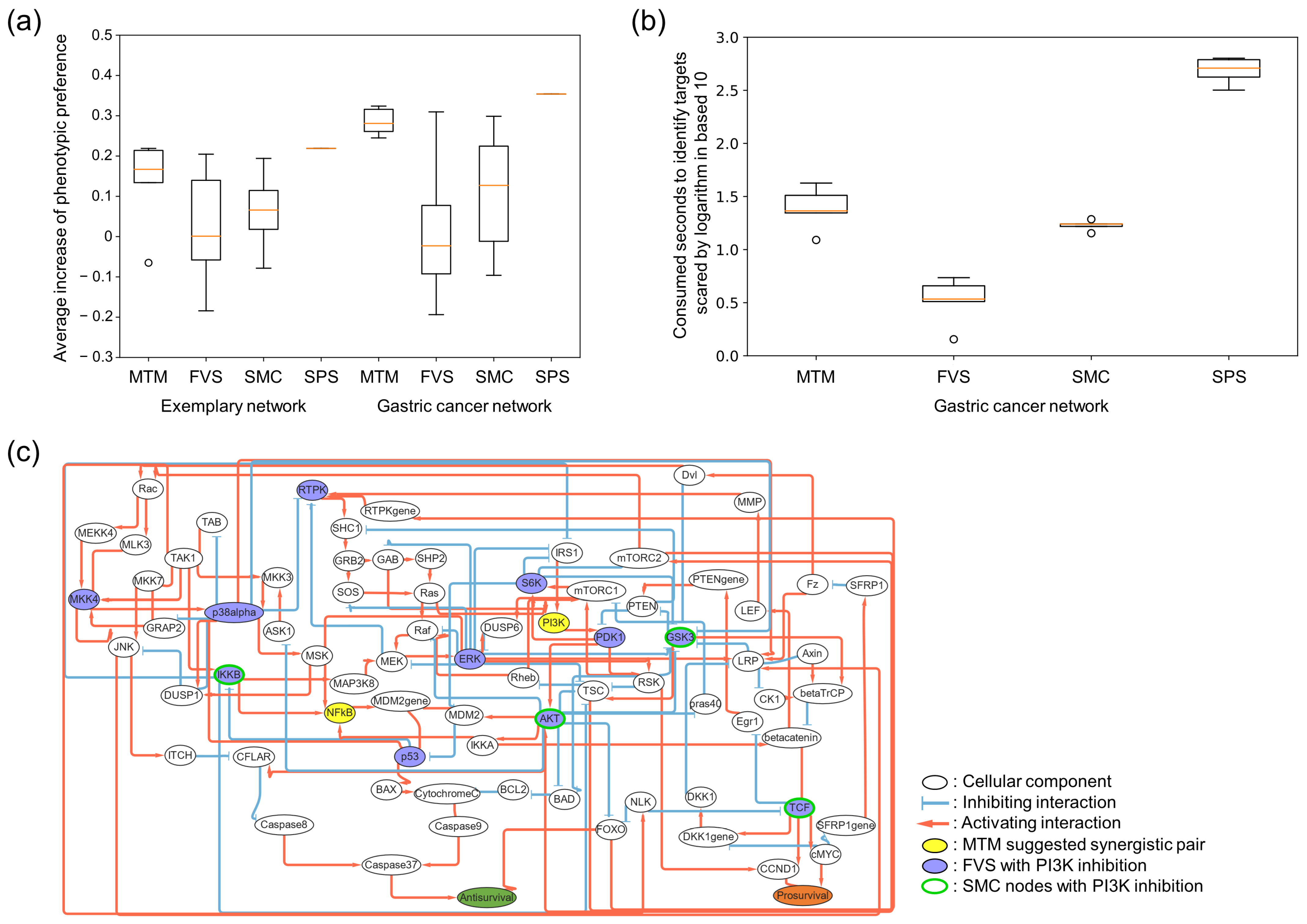
The general control target search algorithms focus on modulating every node state within a network converging to a desired state. This approach often necessitates the perturbation of a substantial number of target nodes for proper network controls. However, to control the phenotype of a cancer cell to a specific desired state, it is not imperative to regulate every node within the network. Instead, modulating a few marker nodes that determine the phenotype is sufficient. Moreover, simultaneously controlling a large number of target nodes is nearly infeasible. These biological aspects underscore that effective control strategy of biological networks should prioritize control of phenotypic marker nodes through a realistic number of target nodes. In our study, we focused on the biological feasibility of the control targets identified. Our approach is based on two key considerations. First, the challenge in cancer therapy is not the identification of new drug target genes, but rather addressing the prevalent issue of drug resistance in well-established target drugs. To this end, the MTM algorithm pinpoints the most synergistic drug targets within given perturbations of target nodes. Second, given the impracticality of controlling multiple genes or biological molecules within a cell, we limited the number of control targets to two. As a result, the MTM can show practical applicability on target identifications to overcome drug resistance in cancers, enhanced over prior control theories.
Several motif-based network control theories exist, and identifying targets regulating each of these motifs within a network is crucial to govern the entire network [
31]. In addition, numerous system biological strategies have been developed to measure phenotypic changes according to modified expressions of a gene set [
32]. Together with constructed Boolean models of cancer, in silico simulations, which implement those control theories that aid in predicting drug responses and interpret molecular mechanisms, have been widely used in various targeted cancer therapies [
33]. A common way to regulate the entire gene set is by identifying their master regulator, a gene that regulates certain gene sets, as a control target [
34]. However, heterogeneous responses of the gene sets and multi-stable motifs can dramatically reduce the control effects. Our results have revealed that these conflicts between the motifs, rather than the efficacy of a certain control target, give rise to the heterogeneous drug responses of the given anti-cancer drug. Thus, our MTM-based network control strategy can identify novel and effective synergistic pairs to common pharmacological targets that can reduce heterogeneous drug responses.
Experimental validations on control targets identified by analyzing cellular network models are crucial for substantiating in silico studies such as the MTM. However, measuring the dynamics of the mutual inhibitions between feedback loops proposed in this study requires extensive labor and resources. Therefore, instead of in-house experiments, we opted for an indirect validation method. We assessed whether the MTM identifies combinatorial targets mentioned in previous research utilizing two network models with extensive experimental validations [
21,
35]. Additionally, we referenced supporting experimental publications for a novel combination not covered in the studies [
22,
23]. While these studies may not be fully oriented to reproducing the mutual state conflict between positive feedback loops, the consistency between the MTM analysis results and actual drug responsiveness of cancer cell lines suggest their validity. Uncertainties in this study can be addressed through further analysis of various cancer cell models recapitulating network dynamics with a focus on positive feedback loops and supplementing time-series experimental data on network dynamics associated with drug response [
36]. In clinical practice, despite the identification of major cancer-related genes and the development of targeted drugs for each mutation, adaptive resistance remains as a challenge with single-drug treatment. The MTM, initially designed to identify combination targets that minimize adaptive resistance for specific anticancer drugs, leverages the inherent drug response dynamics of cancer cell networks with minimal intervention. This approach can mitigate drug overuse, which can lead to toxicity issues, making MTM highly applicable in clinical settings [
37].
In this study, we applied the MTM specifically to single-node perturbations induced by small-molecule inhibitors. However, it is important to note that many cancer treatments such as lipid nanoparticles or siRNAs/mRNAs typically influence multiple molecular targets at once. This results in more significant changes in network states than what is observed with small-molecule treatments. Despite the complexity introduced by treatments that simultaneously affect various molecules within a network, the MTM remains applicable to identify the most synergistic target pair reducing heterogeneous responses. The key to its applicability lies in the fact that regardless of how many molecules are affected by the perturbation, the attractor landscape of the cancer cell network undergoes a single but significant change due to the treatment. This change prompts state transitions within the network, all of which can be effectively analyzed using the MTM.
In biological networks, heterogeneous cellular responses to external stimuli are commonplace. Our MTM-based target identification approach, which reduces competitive stabilization, offers a robust method for analyzing network dynamics. The heterogeneous cellular responses common in biological networks to external signals make our MTM-based target identification strategy versatile for analyzing network dynamics. This approach is effective across various network models, whether deterministic or stochastic. This is because iterative simulations with numerous random initial states can bring randomness of the stochastic nature of biological networks to synchronous Boolean or ordinary differential equation (ODE) networks with deterministic logics. For instance, we have shown that extracting the MTMs and identifying synergistic targets based on state-flipping frequencies are also effectively applicable in synchronous Boolean network models (see
Supplementary Figure S5 for details) [
35]. Thus, competitive stabilization of a network state is not merely an artifact of stochastic or asynchronous updating of logic of Boolean models.
Two questions arise from our findings. First, two of the suggested targets by the MTM may not be interchangeable, even though it suggests combinatorial synergistic targets of a given target drug. In addition, the optimal synergistic pair of a specific target suggested by the MTM is designated to the given target and can be changed when the identified synergistic target is reversely assigned as a new given target. However, it is comprehensive, since synergistic target pairs in cancer therapies often play different roles in regulating cell states [
38]. Second, some of the synergistic pairs for a certain drug target, which is located relatively lower in a hierarchy from the network, cannot be identified. However, this aligns with biological expectations, since common molecules targeted by cancer drugs generally reside at higher hierarchies in the network to regulate a wide range of downstream molecules [
39] (see
Supplementary Figure S6 for further details). Therefore, our strategy of identifying synergistic targets using the MTM is generally applicable to any modeling scheme without compromising the biological insights for cancer therapeutics.
4. Materials and Methods
4.1. Boolean Network Models and Simulation Schemes
We employed Boolean models with asynchronously updates. In this framework, variables can only be 1 or 0 to represent the activity of the corresponding biological component as “active” or “inactive”, respectively. Each Boolean network component and its relationship with other nodes can be represented using Boolean operators AND, OR, and NOT. The logical regulatory equation assigned to each component updates the state of corresponding components and causes the state flips between 1 and 0. During each simulation step, one of the variables is randomly selected and updated according to its logical regulatory equation. The stochastic spreading of serial molecular state flips through the network is implemented in Boolean modeling by asynchronously updating a state of randomly selected single node for each step of network state change. In the case of the gastric cancer model [
21], we followed multileveled variables (named prosurvival, antisurvival, Caspase3/7, and CCND1) and their corresponding logical formulae.
4.2. Identification of Every Possible Attractor
To identify every possible attractor in a Boolean network, we implemented the algorithm suggested by He et al. This algorithm identifies attractors from a simplified Boolean network by determining constant nodes according to the network logic, perturbation inputs and initial states [
40]. We hard-coded such an algorithm with MATLAB R2021a. All the identified attractors were validated by verifying whether they remained within the identified states during network simulations.
4.3. Calculation of Phenotypic Preference for Each Attractor
To determine the phenotypic preference of each attractor, we first established the phenotypic marker nodes and desirable network states of each model. For attractors that remain within a single state, the corresponding state value of the marker node directly represents the phenotype of that attractor. Conversely, for those with multiple states, such as a complex attractor in an asynchronously updating Boolean modeling scheme, the phenotype is represented by the average state value of marker nodes across these states. In case of the gastric cancer model suggested by Flobak et al. [
21], we adhered to the phenotypic outputs delineated by the authors. We also defined that the state of the antisurvival node has a higher value than that of the prosurvival node as our desirable attractor.
4.4. Calculation of State Transition and MTM Extraction
To induce the state transitions of network models, we pinned the corresponding target node state as 1 (ON) or 0 (OFF), ensuring their states remained fixed during the simulations. Then, we iteratively simulated the state transitions by using every possible attractor of the model before and after the pinning and target perturbation as initial and final states, respectively. Every state transition from the initial states to reachable attractors from the perturbed network is then merged into a MTM.
4.5. Calculation of State-Flipping Frequency for Each Node in the MTM
The state-flipping frequency of each node within an MTM is calculated by following three steps. First, state transition probabilities of each transition within the MTM are calculated by assuming that state transition probabilities for consecutive states are evenly distributed in every branch. Second, the MTM is factorized to state transition paths, each anchored by attractors at both ends. Phenotypic preferences of each path are assigned by comparing relative preferences of the both attractors. Third, we calculate the weighted flipping frequency of each node by adding every multiple of state-flipping numbers from the state transition probability of the path and the phenotypic preference, as well as state transition types. The weighted probability of each state transition path is calculated by multiplying the factor of corresponding transient states according to their outgoing edges. The phenotypic preferences are then multiplied by 1 if the path is desirable or −1 if undesirable. A factor of 1 is multiplied in the state transition types if the transition is included within the regulated attractor landscape (regulated transitions), or a factor of −1 is multiplied if the transition is included within the original attractor landscape (reversal transitions).
4.6. Estimating Significance of Each Transition Path Using Approximated Basins of Attraction
Due to the computational complexity of calculating the basin size of each attractor in an asynchronously updated Boolean model, we employed an approximation method. We estimated the basin of each attractor by tracing a number of converged attractors from a pool of sampled initial states. To sample the initial states, we randomly choose the state of each node in the network between 1 and 0 in uniform distribution. We choose 10,000 non-overlapping initial states and trace converged attractors from each initial state to approximate the basin size of each attractor. To calculate the significance of a specific transition path, the ratio for the number of converged initial states to the starting attractor within the path over 10,000 initial states is multiplied by the weighted flipping frequency of that path.
4.7. Identification of Multi-Stable Motifs and Interconnecting Nodes
After calculating every possible attractor from the original attractor landscape as well as every reachable attractor from these attractors once regulated, we identified network motifs, which are SCCs, that possess at least two distinct stable states in attractors. The existence and a parametric region of multi-stability from the identified network motifs is then measured with BioSwitch, developed by Yordanov et al. [
41], which computes bifurcation diagrams of network motifs from the limit points. The interconnecting nodes between the multi-stable motifs are identified by the PathLinker algorithm, which connects source nodes to target nodes on a given network structure by calculating k-shortest paths, developed by Gil et al. [
42].
4.8. Determination of Control Type for the MTM Identified Synergistic Targets
To hinder serial state flip propagation between multi-stable motifs, a proper control type of ON or OFF state should be selected for pinning the synergistic target identified by the MTM. If the control effect of the given target node affects the identified synergistic target by turning it OFF, it has to be pinned to ON state for interfering its propagation, and vice versa. According to the control type of the given target node and the signs of connected interlinks between motifs and the identified target, the control effect of the given perturbation propagated to the synergistic target node is calculated. For this, we perform signal flow analysis to estimate signal propagation after perturbation using only topological information of the network [
43]. As a tendency for an altered state of the identified synergistic target to be ON or OFF is calculated based on the given control node using signal flow analysis, the control type of the synergistic target can be determined as the opposite of this state.
4.9. Identification of Synergistic Target Pairs Using Network Control Theories
We implemented the original algorithms of FVS [
24] and SMC [
25] using hard-coded codes of Python 3.7.6. To compare the control effectiveness of the known control target search algorithm with the MTM, we limited the number of control target nodes to two. This adaptation was necessary because original algorithms often suggest controlling more than two nodes. We evaluated the control efficacy of every possible two-node pair selected from these suggested target lists and calculated their average control effectiveness. For instance, if a strategy proposes controlling nodes A, B, and C, we assessed the control effect on phenotypic nodes when controlling A and B, B and C, and C and A, respectively, to derive an average efficacy for two-node controls. To calculate average control effectiveness of target nodes suggested by the FVS, every possible two-node pair in the suggested target nodes is regulated and their corresponding phenotypic changes of attractors are measured. To calculate the average control effectiveness of two-node pairs suggested by the SMC, every succession path to desired steady states in a succession diagram is selected. Then, every possible two-node pair suggested from those succession paths is regulated and their corresponding phenotypic changes of attractors are measured. In the case of the MTM, five synergistic targets with the highest weighted flipping frequencies were selected.
4.10. Curation of Biological Network Structure from the OmniPath Database
To extract the biological network structures, we downloaded the OmniPath network structural data file from the archives (
https://archive.omnipathdb.org/ (accessed on 28 March 2024)) [
44]. The downloaded versions of two interaction data files were uploaded on 14 June 2018, and 26 April 2019. Those two data files were merged, and interactions without signs or directions were removed. Then, the interactions were cross-validated by leaving the links with confidence level A of the DoRothEA signed target gene–transcription factor interaction data [
45]. Finally, the network with the largest number of connected components, with 3877 nodes and 10,814 links, was selected as a curated OmniPath network structure.
4.11. Randomization of Network Structure While Preserving Degree Distributions
To randomize link connections between the nodes from the curated OmniPath network structure, we first selected two non-overlapping nodes in pair and their outgoing links, as well as their corresponding target node. We then swapped these target nodes from each pair for a sufficient number of iterations. We discretely increased the number of iterations from 10% to 50% of a total number of links in the network by 10% increments for repeatedly generating randomized network structures in groups of 10. As a result, 50 randomized network structures were generated.
4.12. Extraction of Multi-Stable Motif Structures from the Curated OmniPath Network
To extract the multi-stable motifs with specific network structures from the curated OmniPath network and its randomized networks, we implemented the “pattern join” method suggested by Patra et al. [
46], which iteratively screens the existence of a specific motif structure by joining smaller motifs within a network. The numbers of specific multi-stable motif structures overlapped from the given network were systematically explored by hard-coded MATLAB R2021a simulations.