Early Gastric Cancers in Central Norway 2001 to 2016—A Population-Based Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
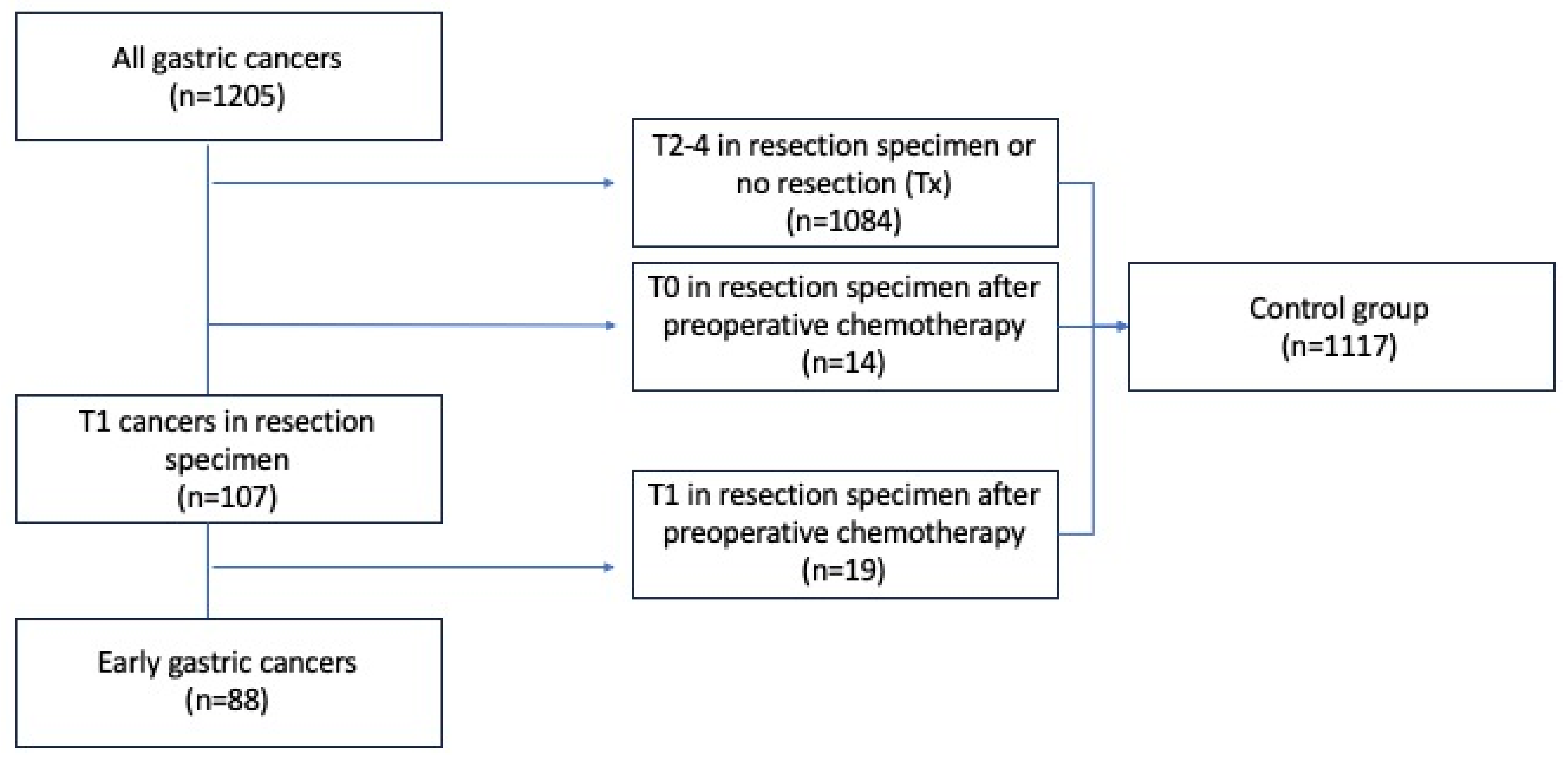
2.2. Early Gastric Cancer and Control Groups
2.3. Data Collection and Variables
2.4. Histopathological and Immunohistochemical Analyses of EGCs
2.5. Statistical Analysis
2.6. Ethics
3. Results
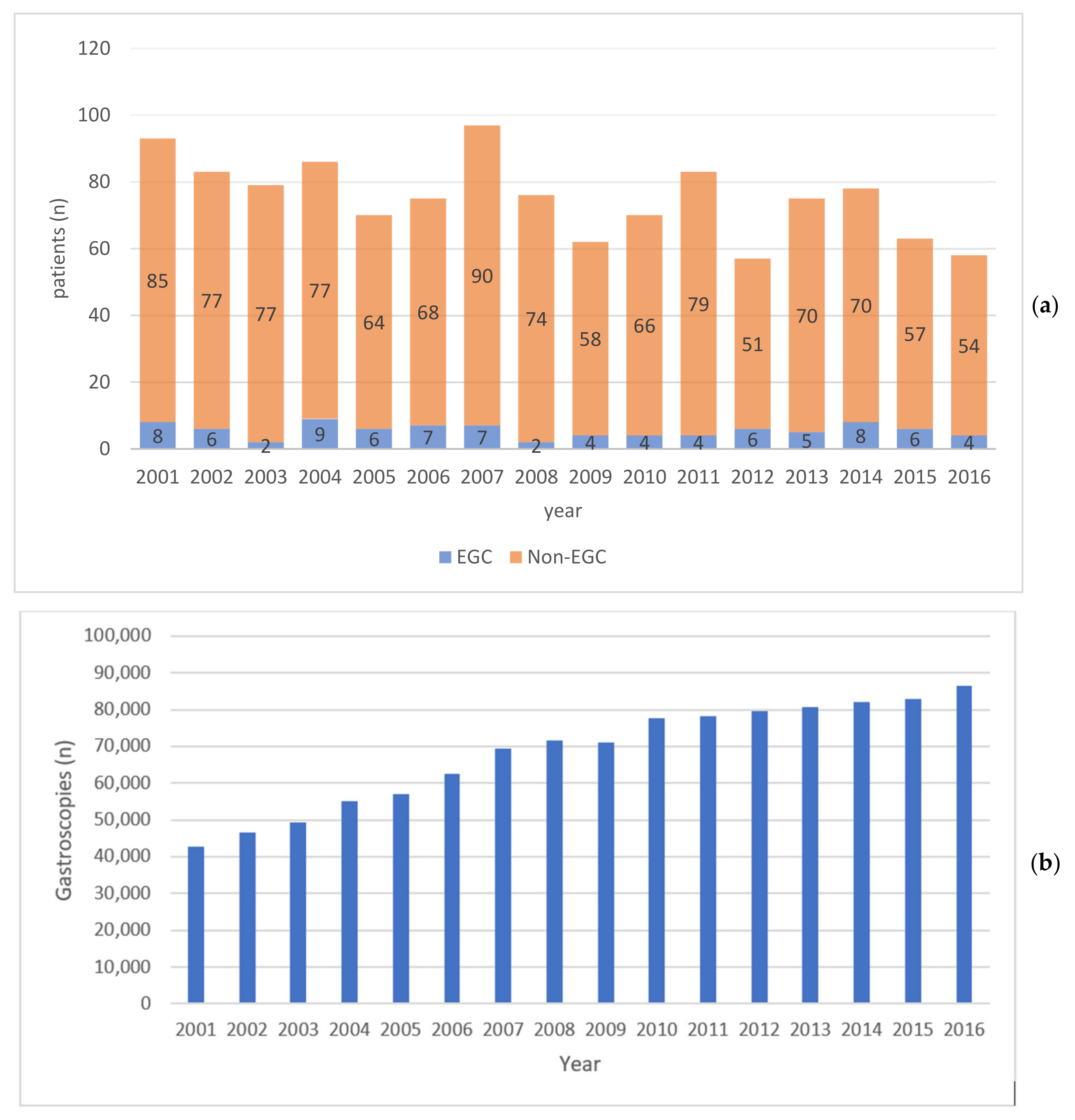
3.1. Annual Number of EGC and Gastric Cancer
3.2. Patient Characteristics
3.3. Tumor Location and (y)pTNM Stage
3.4. Lauren Classification
3.5. Symptoms at Diagnosis and Findings at Upper Endoscopy
3.6. Findings at Pre-Operative EUS and CT
3.7. Histopathological Findings and eCura Risk Stratification in N0 and N+ EGC Patients
3.8. Recurrence of Cancer and Cause of Death in EGC Patients
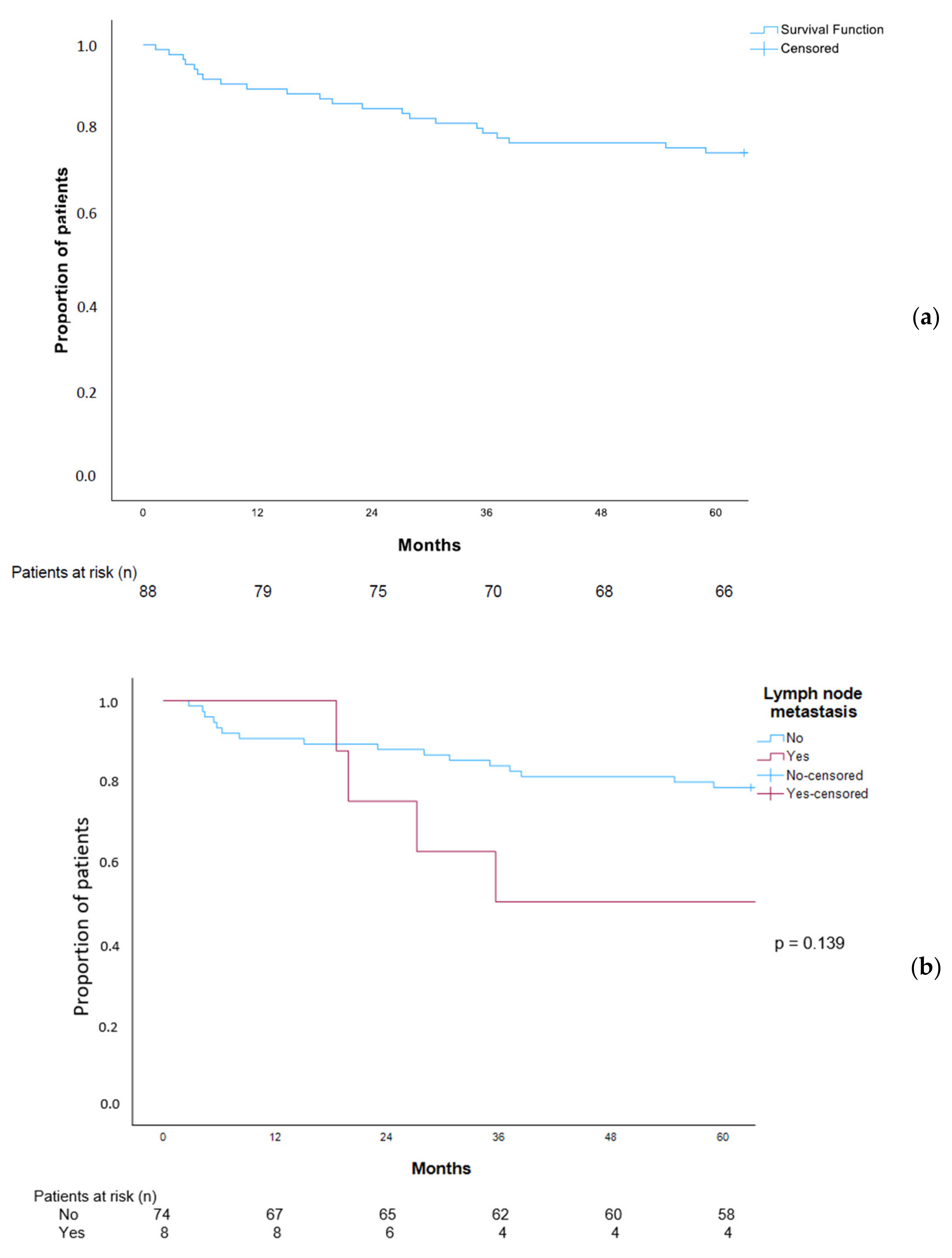
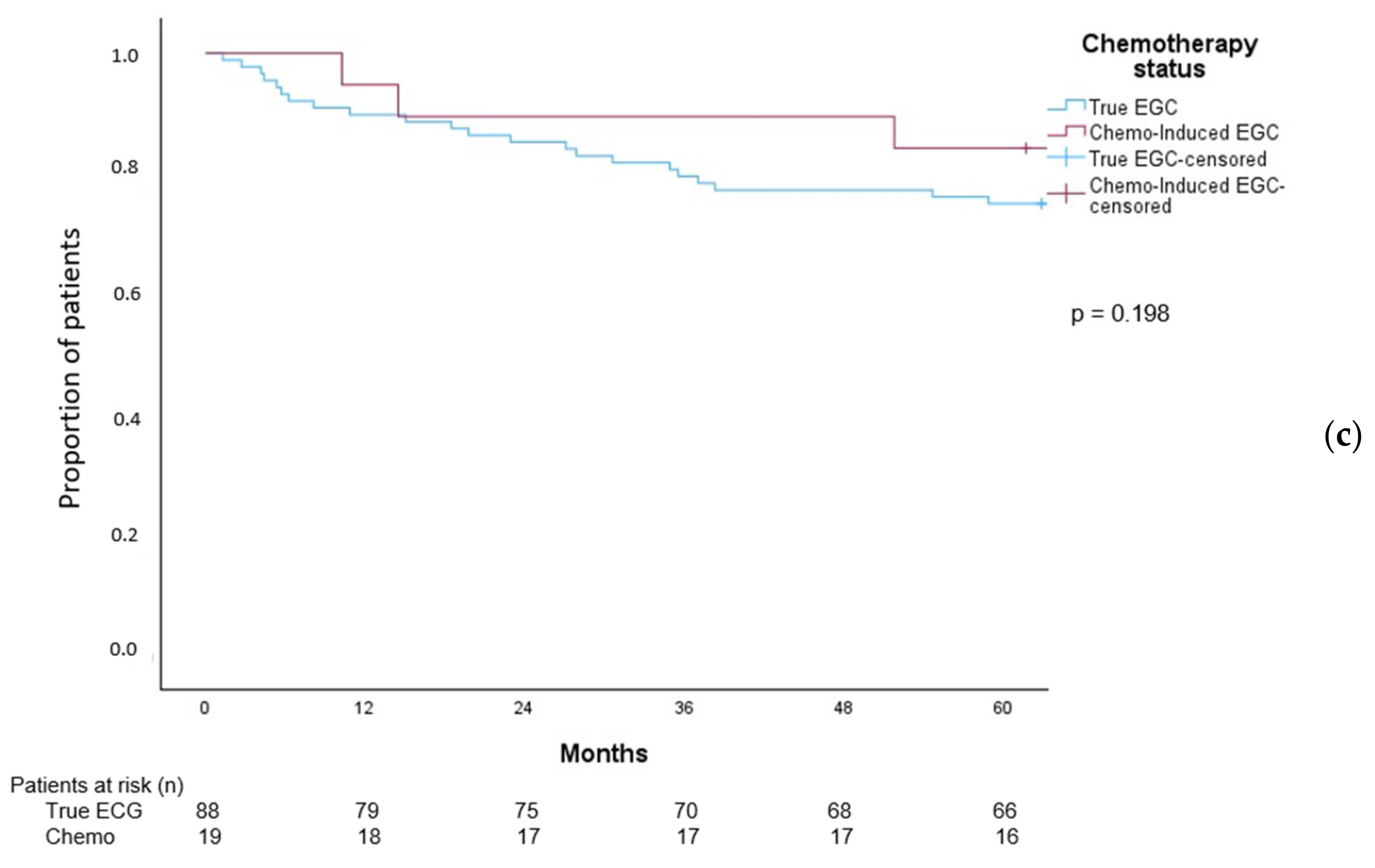
3.9. Overall Survival in Patients with EGC Patients
4. Discussion
4.1. EGC and GC per Year
4.2. EGC Patient and Tumor Characteristics
4.3. EGC, Lymph Node Metastases, and Distant Metastases
4.4. EGC and Imaging
4.5. Survival in EGC Patients
4.6. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| CT | computed tomography |
| eCura | Endoscopic curability |
| EGC | Early gastric cancer |
| ESD | Endoscopic submucosal dissection |
| EUS | Endoscopic ultrasound |
| GI | Gastrointestinal |
| IHC | Immunohistochemical |
| LNM | Lymph node metastases |
| NPR | Norwegian patient registry |
| NUD | Non-numerical unstructured data |
| SM | Submucosal |
References
- International Agency for Reseach on Cancer WHO. 2024. Available online: https://gco.iarc.fr/today/en/dataviz/bars?mode=cancer&group_populations=1&cancers=7&types=0&sort_by=value0&key=total (accessed on 13 March 2024).
- Cancer Registry of Norway. Cancer in Norway 2021—Cancer Incidence, Mortality, Survival and Prevalence in Norway; Cancer Registry of Norway: Oslo, Norway, 2022. [Google Scholar]
- Laurén, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. Acta Pathol. Microbiol. Scand. 1965, 6, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Henson, D.E.; Dittus, C.; Younes, M.; Nguyen, H.; Albores-Saavedra, J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: Increase in the signet ring cell type. Arch. Pathol. Lab. Med. 2004, 128, 765–770. [Google Scholar] [CrossRef]
- Anderson, W.F.; Rabkin, C.S.; Turner, N.; Fraumeni, J.F., Jr.; Rosenberg, P.S.; Camargo, M.C. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J. Natl. Cancer Inst. 2018, 110, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, D.; Allum, W.H.; Stenning, S.P.; Thompson, J.N.; Van de Velde, C.J.; Nicolson, M.; Scarffe, J.H.; Lofts, F.J.; Falk, S.J.; Iveson, T.J.; et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N. Engl. J. Med. 2006, 355, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bringeland, E.A.; Wasmuth, H.H.; Mjones, P.; Myklebust, T.A.; Gronbech, J.E. A population-based study on incidence rates, Lauren distribution, stage distribution, treatment, and long-term outcomes for gastric adenocarcinoma in Central Norway 2001–2011. Acta Oncol. 2017, 56, 39–45. [Google Scholar] [CrossRef]
- Bringeland, E.A.; Wasmuth, H.H.; Johnsen, G.; Johnsen, T.B.; Juel, I.S.; Mjones, P.; Uggen, P.E.; Ystgaard, B.; Gronbech, J.E. Outcomes among patients treated for gastric adenocarcinoma during the last decade. J. Surg. Oncol. 2013, 107, 752–757. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Bardot, A.; Rutherford, M.J.; Ferlay, J.; Little, A.; Møller, B.; Bucher, O.; De, P.; Woods, R.R.; et al. International variation in oesophageal and gastric cancer survival 2012–2014: Differences by histological subtype and stage at diagnosis (an ICBP SURVMARK-2 population-based study). Gut 2022, 71, 1532–1543. [Google Scholar] [CrossRef]
- Januszewicz, W.; Turkot, M.H.; Malfertheiner, P.; Regula, J. A Global Perspective on Gastric Cancer Screening: Which Concepts Are Feasible, and When? Cancers 2023, 15, 664. [Google Scholar] [CrossRef]
- Yang, K.; Lu, L.; Liu, H.; Wang, X.; Gao, Y.; Yang, L.; Li, Y.; Su, M.; Jin, M.; Khan, S. A comprehensive update on early gastric cancer: Defining terms, etiology, and alarming risk factors. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 255–273. [Google Scholar] [CrossRef]
- Murakami, T. Pathomorphological diagnosis, definition and gross classification of early gastric cancer. Gann Monogr. Cancer Res. 1971, 11, 53–66. [Google Scholar]
- Schlemper, R.J.; Riddell, R.H.; Kato, Y.; Borchard, F.; Cooper, H.S.; Dawsey, S.M.; Dixon, M.F.; Fenoglio-Preiser, C.M.; Fléjou, J.F.; Geboes, K.; et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000, 47, 251–255. [Google Scholar] [CrossRef]
- Schlemper, R.J.; Itabashi, M.; Kato, Y.; Lewin, K.J.; Riddell, R.H.; Shimoda, T.; Sipponen, P.; Stolte, M.; Watanabe, H.; Takahashi, H.; et al. Differences in diagnostic criteria for gastric carcinoma between Japanese and western pathologists. Lancet 1997, 349, 1725–1729. [Google Scholar] [CrossRef]
- Gotoda, T.; Yanagisawa, A.; Sasako, M.; Ono, H.; Nakanishi, Y.; Shimoda, T.; Kato, Y. Incidence of lymph node metastasis from early gastric cancer: Estimation with a large number of cases at two large centers. Gastric Cancer 2000, 3, 219–225. [Google Scholar] [CrossRef]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer 2017, 20, 1–19. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Koike, T.; Uno, K.; Asano, N.; Imatani, A.; Masamune, A. Is Additional Gastrectomy Required for Elderly Patients after Endoscopic Submucosal Dissection with Endoscopic Curability C-2 for Early Gastric Cancer? Digestion 2022, 103, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Makimoto, S.; Mushiake, Y.; Takami, T.; Shintani, H.; Kataoka, N.; Yamaguchi, T.; Oura, S. Evaluation of additional gastrectomy after noncurative endoscopic submucosal dissection for early gastric cancer. BMC Surg. 2022, 22, 352. [Google Scholar] [CrossRef]
- Toyokawa, T.; Ohira, M.; Tanaka, H.; Minamino, H.; Sakurai, K.; Nagami, Y.; Kubo, N.; Yamamoto, A.; Sano, K.; Muguruma, K.; et al. Optimal management for patients not meeting the inclusion criteria after endoscopic submucosal dissection for gastric cancer. Surg. Endosc. 2016, 30, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- Sandø, A.D.; Fougner, R.; Grønbech, J.E.; Bringeland, E.A. The value of restaging CT following neoadjuvant chemotherapy for resectable gastric cancer. A population based study. World J. Surg. Oncol. 2021, 19, 212. [Google Scholar] [CrossRef] [PubMed]
- Bringeland, E.A.; Wasmuth, H.H.; Fougner, R.; Mjones, P.; Gronbech, J.E. Impact of perioperative chemotherapy on oncological outcomes after gastric cancer surgery. Br. J. Surg. 2014, 101, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Helsedirektoratet Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av kreft i magesekken (ventrikkelkreft). Available online: https://www.helsedirektoratet.no/retningslinjer/kreft-i-magesekken-handlingsprogram (accessed on 26 February 2024).
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hirano, M.; Esaki, M.; et al. A Scoring System to Stratify Curability after Endoscopic Submucosal Dissection for Early Gastric Cancer: “eCura system”. Am. J. Gastroenterol. 2017, 112, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. International Union Against Cancer (UICC) TNM Classification of Malignant Tumors, 7th ed.; Wiley-Blackwell: Oxford, UK, 2009. [Google Scholar]
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011, 14, 101–112. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Ilic, M.; Ilic, I. Epidemiology of stomach cancer. World J. Gastroenterol. 2022, 28, 1187–1203. [Google Scholar] [CrossRef] [PubMed]
- Hamashima, C. Cancer screening guidelines and policy making: 15 years of experience in cancer screening guideline development in Japan. Jpn. J. Clin. Oncol. 2018, 48, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Hollowood, A. Early gastric cancer: Diagnosis and less invasive treatments. Scand. J. Surg. 2006, 95, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Pimenta-Melo, A.R.; Monteiro-Soares, M.; Libanio, D.; Dinis-Ribeiro, M. Missing rate for gastric cancer during upper gastrointestinal endoscopy: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.; Bringeland, E.A.; Qvigstad, G.; Fossmark, R. Gastric Cancers Missed at Upper Endoscopy in Central Norway 2007 to 2016-A Population-Based Study. Cancers 2021, 13, 5628. [Google Scholar] [CrossRef] [PubMed]
- Capelle, L.G.; Haringsma, J.; de Vries, A.C.; Steyerberg, E.W.; Biermann, K.; van Dekken, H.; Kuipers, E.J. Narrow band imaging for the detection of gastric intestinal metaplasia and dysplasia during surveillance endoscopy. Dig. Dis. Sci. 2010, 55, 3442–3448. [Google Scholar] [CrossRef]
- Bergquist, J.R.; Leiting, J.L.; Habermann, E.B.; Cleary, S.P.; Kendrick, M.L.; Smoot, R.L.; Nagorney, D.M.; Truty, M.J.; Grotz, T.E. Early-onset gastric cancer is a distinct disease with worrisome trends and oncogenic features. Surgery 2019, 166, 547–555. [Google Scholar] [CrossRef]
- Pessorrusso, F.C.S.; Felipe-Silva, A.; Jacob, C.E.; Ramos, M.; Ferreira, V.A.A.; de Mello, E.S.; Zilberstein, B.; Ribeiro, U., Jr.; Maluf-Filho, F. Risk assessment of lymph node metastases in early gastric adenocarcinoma fulfilling expanded endoscopic resection criteria. Gastrointest. Endosc. 2018, 88, 912–918. [Google Scholar] [CrossRef]
- Pereira, M.A.; Ramos, M.; Dias, A.R.; Faraj, S.F.; Yagi, O.K.; Safatle-Ribeiro, A.V.; Maluf-Filho, F.; Zilberstein, B.; Cecconello, I.; de Mello, E.S.; et al. Risk Factors for Lymph Node Metastasis in Western Early Gastric Cancer After Optimal Surgical Treatment. J. Gastrointest. Surg. 2018, 22, 23–31. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, S.H.; Park, J.M.; Park, C.S.; Park, K.S.; Kim, E.S.; Cho, K.B. Prediction of the indication criteria for endoscopic resection of early gastric cancer. World J. Gastroenterol. 2015, 21, 11160–11167. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Gong, E.J.; Chung, E.J.; Park, H.W.; Bae, S.E.; Kim, E.H.; Kim, J.; Do, Y.S.; Kim, T.H.; Chang, H.S.; et al. The Characteristics and Prognosis of Diffuse-Type Early Gastric Cancer Diagnosed during Health Check-Ups. Gut Liver 2017, 11, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Paredes, O.; Baca, C.; Cruz, R.; Paredes, K.; Luque-Vasquez, C.; Chavez, I.; Taxa, L.; Ruiz, E.; Berrospi, F.; Payet, E. Predictive factors of lymphatic metastasis and evaluation of the Japanese treatment guidelines for endoscopic resection of early gastric cancer in a high-volume center in Perú. Heliyon 2023, 9, e16293. [Google Scholar] [CrossRef]
- Ahmad, R.; Setia, N.; Schmidt, B.H.; Hong, T.S.; Wo, J.Y.; Kwak, E.L.; Rattner, D.W.; Lauwers, G.Y.; Mullen, J.T. Predictors of Lymph Node Metastasis in Western Early Gastric Cancer. J. Gastrointest. Surg. 2016, 20, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Bausys, R.; Bausys, A.; Vysniauskaite, I.; Maneikis, K.; Klimas, D.; Luksta, M.; Strupas, K.; Stratilatovas, E. Risk factors for lymph node metastasis in early gastric cancer patients: Report from Eastern Europe country- Lithuania. BMC Surg. 2017, 17, 108. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, M.; Kamisako, T.; Yutani, I.; Kudo, M.; Shigeoka, H.; Tanaka, A.; Okuno, K.; Yasutomi, M. Two cases of histopathologically advanced (stage IV) early gastric cancers. Tumori 2001, 87, 191–195. [Google Scholar] [CrossRef]
- Zhao, X.; Cai, A.; Xi, H.; Chen, L.; Peng, Z.; Li, P.; Liu, N.; Cui, J.; Li, H. Predictive Factors for Lymph Node Metastasis in Undifferentiated Early Gastric Cancer: A Systematic Review and Meta-analysis. J. Gastrointest. Surg. 2017, 21, 700–711. [Google Scholar] [CrossRef]
- Pyo, J.H.; Lee, H.; Min, Y.W.; Min, B.H.; Lee, J.H.; Kim, K.M.; Kim, H.; Kim, K.; Kim, J.J. Feasibility of Endoscopic Resection in Early Gastric Cancer with Lymphovascular Invasion. Ann. Surg. Oncol. 2019, 26, 449–455. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Li, S.; Bai, F.; Xie, H.; Shan, H.; Liu, Z.; Ma, T.; Tang, X.; Tang, H.; et al. Risk Factors of Lymph Node Metastasis and Its Prognostic Significance in Early Gastric Cancer: A Multicenter Study. Front. Oncol. 2021, 11, 649035. [Google Scholar] [CrossRef]
- Hatta, W.; Gotoda, T.; Kanno, T.; Yuan, Y.; Koike, T.; Moayyedi, P.; Masamune, A. Prevalence and risk factors for lymph node metastasis after noncurative endoscopic resection for early gastric cancer: A systematic review and meta-analysis. J. Gastroenterol. 2020, 55, 742–753. [Google Scholar] [CrossRef]
- Du, M.Z.; Gan, W.J.; Yu, J.; Liu, W.; Zhan, S.H.; Huang, S.; Huang, R.P.; Guo, L.C.; Huang, Q. Risk factors of lymph node metastasis in 734 early gastric carcinoma radical resections in a Chinese population. J. Dig. Dis. 2018, 19, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Lee, H.; Lee, H.H.; Lee, J.H.; Jun, K.H.; Kim, J.J.; Song, K.Y.; Kim, D.J. A Nomogram for Predicting Extraperigastric Lymph Node Metastasis in Patients With Early Gastric Cancer. J. Gastric Cancer 2023, 23, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Hatta, W.; Gotoda, T.; Oyama, T.; Kawata, N.; Takahashi, A.; Yoshifuku, Y.; Hoteya, S.; Nakagawa, M.; Hirano, M.; Esaki, M.; et al. Is the eCura system useful for selecting patients who require radical surgery after noncurative endoscopic submucosal dissection for early gastric cancer? A comparative study. Gastric Cancer 2018, 21, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Gotoda, T. Endoscopic resection of early gastric cancer. Gastric Cancer 2007, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hallinan, J.T.; Venkatesh, S.K. Gastric carcinoma: Imaging diagnosis, staging and assessment of treatment response. Cancer Imaging 2013, 13, 212–227. [Google Scholar] [CrossRef]
- Kim, J.W.; Shin, S.S.; Heo, S.H.; Lim, H.S.; Lim, N.Y.; Park, Y.K.; Jeong, Y.Y.; Kang, H.K. The role of three-dimensional multidetector CT gastrography in the preoperative imaging of stomach cancer: Emphasis on detection and localization of the tumor. Korean J. Radiol. 2015, 16, 80–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ma, T.; Li, X.; Zhang, T.; Duan, M.; Ma, Q.; Cong, L.; Huang, Z.; Wang, X.; Chen, Y. Effect of visceral adipose tissue on the accuracy of preoperative T-staging of gastric cancer. Eur. J. Radiol. 2022, 155, 110488. [Google Scholar] [CrossRef] [PubMed]
- Yada, T.; Yokoi, C.; Uemura, N. The current state of diagnosis and treatment for early gastric cancer. Diagn. Ther. Endosc. 2013, 2013, 241320. [Google Scholar] [CrossRef]
- Waddingham, W.; Nieuwenburg, S.A.V.; Carlson, S.; Rodriguez-Justo, M.; Spaander, M.; Kuipers, E.J.; Jansen, M.; Graham, D.G.; Banks, M. Recent advances in the detection and management of early gastric cancer and its precursors. Frontline Gastroenterol. 2021, 12, 322–331. [Google Scholar] [CrossRef]
- Probst, A.; Schneider, A.; Schaller, T.; Anthuber, M.; Ebigbo, A.; Messmann, H. Endoscopic submucosal dissection for early gastric cancer: Are expanded resection criteria safe for Western patients? Endoscopy 2017, 49, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, M.; Notsuka, T.; Tomoda, H. Recurrence of early gastric cancer. Semin. Surg. Oncol. 1991, 7, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Sasako, M.; Kinoshita, T.; Maruyama, K. Recurrence of early gastric cancer. Follow-up of 1475 patients and review of the Japanese literature. Cancer 1993, 72, 3174–3178. [Google Scholar] [CrossRef]
- Itoh, H.; Oohata, Y.; Nakamura, K.; Nagata, T.; Mibu, R.; Nakayama, F. Complete ten-year postgastrectomy follow-up of early gastric cancer. Am. J. Surg. 1989, 158, 14–16. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, Y.; Ishida, A.; Hayashi, K.; Kaneta, Y.; Watanabe, H.; Kano, K.; Furuta, M.; Takahashi, K.; Fujikawa, H.; Yamada, T.; et al. Feasibility of gastric endoscopic submucosal dissection in elderly patients aged ≥ 80 years. World J. Gastrointest. Endosc. 2022, 14, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Kishida, Y.; Takizawa, K.; Kakushima, N.; Kawata, N.; Yoshida, M.; Yabuuchi, Y.; Yamamoto, Y.; Ito, S.; Imai, K.; Hotta, K.; et al. Endoscopic submucosal dissection versus surgery in elderly patients with early gastric cancer of relative indication for endoscopic resection. Dig. Endosc. 2022, 34, 497–507. [Google Scholar] [CrossRef]
- Levenson, G.; Voron, T.; Paye, F.; Balladur, P.; Debove, C.; Chafai, N.; De Dios, A.G.; Lefevre, J.H.; Parc, Y. Tumor downstaging after neoadjuvant chemotherapy determines survival after surgery for gastric adenocarcinoma. Surgery 2021, 170, 1711–1717. [Google Scholar] [CrossRef]
- Prasad, P.; Sivaharan, A.; Navidi, M.; Fergie, B.H.; Griffin, S.M.; Phillips, A.W. Significance of neoadjuvant downstaging in gastric adenocarcinoma. Surgery 2022, 172, 593–601. [Google Scholar] [CrossRef]
- Sandø, A.D.; Grønbech, J.E.; Bringeland, E.A. Does the ypTNM-stage adequately predict long-term survival rates in gastric cancer patients receiving neoadjuvant chemotherapy followed by radical resection? Acta Oncol. 2023, 62, 1846–1853. [Google Scholar] [CrossRef]
| Variable | Entire Cohort | EGC | Non-EGC | p-Value |
|---|---|---|---|---|
| Patients, n (%) | 1205 | 88 (7.3) | 1117 (92.7) | |
| Age at diagnosis, years | 0.137 | |||
| Median (range) | 75 (21−99) | 77 (43−91) | 74 (21−99) | |
| Sex, n (%) | 0.294 | |||
| Male | 773 (64.1) | 61 (69.3) | 712 (63.7) | |
| Cancer location, n (%) | <0.001 | |||
| Cardia | 337 (28.0) | 13 (14.8) | 324 (29.0) | |
| Corpus | 328 (27.2) | 32 (36.4) | 296 (26.5) | |
| Antrum | 392 (32.5) | 43 (48.9) | 349 (31.2) | |
| Diffuse | 142 (11.8) | 0 (0.0) | 142 (12.7) | |
| Not recorded | 6 (0.5) | 0 (0.0) | 6 (0.5) | |
| (y)pT stage, n (%) | ||||
| T0 | 14 (1.2) | - | 14 (1.3) | |
| T1 | 107 (8.9) | 88 (100.0) | 19 (1.7) | |
| T2 | 55 (4.6) | - | 55 (4.9) | |
| T3 | 178 (14.8) | - | 178 (16.0) | |
| T4a | 204 (17.0) | - | 204 (18.3) | |
| T4b | 82 (6.8) | - | 82 (7.4) | |
| Tx | 560 (46.7) | - | 560 (50.4) | |
| (y)pN stage, n (%) | <0.001 | |||
| N0 | 268 (22.4) | 74 (84.1) | 189 (17.0) | |
| N1 | 101 (8.4) | 3 (3.4) | 98 (8.8) | |
| N2 | 106 (8.9) | 4 (4.5) | 102 (9.2) | |
| N3 | 120 (10.0) | 1 (1.1) | 119 (10.7) | |
| Nx | 602 (50.3) | 6 (6.8) | 601 (54.2) | |
| (y)pM stage, n (%) | <0.001 | |||
| M0 | 605 (50.2) | 88 (100.0) | 520 (46.6) | |
| M1 | 515 (42.7) | 0 (0.0) | 515 (46.1) | |
| Mx | 85 (7.1) | 0 (0.0) | 82 (7.3) | |
| Lauren classification, n (%) | <0.001 | |||
| Diffuse | 365 (30.3) | 17 (19.5) | 348 (31.2) | |
| Intestinal | 557 (46.3) | 64 (73.6) | 493 (44.1) | |
| Mixed diffuse/intestinal | 132 (11.0) | 4 (4.6) | 128 (11.5) | |
| Cancer NUD/No biopsy | 150 (12.5) | 2 (2.3) | 148 (13.2) |
| Variable | |
|---|---|
| Symptoms at time of diagnosis (total n = 88), n (%) | |
| No symptoms | 10 (11.4) |
| Dysphagia | 8 (9.1) |
| Epigastric pain | 37 (42.0) |
| GI-bleeding/anemia | 35 (39.8) |
| Vomiting/nausea | 15 (17.0) |
| Acid reflux | 11 (12.5) |
| Weight loss/reduced general health | 17 (19.3) |
| No information | 2 (2.3) |
| Findings at upper endoscopy (total n = 83), n (%) | |
| Inflammation | 27 (32.5) |
| Ulcer | 43 (51.8) |
| Polyp or tumor | 40 (48.2) |
| EUS T-staging (total n = 15), n (%) | |
| T0 | 4 (26.7) |
| T1 | 5 (33.3) |
| T2 | 5 (33.3) |
| T3 | 1 (6.7) |
| T4 | 0 (0.0) |
| EUS N-staging | |
| N0 | 13 (86.7) |
| N1 or more | 2 (13.3) |
| Pre-operative imaging | |
| CT | 81 (92.0) |
| Ultrasound | 2 (2.3) |
| No imaging | 5 (5.7) |
| CT T-staging, n (%) | |
| T0 | 16 (19.8) |
| T1 | 2 (2.5) |
| T2 | 12 (14.8) |
| T3 | 5 (6.2) |
| T4 | 2 (2.5) |
| Tx | 44 (54.3) |
| CT N-staging, n (%) | |
| N0 | 71 (87.7) |
| N1 | 7 (8.6) |
| N2 | 1 (1.2) |
| N3 | 0 (0.0) |
| Nx | 2 (2.5) |
| CT M-staging, n (%) | |
| M0 | 78 (96.3) |
| M1 | 0 (0.0) |
| Mx | 3 (3.7) |
| Variable | Total | EGC N0 | EGC N+ | p-Value |
|---|---|---|---|---|
| Patients, n (%) | 82 (100.0) | 74 (90.8) | 8 (9.2) | |
| Differentiation, n (%) | 0.558 | |||
| Poor | 36 (43.9) | 31 (41.9) | 5 (62.5) | |
| Moderately | 44 (53.7) | 41 (55.4) | 3 (37.5) | |
| Uncertain | 2 (2.4) | 2 (2.7) | 0 (0.0) | |
| Lauren, n (%) | 0.272 | |||
| Diffuse | 17 (20.7) | 15 (20.3) | 2 (25.0) | |
| Intestinal | 59 (72.0) | 54 (73.0) | 5 (62.5) | |
| Mixed | 4 (4.9) | 4 (5.4) | 0 (0.0) | |
| Cancer NUD/no biopsy | 2 (2.4) | 1 (1.4) | 1 (12.5) | |
| Depth of invasion, n (%) | 0.879 | |||
| M | 28 (34.5) | 26 (35.6) | 2 (25.0) | |
| SM1 | 13 (16.0) | 12 (16.4) | 1 (12.5) | |
| SM2 | 39 (48.1) | 34 (46.6) | 5 (62.5) | |
| Uncertain | 2 (1.2) | 2 (1.4) | 0 (0.0) | |
| Tumor size, mm | ||||
| Median, (range) a | 20 (4–90) | 20 (4–90) | 22.5 (10–60) | 0.401 |
| SM depth, µm | ||||
| Median, (range) | 2000 (0–9000) | 2000 (0–9000) | 2000 (400–2800) | 0.794 |
| Ulceration, n (%) | ||||
| None | 32 (39.0) | 30 (39.2) | 2 (25.0) | 0.145 |
| Erosion | 30 (36.6) | 28 (37.8) | 2 (25.0) | |
| Ulceration | 17 (20.7) | 13 (17.6) | 4 (50.0) | |
| Uncertain | 3 (3.7) | 3 (4.1) | 0 (0.0) | |
| Vascular invasion, n (%) | ||||
| None | 51 (63.3) | 47 (63.5) | 4 (50.0) | |
| Lymphatic | 17 (21.8) | 15 (21.4) | 2 (25.0) | |
| Arterial | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Venous | 12 (15.4) | 10 (14.3) | 2 (25.0) | |
| Uncertain | 2 (2.4) | 2 (2.7) | 0 (0.0) | |
| eCura scores, n (%) | ||||
| Low risk | 53 (64.6) | 48 (64.9) | 5 (62.5) | |
| Intermediate risk | 16 (19.5) | 14 (18.9) | 2 (25.0) | |
| High risk | 10 (12.2) | 9 (12.2) | 1 (12.5) | |
| Incomplete data | 3 (3.7) | 3 (4.1) | 0 (0.0) |
| Variable | |
|---|---|
| Recurrence (available for n = 86), n (%) | 7 (8.1) |
| Time to recurrence, months. Median (range). | 29 (14–124) |
| Died during follow-up, n (%) | 55 (62.5) |
| Cause of death, n (%) | |
| Cancer recurrence | 7 (12.7) |
| Not cancer-related | 43 (78.2) |
| Postoperative complications | 5 (9.1) |
| Cause of death within 5 years, n (%) | |
| Cancer recurrence | 4 (18.2) |
| Not cancer related | 13 (59.1) |
| Postoperative complications | 5 (22.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kvamme, C.J.; Stillingen, T.L.; Sandø, A.D.; Mjønes, P.; Bringeland, E.A.; Fossmark, R. Early Gastric Cancers in Central Norway 2001 to 2016—A Population-Based Study. Cancers 2024, 16, 1222. https://doi.org/10.3390/cancers16061222
Kvamme CJ, Stillingen TL, Sandø AD, Mjønes P, Bringeland EA, Fossmark R. Early Gastric Cancers in Central Norway 2001 to 2016—A Population-Based Study. Cancers. 2024; 16(6):1222. https://doi.org/10.3390/cancers16061222
Chicago/Turabian StyleKvamme, Camilla J., Thomas L. Stillingen, Alina D. Sandø, Patricia Mjønes, Erling A. Bringeland, and Reidar Fossmark. 2024. "Early Gastric Cancers in Central Norway 2001 to 2016—A Population-Based Study" Cancers 16, no. 6: 1222. https://doi.org/10.3390/cancers16061222
APA StyleKvamme, C. J., Stillingen, T. L., Sandø, A. D., Mjønes, P., Bringeland, E. A., & Fossmark, R. (2024). Early Gastric Cancers in Central Norway 2001 to 2016—A Population-Based Study. Cancers, 16(6), 1222. https://doi.org/10.3390/cancers16061222






