Systemic Chemotherapy in Colorectal Peritoneal Metastases Treated with Cytoreductive Surgery: Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
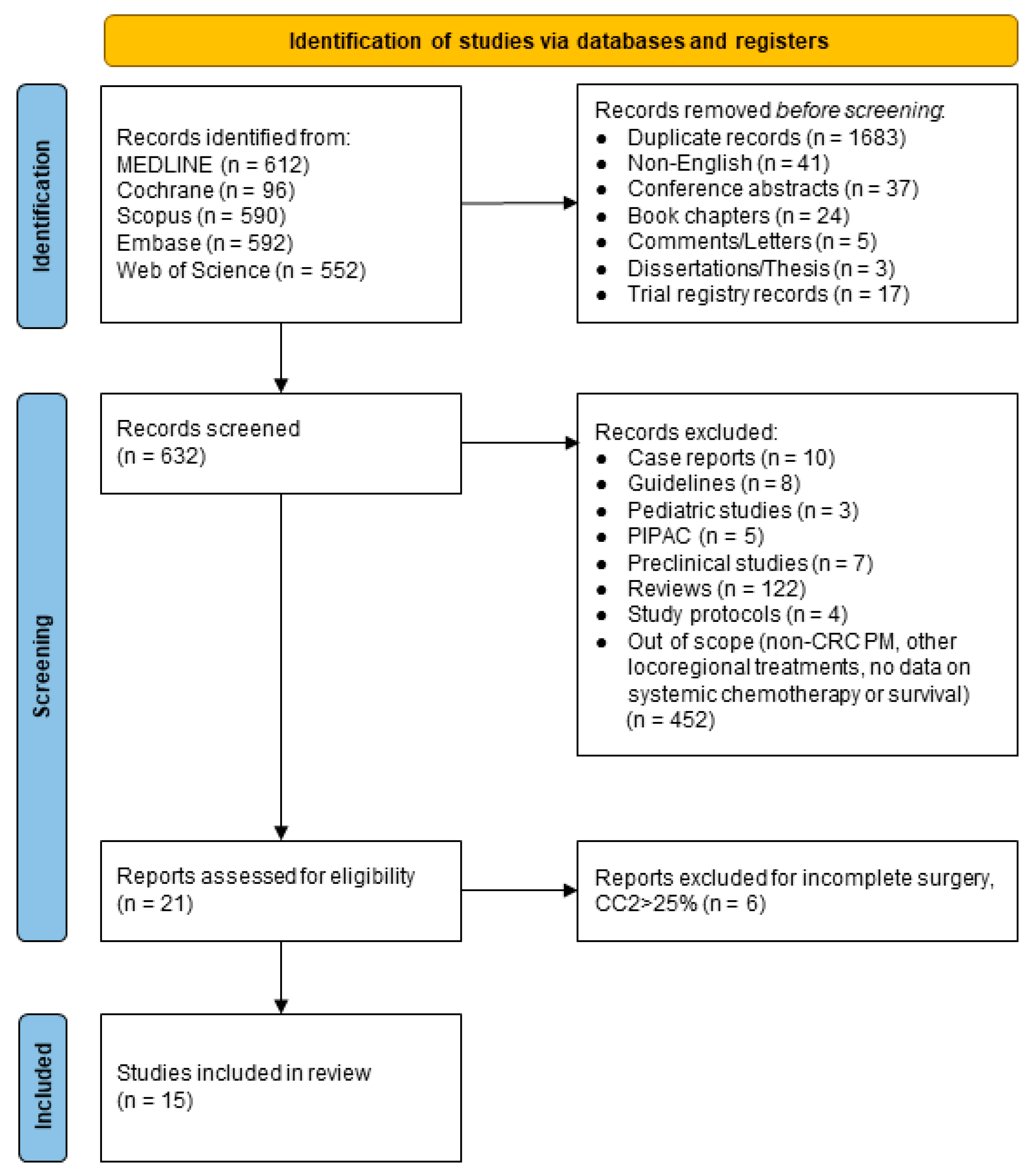
2.2. Search Strategy
2.3. Selection Criteria and Outcome Measures
2.4. Data Extraction
2.5. Statistical Analysis
2.6. Quality Assessment of Retrieved Articles
3. Results
3.1. Study and Patient Characteristics
3.2. Outcome Measures
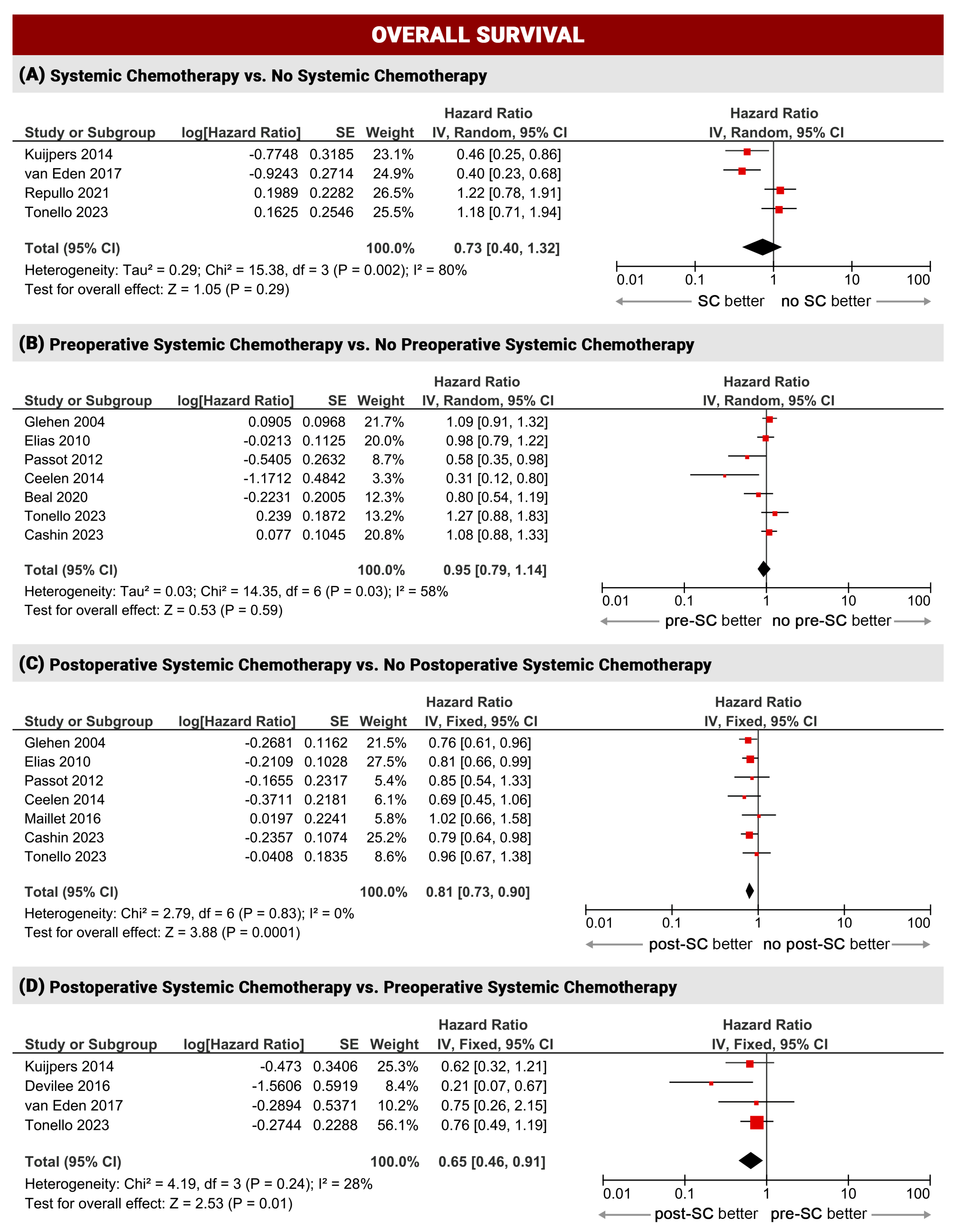
3.2.1. Overall Survival
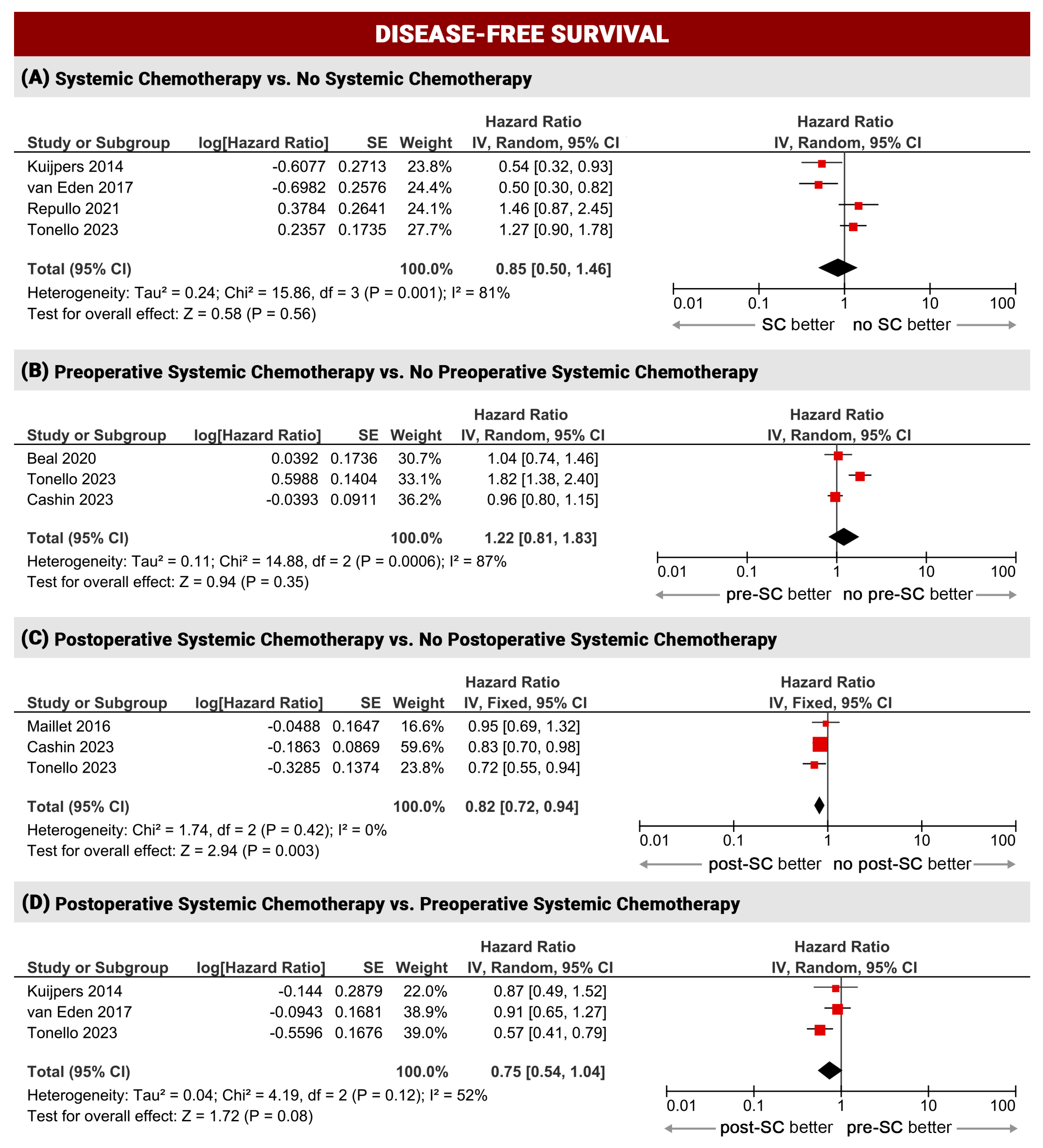
3.2.2. Disease-Free Survival
3.2.3. Postoperative Complications
3.3. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Segelman, J.; Granath, F.; Holm, T.; MacHado, M.; Mahteme, H.; Martling, A. Incidence, Prevalence and Risk Factors for Peritoneal Carcinomatosis from Colorectal Cancer. Br. J. Surg. 2012, 99, 699–705. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Prognosis of Patients with Peritoneal Metastatic Colorectal Cancer given Systemic Therapy: An Analysis of Individual Patient Data from Prospective Randomised Trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) Database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Goldman, C.D.; Pockaj, B.A.; Nelson, G.D.; Goldberg, R.M.; Pitot, H.C.; Grothey, A.; Alberts, S.R.; Sargent, D.J. Treatment of Colorectal Peritoneal Carcinomatosis with Systemic Chemotherapy: A Pooled Analysis of North Central Cancer Treatment Group Phase III Trials N9741 and N9841. J. Clin. Oncol. 2012, 30, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Kwiatkowski, F.; Sugarbaker, P.H.; Elias, D.; Levine, E.A.; De Simone, M.; Barone, R.; Yonemura, Y.; Cavaliere, F.; Quenet, F.; et al. Cytoreductive Surgery Combined with Perioperative Intraperitoneal Chemotherapy for the Management of Peritoneal Carcinomatosis from Colorectal Cancer: A Multi-Institutional Study. J. Clin. Oncol. 2004, 22, 3284–3292. [Google Scholar] [CrossRef]
- Elias, D.; Lefevre, J.H.; Chevalier, J.; Brouquet, A.; Marchal, F.; Classe, J.M.; Ferron, G.; Guilloit, J.M.; Meeus, P.; Goere, D.; et al. Complete Cytoreductive Surgery plus Intraperitoneal Chemohyperthermia with Oxaliplatin for Peritoneal Carcinomatosis of Colorectal Origin. J. Clin. Oncol. 2009, 27, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.D.; Black, D.; Savady, R.; Sugarbaker, P.H. Systematic Review on the Efficacy of Cytoreductive Surgery Combined with Perioperative Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis from Colorectal Carcinoma. J. Clin. Oncol. 2006, 24, 4011–4019. [Google Scholar] [CrossRef] [PubMed]
- Waite, K.; Youssef, H. The Role of Neoadjuvant and Adjuvant Systemic Chemotherapy with Cytoreductive Surgery and Heated Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Systematic Review. Ann. Surg. Oncol. 2017, 24, 705–720. [Google Scholar] [CrossRef]
- Passot, G.; Vaudoyer, D.; Cotte, E.; You, B.; Isaac, S.; Noel Gilly, F.; Mohamed, F.; Glehen, O. Progression Following Neoadjuvant Systemic Chemotherapy May Not Be a Contraindication to a Curative Approach for Colorectal Carcinomatosis. Ann. Surg. 2012, 256, 125–129. [Google Scholar] [CrossRef]
- Gilly, F.N. Phase II Studies: International Registry of Colorectal Carcinomatosis. Eur. J. Surg. Oncol. 2006, 32, 648–654. [Google Scholar] [CrossRef]
- Eveno, C.; Passot, G.; Goéré, D.; Soyer, P.; Gayat, E.; Glehen, O.; Elias, D.; Pocard, M. Bevacizumab Doubles the Early Postoperative Complication Rate after Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Peritoneal Carcinomatosis of Colorectal Origin. Ann. Surg. Oncol. 2014, 21, 1792–1800. [Google Scholar] [CrossRef]
- Glockzin, G.; Zeman, F.; Croner, R.S.; Konigsrainer, A.; Pelz, J.; Strohlein, M.A.; Rau, B.; Arnold, D.; Koller, M.; Schlitt, H.J.; et al. Perioperative Systemic Chemotherapy, Cytoreductive Surgery, and Hyperthermic Intraperitoneal Chemotherapy in Patients with Colorectal Peritoneal Metastasis: Results of the Prospective Multicenter Phase 2 COMBATAC Trial. Clin. Colorectal. Cancer 2018, 17, 285–296. [Google Scholar] [CrossRef]
- Rovers, K.P.; Bakkers, C.; Nienhuijs, S.W.; Burger, J.W.A.; Creemers, G.M.; Thijs, A.M.J.; Brandt-Kerkhof, A.R.M.; Madsen, E.V.E.; van Meerten, E.; Tuynman, J.B.; et al. Perioperative Systemic Therapy vs Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Alone for Resectable Colorectal Peritoneal Metastases: A Phase 2 Randomized Clinical Trial. JAMA Surg. 2021, 156, 710–720. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tierney, J.F.; Stewart, L.A.; Ghersi, D.; Burdett, S.; Sydes, M.R. Practical Methods for Incorporating Summary Time-to-Event Data into Meta-Analysis. Trials 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Hebert, A.E.; Kreaden, U.S.; Yankovsky, A.; Guo, D.; Li, Y.; Lee, S.H.; Liu, Y.; Soito, A.B.; Massachi, S.; Slee, A.E. Methodology to Standardize Heterogeneous Statistical Data Presentations for Combining Time-to-Event Oncologic Outcomes. PLoS ONE 2022, 17, 0263661. [Google Scholar] [CrossRef] [PubMed]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the Mean and Variance from the Median, Range, and the Size of a Sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ Br. Med. J. 2003, 327, 557. [Google Scholar] [CrossRef]
- Wallace, B.C.; Lajeunesse, M.J.; Dietz, G.; Dahabreh, I.J.; Trikalinos, T.A.; Schmid, C.H.; Gurevitch, J. OpenMEE: Intuitive, Open-Source Software for Meta-Analysis in Ecology and Evolutionary Biology. Methods Ecol. Evol. 2017, 8, 941–947. [Google Scholar] [CrossRef]
- Maillet, M.; Glehen, O.; Lambert, J.; Goere, D.; Pocard, M.; Msika, S.; Passot, G.; Elias, D.; Eveno, C.; Sabate, J.M.; et al. Early Postoperative Chemotherapy after Complete Cytoreduction and Hyperthermic Intraperitoneal Chemotherapy for Isolated Peritoneal Carcinomatosis of Colon Cancer: A Multicenter Study. Ann. Surg. Oncol. 2016, 23, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Elias, D.; Gilly, F.; Boutitie, F.; Quenet, F.; Bereder, J.-M.; Mansvelt, B.; Lorimier, G.; Dubè, P.; Glehen, O. Peritoneal Colorectal Carcinomatosis Treated with Surgery and Perioperative Intraperitoneal Chemotherapy: Retrospective Analysis of 523 Patients from a Multicentric French Study. J. Clin. Oncol. 2010, 28, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, W.; Van Nieuwenhove, Y.; Putte, D.V.; Pattyn, P. Neoadjuvant Chemotherapy with Bevacizumab May Improve Outcome after Cytoreduction and Hyperthermic Intraperitoneal Chemoperfusion (HIPEC) for Colorectal Carcinomatosis. Ann. Surg. Oncol. 2014, 21, 3023–3028. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, A.M.; Mehta, A.M.; Boot, H.; van Leerdam, M.E.; Hauptmann, M.; Aalbers, A.G.; Verwaal, V.J. Perioperative Systemic Chemotherapy in Peritoneal Carcinomatosis of Lymph Node Positive Colorectal Cancer Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Oncol. 2014, 25, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Devilee, R.A.; Simkens, G.A.; van Oudheusden, T.R.; Rutten, H.J.; Creemers, G.J.; Ten Tije, A.J.; de Hingh, I.H. Increased Survival of Patients with Synchronous Colorectal Peritoneal Metastases Receiving Preoperative Chemotherapy before Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Ann. Surg. Oncol. 2016, 23, 2841–2848. [Google Scholar] [CrossRef] [PubMed]
- van Eden, W.J.; Kok, N.F.; Jozwiak, K.; Lahaye, M.L.; Beets, G.L.; van Leerdam, M.E.; Boot, H.; Aalbers, A.G. Timing of Systemic Chemotherapy in Patients with Colorectal Peritoneal Carcinomatosis Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Dis. Colon Rectum 2017, 60, 477–487. [Google Scholar] [CrossRef]
- Beal, E.W.; Suarez-Kelly, L.P.; Kimbrough, C.W.; Johnston, F.M.; Greer, J.; Abbott, D.E.; Pokrzywa, C.; Raoof, M.; Lee, B.; Grotz, T.E.; et al. Impact of Neoadjuvant Chemotherapy on the Outcomes of Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Peritoneal Metastases: A Multi-Institutional Retrospective Review. J. Clin. Med. 2020, 9, 748. [Google Scholar] [CrossRef]
- Repullo, D.J.; Barbois, S.; Leonard, D.; Bohlok, A.; Van den Audenaeren, E.T.; Hendlisz, A.; Van den Eynde, M.; Donckier, V.; Kartheuser, A.; Liberale, G. The Absence of Benefit of Perioperative Chemotherapy in Initially Resectable Peritoneal Metastases of Colorectal Cancer Origin Treated with Complete Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: A Retrospective Analysis. Eur. J. Surg. Oncol. 2021, 47, 1661–1667. [Google Scholar] [CrossRef]
- Hanna, D.N.; Macfie, R.; Ghani, M.O.; Hermina, A.; Mina, A.; Cha, D.E.; Bailey, C.E.; Cohen, N.; Labow, D.; Golas, B.; et al. A Total Neoadjuvant Chemotherapy Approach Is Associated with Improved Recurrence-Free Survival in Patients with Colorectal Peritoneal Metastases Undergoing Cytoreductive Surgery and HIPEC. J. Surg. Oncol. 2023, 127, 442–449. [Google Scholar] [CrossRef]
- Baratti, D.; Kusamura, S.; Iusco, D.; Bonomi, S.; Grassi, A.; Virzi, S.; Leo, E.; Deraco, M. Postoperative Complications after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy Affect Long-Term Outcome of Patients with Peritoneal Metastases from Colorectal Cancer: A Two-Center Study of 101 Patients. Dis. Colon Rectum 2014, 57, 858–868. [Google Scholar] [CrossRef]
- Cashin, P.H.; Esquivel, J.; Larsen, S.G.; Liauw, W.; Alzahrani, N.A.; Morris, D.L.; Kepenekian, V.; Sourrouille, I.; Dumont, F.; Tuech, J.J.; et al. Perioperative Chemotherapy in Colorectal Cancer with Peritoneal Metastases: A Global Propensity Score Matched Study. EClinicalMedicine 2023, 55, 101746. [Google Scholar] [CrossRef]
- Tonello, M.; Baratti, D.; Sammartino, P.; Di Giorgio, A.; Robella, M.; Sassaroli, C.; Framarini, M.; Valle, M.; Macri, A.; Graziosi, L.; et al. Is Systemic Chemotherapy Useful in Patients Treated with Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for Colorectal Peritoneal Metastases? A Propensity-Score Analysis. Ann. Surg. Oncol. 2023, 31, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, F.J.; Schuitevoerder, D.; Plana, A.; Sherman, S.K.; White, M.G.; Baumgartner, J.M.; Choudry, M.H.A.; Abbott, D.E.; Barone, R.M.; Berri, R.N.; et al. The Chicago Consensus on Peritoneal Surface Malignancies: Management of Colorectal Metastases. Ann. Surg. Oncol. 2020, 27, 1761–1767. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive Surgery plus Hyperthermic Intraperitoneal Chemotherapy versus Cytoreductive Surgery Alone for Colorectal Peritoneal Metastases (PRODIGE 7): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef]
- NCCN Guidelines. 2022. Available online: https://www.Nccn.Org/Guidelines/Guidelines-Detail?Category=1&id=1428 (accessed on 5 February 2024).
- Passot, G.; You, B.; Boschetti, G.; Fontaine, J.; Isaac, S.; Decullier, E.; Maurice, C.; Vaudoyer, D.; Gilly, F.N.; Cotte, E.; et al. Pathological Response to Neoadjuvant Chemotherapy: A New Prognosis Tool for the Curative Management of Peritoneal Colorectal Carcinomatosis. Ann. Surg. Oncol. 2014, 21, 2608–2614. [Google Scholar] [CrossRef] [PubMed]
- Hompes, D.; Aalbers, A.; Boot, H.; van Velthuysen, M.L.; Vogel, W.; Prevoo, W.; van Tinteren, H.; Verwaal, V. A Prospective Pilot Study to Assess Neoadjuvant Chemotherapy for Unresectable Peritoneal Carcinomatosis from Colorectal Cancer. Colorectal. Dis. 2014, 16, O264–O272. [Google Scholar] [CrossRef]
- Van Gestel, Y.R.B.M.; Thomassen, I.; Lemmens, V.E.P.P.; Pruijt, J.F.M.; Van Herk-Sukel, M.P.P.; Rutten, H.J.T.; Creemers, G.J.; Hingh, I.H.J.T. De Metachronous Peritoneal Carcinomatosis after Curative Treatment of Colorectal Cancer. Eur. J. Surg. Oncol. 2014, 40, 963–969. [Google Scholar] [CrossRef]
- van Gestel, Y.R.B.M.; de Hingh, I.H.J.T.; van Herk-Sukel, M.P.P.; van Erning, F.N.; Beerepoot, L.V.; Wijsman, J.H.; Slooter, G.D.; Rutten, H.J.T.; Creemers, G.J.M.; Lemmens, V.E.P.P. Patterns of Metachronous Metastases after Curative Treatment of Colorectal Cancer. Cancer Epidemiol. 2014, 38, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Des Guetz, G.; Nicolas, P.; Perret, G.Y.; Morere, J.F.; Uzzan, B. Does Delaying Adjuvant Chemotherapy after Curative Surgery for Colorectal Cancer Impair Survival? A Meta-Analysis. Eur. J. Cancer 2010, 46, 1049–1055. [Google Scholar] [CrossRef]
- Cleeland, C.S. Symptom Burden: Multiple Symptoms and Their Impact as Patient-Reported Outcomes. J. Natl. Cancer Inst. Monogr. 2007, 2007, 16–21. [Google Scholar] [CrossRef]
- Simkens, G.A.; Rovers, K.P.; Van Oudheusden, T.R.; Nienhuijs, S.W.; Rutten, H.J.; Hingh, I.H. De Major Influence of Postoperative Complications on Costs of Cytoreductive Surgery and HIPEC in Patients with Colorectal Peritoneal Metastases. Medicine 2018, 97, e0042. [Google Scholar] [CrossRef] [PubMed]
- Bushati, M.; Rovers, K.P.; Sommariva, A.; Sugarbaker, P.H.; Morris, D.L.; Yonemura, Y.; Quadros, C.A.; Somashekhar, S.P.; Ceelen, W.; Dubé, P.; et al. The Current Practice of Cytoreductive Surgery and HIPEC for Colorectal Peritoneal Metastases: Results of a Worldwide Web-Based Survey of the Peritoneal Surface Oncology Group International (PSOGI). Eur. J. Surg. Oncol. 2018, 44, 1942–1948. [Google Scholar] [CrossRef] [PubMed]
- Esquivel, J.; Sticca, R.; Sugarbaker, P.; Levine, E.; Yan, T.D.; Alexander, R.; Baratti, D.; Bartlett, D.; Barone, R.; Barrios, P.; et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in the Management of Peritoneal Surface Malignancies of Colonic Origin: A Consensus Statement. Ann. Surg. Oncol. 2007, 14, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Glehen, O.; Cotte, E.; Schreiber, V.; Sayag-Beaujard, A.C.; Vignal, J.; Gilly, F.N. Intraperitoneal Chemohyperthermia and Attempted Cytoreductive Surgery in Patients with Peritoneal Carcinomatosis of Colorectal Origin. Br. J. Surg. 2004, 91, 747–754. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, Y.; Liang, J.; Pei, W.; Zhou, Z. Neoadjuvant Chemotherapy Followed by Hyperthermic Intraperitoneal Chemotherapy for Patients with Colorectal Peritoneal Metastasis: A Retrospective Study of Its Safety and Efficacy. World J. Surg. Oncol. 2021, 19, 151. [Google Scholar] [CrossRef]
- Rovers, K.P.; Simkens, G.A.; Punt, C.J.; van Dieren, S.; Tanis, P.J.; de Hingh, I.H. Perioperative Systemic Therapy for Resectable Colorectal Peritoneal Metastases: Sufficient Evidence for Its Widespread Use? A Critical Systematic Review. Crit. Rev. Oncol. Hematol. 2017, 114, 53–62. [Google Scholar] [CrossRef]

| First Author, Year | Study Design (Centers n) | Study Period, Country | Sample Size (n) | PCI | CC0-1 (%) | Liver M+ (%) | SC Regimens | Target Therapy (%) | HIPEC Regimen |
|---|---|---|---|---|---|---|---|---|---|
| Glehen, 2004 [5] | retrospective multicentric (28) | 1987–2002 Worldwide | 506 | n.r. | 75 | 12 | FOLFOX, FOLFIRI, FOLFOXIRI | n.r. | MMC, CIS-MMC, OX |
| Elias, 2010 [22] | retrospective multicentric (23) | 1990–2007 Belgium, Canada, France, Switzerland | 523 | 10 | 95 | 15 | FOLFOX, FOLFIRI, FOLFOXIRI | 0 | MMC, CIS-MMC, OX (±IRI) |
| Passot, 2012 [9] | retrospective single center | 1991–2010 France | 120 | 8 | 86 | 0 | FOLFOX, FOLFIRI | 19 | MMC, MMC-OX, MMC-IRI |
| Baratti, 2014 [30] | retrospective multicentric (2) | 2004–2012 Italy | 101 | 10 | 98 | 8 | CAPOX, FOLFOX | 22 | CIS-MMC |
| Ceelen, 2014 [23] | retrospective single center | 2002–2012 Belgium | 166 | n.r. | 87 | n.r. | FOLFOX, FOLFIRI | 42 | MMC, OX |
| Kuijpers, 2014 [24] | retrospective single center | 2004–2012 The Netherlands | 71 | n.r. | 100 | n.r. | CAPOX, FOLFOX | n.r. | MMC |
| Devilee, 2016 [25] | retrospective single center | 2007–2014 The Netherlands | 91 | 100 | 20 | CAPOX, FOLFOX | 28 | n.r. | |
| Maillet, 2016 [21] | prospective multicenter (4) | 2004–2012 France | 231 | 9 | 100 | 5 | 5-FU, FOLFOX, FOLFIRI | 34 | OX, OX-IRI, MMC |
| van Eden, 2017 [26] | retrospective single center | 2004–2015 The Netherlands | 280 | n.r. | 100 | 6 | CAPOX, FOLFOX | n.r. | MMC, OX |
| Beal, 2020 [27] | retrospective multicentric (12) | 2000–2017 USA | 298 | 13 | 88 | 0 | CAPOX, FOLFOX FOLFIRI, FOLFOXIRI | 54 | MMC, OX |
| Repullo, 2021 [28] | retrospective multicentric (2) | 2008–2017 Belgium | 125 | 6 | 100 | 30 | FOLFOX, FOLFIRI, 5-FU-CAPE | 58 | MMC, OX |
| Rovers, 2021 [13] | RCT (9) | 2012–2017 The Netherlands | 79 | 9 | 87 | 0 | CAPOX, FOLFIRI, FOLFOX | 98 | MMC, OX |
| Hanna, 2022 [29] | retrospective multicentric (2) | 2011–2019 USA | 79 | 11 | 93 | 17 | FOLFOX, FOLFIRI, CAPOX | 63 | MMC |
| Cashin, 2023 [31] | retrospective multicentric (39) | 1991–2018 Worldwide | 1486 | 10 | 99 | 13 | n.r. | n.r. | CIS, IRI, MMC, OX-IRI |
| Tonello, 2023 [32] | retrospective multicentric (13) | 1997–2017 Italy | 367 | 9 | 100 | 0 | FOLFOX, FOLFIRI, FOLFOXIRI | 58 | CIS-MMC, OX |
| SC | No SC | Pre-SC | Post-SC | Peri-SC | No Pre-SC | No Post-SC | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | OS | DFS | C | n | OS | DFS | C | n | OS | DFS | C | n | OS | DFS | C | n | OS | DFS | C | n | OS | DFS | C | n | OS | DFS | C | |
| Glehen, 2004 [5] | - | - | - | - | - | - | - | - | 275 | 19 | - | - | 204 | 25 | - | - | - | - | - | - | 231 | 20 | - | - | 302 | 16 | - | - |
| Elias, 2010 [22] | - | - | - | - | - | - | - | - | 370 | 30 | - | - | 232 | 31 | - | - | - | - | - | - | 153 | 30 | - | - | 291 | 27 | - | - |
| Passot, 2012 [9] | - | - | - | - | - | - | - | - | 90 | 37 | - | - | 77 | 36 | - | - | - | - | - | - | 30 | 24 | - | - | 43 | 14 | - | - |
| Baratti, 2014 [30] | - | - | - | - | - | - | - | - | 60 | - | - | 19 | - | - | - | - | - | - | - | - | 41 | - | - | 5 | - | - | - | - |
| Ceelen, 2014 [23] | - | - | - | - | - | - | - | - | 61 | 29 | - | - | 83 | 30 | - | - | - | - | - | - | 105 | 25 | - | - | 83 | 22 | - | - |
| Kuijpers, 2014 [24] | 55 | 3 | 15 | - | 16 | 14 | 4 | - | 25 | 27 | 13 | - | 32 | 24 | 14 | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Devilee, 2016 [25] | - | - | - | - | - | - | - | - | - | - | - | - | 66 | 39 | - | 11 | 25 | n.e. | - | 6 | 25 | - | - | - | - | - | - | - |
| Maillet, 2016 [21] | - | - | - | - | - | - | - | - | - | - | - | - | 151 | 43 | 13 | 42 | - | - | - | - | - | - | - | - | 70 | 50 | 10 | 25 |
| van Eden, 2017 [26] | 247 | 41 | 22 | 76 | 33 | 34 | 17 | 8 | - | - | - | - | 169 | 43 | 22 | 53 | 78 | 37 | 20 | 23 | - | - | - | - | - | - | - | - |
| Beal, 2020 [27] | - | - | - | - | - | - | - | - | 196 | 33 | 14 | 47 | - | - | - | - | - | - | - | - | 102 | 22 | 13 | 19 | - | - | - | - |
| Repullo, 2021 [28] | - | - | - | - | 56 | 72 | 17 | 15 | - | - | - | - | - | - | - | - | 69 | 43 | 11 | 12 | - | - | - | - | - | - | - | - |
| Rovers, 2021 [13] | - | - | - | - | - | - | - | - | 37 | - | - | 8 | - | - | - | - | - | - | - | - | 42 | - | - | 14 | - | - | - | - |
| Hanna, 2022 [29] | - | - | - | - | - | - | - | - | 34 | 78 | 30 | - | - | - | - | - | 45 | 61 | 12 | - | - | - | - | - | - | - | - | - |
| Cashin, 2023 [31] | - | - | - | - | - | - | - | - | 354 | 35 | 12 | 92 | 389 | 46 | 13 | 143 | - | - | - | - | 354 | 37 | 13 | 107 | 389 | 37 | 11 | 159 |
| Tonello, 2023 [32] | 294 | 38 | 13 | 55 | 73 | 55 | 18 | 8 | 119 | 36 | 9 | 32 | 106 | 43 | 16 | 12 | 69 | 38 | 14 | 11 | 179 | 51 | 17 | 20 | 192 | 39 | 12 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tonello, M.; Cenzi, C.; Pizzolato, E.; Fiscon, R.; Del Bianco, P.; Pilati, P.; Sommariva, A. Systemic Chemotherapy in Colorectal Peritoneal Metastases Treated with Cytoreductive Surgery: Systematic Review and Meta-Analysis. Cancers 2024, 16, 1182. https://doi.org/10.3390/cancers16061182
Tonello M, Cenzi C, Pizzolato E, Fiscon R, Del Bianco P, Pilati P, Sommariva A. Systemic Chemotherapy in Colorectal Peritoneal Metastases Treated with Cytoreductive Surgery: Systematic Review and Meta-Analysis. Cancers. 2024; 16(6):1182. https://doi.org/10.3390/cancers16061182
Chicago/Turabian StyleTonello, Marco, Carola Cenzi, Elisa Pizzolato, Riccardo Fiscon, Paola Del Bianco, Pierluigi Pilati, and Antonio Sommariva. 2024. "Systemic Chemotherapy in Colorectal Peritoneal Metastases Treated with Cytoreductive Surgery: Systematic Review and Meta-Analysis" Cancers 16, no. 6: 1182. https://doi.org/10.3390/cancers16061182
APA StyleTonello, M., Cenzi, C., Pizzolato, E., Fiscon, R., Del Bianco, P., Pilati, P., & Sommariva, A. (2024). Systemic Chemotherapy in Colorectal Peritoneal Metastases Treated with Cytoreductive Surgery: Systematic Review and Meta-Analysis. Cancers, 16(6), 1182. https://doi.org/10.3390/cancers16061182