Simple Summary
There are several rare types of hematologic malignancies and germline predispositions syndromes in children and adolescent young adults. In this descriptive review, we briefly describe rare hematologic malignancies, myelodysplastic neoplasms, and predispositions syndromes in children for which we have cohort outcome data and summarize emerging concepts in pathogenesis, diagnosis, prognostication, and treatment.
Abstract
There are a variety of rare hematologic malignancies and germline predispositions syndromes that occur in children and adolescent young adults (AYAs). These entities are important to recognize, as an accurate diagnosis is essential for risk assessment, prognostication, and treatment. This descriptive review summarizes rare hematologic malignancies, myelodysplastic neoplasms, and germline predispositions syndromes that occur in children and AYAs. We discuss the unique biology, characteristic genomic aberrations, rare presentations, diagnostic challenges, novel treatments, and outcomes associated with these rare entities.
1. Introduction
Acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) are the predominant types of leukemia in children (age 0 to 14 years) and adolescent young adults (AYAs) (age 15 to 39 years). Myelodysplastic neoplasms, de novo or secondary to predisposition syndromes, carry a risk of progression to AML. Additionally, germline predisposition syndromes may progress to other myeloid neoplasms or ALL. In children and AYAs, there are several rare myelodysplastic neoplasms, AML, and predisposition syndromes that occur in <5% of cases, but are important to recognize due to their unique presentation, poor outcomes, and the potential for novel therapy. This descriptive review focuses on these rare entities (Figure 1), highlighting genomic and molecular features, unique presentations, diagnostic challenges, outcomes, and current treatment approaches, as well as potential novel therapies.
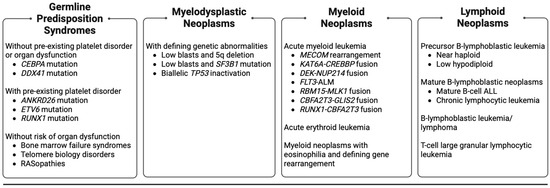
Figure 1.
Rare (occurring in <5% of cases in children and AYAs) hematologic malignancies, myelodysplastic neoplasms, and germline predisposition syndromes in children and AYAs included for review. AYAs: adolescent young adults. ALL: acute lymphoblastic leukemia.
2. Myelodysplastic Neoplasms, Rare Entities
2.1. Myelodysplastic Neoplasms
Myelodysplastic neoplasms (abbreviated MDS by the World Health Organization (WHO)) are a heterogenous group of clonal hematologic disorders characterized by morphologic dysplasia of one or more cell lineages and ineffective hematopoiesis, leading to peripheral blood cytopenia and an increased but variable propensity for transformation into AML [1,2]. An overview of their molecular pathogenesis is summarized in Figure 2. The term myelodysplastic neoplasms replaced myelodysplastic syndromes in the WHO 2022 classification of hematopoietic and lymphoid tumors, underscoring their neoplastic nature [2].
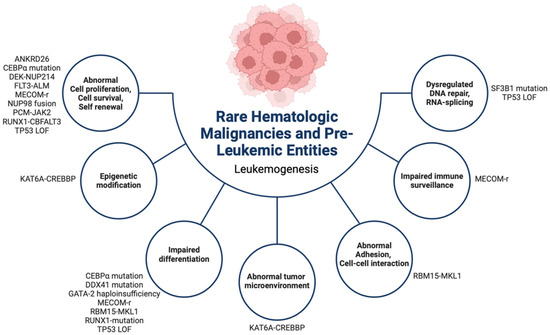
Figure 2.
Summary of the leukemogenesis of rare (occurring in <5% of cases in children and AYAs) hematologic malignancies, myelodysplastic neoplasms, and germline predisposition syndromes in children and AYAs. AYAs: adolescent young adults. r: rearrangement, LOF: loss of function.
MDS are rare in children, accounting for <5% of all hematologic neoplasms, and are extremely variable in terms of clinical features, disease progression, and outcomes [3,4]. The term “childhood MDS” defines a category of diseases biologically distinct from adult MDS [5]. Childhood MDS may be primary (de novo) or secondary, with the latter associated with predisposing conditions such as germline or somatic mutations, inherited bone marrow failure syndromes, or prior chemotherapy or radiation therapy [4]. The median age at diagnosis is 7–8 years, but MDS may present in children of any age with an approximately equal male-to-female ratio [4]. Presenting symptoms reflect persistent peripheral blood cytopenias and commonly include fatigue, bleeding, fever, and infections [4,6,7]. Hepatomegaly is usually absent; splenomegaly is rare; and lymphadenopathy is uncommon outside of concurrent infection [4,7]. Approximately 20% of children may be asymptomatic at presentation [7].
Diagnosing MDS is often challenging and requires a correlation of clinical features, peripheral blood values, bone marrow evaluation(s), cytogenetics, and molecular results [4,6]. The recommended threshold for the degree of morphologic dysplasia is 10% for each cell lineage, and the cytopenias are defined as hemoglobin < 13 g/dL (males) and <12 g/dL (females), absolute neutrophil count < 1.8 × 109/L, and platelets < 150 × 109/L [2]. Immunophenotyping via flow cytometry has shown variable success in diagnosing childhood MDS and may aid in approximately 10% of cases when immunophenotypic aberrations are present [8,9,10]. Immunohistochemistry may be helpful in confirming the diagnosis [4]. Cytogenetic and molecular testing have become increasingly important in the diagnosis and classification and include identifying predisposing somatic mutations and karyotype abnormalities and revealing a distinct mutational landscape of childhood MDS involving somatic mutations in SETBP1, ASXL1, RUNX1, and the RAS pathway [5,11].
The WHO classifies MDS as those having defining genetic abnormalities (“myelodysplastic neoplasms with defining genetic abnormalities”) or defining morphology (“myelodysplastic neoplasms morphologically defined”) [2] (Table 1). MDS is stratified based on the risk of leukemic transformation [6]. Disease-related risk and prognostication in adults are commonly assessed using the Revised International Prognostic Scoring System (IPSS-R), but this tool has limited utility in children and AYAs [12,13] (Table 2, Table 3 and Table 4). In general, children and AYAs with MDS without increased blasts (i.e., <5% blasts) have a better prognosis than those with increased blasts [4]. Additional poor prognostic features include monosomy 7 (associated with progression to more advanced MDS) [7], SETBP1 mutations, and CD7 expression of myeloid blasts (both associated with decreased overall survival) [14,15].

Table 1.
WHO 2022 classification of myelodysplastic neoplasms (modified from Khoury et al. [2]).

Table 2.
Revised International Scoring System (IPSS-R) for evaluating cytogenetic risk in adult MDS (modified from Greenberg et al. [12] and Marques et al. [6]).

Table 3.
Revised International Scoring System (IPSS-R) adult MDS prognostic scoring system (modified from Greenberg et al. [12] and Marques et al. [6]).

Table 4.
Revised International Scoring System (IPSS-R) adult MDS risk stratification (modified from Greenberg et al. [12] and Marques et al. [6]).
Treatment of MDS in children and AYAs is challenging due to limited treatment options and the lack of consensus on optimal management. Treatment selection relies on the diagnosis, cytogenetic and molecular features, and clinical scenario. Current treatment strategies include observation (watch-and-wait), immunosuppressive therapy (IST), and allogeneic hematopoietic stem cell transplant (allo-HSCT) [4]. Allo-HSCT is the only curative treatment for childhood MDS, but due to associated morbidities, it is not offered as first-line therapy for all patients. It is routinely offered to patients with excess blasts, monosomy 7, del(7q), complex karyotype, transfusion-dependence, severe neutropenia, or MDS secondary to chemotherapy or radiation therapy [16]. Improved understanding of the molecular pathogenesis of childhood MDS and the identification of recurrent somatic driver mutations offer potential for the development of targeted therapies.
2.2. MDS with Low Blasts and 5q Deletion
2.2.1. Pathologic and Cytogenetic Features
MDS with low blasts and 5q deletion occurs predominately in middle aged to elderly females [17], with fewer than 10 cases previously reported in children and AYAs [18,19,20,21,22,23,24,25]. It was first described by Van den Berghe et al. in 1974 [26], and further defined by Sokal et al. in 1975 [27]. The deleted region in the long arm of chromosome 5 contains genes important for hematopoiesis, leading to defective erythropoiesis and anemia [28,29]. The deletion can occur in isolation (5q syndrome) or in combination with other genetic abnormalities, the most common of which are mutations in TP53 [30]. Isolated del(5q) is associated with a better prognosis, slower disease progression, a low risk of transformation to AML, and usually a favorable response to treatment [17,18,22,31,32].
2.2.2. Treatment
Lenalidomide is an immunomodulatory agent that selectively suppresses the del(5q) clone [29]. Lenalidomide monotherapy has been evaluated in phase 2 and phase 3 trials in adults with low- and intermediate-1 risk del(5q) MDS, and outcomes have been compared to adults with non-del(5q) MDS with the same IPSS risk score [33,34,35]. In these trials, patients treated with lenalidomide had a significant erythroid response and a higher complete and partial cytogenetic response, with an improvement in median hemoglobin concentration resulting in a decreased transfusion requirement [33,34]. In adults with higher risk del(5q) MDS (i.e., intermediate-2 and high-risk per IPSS risk score), lenalidomide monotherapy resulted in only modest improvements in erythroid response and remission [36,37]. Improved results for adults with higher risk MDS were seen with lenalidomide and azacytidine combination therapy [38,39,40]. Contrary to isolated del(5q), adults with concomitant del(5q) and TP53 mutations tend to have an inferior response to lenalidomide and an overall worse prognosis [37,41,42]. Despite promising results in adults, lenalidomide has not been investigated or utilized in the treatment of children and AYAs with del(5q) MDS.
Currently, there is no standard of care therapy for children and AYAs with del(5q) MDS. Treatment strategies have included observation [24], chemotherapy with steroids [21,25] or erythropoietin [20], and HSCT [18,19,22].
2.3. MDS with Low Blasts and SF3B1 Mutation
2.3.1. Pathologic and Cytogenetic Features
Though well described in adult MDS, SF3B1 mutations are rare in childhood MDS [4,5,14,43], with a solitary case report in a 17-year-old patient with Fanconi Anemia and MDS with ring sideroblasts and multilineage dysplasia [44]. SF3B1 is the most common somatic spliceosome machinery gene mutation in adult MDS [4,45,46] and is associated with a highly homogenous phenotype characterized by erythroid dysplasia, ring sideroblasts, ineffective erythropoiesis, and often normal or nearly normal platelet and neutrophil counts [46,47,48]. Adults with SF3B1-mutated MDS are more often female, significantly older at the age of onset, and have a favorable outcome and lower risk of progression to AML [46,48,49,50,51]. In adults, the detection of SF3B1 mutations is a favorable prognostic indicator associated with low IPSS-R categories and adult MDS with ring sideroblasts [46]. Due to the paucity of reported cases, the prognostic significance of SF3B1-mutated MDS in children and AYAs is unknown. In a cohort of 53 pediatric patients aged 11 months to 17 years with AML (52 with de novo AML, 1 with secondary AML), no recurrent SF3B1 mutations were identified, suggesting that SF3B1 mutations are unlikely to be a leukemogenic driver in childhood AML [52].
2.3.2. Treatment
With the paucity of reported cases, there is no standard of care therapy for children and AYAs with MDS with low blasts and the SF3B1 mutation. In in vitro and ex vivo studies in SF3B1-mutated AML, SF3B1 modulators have shown potential as novel therapeutic targets in FLT3-internal tandem duplication (ITD) AML cells with a high allelic ratio and/or long ITD length [53]. Future studies are needed to elucidate the role of SF3B1 modulators in the treatment of SF3B1-mutated MDS.
2.4. MDS with Biallelic TP53 Inactivation
2.4.1. Pathologic and Cytogenetic Features
The TP53 tumor suppressor gene functions as a negative regulator of cell proliferation. In MDS, TP53 inactivation by mutation or deletion is associated with high-risk disease, rapid progression to AML, and poor prognosis in AYAs and adults [54]. TP53 aberrations can be mono- or biallelic, each representing a subset of patients with distinct clinical phenotypes and outcomes [55]. Biallelic TP53 inactivation results in loss of function of the TP53 tumor suppressor gene and is a common and poor prognostic finding in adults with complex karyotypic MDS [54,55]. In general, TP53 abnormalities are reported at low frequencies in childhood MDS [56,57,58], and biallelic TP53 aberrations have not been described in the pathogenesis of childhood MDS [5,14]. However, biallelic TP53 aberrations have been described in pediatric B-cell non-Hodgkin lymphoma and pleuropulmonary blastoma [59,60].
2.4.2. Treatment
Due to the paucity of reported pediatric or AYA cases, there is no standard of care therapy for children and AYAs with MDS with biallelic TP53 inactivation, but HSCT may be considered.
3. Myeloid Neoplasms, Rare Entities
3.1. AML
AML is relatively rare in children and AYAs, representing approximately 15% of acute leukemias. Despite maximally intensified chemotherapy and increased implementation of upfront HSCT for high-risk disease, the overall survival is in the 50 to 70% range, with approximately 40% of patients with de novo AML ultimately experiencing a relapse [61,62]. Large-scale, comprehensive genomic sequencing studies have improved the understanding of AML biology, risk stratification, prognostication, and treatment. AML was historically classified morphology using the French-American-British (FAB) classification (Table 5). AML is now risk-stratified by a combination of defining genetic features [2] (Table 6) and response to treatment. The diagnostic workup includes bone marrow morphology, flow cytometry immunophenotyping, cytogenetic studies (karyotyping and fluorescence in situ hybridization (FISH)), and comprehensive sequencing. Anthracyclines and cytarabine are key components of AML therapy for low- and intermediate-risk diseases, and consolidation with HSCT for high-risk diseases [61].

Table 5.
French-American-British classification of acute myeloid leukemia.

Table 6.
WHO 2022 classification of acute myeloid leukemia.
Efforts are ongoing to identify and develop effective targeted treatments, including small molecule inhibitors, cell surface antibody drug conjugates, and chimeric antigen receptor T cells (CAR-T), for use in upfront and relapsed/refractory settings [62,63,64,65,66,67,68]. Active targeted therapy trials in children and AYAs with AML are summarized in Table 7. (Table 7). The current Children’s Oncology Group AAML1831 trial (NCT04293562) has incorporated gilteritinib, a FLT3 inhibitor, in combination with standard chemotherapy and as maintenance therapy for all patients with FLT3 activating mutations or FLT3-internal tandem duplication (ITD) with a high allelic ratio (>0.1). The AAML1831 trial has also incorporated upfront gemtuzumab ozogamicin (CD33-targeted therapy) with conventional induction chemotherapy.

Table 7.
Genomic and cell surface targets in pediatric AML currently under clinical study.
Briefly described below are subtypes of AML with rare genetic alterations. In general, treatment of these rare entities is per the current standard of care for AML therapy, with additions or exceptions noted below.
3.2. AML with MECOM Rearrangement
3.2.1. Pathologic and Cytogenetic Features
The MDS1 and EVI1 complex locus protein (MECOM) gene (previously known as EVI1) is located on chromosome 3q26 and encodes a zinc-finger protein transcriptional regulator that is critical for hematopoiesis and hematopoietic stem cell self-renewal [69]. MECOM aberrations (i.e., overexpression, rearrangements, mutations) have been associated with poor prognosis of pediatric and adult myeloproliferative neoplasms [70,71,72,73,74,75,76,77,78,79,80]. MECOM rearrangements have been rarely reported in pediatric patients. The translocation of t(3;21)(q26;q22) results in the fusion of MECOM with RUNX1 and has been reported in a pediatric patient with acute myelomonocytic leukemia [81], acute monoblastic leukemia [82], and secondary MDS/AML after treatment for T-cell ALL [83]. Additional MECOM fusions have been reported in pediatric patients, including MECOM-RPN1 in a patient with AML with megakaryocytic differentiation [84] and EIF4A2-MECOM in a patient with severe congenital neutropenia and secondary AML [85]. Patients with these rare MECOM fusions ranged in age from 0 to 13 years.
In children and AYAs with AML, MECOM overexpression has been reported in up to 30% of patients [79,80,86,87]. Its frequency is significantly higher among younger patients (<10 years) with intermediate or high-risk AML, complex karyotypes, and MLL (11q23) abnormalities [79,80,86,87]. MECOM overexpression is an unfavorable prognostic factor that is associated with a lower event-free survival (EFS) (37.3–40% versus 50–68.4%) and overall survival (OS) (38.7–51% versus 68–78.9%), as well as a higher cumulative incidence of relapse [80,86].
3.2.2. Treatment
Treatment strategies in children with AML with MECOM rearrangement have included chemotherapy and allo-HSCT [80,81]. With limited patient cases, there is no established standard of care. On AAML1831 (NCT04293562) (Table 7), MECOM-RPN1 and MECOM-RUNX1 fusions are classified as unfavorable prognostic markers, and AML patients harboring these fusions are treated per the high-risk arm (two induction cycles and one intensification chemotherapy cycle followed by allo-HSCT). All other MECOM aberrations are considered neutral prognostic markers, and the standard treatment consists of five cycles of chemotherapy (two induction cycles and three intensification cycles; Footnote 3, Table 7) if there is no evidence of residual AML (measurable residual disease (MRD) < 0.05%) at the end of induction 1, or three cycles of chemotherapy (two induction cycles and one intensification cycle) followed by allo-HSCT if there is evidence of residual AML (MRD ≥ 0.05%) at the end of induction 1 (Footnote 3, Table 7).
3.2.3. MECOM-Associated Syndrome
MECOM-associated syndrome is a rare entity, reported in approximately 50 families, and is characterized by MECOM mutations, autosomal dominant amegakaryocytic thrombocytopenia, progressive bone marrow failure, pancytopenia, and radioulnar synostosis [88,89]. Additional forearm and hand anomalies, cardiac and renal anomalies, hearing loss, and/or B-cell deficiencies may also be present [89]. Due to congenital thrombocytopenia and progressive pancytopenia, allo-HSCT is often required in infancy [88,89]. Allo-HSCT with reduced-intensity conditioning has been shown to be both feasible and efficacious in infants with MECOM-associated syndrome [90]. One report linked MECOM mutations with hereditary hematologic malignancies, after two out of four family members with MECOM mutations and radioulnar synostosis developed MDS in adulthood [91].
3.3. AML with KAT6A-CREBBP Fusion
3.3.1. Pathologic and Cytogenetic Features
The translocation t(8;16)(p11;p13) results in fusion of KAT6A (lysine acetyltransferase 6A, also known as the monocytic leukemic zinc-finger (MOZ)) to CREB-binding protein (CREBBP or CBP). Both KAT6A and CREBBP have histone acetyltransferase (HAT) activity and act as transcriptional co-activators [92]. In addition to its activity as a HAT, KAT6A is involved in the transcriptional regulation of several transcription factors with hematopoietic specificity [93]. KAT6A was first identified as a fusion partner of CREBBP in the context of AML, with the fusion (KAT6A-CREBBP) functioning as a transcriptional co-activator that promotes leukemogenesis [94,95,96]. An international retrospective study identified and characterized 62 children and AYAs age 0 to 18 years with KAT6A-CREBBP [97]. Patients were typically very young at diagnosis (median age 1.2 years; 50% of the cases were <2 years of age at diagnosis), often presenting within the first month of life [97]. In this cohort, outcomes were similar to other pediatric AML cases, outside of spontaneous remissions in a subset of young infants [97].
3.3.2. Treatment
KAT6A-CREBBP fusion is considered an unfavorable prognostic marker (high-risk) on AAML1831 (NCT04293562), and the current standard of care would include two induction chemotherapy cycles and one intensification cycle, followed by a consolidative allo-HSCT [97]. Several targeted therapies are currently being developed that specifically inhibit KAT6 [98].
3.4. AML with DEK-NUP214 Fusion
3.4.1. Pathologic and Cytogenetic Features
Translocation t(6;9)(p22;q34) results in the DEK-NUP214 fusion gene [99]. This DEK-NUP214 fusion protein is a nucleoporin with altered nuclear protein transport. Increased expression of the DEK-NUP214 fusion protein is specific to myeloid lineage cells, underscoring its association with AML, though its role in myeloid leukemogenesis is not completely understood [100]. DEK-NUP214 fusion has been reported in both AML and MDS in children and AYAs [101,102,103,104,105,106]. A report from the Children’s Oncology Group (2839 total patients, age range 0 to 29.8 years, treated on six consecutive AML trials) identified only 48 cases (1.7%) of the DEK-NUP214 fusion [107]. DEK-NUP214 fusion was associated with an older age (median age 12.6 years versus 8.9 years, p < 0.001), compared to DEK-NUP214 negative AML patients, and the FAB morphology subtype M2 (p = 0.03) [107]. Patients with DEK-NUP214 positive AML had outcomes similar to those of patients with AML with poor prognostic features, such as –7 or 5/del5q. Compared to patients with DEK-NUP214 negative AML, patients with DEK-NUP214 positive AML had a lower complete remission (CR) (67% versus 79%, p = 0.04), lower OS (39% versus 57%, p = 0.03), and higher relapse rates (64% versus 42%, p = 0.04) [107]. Both DEK-NUP214-positive AML and MDS are also highly associated with a co-occurring FLT3-ITD mutation [102,105,106,108,109]. In the Children’s Oncology Group cohort, however, outcomes in the DEK-NUP214 positive AML group were independent of FLT3-ITD, with no significant difference in the OS between the FLT3-ITD-positive and -negative groups (40% and 27%, respectively, p > 0.9) [107]. A high risk of relapse and poor overall survival have also been reported in international cohorts of children and AYAs with AML [106].
3.4.2. Treatment
DEK-NUP214 is considered an unfavorable prognostic marker, and patients harboring this mutation should be treated per high-risk AML protocols (two induction cycles and one intensification cycle followed by allo-HSCT) (Footnote 3, Table 7).
3.5. AML with FLT3-ALM
3.5.1. Pathologic and Cytogenetic Features
Somatic mutations of FLT3 are among the most common mutations in AML, with a prevalence of 10 to 20% in children and AYAs with AML, including acute promyelocytic leukemia (APL) [110,111,112,113,114]. FLT3 mutations include internal tandem duplication (ITD; FLT3-ITD) and missense mutations in the activation loop of the tyrosine kinase domain (TKD; FLT3-ALM or FLT3-TKD), with the former being more common [108]. These genetic aberrations result in the constitutive activation of the FLT3 receptor, resulting in increased cellular proliferation and leukemogenesis [115,116]. In a large cohort of 630 pediatric patients age < 21 years with de novo AML, FLT3-ITD was detected in 12% of patients (age 0.6–19.8 years) and FLT-ALM in 6.7% (age 0.3–19.7 years) [117]. In this cohort of children and AYAs, the progression-free survival (PFS) and OS of patients with FLT3-ITD were inferior to those of FLT3-wild type (WT) (31% versus 55%, p < 0.001), but the PFS and OS were similar in patients with FLT3-ALM compared to FLT3-WT (51% versus 55%, respectively, p = 0.8). A report from the Children’s Oncology Group of 104 pediatric patients age < 21 years with APL identified FLT3-ALM in 14% of the patients. In this cohort, patients with FLT3-ALM and FLT3-ITD had similar outcomes, including similar rates of induction death, complete remission, EFS, and OS [114].
3.5.2. Treatment
Multiple FLT3 inhibitors have been developed for use in FLT3-mutated AML, classified by receptor binding and the mutation(s) they are active against. The multi-center Children’s Oncology Group AAML1031 trial investigated the feasibility and efficacy of incorporating sorafenib (a multi-kinase tyrosine kinase inhibitor (TKI)) when combined with standard AML chemotherapy in children and AYAs (age < 30 years) with de novo AML and FLT3-ITD with a high allelic ratio (>0.4) [118]. Compared to a nonrandomized control group, patients in the sorafenib-treated cohort had significantly improved EFS (55.9% versus 31.9%, p = 0.001) and lower relapse risk (17.6% versus 44.1%, p = 0.012) [118]. AML1031 also demonstrated that post-transplant sorafenib maintenance therapy for one year is feasible [119]. Midostaurin and gilteritinib are also TKIs used in FLT3-ITD adult patients, with the latter targeted more specifically against FLT3. Studies in adults utilizing midostaurin and gilteritinib, including small cohorts of patients with FLT3-ALM, have demonstrated improved treatment responses [120,121]. The RATIFY trial (NCT00651261) investigated the addition of midostaurin to chemotherapy in newly diagnosed FLT3-mutated adult patients. A sub-analysis of patients with FLT3-ALM, including patients aged 19.3 to 59.9 years (median age 48.8 years), showed longer EFS in the midostaurin-treated patients (45.2% versus 30.1%, p = 0.044, compared to the placebo arm). There were no further sub-analyses comparing AYAs and adult patients in this study [122]. Currently, the AAML1831 trial is investigating gilteritinib in combination with standard chemotherapy and 1-year maintenance chemotherapy after allo-HSCT or after completion of induction and intensification chemotherapy in children and AYAs age < 22 years with FLT3 activating mutations or FLT3-ITD with a high allelic ratio (>0.1) (Footnote 3, Table 7).
Recently, FLT3 has been targeted with CAR-T therapy. Preclinical in vitro data demonstrate the cytotoxic effects of targeted CAR-T cells on AML cell lines and primary AML cells, as well as a survival benefit in mouse xenograft models [67].
3.6. AML with Megakaryoblastic Differentiation, RBM15-MKL1 Fusion
3.6.1. Pathologic and Cytogenetic Features
RBM15-MKL1 (previously known as OTT/MAL) translocation t(1;22)(p13;q13) fuses the RNA-binding motif protein (RBM15) gene on chromosome 1 with the megakaryoblastic leukemia-1 (MKL1) gene on chromosome 22. RBM15-MKL1 is a recurrent genetic aberration unique to non-Down syndrome acute megakaryoblastic leukemia (non-DS AMKL), occurring in up to 13% of cases [123,124,125,126]. AMKL is an AML subtype in which cell morphology resembles abnormal megakaryoblasts. Case reports of a TERT promotor variant in a young child with RBM15-MKL1-positive non-DS AMKL [127], a 3-way variant translocation (t(1;7;22)(p13;q21;q13)) in a neonate with non-DS AMKL [128], and a 4-way variant translocation (t(1;22;17;18)(p13;q13;q22;q12)) in an infant with non-DS AMKL [129] have been published. AMKL is rare in adults but accounts for 4–15% of newly diagnosed childhood AML [130]. Patients typically present at age < 2 years with anemia, thrombocytopenia, and organomegaly [131]. Clinical outcomes of RBM15-MKL1-positive non-DS AMKL in children and AYAs vary among studies. Inaba et al. and Schweitzer et al. describe intermediate event-free survival (EFS) and overall survival (OS) of 38–50% and 56–63%, respectively [124,132], whereas better outcomes (59% EFS, 70% OS) have been described by de Rooij et al. [125]. In these studies, patients were all < 18 years old, and the investigators speculated that differences in supportive care might have biased the outcome [124,125].
3.6.2. Treatment
RBM15-MKL1 fusion is considered a neutral prognostic marker on AAML1831, and the standard of care would include chemotherapy with or without allo-HSCT, pending response to induction chemotherapy (Footnote 3, Table 7). There are no known specific novel inhibitors targeting RBM15-MKL1 specifically.
3.7. AML with Megakaryoblastic Differentiation, CBFA2T3-GLIS2 Fusion
3.7.1. Pathologic and Cytogenetic Features
CBFA2T3-GLIS2 fusion characterizes an AML subtype exclusive to pediatrics, accounting for approximately 5% of all pediatric AML and primarily found in non-DS AMKL in infants and AML in early childhood, with most patients age < 5 years [130,133,134]. The presence of the CBFA2T3-GLIS2 fusion is associated with aggressive disease and a dismal prognosis, with OS ranging from 15 to 30% and EFS of 38% [84,125,133,134,135]. The CBFA2T3-GLIS2 fusion protein promotes leukemogenesis by upregulating transcription factors that ultimately result in enhanced self-renewal and inhibition of cell differentiation [133]. The fusion results from the cryptic inversion of chromosome 16, which is often missed in morphology and cytogenetic studies. Thus, diagnosis relies on a high CD56 expression, dim/negative CD45, CD38, and HLA-DR expression (termed RAM phenotype) [84,136]. Concomitant cytogenetic abnormalities are rare in patients with CBFA2T3-GLIS2 fusion AML [135,137].
3.7.2. Treatment
Given their poor prognosis, CBFA2T3-GLIS2 fusion-positive patients are allocated to high-risk arms of pediatric treatment protocols, including AAML1831 (NCT04293562), and are candidates for allo-HSCT if negative MRD and complete remission are achieved. The CBFA2T3-GLIS2 fusion is an attractive option for targeted therapies. In pre-clinical studies, CD56-directed antibody-drug conjugates effectively targeted CBFA2T3-GLIS2 fusion-positive AML blasts [137]. Additionally, Le et al. demonstrated the efficacy of CAR-T directed against cell surface folate receptor alpha in vitro and in mouse xenograft models [68].
3.8. AML with RUNX1-CBFA2T3 Fusion
3.8.1. Pathologic and Cytogenetic Features
RUNX1-CBFA2T3 fuses RUNX1 on chromosome 16 and CBFA2T3 on chromosome 21 (t(16;21)(q24;q22)) and is a rare translocation described in de novo and therapy-related AML. In pediatric patients, it has been primarily described in children and AYAs aged 1 to 39 years [138,139,140,141,142,143,144,145]. Many patients with RUNx1-CBFA2T3 had M1, M2, or M4 FAB subtypes, and a subset of these patients were found to have eosinophilia [140,141,142,144,146], but accumulation and evaluation of additional cases are needed to determine if these patients represent a clinically significant distinct subgroup. In an international cohort of 23 children and AYAs (median age 6.8 years, age range 1 to 17 years) with RUNX1-CBFA2T3, the fusion was associated with an overall favorable outcome with EFS and OS of 77% and 81%, respectively, and a 0% 4-year cumulative incidence of relapse [138].
3.8.2. Treatment
Therapy has included chemotherapy with or without consolidative allo-HSCT. RUNX1-CBFA2T3 fusion is considered a neutral prognostic marker, and the standard of care would include chemotherapy with or without allo-HSCT, depending on the response to induction chemotherapy. Given favorable outcomes, consideration should be given to risk-stratifying RUNX1-CBFA2T3 to standard-risk AML therapy.
3.9. Acute Erythroid Leukemia
3.9.1. Pathologic and Cytogenetic Features
Acute erythroid leukemia (AEL) is a rare subtype of AML characterized by the proliferation of immature erythroblasts in the bone marrow and peripheral blood. AEL is more common in adults but rarely occurs in children and AYAs, accounting for <5% of all AML cases [147,148]. The diagnosis of AEL is based on the presence of at least 50% erythroid precursors in the bone marrow and/or peripheral blood, along with evidence of dysplasia of ≥10% of the cells in two or more hematopoietic lineages. The genetic basis of AEL remains poorly defined, but both adult and pediatric cases show enrichment of NUP98 rearrangements, as well as a variety of other somatic mutations [149,150,151,152]. The Children’s Oncology Group reported recurrent NUP98 rearrangements in approximately 32% of pediatric AEL cases [149]. AEL is associated with a poor prognosis, with OS and EFS each accounting for approximately 20% of pure erythroid leukemia [149].
3.9.2. Treatment
Given the poor prognosis of this entity, HSCT is preferred.
3.10. Myeloid Neoplasms with Eosinophilia and Defining Gene Rearrangement
3.10.1. Pathologic and Cytogenetic Features
This rare group of myeloid malignancies was previously known as chronic eosinophilic leukemia (CEL) or idiopathic hypereosinophilic syndrome (HES). They are characterized by a clonal proliferation of myeloid and eosinophilic cells and are associated with gene fusions involving PDGRA, PDGFRB, FGFR1, or a protein tyrosine kinase such as JAK2 (e.g., PCM-JAK2), resulting in constitutively active tyrosine kinase and proliferation of the abnormal clone [153]. Clinicopathologic presentation is heterogenous. Leukocytosis with eosinophilia, anemia, hepatosplenomegaly, lymphadenopathy, and skin lesions (rashes, ulcers) have been reported in children aged 0 to 14 years [153].
3.10.2. Treatment
Treatment often involves a tyrosine kinase inhibitor (TKI) with or without chemotherapy and/or HSCT [153].
4. Syndromes Predisposing to Myelodysplastic and Myeloid Neoplasms, Rare Entities
4.1. Syndromes Predisposing to Myelodysplastic and Myeloid Neoplasms
A variety of syndromes with germline mutations may predispose to MDS and/or myeloid leukemias. Broadly, these may be classified as germline predisposition syndromes with or without pre-existing platelet disorders or a risk of organ dysfunction (Table 8). Constitutional pathogenic variants in DDX41, ETV6, CEBPA, RUNX1, ANKRD26, and GATA2 are especially prone to an increased risk of developing hematologic malignancies [154]. The clinical presentation of the syndromes is variable but may include isolated cytopenias or pancytopenia, with or without bone marrow failure [155]. Accurate diagnosis of inherited predisposition syndromes prior to myelodysplastic or leukemic transformation is paramount for management, treatment, and family genetic counseling [155]. Allo-HSCT is often considered for a cure. International best practice consensus guidelines detailing recommendations on the HSCT timeline, genomic assessment, donor selection, and genetic counseling have been published [154]. Briefly described below are the rare entities among these syndromes.

Table 8.
Syndromes with germline mutations predisposing to myelodysplastic and/or myeloid neoplasms.
4.2. Myeloid Neoplasms with Germline Predisposition without a Pre-Existing Platelet Disorder or Risk of Organ Dysfunction with CEBPA and DDX41 Mutations
These rare disorders are characterized by either CEBPA or DDX41 mutations and are often inherited in families without a pre-existing platelet disorder or organ dysfunction [156,157,158,159]. Patients with familial AML with germline CEBPA mutations are typically younger at presentation than patients with de novo disease, with a median age of 25 years and without antecedent MDS or cytopenias [158]. However, age at onset is variable, occurring at 1.8 to 62 years [157,158]. Both CEBPA and DDX41 mutations are associated with an increased risk of developing AML and MDS (DDX41 mutations) [158,159,160,161].
4.3. Myeloid Neoplasms with Germline Predisposition and Pre-Existing Platelet Disorders with RUNX1, ANKRD26, and ETV6 Mutations
These germline mutations are associated with thrombocytopenia as well as an increased risk of developing MDS or AML. RUNX1 mutations are associated with familial platelet disorder (FPD) with a predisposition to AML [157,161,162,163,164], while ANKRD26 and ETV6 mutations are associated with inherited thrombocytopenia and a predisposition to developing MDS and AML [162,165,166]. RUNX1-mutated families are heterogenous in mutation profile and phenotype [164]. In general, patients often have thrombocytopenia (platelet counts range from 70–145 × 109/L) and associated mucosal bleeding, as well as eczema or psoriasis [161]. Childhood onset (age < 18 years) malignancy is observed in 50% of families with RUNX1 mutations, although the median age (29 years) of onset is in young adulthood [161]. Treatment includes chemotherapy and allo-HSCT.
Patients with ANKRD26-related thrombocytopenia also present at variable ages, ranging from age 10 to 75 years with thrombocytopenia (platelet counts range from 8.5–85 × 109/L), mucosal bleeding, and menorrhagia; life-threatening hemorrhages are rare [165]. In addition to AML and MDS, germline ANKRD26 mutations have been less often associated with chronic myelogenous leukemia and chronic lymphocytic leukemia [165].
Patients with germline ETV6 mutations similarly present with thrombocytopenia and mucosal bleeding, though some patients also have anemia and/or neutropenia at presentation [166]. In addition to MDS and AML, patients with germline ETV6 mutations have developed other hematologic malignancies (i.e., pre-B cell ALL, mixed-phenotype acute leukemia, chronic myelomonocytic leukemia, multiple myeloma), as well as skin and colorectal cancer [166].
4.4. Myeloid Neoplasms with Germline Predisposition and Risk of Organ Dysfunctions
Children and AYAs with genetic-based syndromes including, but not limited to, inherited bone marrow failure syndromes (BMFS), telomere biology disorders (e.g., dyskeratosis congenita), neurofibromatosis type 1 (NF1), Noonan syndrome, or Noonan syndrome-like disorders, and Trisomy 21 have an increased predisposition to developing myeloid neoplasms [162]. These syndromes may affect several organ systems as well as hematopoiesis, underscoring their variable phenotypic presentation and progression to malignancy. Inherited BMFS may predispose to MDS and AML, whereas RASopathies such as NF1 and Noonan/Noonan-like syndromes may predispose to juvenile myelomonocytic leukemia (JMML). Spontaneous or germline mutations in the GATA2 gene may disrupt normal hematopoiesis and are associated with the development of myeloid neoplasia, including AML, MDS, and chronic myelomonocytic leukemia [161,167].
5. B-Cell Lymphoid Proliferations, Rare Entities
5.1. B-Cell Lymphoid Neoplasms
ALL is the most common pediatric malignancy [168]. B-cell ALL accounts for 75–80% of cases [168] and commonly presents with cytopenias and associated symptoms due to bone marrow involvement by leukemic blasts, as well as constitutional symptoms such as fever. Extramedullary involvement of the central nervous system (CNS) or testis in males may also occur. Similar to AML, diagnostic workup includes bone marrow morphology, flow cytometry, immunophenotyping, cytogenetic studies (karyotyping and FISH), and comprehensive sequencing in high-risk patients. Risk stratification and prognostication include age, white blood cell count (WBC), genomic aberrations, and response to induction chemotherapy [169]. WHO classification emphasizes the critical role of genomic aberrations in risk stratification and prognostication in ALL [170].
Highlighted below are rare precursor and mature B-cell ALL subtypes. Currently open Children’s Oncology Group trials, AALL1731 (standard risk, patient age > 1 to ≤31 years) (NCT03914625) and AALL1732 (high risk, patient age > 1 to ≤25 years) (NCT03959085) are incorporating targeted therapies: blinatumomab (bi-specific anti-CD19 and anti-CD3 fusion protein) in AALL1731 and inotuzumab ozogamicin (anti-CD22 antibody) in AALL1732, respectively. Both blinatumomab and inotuzumab ozogamicin have been used in the relapsed/refractory setting [171,172]. There is increasing interest in investigating the use of CD19 CAR-T earlier in treatment for very high risk patients, patients at high risk of relapse, and patients in their first relapse [173,174].
5.2. Near Haploid ALL (24 to 30 Chromosomes)
5.2.1. Pathologic and Cytogenetic Features
Near-haploid ALL is a rare subtype of B-cell ALL that accounts for approximately 2–4% of cases and is usually associated with a loss or absence of multiple chromosomes, most commonly chromosomes X, Y, 6, 7, 8, 9, 17, 18, and 20 [175,176]. The role of chromosome loss in leukemogenesis has not been elucidated. Near-haploidy presents at younger ages (median age 5 years, range 1–19 years) [176]. Both near-haploidy and low-hypodiploid groups present with approximately equal incidence in males and females and with a relatively low WBC of <50 × 109/L [176]. Near-haploid ALL has been shown to harbor a distinct mutational profile as compared to low-hypodiploidy [177,178]. Greater than 70% of near-haploidy cases harbor activating receptor tyrosine kinase (RTK) and RAS singling alterations, with additional common somatic alterations involving histone modifiers, NF1, CREBBP, CDKN2A/B, IKZF3, and PAG1 [175,177]. Near-haploidy is a poor prognostic marker (EFS approximately 28%, OS approximately 34%) associated with induction failure and an increased risk of relapse [178,179].
5.2.2. Treatment
Due to poor outcomes, treatment includes intensified chemotherapy and HSCT [176,179]. In relapse or refractory settings, CD19 and CD22-targeting immunotherapy and anti-CD19 CAR-T therapy have been employed [180]. Holmfeldt et al. investigated the sensitivity of hypodiploid ALL cell lines and xenografts to MEK, PI3K, and PI3K/mTOR inhibitors [177]. Both PI3K and PI3K/mTOR inhibitors decreased tumor proliferation, making PI3K pathway inhibition a promising potential treatment option [177].
5.3. Low Hypodiploid ALL (31 to 39 Chromosomes)
5.3.1. Pathologic and Cytogenetic Features
Low hypodiploid is also a rare subtype of B-cell ALL, accounting for <3% of all cases [175], and is usually associated with retention of disomies X/Y, 1, 5, 6, 8, 10, 11, 14, 18, 19, 21, and 22 [176]. As with near haploidy, the selective advantage of chromosome loss in leukemogenesis is poorly understood. Low hypodiploidy occurs at all ages, but with a higher median age (age 15 years), and the incidence increases with age [176,178]. As outlined above, its gender incidence and the presenting WBC at diagnosis are similar to those of near-haploid ALL, but its genomic landscape is distinct [175,176,177]. Genomic alterations in TP53 are common and a hallmark of low hypodiploid ALL, with greater than 90% of cases harboring loss-of-function mutations in TP53 [181]. Importantly, TP53 alterations in low hypodiploid ALL have been detected in nontumor cells in almost half of pediatric cases, suggesting an association with Li-Fraumeni syndrome [177]. Additional genomic alterations include RB1 and IKZF2 [177,182]. Outcomes are poor, with EFS and OS approximately 37% and 40%, respectively [179].
5.3.2. Treatment
Similar to ALL with near-haploidy, treatment includes intensified chemotherapy and HSCT. CAR-T may also be considered in the relapsed/refractory setting and in patients with relapsed/refractory B-cell ALL harboring TP53 alterations. Interestingly, decitabine may improve CAR-T cell efficacy [183]. Hypodiploid ALL clones have aberrant RAS and PI3K signaling, and PI3K and PI3K/mTOR inhibitors may be an intriguing novel treatment option [177]. Given the high frequency of TP53 mutations and their presence in nontumor cells, patients should be evaluated for germline TP53 mutations and Li-Fraumeni syndrome and, if identified, offered genetic counseling [176,177].
5.4. Mature B-Cell ALL
5.4.1. Pathologic and Cytogenetic Features
Mature B-cell ALL is a rare subtype of B-cell ALL characterized by the presence of mature-appearing B-cells with surface IgM with light-chain restriction in the absence of immature B-cell antigens and FAB-L3 morphology [184]. It is often associated with t(8;14) or its variants and is molecularly characterized by MYC aberrations, including MYC overexpression, rearrangements, and fusion gene products (C-MYC/IGL or IGK), which result in leukemogenesis [184]. Based on these features, mature B-cell ALL is often considered the leukemic phase of Burkitt lymphoma. Patients are predominately male, with a median age of 9 years at presentation, and tend to have favorable outcomes (OS greater than 80%) [185].
Rare cases of mature B-cell ALL with KMT2A rearrangement have been reported in children and AYAs age 0 to 24 years, with the vast majority being age < 2 years and presenting with extramedullary involvement of the liver, spleen, skin, kidney, and occasionally the central nervous system [184,186]. Given the small numbers and varying treatment approaches, outcomes cannot be generalized.
5.4.2. Treatment
Cases of mature B-cell ALL have been treated with standard lymphoma protocols [184,186]. Case reports of children, AYAs, and adults aged 2 to 78 years using CAR-T therapy for relapsed Burkitt lymphoma have been published [187].
5.5. B-Lymphoblastic Leukemia/Lymphoma
5.5.1. Pathologic and Cytogenetic Features
B-lymphoblastic leukemia/lymphoma (LBL/L) is an aggressive subtype of leukemia/lymphoma that can be associated with specific genetic abnormalities, such as a translocation between the TCF3 gene on chromosome 19 and the PBX1 gene on chromosome X or a translocation of the IGH gene on chromosome 14 and IL3, resulting in the fusion gene product TCF3-PBX1 or IGH-IL3, respectively. In early studies, B-LBL/L with TCF3-PBX1 showed a poor response to chemotherapy. However, with more recent chemotherapy, it has shown an improved prognosis, albeit with an increased risk of CNS relapse [169,188].
5.5.2. Treatment
Treatment typically consists of chemotherapy [188].
5.6. Chronic Lymphocytic Leukemia
5.6.1. Pathologic and Cytogenetic Features
Chronic lymphocytic leukemia (CLL) is a lymphoproliferative disorder characterized by the accumulation of monoclonal B cells in the blood, bone marrow, or lymphoid tissues [189]. It predominantly occurs in older adults (median age 72 years at diagnosis) and is very uncommon in children and AYAs, with fewer than 10 previously reported cases [189,190]. The presentation of CLL in childhood may be associated with organomegaly or lymphadenopathy, as opposed to hyperleukocytosis (as in adults) [190]. CLL may be associated with specific genetic abnormalities such as Trisomy 12 or TP53 gene mutations [189], which may contribute to leukemogenesis. The outcomes of CLL in children are favorable, with reported CR rates of 44% and overall response rates of 95% [191].
5.6.2. Treatment
Standard treatment consists of chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximab [189,191]. In the relapsed/refractory setting, CD19 CAR-T therapy has been demonstrated to be efficacious in adults [192,193].
6. T-Cell Lymphoid Proliferations, Rare Entities
6.1. T-Cell Large Granular Lymphocytic Leukemia
6.1.1. Pathologic and Cytogenetic Features
T-cell large granular lymphocytic leukemia (T-LGL) is a rare, classically clinically indolent disease of adults, with fewer than 10 cases reported in children and AYAs (age range 2 to 13 years) [194,195,196,197,198]. LGL is a mature, peripheral T-cell neoplasm that develops due to clonal proliferation of cytotoxic (CD8+) T cells that infiltrate organs such as bone marrow, liver, and spleen, resulting in cytopenias. LGL is also associated with dysregulation of or abnormalities involving the immune system and presents with coexisting autoimmune phenomena, such as rheumatoid arthritis, autoimmune neutropenia, or thrombocytopenia [196,197,198,199,200]. It has also rarely been described in adult solid organ transplant recipients [201].
Antigenic and cytokine stimulation of multiple molecular pathways has been implicated in the pathogenesis of T-LGL, including JAK/STAT, PI3K/AKT, FAS/FAS-L, RAS, NF-KB, and the sphingolipid rheostat pathway [202]. Lymphocytes in T-LGL may be difficult to morphologically distinguish from non-neoplastic cytotoxic lymphocytes; thus, diagnosis relies heavily on molecular techniques such as polymerase chain reaction (PCR) utilizing a probe for T-cell receptor (TCR)γ for diagnosis and flow cytometry for confirmatory immunophenotyping [200].
6.1.2. Treatment
Treatment is indicated for patients with symptomatic anemia, neutropenia with recurrent infections, and/or autoimmune conditions [203]. Immunosuppressive therapy (commonly methotrexate, cyclosporine, and cyclophosphamide) often mitigates sequelae but may or may not result in cure [194,203]. In addition to immunosuppressive therapy, chemotherapy or HSCT may also be utilized [194,200,204,205,206]. In the relapsed/refractory setting, allo-HSCT successfully induced remission in a 13-year-old, suggesting allo-HSCT be considered for a definitive cure in children [194]. Molecular pathways implicated in T-LGL pathogenesis offer potential for targeted therapeutic approaches [203], though none have been studied in children and AYAs.
7. Conclusions
Described here are rare hematologic malignancies, myelodysplastic neoplasms, and predisposition syndromes in children and AYAs occurring with a low (<5%) frequency. Collectively, they are a heterogenous group, each of which often has a unique and complex genetic basis and clinical phenotype. Recognition and accurate diagnosis of these entities are important and may direct treatment choices and impact outcomes. Specific molecular lesions, genomic alterations, or unique immunophenotypes may help identify novel treatment approaches in these patients. Treatment usually involves conventional chemotherapy, immunotherapy, CAR-T therapy, HSCT, molecular targeted therapies, or a combination of the above. There is often a lack of consensus on optimal management, so the treatment may need to be individualized. Despite recent advances in classification schema, diagnostic methods, and treatment landscape, there is still much to learn about these rare hematologic malignancies and pre-leukemic entities.
Author Contributions
Conceptualization, S.B.; methodology, S.B. and A.B.; software, S.B. and A.B.; validation, S.B. and A.B.; formal analysis, S.B. and A.B.; investigation, S.B. and A.B.; resources, S.B. and A.B.; data curation, S.B. and A.B.; writing—original draft preparation, S.B. and A.B.; writing—review and editing, S.B. and A.B.; visualization, S.B. and A.B.; supervision, S.B.; project administration, S.B. and A.B.; funding acquisition, S.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interests.
References
- Orkin, S.H.; Nathan, D.G.; Ginsburg, D.; Look, A.T.; Fisher, D.E.; Lux, S. Nathan and Oski’s Hematology and Oncology of Infancy and Childhood; Elsevier Health Sciences: Philedelphia, PA, USA, 2014. [Google Scholar]
- Khoury, J.D.; Solary, E.; Abla, O.; Akkari, Y.; Alaggio, R.; Apperley, J.F.; Bejar, R.; Berti, E.; Busque, L.; Chan, J.K.C.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Myeloid and Histiocytic/Dendritic Neoplasms. Leukemia 2022, 36, 1703–1719. [Google Scholar] [CrossRef]
- Malcovati, L.; Porta, M.G.; Pascutto, C.; Invernizzi, R.; Boni, M.; Travaglino, E.; Passamonti, F.; Arcaini, L.; Maffioli, M.; Bernasconi, P.; et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: A basis for clinical decision making. J. Clin. Oncol. 2005, 23, 7594–7603. [Google Scholar] [CrossRef]
- Chisholm, K.M.; Bohling, S.D. Childhood Myelodysplastic Syndrome. Clin. Lab. Med. 2023, 43, 639–655. [Google Scholar] [CrossRef]
- Pastor, V.; Hirabayashi, S.; Karow, A.; Wehrle, J.; Kozyra, E.J.; Nienhold, R.; Ruzaike, G.; Lebrecht, D.; Yoshimi, A.; Niewisch, M.; et al. Mutational landscape in children with myelodysplastic syndromes is distinct from adults: Specific somatic drivers and novel germline variants. Leukemia 2017, 31, 759–762. [Google Scholar] [CrossRef]
- Marques, F.K.; Sabino, A.P. Myelodysplastic neoplasms: An overview on diagnosis, risk-stratification, molecular pathogenesis, and treatment. Biomed. Pharmacother. 2022, 156, 113905. [Google Scholar] [CrossRef]
- Kardos, G.; Baumann, I.; Passmore, S.J.; Locatelli, F.; Hasle, H.; Schultz, K.R.; Starý, J.; Schmitt-Graeff, A.; Fischer, A.; Harbott, J.; et al. Refractory anemia in childhood: A retrospective analysis of 67 patients with particular reference to monosomy 7. Blood 2003, 102, 1997–2003. [Google Scholar] [CrossRef]
- Aalbers, A.M.; van den Heuvel-Eibrink, M.M.; de Haas, V.; Te Marvelde, J.G.; de Jong, A.X.; van der Burg, M.; Dworzak, M.; Hasle, H.; Locatelli, F.; De Moerloose, B.; et al. Applicability of a reproducible flow cytometry scoring system in the diagnosis of refractory cytopenia of childhood. Leukemia 2013, 27, 1923–1925. [Google Scholar] [CrossRef]
- Aalbers, A.M.; van den Heuvel-Eibrink, M.M.; Baumann, I.; Dworzak, M.; Hasle, H.; Locatelli, F.; De Moerloose, B.; Schmugge, M.; Mejstrikova, E.; Nováková, M.; et al. Bone marrow immunophenotyping by flow cytometry in refractory cytopenia of childhood. Haematologica 2015, 100, 315–323. [Google Scholar] [CrossRef]
- Chisholm, K.M.; Xu, M.; Davis, B.; Ogi, A.; Pacheco, M.C.; Geddis, A.E.; Tsuchiya, K.D.; Rutledge, J.C. Evaluation of the Utility of Bone Marrow Morphology and Ancillary Studies in Pediatric Patients Under Surveillance for Myelodysplastic Syndrome. Am. J. Clin. Pathol. 2018, 149, 499–513. [Google Scholar] [CrossRef]
- Haferlach, T.; Nagata, Y.; Grossmann, V.; Okuno, Y.; Bacher, U.; Nagae, G.; Schnittger, S.; Sanada, M.; Kon, A.; Alpermann, T.; et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia 2014, 28, 241–247. [Google Scholar] [CrossRef]
- Greenberg, P.L.; Tuechler, H.; Schanz, J.; Sanz, G.; Garcia-Manero, G.; Solé, F.; Bennett, J.M.; Bowen, D.; Fenaux, P.; Dreyfus, F.; et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012, 120, 2454–2465. [Google Scholar] [CrossRef] [PubMed]
- Hasle, H.; Baumann, I.; Bergsträsser, E.; Fenu, S.; Fischer, A.; Kardos, G.; Kerndrup, G.; Locatelli, F.; Rogge, T.; Schultz, K.R.; et al. The International Prognostic Scoring System (IPSS) for childhood myelodysplastic syndrome (MDS) and juvenile myelomonocytic leukemia (JMML). Leukemia 2004, 18, 2008–2014. [Google Scholar] [CrossRef]
- Schwartz, J.R.; Ma, J.; Lamprecht, T.; Walsh, M.; Wang, S.; Bryant, V.; Song, G.; Wu, G.; Easton, J.; Kesserwan, C.; et al. The genomic landscape of pediatric myelodysplastic syndromes. Nat. Commun. 2017, 8, 1557. [Google Scholar] [CrossRef]
- Veltroni, M.; Sainati, L.; Zecca, M.; Fenu, S.; Tridello, G.; Testi, A.M.; Merlone, A.D.; Buldini, B.; Leszl, A.; Lo Nigro, L.; et al. Advanced pediatric myelodysplastic syndromes: Can immunophenotypic characterization of blast cells be a diagnostic and prognostic tool? Pediatr. Blood Cancer 2009, 52, 357–363. [Google Scholar] [CrossRef]
- Locatelli, F.; Strahm, B. How I treat myelodysplastic syndromes of childhood. Blood 2018, 131, 1406–1414. [Google Scholar] [CrossRef]
- Boultwood, J.; Lewis, S.; Wainscoat, J. The 5q-syndrome. Blood 1994, 84, 3253–3260. [Google Scholar] [CrossRef]
- Antillón, F.; Raimondi, S.C.; Fairman, J.; Liang, H.; Nagarajan, L.; Head, D.; Ribeiro, R.C. 5q- in a child with refractory anemia with excess blasts: Similarities to 5q- syndrome in adults. Cancer Genet. Cytogenet. 1998, 105, 119–122. [Google Scholar] [CrossRef]
- Chantrain, C.; Vermylen, C.; Michaux, L.; Brichard, B.; Cornu, G. Clonal monosomy 7 and 5q--in a child with myelodysplastic syndrome. Pediatr. Hematol. Oncol. 2000, 17, 505–509. [Google Scholar] [CrossRef]
- Uyttebroeck, A.; Brock, P.; De Groote, B.; Renard, M.; Dal Cin, P.; Van den Berghe, H.; Casteels-Van Daele, M. 5q- syndrome in a child. Cancer Genet. Cytogenet. 1995, 80, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Sugita, K.; Kaneko, T.; Sekine, Y.; Taguchi, N.; Miyauchi, J. Mast cell leukemia evolved from RAEB-T (5q-syndrome) in a 12 year-old girl. Rinsho Ketsueki 1996, 37, 430–436. [Google Scholar] [PubMed]
- Pitman, S.D.; Victorio, A.; Rowsell, E.; Morris, J.; Wang, J. 5q- syndrome in a child with slowly progressive pancytopenia: A case report and review of the literature. J. Pediatr. Hematol. Oncol. 2006, 28, 115–119. [Google Scholar] [CrossRef]
- Elkattawy, S.; Ayad, S.; El-Feki, I.; Guo, X.; Appiah-Kubi, E.; Talpur, A.; Kessler, W. 5q Deletion Myelodysplastic Syndrome in a Young Male Patient. Cureus 2021, 13, e17466. [Google Scholar] [CrossRef]
- Adams, R.H.; Lemons, R.S.; Thangavelu, M.; Le Beau, M.M.; Christensen, R.D. Interstitial deletion of chromosome 5, del(5q), in a newborn with Down syndrome and an unusual hematologic disorder. Am. J. Hematol. 1989, 31, 273–279. [Google Scholar] [CrossRef]
- Shikano, T.; Ishikawa, Y.; Anakura, M. Myelodysplastic syndrome with partial deletion of the long arm of chromosome 5: First report of a case in a child. Acta Paediatr. Jpn. 1992, 34, 539–542. [Google Scholar] [CrossRef]
- Van den Berghe, H.; Cassiman, J.J.; David, G.; Fryns, J.P.; Michaux, J.L.; Sokal, G. Distinct haematological disorder with deletion of long arm of no. 5 chromosome. Nature 1974, 251, 437–438. [Google Scholar] [CrossRef]
- Sokal, G.; Michaux, J.L.; Van Den Berghe, H.; Cordier, A.; Rodhain, J.; Ferrant, A.; Moriau, M.; De Bruyere, M.; Sonnet, J. A new hematologic syndrome with a distinct karyotype: The 5 q--chromosome. Blood 1975, 46, 519–533. [Google Scholar] [CrossRef]
- Adema, V.; Bejar, R. What lies beyond del(5q) in myelodysplastic syndrome? Haematologica 2013, 98, 1819–1821. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, M.R.; Besa, E.C. Myelodysplastic syndromes with 5q deletion: Pathophysiology and role of lenalidomide. Ann. Hematol. 2014, 93, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Hosono, N.; Makishima, H.; Mahfouz, R.; Przychodzen, B.; Yoshida, K.; Jerez, A.; LaFramboise, T.; Polprasert, C.; Clemente, M.J.; Shiraishi, Y.; et al. Recurrent genetic defects on chromosome 5q in myeloid neoplasms. Oncotarget 2017, 8, 6483–6495. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Robinson, W.A.; Hamstra, R.D.; Wallner, S.F. Macrocytic anemia, thrombocytosis and nonlobulated megakaryocytes: The 5q-syndrome, a distinct entity. Am. J. Med. 1979, 66, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Van den Berghe, H.; Vermaelen, K.; Mecucci, C.; Barbieri, D.; Tricot, G. The 5q-anomaly. Cancer Genet. Cytogenet. 1985, 17, 189–255. [Google Scholar] [CrossRef]
- List, A.; Dewald, G.; Bennett, J.; Giagounidis, A.; Raza, A.; Feldman, E.; Powell, B.; Greenberg, P.; Thomas, D.; Stone, R.; et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N. Engl. J. Med. 2006, 355, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Fenaux, P.; Giagounidis, A.; Selleslag, D.; Beyne-Rauzy, O.; Mufti, G.; Mittelman, M.; Muus, P.; Te Boekhorst, P.; Sanz, G.; Del Cañizo, C.; et al. A randomized phase 3 study of lenalidomide versus placebo in RBC transfusion-dependent patients with Low-/Intermediate-1-risk myelodysplastic syndromes with del5q. Blood 2011, 118, 3765–3776. [Google Scholar] [CrossRef]
- Raza, A.; Reeves, J.A.; Feldman, E.J.; Dewald, G.W.; Bennett, J.M.; Deeg, H.J.; Dreisbach, L.; Schiffer, C.A.; Stone, R.M.; Greenberg, P.L.; et al. Phase 2 study of lenalidomide in transfusion-dependent, low-risk, and intermediate-1 risk myelodysplastic syndromes with karyotypes other than deletion 5q. Blood 2008, 111, 86–93. [Google Scholar] [CrossRef]
- Adès, L.; Boehrer, S.; Prebet, T.; Beyne-Rauzy, O.; Legros, L.; Ravoet, C.; Dreyfus, F.; Stamatoullas, A.; Chaury, M.P.; Delaunay, J.; et al. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: Results of a phase 2 study. Blood 2009, 113, 3947–3952. [Google Scholar] [CrossRef] [PubMed]
- Möllgård, L.; Saft, L.; Treppendahl, M.B.; Dybedal, I.; Nørgaard, J.M.; Astermark, J.; Ejerblad, E.; Garelius, H.; Dufva, I.H.; Jansson, M.; et al. Clinical effect of increasing doses of lenalidomide in high-risk myelodysplastic syndrome and acute myeloid leukemia with chromosome 5 abnormalities. Haematologica 2011, 96, 963–971. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Platzbecker, U.; Braulke, F.; Kündgen, A.; Götze, K.; Bug, G.; Schönefeldt, C.; Shirneshan, K.; Röllig, C.; Bornhäuser, M.; Naumann, R.; et al. Sequential combination of azacitidine and lenalidomide in del(5q) higher-risk myelodysplastic syndromes or acute myeloid leukemia: A phase I study. Leukemia 2013, 27, 1403–1407. [Google Scholar] [CrossRef]
- Scherman, E.; Malak, S.; Perot, C.; Gorin, N.C.; Rubio, M.T.; Isnard, F. Interest of the association azacitidine-lenalidomide as frontline therapy in high-risk myelodysplasia or acute myeloid leukemia with complex karyotype. Leukemia 2012, 26, 822–824. [Google Scholar] [CrossRef][Green Version]
- Sekeres, M.A.; Tiu, R.V.; Komrokji, R.; Lancet, J.; Advani, A.S.; Afable, M.; Englehaupt, R.; Juersivich, J.; Cuthbertson, D.; Paleveda, J.; et al. Phase 2 study of the lenalidomide and azacitidine combination in patients with higher-risk myelodysplastic syndromes. Blood 2012, 120, 4945–4951. [Google Scholar] [CrossRef][Green Version]
- Kantarjian, H.; O’Brien, S.; Ravandi, F.; Borthakur, G.; Faderl, S.; Bueso-Ramos, C.; Abruzzo, L.; Pierce, S.; Shan, J.; Issa, J.P.; et al. The heterogeneous prognosis of patients with myelodysplastic syndrome and chromosome 5 abnormalities: How does it relate to the original lenalidomide experience in MDS? Cancer 2009, 115, 5202–5209. [Google Scholar] [CrossRef]
- Jädersten, M.; Saft, L.; Pellagatti, A.; Göhring, G.; Wainscoat, J.S.; Boultwood, J.; Porwit, A.; Schlegelberger, B.; Hellström-Lindberg, E. Clonal heterogeneity in the 5q- syndrome: p53 expressing progenitors prevail during lenalidomide treatment and expand at disease progression. Haematologica 2009, 94, 1762–1766. [Google Scholar] [CrossRef]
- Hirabayashi, S.; Flotho, C.; Moetter, J.; Heuser, M.; Hasle, H.; Gruhn, B.; Klingebiel, T.; Thol, F.; Schlegelberger, B.; Baumann, I.; et al. Spliceosomal gene aberrations are rare, coexist with oncogenic mutations, and are unlikely to exert a driver effect in childhood MDS and JMML. Blood 2012, 119, e96–e99. [Google Scholar] [CrossRef][Green Version]
- Boles, B.; Shiel, M.; Gardner, J.A.; Conant, J.L. Pediatric Myelodysplastic Syndrome with SF3B1 Mutation. J. Assoc. Genet. Technol. 2023, 49, 69–72. [Google Scholar]
- Hosono, N. Genetic abnormalities and pathophysiology of MDS. Int. J. Clin. Oncol. 2019, 24, 885–892. [Google Scholar] [CrossRef]
- Malcovati, L.; Stevenson, K.; Papaemmanuil, E.; Neuberg, D.; Bejar, R.; Boultwood, J.; Bowen, D.T.; Campbell, P.J.; Ebert, B.L.; Fenaux, P.; et al. SF3B1-mutant MDS as a distinct disease subtype: A proposal from the International Working Group for the Prognosis of MDS. Blood 2020, 136, 157–170. [Google Scholar] [CrossRef]
- Yoshida, K.; Sanada, M.; Shiraishi, Y.; Nowak, D.; Nagata, Y.; Yamamoto, R.; Sato, Y.; Sato-Otsubo, A.; Kon, A.; Nagasaki, M.; et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 2011, 478, 64–69. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Cazzola, M.; Boultwood, J.; Malcovati, L.; Vyas, P.; Bowen, D.; Pellagatti, A.; Wainscoat, J.S.; Hellstrom-Lindberg, E.; Gambacorti-Passerini, C.; et al. Somatic SF3B1 mutation in myelodysplasia with ring sideroblasts. N. Engl. J. Med. 2011, 365, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Malcovati, L.; Papaemmanuil, E.; Bowen, D.T.; Boultwood, J.; Della Porta, M.G.; Pascutto, C.; Travaglino, E.; Groves, M.J.; Godfrey, A.L.; Ambaglio, I.; et al. Clinical significance of SF3B1 mutations in myelodysplastic syndromes and myelodysplastic/myeloproliferative neoplasms. Blood 2011, 118, 6239–6246. [Google Scholar] [CrossRef] [PubMed]
- Visconte, V.; Makishima, H.; Maciejewski, J.P.; Tiu, R.V. Emerging roles of the spliceosomal machinery in myelodysplastic syndromes and other hematological disorders. Leukemia 2012, 26, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Chan, O.; Ali, N.A.; Sallman, D.; Padron, E.; Lancet, J.; Komrokji, R. Therapeutic Outcomes and Prognostic Impact of Gene Mutations Including TP53 and SF3B1 in Patients with Del(5q) Myelodysplastic Syndromes (MDS). Clin. Lymphoma Myeloma Leuk. 2022, 22, e467–e476. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.W.; Kim, H.R.; Baek, H.J.; Kook, H.; Cho, D.; Shin, J.H.; Suh, S.P.; Ryang, D.W.; Shin, M.G. Alteration of the SETBP1 gene and splicing pathway genes SF3B1, U2AF1, and SRSF2 in childhood acute myeloid leukemia. Ann. Lab. Med. 2015, 35, 118–122. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van der Werf, I.; Wojtuszkiewicz, A.; Yao, H.; Sciarrillo, R.; Meggendorfer, M.; Hutter, S.; Walter, W.; Janssen, J.; Kern, W.; Haferlach, C.; et al. SF3B1 as therapeutic target in FLT3/ITD positive acute myeloid leukemia. Leukemia 2021, 35, 2698–2702. [Google Scholar] [CrossRef] [PubMed]
- Haase, D.; Stevenson, K.E.; Neuberg, D.; Maciejewski, J.P.; Nazha, A.; Sekeres, M.A.; Ebert, B.L.; Garcia-Manero, G.; Haferlach, C.; Haferlach, T.; et al. TP53 mutation status divides myelodysplastic syndromes with complex karyotypes into distinct prognostic subgroups. Leukemia 2019, 33, 1747–1758. [Google Scholar] [CrossRef] [PubMed]
- Bernard, E.; Nannya, Y.; Hasserjian, R.P.; Devlin, S.M.; Tuechler, H.; Medina-Martinez, J.S.; Yoshizato, T.; Shiozawa, Y.; Saiki, R.; Malcovati, L.; et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat. Med. 2020, 26, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Matsuda, K.; Taira, C.; Sano, K.; Tanaka-Yanagisawa, M.; Yanagisawa, R.; Nakazawa, Y.; Sakashita, K.; Shiohara, M.; Koike, K. Genetic analysis of TP53 in childhood myelodysplastic syndrome and juvenile myelomonocytic leukemia. Leuk. Res. 2011, 35, 1578–1584. [Google Scholar] [CrossRef]
- Jekic, B.; Novakovic, I.; Lukovic, L.; Kuzmanovic, M.; Popovic, B.; Milasin, J.; Bunjevacki, G.; Damnjanovic, T.; Cvjeticanin, S.; Bunjevacki, V. Lack of TP53 and FMS gene mutations in children with myelodysplastic syndrome. Cancer Genet. Cytogenet. 2006, 166, 163–165. [Google Scholar] [CrossRef]
- Praulich, I.; Tauscher, M.; Göhring, G.; Glaser, S.; Hofmann, W.; Feurstein, S.; Flotho, C.; Lichter, P.; Niemeyer, C.M.; Schlegelberger, B.; et al. Clonal heterogeneity in childhood myelodysplastic syndromes--challenge for the detection of chromosomal imbalances by array-CGH. Genes Chromosomes Cancer 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Newman, A.M.; Zaka, M.; Zhou, P.; Blain, A.E.; Erhorn, A.; Barnard, A.; Crossland, R.E.; Wilkinson, S.; Enshaei, A.; De Zordi, J.; et al. Genomic abnormalities of TP53 define distinct risk groups of paediatric B-cell non-Hodgkin lymphoma. Leukemia 2022, 36, 781–789. [Google Scholar] [CrossRef]
- Pugh, T.J.; Yu, W.; Yang, J.; Field, A.L.; Ambrogio, L.; Carter, S.L.; Cibulskis, K.; Giannikopoulos, P.; Kiezun, A.; Kim, J.; et al. Exome sequencing of pleuropulmonary blastoma reveals frequent biallelic loss of TP53 and two hits in DICER1 resulting in retention of 5p-derived miRNA hairpin loop sequences. Oncogene 2014, 33, 5295–5302. [Google Scholar] [CrossRef]
- Elgarten, C.W.; Aplenc, R. Pediatric acute myeloid leukemia: Updates on biology, risk stratification, and therapy. Curr. Opin. Pediatr. 2020, 32, 57–66. [Google Scholar] [CrossRef]
- Pommert, L.; Tarlock, K. The evolution of targeted therapy in pediatric AML: Gemtuzumab ozogamicin, FLT3/IDH/BCL2 inhibitors, and other therapies. Hematol. Am. Soc. Hematol. Educ. Program 2022, 2022, 603–610. [Google Scholar] [CrossRef]
- Dwivedi, A.; Fu, L.; Chien, C.D.; Pouzolles, M.; Shah, N.N.; Taylor, N. Engineering Off-the-Shelf Gamma Delta CAR T Cells for the Treatment of Acute Myeloid Leukemia. Blood 2023, 142, 4827. [Google Scholar] [CrossRef]
- Shah, N.; Azzi, J.; Cooper, B.; Deol, A.; DiiPersio, J.; Koura, D.; McClune, B.; Muffly, L.; Umair Mushtaq, M.; Narayan, R.; et al. Phase 1/2 Study of Donor-Derived Anti-CD33 Chimeric Antigen Receptor Expressing T Cells (VCAR33) in Patients with Relapsed or Refractory Acute Myeloid Leukemia after Allogeneic Hematopoietic Cell Transplantation. In Proceedings of the ASH Annual Meeting & Exposition, San Diego, CA, USA, 11 December 2023. [Google Scholar]
- Calviño, C.; Ceballos, C.; Alfonso, A.; Jauregui, P.; Calleja-Cervantes, M.E.; San Martin-Uriz, P.; Rodriguez-Marquez, P.; Martin-Mallo, A.; Iglesias, E.; Abizanda, G.; et al. Optimization of universal allogeneic CAR-T cells combining CRISPR and transposon-based technologies for treatment of acute myeloid leukemia. Front. Immunol. 2023, 14, 1270843. [Google Scholar] [CrossRef]
- Bhagwat, A.S.; Torres, L.; Shestova, O.; Shestov, M.; Frey, N.V.; Hexner, E.O.; Luger, S.M.; Loren, A.W.; Martin, M.E.; McCurdy, S.R.; et al. Cytokine Release Syndrome Results in Reduced AML Killing By CD123 CAR T Cells. Blood 2023, 142, 217. [Google Scholar] [CrossRef]
- Rohrbacher, L.; Nixdorf, D.; Stadler, H.; Brauchle, B.; Märkl, F.; Philipp, N.; Hänel, G.; Marcinek, A.; Kazerani, M.; Emhardt, A.J.; et al. Two Players, One Goal: BiTE ® Vs CART Targeting FLT3 in AML. Blood 2023, 142, 3444. [Google Scholar] [CrossRef]
- Le, Q.; Hadland, B.; Smith, J.L.; Leonti, A.; Huang, B.J.; Ries, R.; Hylkema, T.A.; Castro, S.; Tang, T.T.; McKay, C.N.; et al. CBFA2T3-GLIS2 model of pediatric acute megakaryoblastic leukemia identifies FOLR1 as a CAR T cell target. J. Clin. Investig. 2022, 132, e157101. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Kurokawa, M. Ecotropic viral integration site 1, stem cell self-renewal and leukemogenesis. Cancer Sci. 2012, 103, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Tang, G.; Hu, S.; Patel, K.P.; Yin, C.C.; Wang, W.; Lin, P.; Toruner, G.A.; Ok, C.Y.; Gu, J.; et al. Deciphering the complexities of MECOM rearrangement-driven chromosomal aberrations. Cancer Genet. 2019, 233–234, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Lugthart, S.; Gröschel, S.; Beverloo, H.B.; Kayser, S.; Valk, P.J.; van Zelderen-Bhola, S.L.; Jan Ossenkoppele, G.; Vellenga, E.; van den Berg-de Ruiter, E.; Schanz, U.; et al. Clinical, molecular, and prognostic significance of WHO type inv(3)(q21q26.2)/t(3;3)(q21;q26.2) and various other 3q abnormalities in acute myeloid leukemia. J. Clin. Oncol. 2010, 28, 3890–3898. [Google Scholar] [CrossRef]
- Lugthart, S.; van Drunen, E.; van Norden, Y.; van Hoven, A.; Erpelinck, C.A.; Valk, P.J.; Beverloo, H.B.; Löwenberg, B.; Delwel, R. High EVI1 levels predict adverse outcome in acute myeloid leukemia: Prevalence of EVI1 overexpression and chromosome 3q26 abnormalities underestimated. Blood 2008, 111, 4329–4337. [Google Scholar] [CrossRef]
- Gröschel, S.; Lugthart, S.; Schlenk, R.F.; Valk, P.J.; Eiwen, K.; Goudswaard, C.; van Putten, W.J.; Kayser, S.; Verdonck, L.F.; Lübbert, M.; et al. High EVI1 expression predicts outcome in younger adult patients with acute myeloid leukemia and is associated with distinct cytogenetic abnormalities. J. Clin. Oncol. 2010, 28, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Baldazzi, C.; Luatti, S.; Zuffa, E.; Papayannidis, C.; Ottaviani, E.; Marzocchi, G.; Ameli, G.; Bardi, M.A.; Bonaldi, L.; Paolini, R.; et al. Complex chromosomal rearrangements leading to MECOM overexpression are recurrent in myeloid malignancies with various 3q abnormalities. Genes Chromosomes Cancer 2016, 55, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Konoplev, S.N.; Wang, X.; Cui, W.; Chen, S.S.; Medeiros, L.J.; Lin, P. De novo acute myeloid leukemia with inv(3)(q21q26.2) or t(3;3)(q21;q26.2): A clinicopathologic and cytogenetic study of an entity recently added to the WHO classification. Mod. Pathol. 2011, 24, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Grimwade, D.; Hills, R.K.; Moorman, A.V.; Walker, H.; Chatters, S.; Goldstone, A.H.; Wheatley, K.; Harrison, C.J.; Burnett, A.K. Refinement of cytogenetic classification in acute myeloid leukemia: Determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010, 116, 354–365. [Google Scholar] [CrossRef]
- De Braekeleer, M.; Le Bris, M.J.; De Braekeleer, E.; Basinko, A.; Morel, F.; Douet-Guilbert, N. 3q26/EVI1 rearrangements in myeloid hemopathies: A cytogenetic review. Future Oncol. 2015, 11, 1675–1686. [Google Scholar] [CrossRef] [PubMed]
- Haas, K.; Kundi, M.; Sperr, W.R.; Esterbauer, H.; Ludwig, W.D.; Ratei, R.; Koller, E.; Gruener, H.; Sauerland, C.; Fonatsch, C.; et al. Expression and prognostic significance of different mRNA 5’-end variants of the oncogene EVI1 in 266 patients with de novo AML: EVI1 and MDS1/EVI1 overexpression both predict short remission duration. Genes Chromosomes Cancer 2008, 47, 288–298. [Google Scholar] [CrossRef]
- Balgobind, B.V.; Lugthart, S.; Hollink, I.H.; Arentsen-Peters, S.T.; van Wering, E.R.; de Graaf, S.S.; Reinhardt, D.; Creutzig, U.; Kaspers, G.J.; de Bont, E.S.; et al. EVI1 overexpression in distinct subtypes of pediatric acute myeloid leukemia. Leukemia 2010, 24, 942–949. [Google Scholar] [CrossRef]
- Ho, P.A.; Alonzo, T.A.; Gerbing, R.B.; Pollard, J.A.; Hirsch, B.; Raimondi, S.C.; Cooper, T.; Gamis, A.S.; Meshinchi, S. High EVI1 expression is associated with MLL rearrangements and predicts decreased survival in paediatric acute myeloid leukaemia: A report from the children’s oncology group. Br. J. Haematol. 2013, 162, 670–677. [Google Scholar] [CrossRef]
- Duan, J.X.; Liu, F.; Chang, L.; Che, G.L.; Yang, Q.X.; Teng, J.; Jian, H.; Liu, X.J.; Lai, S.Y. A primary pediatric acute myelomonocytic leukemia with t(3;21)(q26;q22): A case report. Medicine 2023, 102, e35721. [Google Scholar] [CrossRef]
- Johansson, B.; Fioretos, T.; Garwicz, S.; Heim, S.; Mitelman, F. t(3;21)(q26;q22) with AML1 rearrangement in a de novo childhood acute monoblastic leukaemia. Br. J. Haematol. 1996, 92, 429–431. [Google Scholar] [CrossRef]
- Li, S.; Yin, C.C.; Medeiros, L.J.; Bueso-Ramos, C.; Lu, G.; Lin, P. Myelodysplastic syndrome/acute myeloid leukemia with t(3;21)(q26.2;q22) is commonly a therapy-related disease associated with poor outcome. Am. J. Clin. Pathol. 2012, 138, 146–152. [Google Scholar] [CrossRef]
- Chisholm, K.M.; Smith, J.; Heerema-McKenney, A.E.; Choi, J.K.; Ries, R.E.; Hirsch, B.A.; Raimondi, S.C.; Wang, Y.C.; Dang, A.; Alonzo, T.A.; et al. Pathologic, cytogenetic, and molecular features of acute myeloid leukemia with megakaryocytic differentiation: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2023, 70, e30251. [Google Scholar] [CrossRef]
- Connelly, J.A.; Mody, R.J.; Wu, Y.M.; Robinson, D.R.; Lonigro, R.J.; Vats, P.; Rabban, E.; Anderson, B.; Walkovich, K. Identification of novel MECOM gene fusion and personalized therapeutic targets through integrative clinical sequencing in secondary acute myeloid leukemia in a patient with severe congenital neutropenia: A case report and literature review. Cold Spring Harb. Mol. Case Stud. 2018, 4, a002204. [Google Scholar] [CrossRef]
- Elsherif, M.; Hammad, M.; Hafez, H.; Yassin, D.; Ashraf, M.; Yasser, N.; Lehmann, L.; Elhaddad, A. MECOM gene overexpression in pediatric patients with acute myeloid leukemia. Acta Oncol. 2022, 61, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Jo, A.; Mitani, S.; Shiba, N.; Hayashi, Y.; Hara, Y.; Takahashi, H.; Tsukimoto, I.; Tawa, A.; Horibe, K.; Tomizawa, D.; et al. High expression of EVI1 and MEL1 is a compelling poor prognostic marker of pediatric AML. Leukemia 2015, 29, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Ammeti, D.; Marzollo, A.; Gabelli, M.; Zanchetta, M.E.; Tretti-Parenzan, C.; Bottega, R.; Capaci, V.; Biffi, A.; Savoia, A.; Bresolin, S.; et al. A novel mutation in MECOM affects MPL regulation in vitro and results in thrombocytopenia and bone marrow failure. Br. J. Haematol. 2023, 203, 852–859. [Google Scholar] [CrossRef]
- Germeshausen, M.; Ancliff, P.; Estrada, J.; Metzler, M.; Ponstingl, E.; Rütschle, H.; Schwabe, D.; Scott, R.H.; Unal, S.; Wawer, A.; et al. MECOM-associated syndrome: A heterogeneous inherited bone marrow failure syndrome with amegakaryocytic thrombocytopenia. Blood Adv. 2018, 2, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Irie, M.; Niihori, T.; Nakano, T.; Suzuki, T.; Katayama, S.; Moriya, K.; Niizuma, H.; Suzuki, N.; Saito-Nanjo, Y.; Onuma, M.; et al. Reduced-intensity conditioning is effective for allogeneic hematopoietic stem cell transplantation in infants with MECOM-associated syndrome. Int. J. Hematol. 2023, 117, 598–606. [Google Scholar] [CrossRef]
- Ripperger, T.; Hofmann, W.; Koch, J.C.; Shirneshan, K.; Haase, D.; Wulf, G.; Issing, P.R.; Karnebogen, M.; Schmidt, G.; Auber, B.; et al. MDS1 and EVI1 complex locus (MECOM): A novel candidate gene for hereditary hematological malignancies. Haematologica 2018, 103, e55–e58. [Google Scholar] [CrossRef]
- Dreveny, I.; Deeves, S.E.; Fulton, J.; Yue, B.; Messmer, M.; Bhattacharya, A.; Collins, H.M.; Heery, D.M. The double PHD finger domain of MOZ/MYST3 induces α-helical structure of the histone H3 tail to facilitate acetylation and methylation sampling and modification. Nucleic Acids Res. 2014, 42, 822–835. [Google Scholar] [CrossRef]
- Perez-Campo, F.M.; Costa, G.; Lie-a-Ling, M.; Kouskoff, V.; Lacaud, G. The MYSTerious MOZ, a histone acetyltransferase with a key role in haematopoiesis. Immunology 2013, 139, 161–165. [Google Scholar] [CrossRef]
- Borrow, J.; Stanton, V.P., Jr.; Andresen, J.M.; Becher, R.; Behm, F.G.; Chaganti, R.S.; Civin, C.I.; Disteche, C.; Dubé, I.; Frischauf, A.M.; et al. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat. Genet. 1996, 14, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Troke, P.J.; Kindle, K.B.; Collins, H.M.; Heery, D.M. MOZ fusion proteins in acute myeloid leukaemia. Biochem. Soc. Symp. 2006, 73, 23–39. [Google Scholar] [CrossRef]
- Lamble, A.J.; Gerbing, R.B.; Smith, J.L.; Ries, R.E.; Kolb, E.A.; Alonzo, T.A.; Meshinchi, S. Crebbp Alterations Are Associated with a Poor Prognosis in De Novo AML. Blood 2021, 138, 3451. [Google Scholar] [CrossRef]
- Coenen, E.A.; Zwaan, C.M.; Reinhardt, D.; Harrison, C.J.; Haas, O.A.; de Haas, V.; Mihál, V.; De Moerloose, B.; Jeison, M.; Rubnitz, J.E.; et al. Pediatric acute myeloid leukemia with t(8;16)(p11;p13), a distinct clinical and biological entity: A collaborative study by the International-Berlin-Frankfurt-Münster AML-study group. Blood 2013, 122, 2704–2713. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Leaver, D.J.; Hermans, S.J.; Kelly, G.L.; Brennan, M.S.; Downer, N.L.; Nguyen, N.; Wichmann, J.; McRae, H.M.; Yang, Y.; et al. Inhibitors of histone acetyltransferases KAT6A/B induce senescence and arrest tumour growth. Nature 2018, 560, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Rowley, J.D.; Potter, D. Chromosomal banding patterns in acute nonlymphocytic leukemia. Blood 1976, 47, 705–721. [Google Scholar] [CrossRef] [PubMed]
- Ageberg, M.; Drott, K.; Olofsson, T.; Gullberg, U.; Lindmark, A. Identification of a novel and myeloid specific role of the leukemia-associated fusion protein DEK-NUP214 leading to increased protein synthesis. Genes Chromosomes Cancer 2008, 47, 276–287. [Google Scholar] [CrossRef]
- Gupta, M.; Ashok Kumar, J.; Sitaram, U.; Neeraj, S.; Nancy, A.; Balasubramanian, P.; Abraham, A.; Mathews, V.; Viswabandya, A.; George, B.; et al. The t(6;9)(p22;q34) in myeloid neoplasms: A retrospective study of 16 cases. Cancer Genet. Cytogenet. 2010, 203, 297–302. [Google Scholar] [CrossRef]
- Visconte, V.; Shetty, S.; Przychodzen, B.; Hirsch, C.; Bodo, J.; Maciejewski, J.P.; Hsi, E.D.; Rogers, H.J. Clinicopathologic and molecular characterization of myeloid neoplasms with isolated t(6;9)(p23;q34). Int. J. Lab. Hematol. 2017, 39, 409–417. [Google Scholar] [CrossRef]
- Cuneo, A.; Kerim, S.; Vandenberghe, E.; Van Orshoven, A.; Rodhain, J.; Bosly, A.; Zachee, P.; Louwagie, A.; Michaux, J.L.; Dal Cin, P.; et al. Translocation t(6;9) occurring in acute myelofibrosis, myelodysplastic syndrome, and acute nonlymphocytic leukemia suggests multipotent stem cell involvement. Cancer Genet. Cytogenet. 1989, 42, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Soekarman, D.; von Lindern, M.; Daenen, S.; de Jong, B.; Fonatsch, C.; Heinze, B.; Bartram, C.; Hagemeijer, A.; Grosveld, G. The translocation (6;9) (p23;q34) shows consistent rearrangement of two genes and defines a myeloproliferative disorder with specific clinical features. Blood 1992, 79, 2990–2997. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Yabe, M.; Zhang, X.; Kim, Y.; Wu, X.; Wei, P.; Chi, S.; Zheng, L.; Garcia-Manero, G.; Shao, L.; et al. Myelodysplastic syndrome with t(6;9)(p22;q34.1)/DEK-NUP214 better classified as acute myeloid leukemia? A multicenter study of 107 cases. Mod. Pathol. 2021, 34, 1143–1152. [Google Scholar] [CrossRef]
- Sandahl, J.D.; Coenen, E.A.; Forestier, E.; Harbott, J.; Johansson, B.; Kerndrup, G.; Adachi, S.; Auvrignon, A.; Beverloo, H.B.; Cayuela, J.M.; et al. t(6;9)(p22;q34)/DEK-NUP214-rearranged pediatric myeloid leukemia: An international study of 62 patients. Haematologica 2014, 99, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Tarlock, K.; Alonzo, T.A.; Moraleda, P.P.; Gerbing, R.B.; Raimondi, S.C.; Hirsch, B.A.; Ravindranath, Y.; Lange, B.; Woods, W.G.; Gamis, A.S.; et al. Acute myeloid leukaemia (AML) with t(6;9)(p23;q34) is associated with poor outcome in childhood AML regardless of FLT3-ITD status: A report from the Children’s Oncology Group. Br. J. Haematol. 2014, 166, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Meshinchi, S.; Woods, W.G.; Stirewalt, D.L.; Sweetser, D.A.; Buckley, J.D.; Tjoa, T.K.; Bernstein, I.D.; Radich, J.P. Prevalence and prognostic significance of Flt3 internal tandem duplication in pediatric acute myeloid leukemia. Blood 2001, 97, 89–94. [Google Scholar] [CrossRef]
- Thiede, C.; Steudel, C.; Mohr, B.; Schaich, M.; Schäkel, U.; Platzbecker, U.; Wermke, M.; Bornhäuser, M.; Ritter, M.; Neubauer, A.; et al. Analysis of FLT3-activating mutations in 979 patients with acute myelogenous leukemia: Association with FAB subtypes and identification of subgroups with poor prognosis. Blood 2002, 99, 4326–4335. [Google Scholar] [CrossRef]
- Bolouri, H.; Farrar, J.E.; Triche, T., Jr.; Ries, R.E.; Lim, E.L.; Alonzo, T.A.; Ma, Y.; Moore, R.; Mungall, A.J.; Marra, M.A.; et al. The molecular landscape of pediatric acute myeloid leukemia reveals recurrent structural alterations and age-specific mutational interactions. Nat. Med. 2018, 24, 103–112. [Google Scholar] [CrossRef]
- Shih, L.Y.; Kuo, M.C.; Liang, D.C.; Huang, C.F.; Lin, T.L.; Wu, J.H.; Wang, P.N.; Dunn, P.; Lai, C.L. Internal tandem duplication and Asp835 mutations of the FMS-like tyrosine kinase 3 (FLT3) gene in acute promyelocytic leukemia. Cancer 2003, 98, 1206–1216. [Google Scholar] [CrossRef]
- Au, W.Y.; Fung, A.; Chim, C.S.; Lie, A.K.; Liang, R.; Ma, E.S.; Chan, C.H.; Wong, K.F.; Kwong, Y.L. FLT-3 aberrations in acute promyelocytic leukaemia: Clinicopathological associations and prognostic impact. Br. J. Haematol. 2004, 125, 463–469. [Google Scholar] [CrossRef]
- Gale, R.E.; Hills, R.; Pizzey, A.R.; Kottaridis, P.D.; Swirsky, D.; Gilkes, A.F.; Nugent, E.; Mills, K.I.; Wheatley, K.; Solomon, E.; et al. Relationship between FLT3 mutation status, biologic characteristics, and response to targeted therapy in acute promyelocytic leukemia. Blood 2005, 106, 3768–3776. [Google Scholar] [CrossRef]
- Kutny, M.A.; Moser, B.K.; Laumann, K.; Feusner, J.H.; Gamis, A.; Gregory, J.; Larson, R.A.; Powell, B.L.; Stock, W.; Willman, C.L.; et al. FLT3 mutation status is a predictor of early death in pediatric acute promyelocytic leukemia: A report from the Children’s Oncology Group. Pediatr. Blood Cancer 2012, 59, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kiyoi, H.; Nakano, Y.; Suzuki, R.; Kodera, Y.; Miyawaki, S.; Asou, N.; Kuriyama, K.; Yagasaki, F.; Shimazaki, C.; et al. Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood 2001, 97, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Mizuki, M.; Fenski, R.; Halfter, H.; Matsumura, I.; Schmidt, R.; Müller, C.; Grüning, W.; Kratz-Albers, K.; Serve, S.; Steur, C.; et al. Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood 2000, 96, 3907–3914. [Google Scholar] [CrossRef]
- Meshinchi, S.; Alonzo, T.A.; Stirewalt, D.L.; Zwaan, M.; Zimmerman, M.; Reinhardt, D.; Kaspers, G.J.; Heerema, N.A.; Gerbing, R.; Lange, B.J.; et al. Clinical implications of FLT3 mutations in pediatric AML. Blood 2006, 108, 3654–3661. [Google Scholar] [CrossRef] [PubMed]
- Pollard, J.A.; Alonzo, T.A.; Gerbing, R.; Brown, P.; Fox, E.; Choi, J.; Fisher, B.; Hirsch, B.; Kahwash, S.; Getz, K.; et al. Sorafenib in Combination With Standard Chemotherapy for Children With High Allelic Ratio FLT3/ITD+ Acute Myeloid Leukemia: A Report From the Children’s Oncology Group Protocol AAML1031. J. Clin. Oncol. 2022, 40, 2023–2035. [Google Scholar] [CrossRef]
- Tarlock, K.; Chang, B.; Cooper, T.; Gross, T.; Gupta, S.; Neudorf, S.; Adlard, K.; Ho, P.A.; McGoldrick, S.; Watt, T.; et al. Sorafenib treatment following hematopoietic stem cell transplant in pediatric FLT3/ITD acute myeloid leukemia. Pediatr. Blood Cancer 2015, 62, 1048–1054. [Google Scholar] [CrossRef]
- Stone, R.M.; Mandrekar, S.J.; Sanford, B.L.; Laumann, K.; Geyer, S.; Bloomfield, C.D.; Thiede, C.; Prior, T.W.; Döhner, K.; Marcucci, G.; et al. Midostaurin plus Chemotherapy for Acute Myeloid Leukemia with a FLT3 Mutation. N. Engl. J. Med. 2017, 377, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Perl, A.E.; Martinelli, G.; Cortes, J.E.; Neubauer, A.; Berman, E.; Paolini, S.; Montesinos, P.; Baer, M.R.; Larson, R.A.; Ustun, C.; et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N. Engl. J. Med. 2019, 381, 1728–1740. [Google Scholar] [CrossRef]
- Voso, M.T.; Larson, R.A.; Jones, D.; Marcucci, G.; Prior, T.; Krauter, J.; Heuser, M.; Lavorgna, S.; Nomdedeu, J.; Geyer, S.M.; et al. Midostaurin in patients with acute myeloid leukemia and FLT3-TKD mutations: A subanalysis from the RATIFY trial. Blood Adv. 2020, 4, 4945–4954. [Google Scholar] [CrossRef]
- Gruber, T.A.; Downing, J.R. The biology of pediatric acute megakaryoblastic leukemia. Blood 2015, 126, 943–949. [Google Scholar] [CrossRef]
- Inaba, H.; Zhou, Y.; Abla, O.; Adachi, S.; Auvrignon, A.; Beverloo, H.B.; de Bont, E.; Chang, T.-T.; Creutzig, U.; Dworzak, M.; et al. Heterogeneous cytogenetic subgroups and outcomes in childhood acute megakaryoblastic leukemia: A retrospective international study. Blood 2015, 126, 1575–1584. [Google Scholar] [CrossRef]
- de Rooij, J.D.E.; Masetti, R.; van den Heuvel-Eibrink, M.M.; Cayuela, J.-M.; Trka, J.; Reinhardt, D.; Rasche, M.; Sonneveld, E.; Alonzo, T.A.; Fornerod, M.; et al. Recurrent abnormalities can be used for risk group stratification in pediatric AMKL: A retrospective intergroup study. Blood 2016, 127, 3424–3430. [Google Scholar] [CrossRef]
- Hara, Y.; Shiba, N.; Ohki, K.; Tabuchi, K.; Yamato, G.; Park, M.-j.; Tomizawa, D.; Kinoshita, A.; Shimada, A.; Arakawa, H.; et al. Prognostic impact of specific molecular profiles in pediatric acute megakaryoblastic leukemia in non-Down syndrome. Genes Chromosomes Cancer 2017, 56, 394–404. [Google Scholar] [CrossRef]
- Langenberg-Ververgaert, K.; Renzi, S.; Fuligni, F.; Davidson, S.; Abdelhaleem, M.; Lo, W.; Malkin, D.; Shlien, A.; Shago, M.; Villani, A.; et al. TERT promotor variant associated with poor clinical outcome in a patient with novel RBM15-MKL1 fusion-positive pediatric acute megakaryoblastic leukemia. Pediatr. Blood Cancer 2021, 68, e28542. [Google Scholar] [CrossRef] [PubMed]
- Messiaen, J.; Uyttebroeck, A.; Michaux, L.; Vandenberghe, P.; Boeckx, N.; Jacobs, S.A. t(1;7;22)(p13;q21;q13) is a novel 3-way variant of t(1;22)(p13;q13) neonatal acute megakaryoblastic leukemia: A case report. Mol. Clin. Oncol. 2023, 18, 18. [Google Scholar] [CrossRef]
- Torres, L.; Lisboa, S.; Vieira, J.; Cerveira, N.; Santos, J.; Pinheiro, M.; Correia, C.; Bizarro, S.; Almeida, M.; Teixeira, M.R. Acute megakaryoblastic leukemia with a four-way variant translocation originating the RBM15-MKL1 fusion gene. Pediatr. Blood Cancer 2011, 56, 846–849. [Google Scholar] [CrossRef]
- de Rooij, J.D.; Branstetter, C.; Ma, J.; Li, Y.; Walsh, M.P.; Cheng, J.; Obulkasim, A.; Dang, J.; Easton, J.; Verboon, L.J.; et al. Pediatric non-Down syndrome acute megakaryoblastic leukemia is characterized by distinct genomic subsets with varying outcomes. Nat. Genet. 2017, 49, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.; Dastugue, N.; Haas, O.A.; Harbott, J.; Heerema, N.A.; Huret, J.L.; Landman-Parker, J.; LeBeau, M.M.; Leonard, C.; Mann, G.; et al. Nineteen cases of the t(1;22)(p13;q13) acute megakaryblastic leukaemia of infants/children and a review of 39 cases: Report from a t(1;22) study group. Leukemia 2000, 14, 216–218. [Google Scholar] [CrossRef]
- Schweitzer, J.; Zimmermann, M.; Rasche, M.; von Neuhoff, C.; Creutzig, U.; Dworzak, M.; Reinhardt, D.; Klusmann, J.H. Improved outcome of pediatric patients with acute megakaryoblastic leukemia in the AML-BFM 04 trial. Ann. Hematol. 2015, 94, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Gruber, T.A.; Larson Gedman, A.; Zhang, J.; Koss, C.S.; Marada, S.; Ta, H.Q.; Chen, S.C.; Su, X.; Ogden, S.K.; Dang, J.; et al. An Inv(16)(p13.3q24.3)-encoded CBFA2T3-GLIS2 fusion protein defines an aggressive subtype of pediatric acute megakaryoblastic leukemia. Cancer Cell 2012, 22, 683–697. [Google Scholar] [CrossRef]
- Masetti, R.; Pigazzi, M.; Togni, M.; Astolfi, A.; Indio, V.; Manara, E.; Casadio, R.; Pession, A.; Basso, G.; Locatelli, F. CBFA2T3-GLIS2 fusion transcript is a novel common feature in pediatric, cytogenetically normal AML, not restricted to FAB M7 subtype. Blood 2013, 121, 3469–3472. [Google Scholar] [CrossRef]
- Masetti, R.; Bertuccio, S.N.; Pession, A.; Locatelli, F. CBFA2T3-GLIS2-positive acute myeloid leukaemia. A peculiar paediatric entity. Br. J. Haematol. 2019, 184, 337–347. [Google Scholar] [CrossRef]
- Eidenschink Brodersen, L.; Alonzo, T.A.; Menssen, A.J.; Gerbing, R.B.; Pardo, L.; Voigt, A.P.; Kahwash, S.B.; Hirsch, B.; Raimondi, S.; Gamis, A.S.; et al. A recurrent immunophenotype at diagnosis independently identifies high-risk pediatric acute myeloid leukemia: A report from Children’s Oncology Group. Leukemia 2016, 30, 2077–2080. [Google Scholar] [CrossRef]
- Smith, J.L.; Ries, R.E.; Hylkema, T.; Alonzo, T.A.; Gerbing, R.B.; Santaguida, M.T.; Eidenschink Brodersen, L.; Pardo, L.; Cummings, C.L.; Loeb, K.R.; et al. Comprehensive Transcriptome Profiling of Cryptic CBFA2T3-GLIS2 Fusion-Positive AML Defines Novel Therapeutic Options: A COG and TARGET Pediatric AML Study. Clin. Cancer Res. 2020, 26, 726–737. [Google Scholar] [CrossRef]
- Noort, S.; Zimmermann, M.; Reinhardt, D.; Cuccuini, W.; Pigazzi, M.; Smith, J.; Ries, R.E.; Alonzo, T.A.; Hirsch, B.; Tomizawa, D.; et al. Prognostic impact of t(16;21)(p11;q22) and t(16;21)(q24;q22) in pediatric AML: A retrospective study by the I-BFM Study Group. Blood 2018, 132, 1584–1592. [Google Scholar] [CrossRef] [PubMed]
- Shiba, N.; Yoshida, K.; Hara, Y.; Yamato, G.; Shiraishi, Y.; Matsuo, H.; Okuno, Y.; Chiba, K.; Tanaka, H.; Kaburagi, T.; et al. Transcriptome analysis offers a comprehensive illustration of the genetic background of pediatric acute myeloid leukemia. Blood Adv. 2019, 3, 3157–3169. [Google Scholar] [CrossRef]
- Park, I.J.; Park, J.E.; Kim, H.J.; Jung, H.J.; Lee, W.G.; Cho, S.R. Acute myeloid leukemia with t(16;21)(q24;q22) and eosinophilia: Case report and review of the literature. Cancer Genet. Cytogenet. 2010, 196, 105–108. [Google Scholar] [CrossRef]
- Kawashima, N.; Shimada, A.; Taketani, T.; Hayashi, Y.; Yoshida, N.; Matsumoto, K.; Takahashi, Y.; Kojima, S.; Kato, K. Childhood acute myeloid leukemia with bone marrow eosinophilia caused by t(16;21)(q24;q22). Int. J. Hematol. 2012, 95, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Kondoh, K.; Nakata, Y.; Furuta, T.; Hosoda, F.; Gamou, T.; Kurosawa, Y.; Kinoshita, A.; Ohki, M.; Tomita, Y.; Mori, T. A pediatric case of secondary leukemia associated with t(16;21)(q24;q22) exhibiting the chimeric AML1-MTG16 gene. Leuk. Lymphoma 2002, 43, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Jeandidier, E.; Dastugue, N.; Mugneret, F.; Lafage-Pochitaloff, M.; Mozziconacci, M.J.; Herens, C.; Michaux, L.; Verellen-Dumoulin, C.; Talmant, P.; Cornillet-Lefebvre, P.; et al. Abnormalities of the long arm of chromosome 21 in 107 patients with hematopoietic disorders: A collaborative retrospective study of the Groupe Français de Cytogénétique Hématologique. Cancer Genet. Cytogenet. 2006, 166, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.C.; Kalwinsky, D.K.; Hayashi, Y.; Behm, F.G.; Mirro, J., Jr.; Williams, D.L. Cytogenetics of childhood acute nonlymphocytic leukemia. Cancer Genet. Cytogenet. 1989, 40, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Salomon-Nguyen, F.; Busson-Le Coniat, M.; Lafage Pochitaloff, M.; Mozziconacci, J.; Berger, R.; Bernard, O.A. AML1-MTG16 fusion gene in therapy-related acute leukemia with t(16;21)(q24;q22): Two new cases. Leukemia 2000, 14, 1704–1705. [Google Scholar] [CrossRef]
- Zatkova, A.; Fonatsch, C.; Sperr, W.R.; Valent, P. A patient with de novo AML M1 and t(16;21) with karyotype evolution. Leuk. Res. 2007, 31, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.M.; Begg, C.B. Eastern Cooperative Oncology Group study of the cytochemistry of adult acute myeloid leukemia by correlation of subtypes with response and survival. Cancer Res. 1981, 41, 4833–4837. [Google Scholar] [PubMed]
- Barnard, D.R.; Alonzo, T.A.; Gerbing, R.B.; Lange, B.; Woods, W.G. Comparison of childhood myelodysplastic syndrome, AML FAB M6 or M7, CCG 2891: Report from the Children’s Oncology Group. Pediatr Blood Cancer 2007, 49, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, K.M.; Heerema-McKenney, A.E.; Choi, J.K.; Smith, J.; Ries, R.E.; Hirsch, B.A.; Raimondi, S.C.; Alonzo, T.A.; Wang, Y.C.; Aplenc, R.; et al. Acute erythroid leukemia is enriched in NUP98 fusions: A report from the Children’s Oncology Group. Blood Adv. 2020, 4, 6000–6008. [Google Scholar] [CrossRef] [PubMed]
- Reichard, K.K.; Tefferi, A.; Abdelmagid, M.; Orazi, A.; Alexandres, C.; Haack, J.; Greipp, P.T. Pure (acute) erythroid leukemia: Morphology, immunophenotype, cytogenetics, mutations, treatment details, and survival data among 41 Mayo Clinic cases. Blood Cancer J. 2022, 12, 147. [Google Scholar] [CrossRef]
- Iacobucci, I.; Wen, J.; Meggendorfer, M.; Choi, J.K.; Shi, L.; Pounds, S.B.; Carmichael, C.L.; Masih, K.E.; Morris, S.M.; Lindsley, R.C.; et al. Genomic subtyping and therapeutic targeting of acute erythroleukemia. Nat. Genet. 2019, 51, 694–704. [Google Scholar] [CrossRef]
- Ping, N.; Sun, A.; Song, Y.; Wang, Q.; Yin, J.; Cheng, W.; Xu, Y.; Wen, L.; Yao, H.; Ma, L.; et al. Exome sequencing identifies highly recurrent somatic GATA2 and CEBPA mutations in acute erythroid leukemia. Leukemia 2017, 31, 195–202. [Google Scholar] [CrossRef]
- Saft, L.; Kvasnicka, H.M.; Boudova, L.; Gianelli, U.; Lazzi, S.; Rozman, M. Myeloid/lymphoid neoplasms with eosinophilia and tyrosine kinase fusion genes: A workshop report with focus on novel entities and a literature review including paediatric cases. Histopathology 2023, 83, 829–849. [Google Scholar] [CrossRef]
- Clark, A.; Thomas, S.; Hamblin, A.; Talley, P.; Kulasekararaj, A.; Grinfeld, J.; Speight, B.; Snape, K.; McVeigh, T.P.; Snowden, J.A. Management of patients with germline predisposition to haematological malignancies considered for allogeneic blood and marrow transplantation: Best practice consensus guidelines from the UK Cancer Genetics Group (UKCGG), CanGene-CanVar, NHS England Genomic Laboratory Hub (GLH) Haematological Malignancies Working Group and the British Society of Blood and Marrow Transplantation and cellular therapy (BSBMTCT). Br. J. Haematol. 2023, 201, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Gilad, O.; Dgany, O.; Noy-Lotan, S.; Krasnov, T.; Yacobovich, J.; Rabinowicz, R.; Goldberg, T.; Kuperman, A.A.; Abu-Quider, A.; Miskin, H.; et al. Syndromes predisposing to leukemia are a major cause of inherited cytopenias in children. Haematologica 2022, 107, 2081–2095. [Google Scholar] [CrossRef]
- Mendoza, H.; Podoltsev, N.A.; Siddon, A.J. Laboratory evaluation and prognostication among adults and children with CEBPA-mutant acute myeloid leukemia. Int. J. Lab. Hematol. 2021, 43 (Suppl. S1), 86–95. [Google Scholar] [CrossRef]
- Owen, C.; Barnett, M.; Fitzgibbon, J. Familial myelodysplasia and acute myeloid leukaemia—A review. Br. J. Haematol. 2008, 140, 123–132. [Google Scholar] [CrossRef]
- Tawana, K.; Rio-Machin, A.; Preudhomme, C.; Fitzgibbon, J. Familial CEBPA-mutated acute myeloid leukemia. Semin. Hematol. 2017, 54, 87–93. [Google Scholar] [CrossRef]
- Cheah, J.J.C.; Hahn, C.N.; Hiwase, D.K.; Scott, H.S.; Brown, A.L. Myeloid neoplasms with germline DDX41 mutation. Int. J. Hematol. 2017, 106, 163–174. [Google Scholar] [CrossRef]
- Tawana, K.; Wang, J.; Renneville, A.; Bödör, C.; Hills, R.; Loveday, C.; Savic, A.; Van Delft, F.W.; Treleaven, J.; Georgiades, P.; et al. Disease evolution and outcomes in familial AML with germline CEBPA mutations. Blood 2015, 126, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Hahn, C.N.; Scott, H.S. Secondary leukemia in patients with germline transcription factor mutations (RUNX1, GATA2, CEBPA). Blood 2020, 136, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.S.; Ferrer, A.; Mangaonkar, A.A.; Khan, S.P.; Kohorst, M.A.; Joshi, A.Y.; Hogan, W.J.; Olteanu, H.; Moyer, A.M.; Al-Kali, A.; et al. Spectrum of hematological malignancies, clonal evolution and outcomes in 144 Mayo Clinic patients with germline predisposition syndromes. Am. J. Hematol. 2021, 96, 1450–1460. [Google Scholar] [CrossRef] [PubMed]
- Song, W.J.; Sullivan, M.G.; Legare, R.D.; Hutchings, S.; Tan, X.; Kufrin, D.; Ratajczak, J.; Resende, I.C.; Haworth, C.; Hock, R.; et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat. Genet. 1999, 23, 166–175. [Google Scholar] [CrossRef]
- Brown, A.L.; Arts, P.; Carmichael, C.L.; Babic, M.; Dobbins, J.; Chong, C.E.; Schreiber, A.W.; Feng, J.; Phillips, K.; Wang, P.P.S.; et al. RUNX1-mutated families show phenotype heterogeneity and a somatic mutation profile unique to germline predisposed AML. Blood Adv. 2020, 4, 1131–1144. [Google Scholar] [CrossRef]
- Noris, P.; Perrotta, S.; Seri, M.; Pecci, A.; Gnan, C.; Loffredo, G.; Pujol-Moix, N.; Zecca, M.; Scognamiglio, F.; De Rocco, D.; et al. Mutations in ANKRD26 are responsible for a frequent form of inherited thrombocytopenia: Analysis of 78 patients from 21 families. Blood 2011, 117, 6673–6680. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Churpek, J.E.; Keel, S.B.; Walsh, T.; Lee, M.K.; Loeb, K.R.; Gulsuner, S.; Pritchard, C.C.; Sanchez-Bonilla, M.; Delrow, J.J.; et al. Germline ETV6 mutations in familial thrombocytopenia and hematologic malignancy. Nat. Genet. 2015, 47, 180–185. [Google Scholar] [CrossRef]
- Hahn, C.N.; Chong, C.E.; Carmichael, C.L.; Wilkins, E.J.; Brautigan, P.J.; Li, X.C.; Babic, M.; Lin, M.; Carmagnac, A.; Lee, Y.K.; et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat. Genet. 2011, 43, 1012–1017. [Google Scholar] [CrossRef]
- Brown, P.; Inaba, H.; Annesley, C.; Beck, J.; Colace, S.; Dallas, M.; DeSantes, K.; Kelly, K.; Kitko, C.; Lacayo, N.; et al. Pediatric Acute Lymphoblastic Leukemia, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 81–112. [Google Scholar] [CrossRef]
- Akkari, Y.M.N.; Bruyere, H.; Hagelstrom, R.T.; Kanagal-Shamanna, R.; Liu, J.; Luo, M.; Mikhail, F.M.; Pitel, B.A.; Raca, G.; Shago, M.; et al. Evidence-based review of genomic aberrations in B-lymphoblastic leukemia/lymphoma: Report from the cancer genomics consortium working group for lymphoblastic leukemia. Cancer Genet. 2020, 243, 52–72. [Google Scholar] [CrossRef] [PubMed]
- Alaggio, R.; Amador, C.; Anagnostopoulos, I.; Attygalle, A.D.; Araujo, I.B.O.; Berti, E.; Bhagat, G.; Borges, A.M.; Boyer, D.; Calaminici, M.; et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia 2022, 36, 1720–1748. [Google Scholar] [CrossRef]
- Rheingold, S.R.; Ji, L.; Gore, L.; Xu, X.; Bhojwani, D.; Shah, N.N.; Raetz, E.A.; Loh, M.L.; O’Brien, M.M. Impact of Treatment with Inotuzumab Ozogamicin before or after Chimeric Antigen Receptor T-Cell Therapy in Children with Relapsed/Refractory Acute Lymphoblastic Leukemia. Blood 2023, 142, 2876. [Google Scholar] [CrossRef]
- Hogan, L.E.; Brown, P.A.; Ji, L.; Xu, X.; Devidas, M.; Bhatla, T.; Borowitz, M.J.; Raetz, E.A.; Carroll, A.; Heerema, N.A.; et al. Children’s Oncology Group AALL1331: Phase III Trial of Blinatumomab in Children, Adolescents, and Young Adults With Low-Risk B-Cell ALL in First Relapse. J. Clin. Oncol. 2023, 41, 4118–4129. [Google Scholar] [CrossRef] [PubMed]
- Winestone, L.E.; Bhojwani, D.; Ghorashian, S.; Muffly, L.; Leahy, A.B.; Chao, K.; Steineck, A.; Rössig, C.; Lamble, A.; Maude, S.L.; et al. INSPIRED Symposium Part 4A: Access to CAR T Cell Therapy in Unique Populations with B Cell Acute Lymphoblastic Leukemia. Transplant. Cell Ther. 2024, 30, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Lamble, A.J.; Moskop, A.; Pulsipher, M.A.; Maude, S.L.; Summers, C.; Annesley, C.; Baruchel, A.; Gore, L.; Amrolia, P.; Shah, N. INSPIRED Symposium Part 2: Prevention and Management of Relapse Following Chimeric Antigen Receptor T Cell Therapy for B Cell Acute Lymphoblastic Leukemia. Transplant. Cell Ther. 2023, 29, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Brady, S.W.; Roberts, K.G.; Gu, Z.; Shi, L.; Pounds, S.; Pei, D.; Cheng, C.; Dai, Y.; Devidas, M.; Qu, C.; et al. The genomic landscape of pediatric acute lymphoblastic leukemia. Nat. Genet. 2022, 54, 1376–1389. [Google Scholar] [CrossRef] [PubMed]
- Safavi, S.; Paulsson, K. Near-haploid and low-hypodiploid acute lymphoblastic leukemia: Two distinct subtypes with consistently poor prognosis. Blood 2017, 129, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Holmfeldt, L.; Wei, L.; Diaz-Flores, E.; Walsh, M.; Zhang, J.; Ding, L.; Payne-Turner, D.; Churchman, M.; Andersson, A.; Chen, S.C.; et al. The genomic landscape of hypodiploid acute lymphoblastic leukemia. Nat. Genet. 2013, 45, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Harrison, C.J.; Moorman, A.V.; Broadfield, Z.J.; Cheung, K.L.; Harris, R.L.; Reza Jalali, G.; Robinson, H.M.; Barber, K.E.; Richards, S.M.; Mitchell, C.D.; et al. Three distinct subgroups of hypodiploidy in acute lymphoblastic leukaemia. Br. J. Haematol. 2004, 125, 552–559. [Google Scholar] [CrossRef]
- Nachman, J.B.; Heerema, N.A.; Sather, H.; Camitta, B.; Forestier, E.; Harrison, C.J.; Dastugue, N.; Schrappe, M.; Pui, C.H.; Basso, G.; et al. Outcome of treatment in children with hypodiploid acute lymphoblastic leukemia. Blood 2007, 110, 1112–1115. [Google Scholar] [CrossRef]
- Arpas, T.; Jelinkova, H.; Hrabovsky, S.; Orsulova, M.; Vrzalova, Z.; Navrkalova, V.; Brhelova, E.; Bryjova, L.; Bulikova, A.; Ondrouskova, E.; et al. Very rare near-haploid acute lymphoblastic leukemia resistant to immunotherapy and CAR-T therapy in 19-year-old male patient. Clin. Case Rep. 2022, 10, e05545. [Google Scholar] [CrossRef]
- Mühlbacher, V.; Zenger, M.; Schnittger, S.; Weissmann, S.; Kunze, F.; Kohlmann, A.; Bellos, F.; Kern, W.; Haferlach, T.; Haferlach, C. Acute lymphoblastic leukemia with low hypodiploid/near triploid karyotype is a specific clinical entity and exhibits a very high TP53 mutation frequency of 93%. Genes Chromosomes Cancer 2014, 53, 524–536. [Google Scholar] [CrossRef]
- Safavi, S.; Forestier, E.; Golovleva, I.; Barbany, G.; Nord, K.H.; Moorman, A.V.; Harrison, C.J.; Johansson, B.; Paulsson, K. Loss of chromosomes is the primary event in near-haploid and low-hypodiploid acute lymphoblastic leukemia. Leukemia 2013, 27, 248–250. [Google Scholar] [CrossRef][Green Version]
- Qu, C.; Song, Y.; Yin, J.; Ma, Y.; Kang, L.; Li, Z.; Dai, H.; Zhu, X.; Yu, L.; Wu, D.; et al. Decitabine may improve CAR-T efficacy in refractory/relapsed acute leukemia patients carrying TP53 alterations. Bone Marrow Transplant. 2021, 56, 1710–1713. [Google Scholar] [CrossRef]
- Cui, Y.; Zhou, M.; Zou, P.; Liao, X.; Xiao, J. Mature B cell acute lymphoblastic leukaemia with KMT2A-MLLT3 transcripts in children: Three case reports and literature reviews. Orphanet J. Rare Dis. 2021, 16, 331. [Google Scholar] [CrossRef] [PubMed]
- Worch, J.; Rohde, M.; Burkhardt, B. Mature B-cell lymphoma and leukemia in children and adolescents-review of standard chemotherapy regimen and perspectives. Pediatr. Hematol. Oncol. 2013, 30, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Sajaroff, E.O.; Mansini, A.; Rubio, P.; Alonso, C.N.; Gallego, M.S.; Coccé, M.C.; Eandi-Eberle, S.; Bernasconi, A.R.; Ampatzidou, M.; Paterakis, G.; et al. B-cell acute lymphoblastic leukemia with mature phenotype and MLL rearrangement: Report of five new cases and review of the literature. Leuk. Lymphoma 2016, 57, 2289–2297. [Google Scholar] [CrossRef] [PubMed]
- Kenkel, T.J.; Sridhar, N.; Hammons, L.R.; Hintzke, M.; Shah, N.N. Bone Marrow Aplasia after CAR-T-Cell Therapy for Relapsed/Refractory Burkitt’s Lymphoma. Med. Sci. 2023, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Jeha, S.; Pei, D.; Raimondi, S.C.; Onciu, M.; Campana, D.; Cheng, C.; Sandlund, J.T.; Ribeiro, R.C.; Rubnitz, J.E.; Howard, S.C.; et al. Increased risk for CNS relapse in pre-B cell leukemia with the t(1;19)/TCF3-PBX1. Leukemia 2009, 23, 1406–1409. [Google Scholar] [CrossRef] [PubMed]
- Zenz, T.; Mertens, D.; Küppers, R.; Döhner, H.; Stilgenbauer, S. From pathogenesis to treatment of chronic lymphocytic leukaemia. Nat. Rev. Cancer 2010, 10, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Demir, H.A.; Bayhan, T.; Üner, A.; Kurtulan, O.; Karakuş, E.; Emir, S.; Özyörük, D.; Ceylaner, S. Chronic lymphocytic leukemia in a child: A challenging diagnosis in pediatric oncology practice. Pediatr. Blood Cancer 2014, 61, 933–935. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Fischer, K.; Fingerle-Rowson, G.; Fink, A.M.; Busch, R.; Mayer, J.; Hensel, M.; Hopfinger, G.; Hess, G.; von Grünhagen, U.; et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: A randomised, open-label, phase 3 trial. Lancet 2010, 376, 1164–1174. [Google Scholar] [CrossRef]
- Siddiqi, T.; Maloney, D.G.; Kenderian, S.S.; Brander, D.M.; Dorritie, K.; Soumerai, J.; Riedell, P.A.; Shah, N.N.; Nath, R.; Fakhri, B.; et al. Lisocabtagene Maraleucel (liso-cel) in R/R CLL/SLL: 24-Month Median Follow-up of TRANSCEND CLL 004. Blood 2023, 142, 330. [Google Scholar] [CrossRef]
- Siddiqi, T.; Maloney, D.G.; Kenderian, S.S.; Brander, D.M.; Dorritie, K.; Soumerai, J.; Riedell, P.A.; Shah, N.N.; Nath, R.; Fakhri, B.; et al. Lisocabtagene maraleucel in chronic lymphocytic leukaemia and small lymphocytic lymphoma (TRANSCEND CLL 004): A multicentre, open-label, single-arm, phase 1-2 study. Lancet 2023, 402, 641–654. [Google Scholar] [CrossRef]
- Fries, C.; Evans, A.G.; Cheon, H.; Korones, D.N.; Loughran, T.P., Jr.; Andolina, J.R. Allogeneic Bone Marrow Transplant as a Cure for Refractory T-Cell Large Granular Lymphocytic Leukemia in an Adolescent. J. Pediatr. Hematol. Oncol. 2022, 44, e960–e963. [Google Scholar] [CrossRef]
- Mittal, N.; Loughran, T., Jr.; Rivers, A. T-cell large granular lymphocytic leukemia successfully treated with oral cyclosporine in a child: Case report and review of literature. Pediatr. Blood Cancer 2020, 67, e28487. [Google Scholar] [CrossRef]
- Kondoh, K.; Morimoto, M.; Keino, D.; Oyama, R.; Nagae, C.; Ashikaga, T.; Arai, K.; Nakazawa, A.; Kinoshita, A. T-cell large granular lymphocyte leukemia in a child with anemia and Crohn’s disease. Pediatr. Int. 2013, 55, 111–114. [Google Scholar] [CrossRef]
- Blanchong, C.A.; Olshefski, R.; Kahwash, S. Large granular lymphocyte leukemia: Case report of chronic neutropenia and rheumatoid arthritis-like symptoms in a child. Pediatr. Dev. Pathol. 2001, 4, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, B.J.; Boxer, L.A. Large granular lymphocyte leukemia (LGL) in a child with hyper IgM syndrome and autoimmune hemolytic anemia. Pediatr. Blood Cancer 2008, 50, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Loughran, T.P., Jr. Large granular lymphocytic leukemia: Molecular pathogenesis, clinical manifestations, and treatment. Hematol. Am. Soc. Hematol. Educ. Program 2012, 2012, 652–659. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, Y.J.; Kim, Y.; Kim, H.K.; Lim, J.H.; Jo, J.C. T-large granular lymphocytic leukemia. Blood Res. 2023, 58, S52–S57. [Google Scholar] [CrossRef] [PubMed]
- Alfano, G.; Fontana, F.; Colaci, E.; Mori, G.; Cerami, C.; Messerotti, A.; Potenza, L.; Luppi, M.; Cappelli, G. T-cell large granular lymphocyte leukemia in solid organ transplant recipients: Case series and review of the literature. Int. J. Hematol. 2019, 110, 313–321. [Google Scholar] [CrossRef]
- Rahul, E.; Ningombam, A.; Acharya, S.; Tanwar, P.; Ranjan, A.; Chopra, A. Large granular lymphocytic leukemia: A brief review. Am. J. Blood Res. 2022, 12, 17–32. [Google Scholar] [PubMed]
- Cheon, H.; Dziewulska, K.H.; Moosic, K.B.; Olson, K.C.; Gru, A.A.; Feith, D.J.; Loughran, T.P., Jr. Advances in the Diagnosis and Treatment of Large Granular Lymphocytic Leukemia. Curr. Hematol. Malig. Rep. 2020, 15, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Lamy, T.; Loughran, T.P., Jr. How I treat LGL leukemia. Blood 2011, 117, 2764–2774. [Google Scholar] [CrossRef] [PubMed]
- Loughran, T.P., Jr.; Zickl, L.; Olson, T.L.; Wang, V.; Zhang, D.; Rajala, H.L.; Hasanali, Z.; Bennett, J.M.; Lazarus, H.M.; Litzow, M.R.; et al. Immunosuppressive therapy of LGL leukemia: Prospective multicenter phase II study by the Eastern Cooperative Oncology Group (E5998). Leukemia 2015, 29, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Magnano, L.; Rivero, A.; Matutes, E. Large Granular Lymphocytic Leukemia: Current State of Diagnosis, Pathogenesis and Treatment. Curr. Oncol. Rep. 2022, 24, 633–644. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).