Assessing the New 2020 ESGO/ESTRO/ESP Endometrial Cancer Risk Molecular Categorization System for Predicting Survival and Recurrence
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Molecular Classification
2.3. Assessment of the 2020 ESGO/ESTRO/ESP Classification
2.4. Statistical Analyses
3. Results
3.1. Characteristics of the Population
3.2. Molecular Classification
3.3. Adjuvant Therapy of the Patients
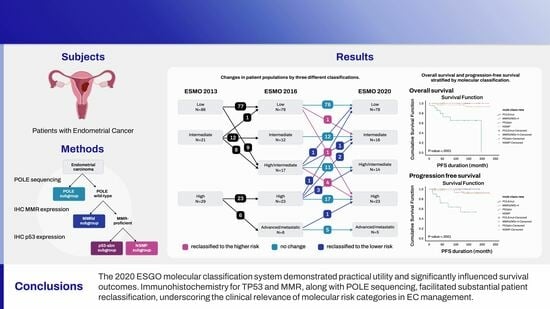
3.4. Shift in Risk Groups between ESMO 2013 and ESMO 2016 Risk Classification
3.5. Shift in Risk Groups between 2016 ESMO and 2020 ESGO
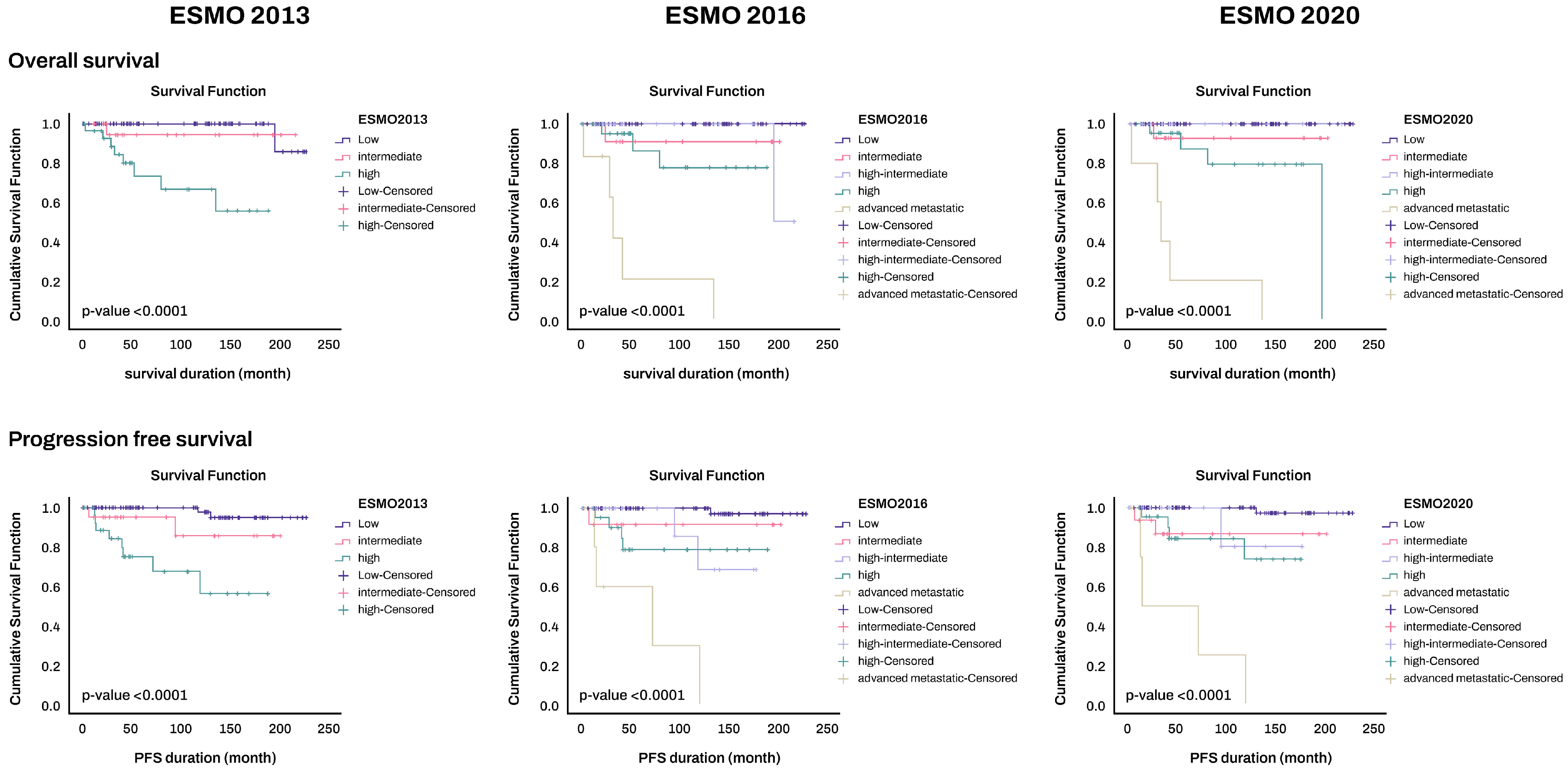
3.6. Prognosis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, B.; Shang, X.; Yan, M.; Li, X.; Wang, W.; Wang, Q.; Zhang, C. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990–2019. Gynecol. Oncol. 2021, 161, 573–580. [Google Scholar] [CrossRef]
- Ha, H.I.; Chang, H.K.; Park, S.J.; Lim, J.; Won, Y.J.; Lim, M.C. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999–2017: Korea Central Cancer Registry. Obstet. Gynecol. Sci. 2021, 64, 444–453. [Google Scholar] [CrossRef]
- Ministry of Health and Welfare, C.C.R. 2021 National Cancer Registry Statistics. Available online: https://www.ncc.re.kr/main.ncc?uri=manage01_2 (accessed on 21 February 2024).
- Korea Central Cancer Registry, National Cancer Center. Annual Report of Cancer Statistics in Korea in 2020. Available online: https://www.ncc.re.kr/cancerStatsView.ncc?bbsnum=638&searchKey=total&searchValue=&pageNum=1 (accessed on 30 November 2023).
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network; Kandoth, C.; Schultz, N.; Cherniack, A.D.; Akbani, R.; Liu, Y.; Shen, H.; Robertson, A.G.; Pashtan, I.; Shen, R.; et al. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef]
- Raffone, A.; Travaglino, A.; Gabrielli, O.; Micheli, M.; Zuccala, V.; Bitonti, G.; Camastra, C.; Gargiulo, V.; Insabato, L.; Zullo, F. Clinical features of ProMisE groups identify different phenotypes of patients with endometrial cancer. Arch. Gynecol. Obstet. 2021, 303, 1393–1400. [Google Scholar] [CrossRef]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Kommoss, F.; Grevenkamp, F.; Karnezis, A.; et al. Final validation of the ProMisE molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of ProMisE: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Radiother. Oncol. 2021, 154, 327–353. [Google Scholar] [CrossRef]
- Hong, J.H.; Cho, H.W.; Ouh, Y.T.; Lee, J.K.; Chun, Y. Lymphocyte activation gene (LAG)-3 is a potential immunotherapeutic target for microsatellite stable, programmed death-ligand 1 (PD-L1)-positive endometrioid endometrial cancer. J. Gynecol. Oncol. 2023, 34, e18. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Int. J. Gynecol. Cancer. 2016, 26, 2–30. [Google Scholar] [CrossRef]
- Colombo, N.; Preti, E.; Landoni, F.; Carinelli, S.; Colombo, A.; Marini, C.; Sessa, C.; ESMO Guidelines Working Group. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24 (Suppl. S6), vi33–vi38. [Google Scholar] [CrossRef]
- Clarke, B.A.; Gilks, C.B. Endometrial carcinoma: Controversies in histopathological assessment of grade and tumour cell type. J. Clin. Pathol. 2010, 63, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial cancer. Nat. Rev. Dis. Primers 2021, 7, 88. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Bendifallah, S.; Ouldamer, L.; Lavoue, V.; Canlorbe, G.; Raimond, E.; Coutant, C.; Graesslin, O.; Touboul, C.; Collinet, P.; Daraï, E.; et al. Patterns of recurrence and outcomes in surgically treated women with endometrial cancer according to ESMO-ESGO-ESTRO Consensus Conference risk groups: Results from the FRANCOGYN study Group. Gynecol. Oncol. 2017, 144, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef]
- Imboden, S.; Nastic, D.; Ghaderi, M.; Rydberg, F.; Siegenthaler, F.; Mueller, M.D.; Rau, T.T.; Epstein, E.; Carlson, J.W. Implementation of the 2021 molecular ESGO/ESTRO/ESP risk groups in endometrial cancer. Gynecol Oncol. 2021, 162, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Leon-Castillo, A.; Britton, H.; McConechy, M.K.; McAlpine, J.N.; Nout, R.; Kommoss, S.; Brucker, S.Y.; Carlson, J.W.; Epstein, E.; Rau, T.T.; et al. Interpretation of somatic POLE mutations in endometrial carcinoma. J. Pathol. 2020, 250, 323–335. [Google Scholar] [CrossRef]
- He, Y.; Wang, T.; Li, N.; Yang, B.; Hu, Y. Clinicopathological characteristics and prognostic value of POLE mutations in endometrial cancer: A systematic review and meta-analysis. Medicine 2020, 99, e19281. [Google Scholar] [CrossRef]
- McAlpine, J.N.; Chiu, D.S.; Nout, R.A.; Church, D.N.; Schmidt, P.; Lam, S.; Leung, S.; Bellone, S.; Wong, A.; Brucker, S.Y.; et al. Evaluation of treatment effects in patients with endometrial cancer and POLE mutations: An individual patient data meta-analysis. Cancer 2021, 127, 2409–2422. [Google Scholar] [CrossRef]
- McEachron, J.; Zhou, N.; Spencer, C.; Chatterton, C.; Shanahan, L.; Katz, J.; Naegele, S.; Singhal, P.K.; Lee, Y.C. Adjuvant chemoradiation associated with improved outcomes in patients with microsatellite instability-high advanced endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 203–208. [Google Scholar] [CrossRef]
- Bosse, T.; Nout, R.A.; McAlpine, J.N.; McConechy, M.K.; Britton, H.; Hussein, Y.R.; Gonzalez, C.; Ganesan, R.; Steele, J.C.; Harrison, B.T.; et al. Molecular Classification of Grade 3 Endometrioid Endometrial Cancers Identifies Distinct Prognostic Subgroups. Am. J. Surg. Pathol. 2018, 42, 561–568. [Google Scholar] [CrossRef] [PubMed]
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit from Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, S.F.; Bao, W. Molecular subtypes of endometrial cancer: Implications for adjuvant treatment strategies. Int. J. Gynaecol. Obstet. 2024, 164, 436–459. [Google Scholar] [CrossRef] [PubMed]
- Marnitz, S.; Schomig-Markiefka, B. The PORTEC-3 trial for high-risk endometrial cancer: Impact of molecular classification on prognosis and benefit from adjuvant therapy. Strahlenther. Onkol. 2021, 197, 266–268. [Google Scholar] [CrossRef] [PubMed]
- Jumaah, A.S.; Salim, M.M.; Al-Haddad, H.S.; McAllister, K.A.; Yasseen, A.A. The frequency of POLE-mutation in endometrial carcinoma and prognostic implications: A systemic review and meta-analysis. J. Pathol. Transl. Med. 2020, 54, 471–479. [Google Scholar] [CrossRef]
- Horeweg, N.; Nout, R.A.; Jürgenliemk-Schulz, I.M.; Lutgens, L.; Jobsen, J.J.; Haverkort, M.A.D.; Mens, J.W.M.; Slot, A.; Wortman, B.G.; de Boer, S.M.; et al. Molecular Classification Predicts Response to Radiotherapy in the Randomized PORTEC-1 and PORTEC-2 Trials for Early-Stage Endometrioid Endometrial Cancer. J. Clin. Oncol. 2023, 41, 4369–4380. [Google Scholar] [CrossRef]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N.; Endometrial Cancer Staging Subcommittee; et al. FIGO staging of endometrial cancer: 2023. J. Gynecol. Oncol. 2023, 34, e85. [Google Scholar] [CrossRef]


| Variables | N (%) | Molecular Classification of the Tumors | |||
|---|---|---|---|---|---|
| POLEmut | MMRd/MSI-H | P53abn | NSMP | ||
| Number (%) | 136 (100%) | 9 (6.6%) | 25 (18.4%) | 22 (16.2%) | 80 (58.8%) |
| Age (years) | |||||
| <40 | 2 (1.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (2.5%) |
| 40–59 | 63 (46.3%) | 6 (66.7%) | 17 (68.0%) | 6 (27.3%) | 34 (42.5%) |
| >60 | 71 (52.2%) | 3 (33.3%) | 8 (32.0%) | 16 (72.7%) | 44 (55.0%) |
| Parity | |||||
| 0 | 24 (17.6%) | 2 (22.2%) | 2 (8.0%) | 3 (13.0%) | 17 (21.3%) |
| 1 | 25 (18.4%) | 3 (33.3%) | 6 (24.0%) | 5 (22.7%) | 11 (3.8%) |
| 2 | 67 (49.3%) | 4 (44.4%) | 14 (56.0%) | 9 (40.9%) | 40 (50.0%) |
| >3 | 20 (14.7%) | 0 (0.0%) | 3 (12.0%) | 5 (22.7%) | 12 (15.0%) |
| Histology | |||||
| Endometrioid | 122 (89.7%) | 9 (100.0%) | 25 (100.0%) | 8 (36.4%) | 80 (100.0%) |
| Non-Endometrioid | 14 (10.3%) | 0 (0.0%) | 0 (0.0%) | 14 (63.6%) | 0 (0.0%) |
| FIGO stage | |||||
| IA | 91 (66.9%) | 6 (66.7%) | 17 (68.0%) | 7 (31.8%) | 61 (76.3%) |
| IB | 23 (16.9%) | 1 (11.1%) | 4 (16.0%) | 5 (22.7%) | 13 (16.3%) |
| II | 5 (3.7%) | 1 (11.1%) | 2 (8.0%) | 2 (9.1%) | 0 (0.0%) |
| III | 13 (9.6%) | 1 (11.1%) | 2 (8.0%) | 6 (27.3%) | 4 (5.0%) |
| IVA | 1 (0.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.3%) |
| IVB | 3 (2.2%) | 0 (0.0%) | 0 (0.0%) | 2 (9.1%) | 1 (1.3%) |
| FIGO 2023 stage | |||||
| IA1 | 33 (24.3%) | 0 (0.0%) | 7 (28.0%) | 0 (0.0%) | 26 (32.5%) |
| IA2 | 38 (27.9%) | 0 (0.0%) | 8 (32.0%) | 0 (0.0%) | 30 (37.5%) |
| IA3 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| IAmPOLEmut | 8 (5.9%) | 8 (88.9%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| IB | 12 (8.8%) | 0 (0.0%) | 2 (8.0%) | 0 (0.0%) | 10 (12.5%) |
| IC | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| IIA | 2 (2.2%) | 0 (0.0%) | 2 (8.0%) | 0 (0.0%) | 0 (0.0%) |
| IIB | 12 (8.8%) | 0 (0.0%) | 4 (16.0%) | 0 (0.0%) | 8 (10.0%) |
| IIC | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| IICmp53abn | 14 (10.3%) | 0 (0.0%) | 0 (0.0%) | 14 (63.6%) | 0 (0.0%) |
| III | 8 (5.9%) | 0 (0.0%) | 0 (0.0%) | 6 (27.3%) | 2 (2.5%) |
| IIIC | 5 (3.7%) | 1 (11.1%) | 2 (8.0%) | 0 (0.0%) | 2 (2.5%) |
| IVA | 1 (0.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (1.3%) |
| IVB | 3 (2.2%) | 0 (0.0%) | 0 (0.0%) | 2 (9.1%) | 1 (1.3%) |
| Grade | |||||
| Low | 113 (83.1%) | 8 (88.9%) | 22 (88.0%) | 5 (22.7%) | 78(97.5%) |
| High | 23 (16.9%) | 1 (11.1%) | 3 (12.0%) | 17 (77.3%) | 2 (2.5%) |
| Tumor size | 3.91 ± 2.61 | 4.01 ± 2.59 | 3.39 ± 1.95 | 4.68 ± 3.22 | 3.85 ± 2.61 |
| Depth of MI | |||||
| None | 43 (31.6%) | 5 (55.6%) | 7 (28.0%) | 3 (13.6%) | 28 (35.0%) |
| <1/2 | 57 (41.9%) | 4 (44.4%) | 11 (44.0%) | 7 (31.8%) | 35 (43.8%) |
| >1/2 | 36 (26.5%) | 0 (0.0%) | 7 (28.0%) | 12 (54.5%) | 17 (21.3%) |
| LVSI | |||||
| Absent | 103 (75.7%) | 8 (88.9%) | 17 (68.0%) | 10 (45.5%) | 68 (85.0%) |
| Present | 33 (24.3%) | 1 (11.11%) | 8 (32.0%) | 12 (54.5%) | 12 (15.0%) |
| Lymph node | |||||
| Unknown | 22 (16.2%) | 3 (33.3%) | 1 (4.0%) | 6 (27.3%) | 12 (15.0%) |
| None | 107 (78.7%) | 5 (55.6%) | 22 (88.0%) | 15 (68.2%) | 65 (81.3%) |
| Yes | 7 (5.1%) | 1 (11.1%) | 2 (8.0%) | 1 (4.5%) | 3 (3.8%) |
| Residual tumor | 5 (3.7%) | 0 (0.0%) | 0 (0.0%) | 3 (13.6%) | 2 (2.5%) |
| Adjuvant therapy | |||||
| None | 69 (50.7%) | 5 (55.6%) | 14 (56.0%) | 4 (18.2%) | 46 (57.5%) |
| Brachytherapy | 32 (23.5%) | 3 (33.3%) | 6 (24.0%) | 2 (9.1%) | 21 (26.3%) |
| EBRT | 10 (7.4%) | 0 (0.0%) | 2 (8.0%) | 3 (13.6%) | 5 (6.3%) |
| CCRT | 11 (8.1%) | 1 (11.1%) | 1 (4.0%) | 6 (27.3%) | 3 (3.8%) |
| Others | 14 (10.3%) | 0 (0.0%) | 2 (8.0%) | 7 (31.8%) | 5 (6.3%) |
| Recurrence | |||||
| Yes | 12 (8.8%) | 0 (0.0%) | 1 (4.0%) | 7 (31.8%) | 4 (5.0%) |
| None | 124 (91.2%) | 9 (6.6%) | 24 (96.0%) | 15 (68.2%) | 76 (95.0%) |
| Survival | |||||
| Alive | 121 (89.0%) | 9 (100.0%) | 24 (96.0%) | 15 (68.2%) | 73 (91.3%) |
| Death | 10 (7.4%) | 0 (0.0%) | 0 (0.0%) | 7 (31.8%) | 3 (3.8%) |
| Unknown | 5 (3.7%) | 0 (0.0%) | 1 (4.0%) | 0 (1.1%) | 4 (5.0%) |
| Variables | Total, N (%) | None, N (%) | Brachytherapy, N (%) | EBRT, N (%) | CCRT, N (%) | Others, N (%) |
|---|---|---|---|---|---|---|
| Number (%) | 136 (100.0%) | 69 (50.7%) | 32 (23.5%) | 10 (7.4%) | 11 (8.1%) | 14 (10.3%) |
| ESMO 2016 | ||||||
| Low | 78 (57.4%) | 54 (78.3%) | 22 (68.8%) | 2 (20.0%) | 0 (0.0%) | 0 (0.0%) |
| Intermediate | 12 (8.8%) | 3 (4.3%) | 4 (12.5%) | 4 (40.0%) | 1 (9.1%) | 0 (0.0%) |
| High-intermediate | 17 (12.5%) | 10 (14.5%) | 4 (12.5%) | 2 (20.0%) | 0 (0.0%) | 1 (7.1%) |
| High | 23 (16.9%) | 2 (2.9%) | 1 (3.1%) | 2 (20.0%) | 8 (72.7%) | 10 (71.4%) |
| Advanced/metastatic | 6 (4.4%) | 0 (0.0%) | 1 (3.1%) | 0 (0.0%) | 2 (18.2%) | 3 (21.4%) |
| ESGO 2020 | ||||||
| Low | 78 (57.4%) | 52 (75.4%) | 24 (75.0%) | 2 (20.0%) | 0 (0.0%) | 0 (0.0%) |
| Intermediate | 16 (11.8%) | 5 (7.2%) | 4 (12.5%) | 4 (40.0%) | 1 (9.1%) | 2 (14.3%) |
| High-intermediate | 14 (10.3%) | 9 (13.0%) | 2 (6.3%) | 1 (10.0%) | 0 (0.0%) | 2 (14.3%) |
| High | 23 (16.9%) | 3 (4.3%) | 1 (3.1%) | 3 (30.0%) | 8 (72.7%) | 8 (57.1%) |
| Advanced/metastatic | 5 (3.7%) | 0 (0.0%) | 1 (3.1%) | 0 (0.0%) | 2 (18.2%) | 2 (14.3%) |
| ESMO 2016 | ||||||
|---|---|---|---|---|---|---|
| ESGO 2020 | Low | Intermediate | High-Intermediate | High | Advanced Metastatic | Total |
| Low | 76 (97.4%) | 0 (0.0%) | 1 (1.3%) | 1 (1.3%) | 0 (0.0%) | 78 (57.4%) |
| Intermediate | 1 (6.3%) | 12 (75.0%) | 1 (6.3%) | 2 (12.5%) | 0 (0.0%) | 16 (11.8%) |
| High-intermediate | 0 (0.0%) | 0 (0.0%) | 11 (78.6%) | 3 (21.4%) | 0 (0.0%) | 14 (10.3%) |
| High | 1 (4.3%) | 0 (0.0%) | 4 (17.4%) | 17 (73.9%) | 1 (4.3%) | 23 (16.9%) |
| Advanced metastatic | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 5 (100.0%) | 5 (3.7%) |
| Total | 78 (57.4%) | 12 (8.8%) | 17 (12.5%) | 23 (16.9%) | 6 (4.4%) | 136 (100.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouh, Y.-T.; Oh, Y.; Joo, J.; Woo, J.H.; Han, H.J.; Cho, H.W.; Lee, J.K.; Chun, Y.; Lim, M.-n.; Hong, J.H. Assessing the New 2020 ESGO/ESTRO/ESP Endometrial Cancer Risk Molecular Categorization System for Predicting Survival and Recurrence. Cancers 2024, 16, 965. https://doi.org/10.3390/cancers16050965
Ouh Y-T, Oh Y, Joo J, Woo JH, Han HJ, Cho HW, Lee JK, Chun Y, Lim M-n, Hong JH. Assessing the New 2020 ESGO/ESTRO/ESP Endometrial Cancer Risk Molecular Categorization System for Predicting Survival and Recurrence. Cancers. 2024; 16(5):965. https://doi.org/10.3390/cancers16050965
Chicago/Turabian StyleOuh, Yung-Taek, Yoonji Oh, Jinwon Joo, Joo Hyun Woo, Hye Jin Han, Hyun Woong Cho, Jae Kwan Lee, Yikyeong Chun, Myoung-nam Lim, and Jin Hwa Hong. 2024. "Assessing the New 2020 ESGO/ESTRO/ESP Endometrial Cancer Risk Molecular Categorization System for Predicting Survival and Recurrence" Cancers 16, no. 5: 965. https://doi.org/10.3390/cancers16050965
APA StyleOuh, Y.-T., Oh, Y., Joo, J., Woo, J. H., Han, H. J., Cho, H. W., Lee, J. K., Chun, Y., Lim, M.-n., & Hong, J. H. (2024). Assessing the New 2020 ESGO/ESTRO/ESP Endometrial Cancer Risk Molecular Categorization System for Predicting Survival and Recurrence. Cancers, 16(5), 965. https://doi.org/10.3390/cancers16050965