Tracking Cancer: Exploring Heart Rate Variability Patterns by Cancer Location and Progression
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Recruitment and Study Sample
2.3. ANS Function via Heart Rate Variability
2.4. Heart Rate Variability Metrics
2.5. Statistical Analysis
3. Results
3.1. HRV Patterns for All Cancers
3.2. HRV Patterns between Cancer and Non-Cancer Patients
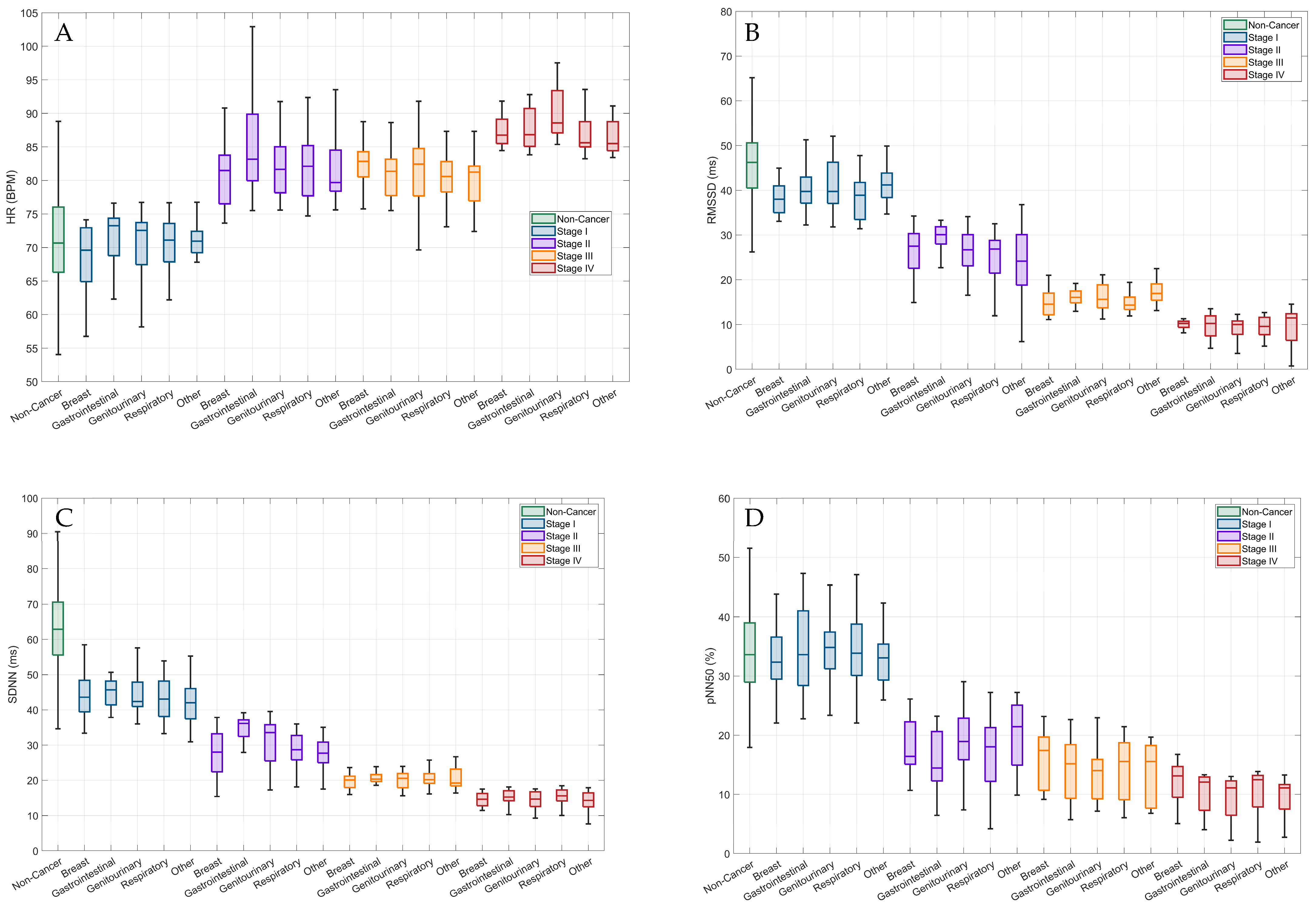
3.3. HRV Patterns by Cancer Location and Stages
4. Discussion
4.1. Practical Application
4.2. Strengths & Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early Detection of Cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Incidence and Relative Survival by Stage at Diagnosis for Common Cancers; Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, GA, USA, 2021.
- Hui, D. Prognostication of Survival in Patients With Advanced Cancer: Predicting the Unpredictable? Cancer Control J. Moffitt Cancer Cent. 2015, 22, 489–497. [Google Scholar] [CrossRef]
- Barzi, A.; Lenz, H.-J.; Quinn, D.I.; Sadeghi, S. Comparative Effectiveness of Screening Strategies for Colorectal Cancer. Cancer 2017, 123, 1516–1527. [Google Scholar] [CrossRef]
- Loud, J.T.; Murphy, J. Cancer Screening and Early Detection in the 21(St) Century. Semin. Oncol. Nurs. 2017, 33, 121–128. [Google Scholar] [CrossRef]
- Philipson, T.J.; Durie, T.; Cong, Z.; Fendrick, A.M. The Aggregate Value of Cancer Screenings in the United States: Full Potential Value and Value Considering Adherence. BMC Health Serv. Res. 2023, 23, 829. [Google Scholar] [CrossRef]
- Alexandraki, I.; Mooradian, A.D. Barriers Related to Mammography Use for Breast Cancer Screening among Minority Women. J. Natl. Med. Assoc. 2010, 102, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Bade, B.C.; Akgün, K.M.; Rose, M.G.; Cain, H.C. Barriers and Facilitators to Lung Cancer Screening and Follow-Up. VA Syst. Lung Cancer 2022, 49, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, M.; Arnold, L.D.; James, A.S. Patients’ Self-Reported Barriers to Colon Cancer Screening in Federally Qualified Health Center Settings. Prev. Med. Rep. 2019, 15, 100896. [Google Scholar] [CrossRef]
- Nagelhout, E.; Comarell, K.; Samadder, N.J.; Wu, Y.P. Barriers to Colorectal Cancer Screening in a Racially Diverse Population Served by a Safety-Net Clinic. J. Community Health 2017, 42, 791–796. [Google Scholar] [CrossRef]
- Escutia-Reyes, D.; de Jesús Garduño-García, J.; Emilio-López-Chávez, G.; Gómez-Villanueva, Á.; Pliego-Carrillo, A.C.; Soto-Piña, A.E.; Reyes-Lagos, J.J. Differences in Heart Rate Variability and Body Composition in Breast Cancer Survivors and Women without Cancer. Sci. Rep. 2021, 11, 14460. [Google Scholar] [CrossRef]
- Guo, Y.; Palmer, J.L.; Strasser, F.; Yusuf, S.W.; Bruera, E. Heart Rate Variability as a Measure of Autonomic Dysfunction in Men with Advanced Cancer. Eur. J. Cancer Care 2013, 22, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Kloter, E.; Barrueto, K.; Klein, S.D.; Scholkmann, F.; Wolf, U. Heart Rate Variability as a Prognostic Factor for Cancer Survival—A Systematic Review. Front. Physiol. 2018, 9, 623. [Google Scholar] [CrossRef] [PubMed]
- Task Force of the European Society of Cardiology the North American Society of Pacing Electrophysiology. Heart Rate Variability. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Hu, S.; Lou, J.; Zhang, Y.; Chen, P. Low Heart Rate Variability Relates to the Progression of Gastric Cancer. World J. Surg. Oncol. 2018, 16, 49. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.; Leadbitter, S.; Miller, G.; Hounat, A.; Kamande, I.; Dolan, R.D.; Horgan, P.G.; Chang, D.K.; Jamieson, N.B.; McMillan, D.C. The Relationship between Heart Rate Variability and TNM Stage, Co-Morbidity, Systemic Inflammation and Survival in Patients with Primary Operable Colorectal Cancer. Sci. Rep. 2023, 13, 8157. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Chen, M.; Wang, J.; Shi, B.; Zhou, Y. Association of Short-Term Heart Rate Variability with Breast Tumor Stage. Front. Physiol. 2021, 12, 678428. [Google Scholar] [CrossRef] [PubMed]
- Magnon, C. Role of the Autonomic Nervous System in Tumorigenesis and Metastasis. Mol. Cell. Oncol. 2015, 2, e975643. [Google Scholar] [CrossRef] [PubMed]
- Horvathova, L.; Mravec, B. Effect of the Autonomic Nervous System on Cancer Progression Depends on the Type of Tumor: Solid Are More Affected Then Ascitic Tumors. Endocr. Regul. 2016, 50, 215–224. [Google Scholar] [CrossRef][Green Version]
- Fadul, N.; Strasser, F.; Palmer, J.L.; Yusuf, S.W.; Guo, Y.; Li, Z.; Allo, J.; Bruera, E. The Association Between Autonomic Dysfunction and Survival in Male Patients with Advanced Cancer: A Preliminary Report. J. Pain Symptom Manage. 2010, 39, 283–290. [Google Scholar] [CrossRef]
- Frye, J.N.; Sutterfield, S.L.; Caldwell, J.T.; Behnke, B.J.; Copp, S.W.; Banister, H.R.; Ade, C.J. Vascular and Autonomic Changes in Adult Cancer Patients Receiving Anticancer Chemotherapy. J. Appl. Physiol. 2018, 125, 198–204. [Google Scholar] [CrossRef]
- Peck, J.; Wishon, M.; Wittels, H.; Hasty, F.; Hendricks, S.; Lee, S.; Wittels, S. COVID-19-Induced Changes in Photoplethysmography. Mil. Med. 2023, 188, e2661–e2669. [Google Scholar] [CrossRef]
- Peck, J.; Wishon, M.J.; Wittels, H.; Lee, S.J.; Hendricks, S.; Davila, H.; Wittels, S.H. Single Limb Electrocardiogram Using Vector Mapping: Evaluation and Validation of a Novel Medical Device. J. Electrocardiol. 2021, 67, 136–141. [Google Scholar] [CrossRef]
- Temme, L.A.; Wittels, H.L.; Wishon, M.J.; St. Onge, P.; McDonald, S.M.; Hecocks, D.; Wittels, S.H. Continuous Physiological Monitoring of the Combined Exposure to Hypoxia and High Cognitive Load in Military Personnel. Biology 2023, 12, 1398. [Google Scholar] [CrossRef] [PubMed]
- Renaghan, E.; Wittels, H.L.; Feigenbaum, L.A.; Wishon, M.J.; Chong, S.; Wittels, E.D.; Hendricks, S.; Hecocks, D.; Bellamy, K.; Girardi, J.; et al. Exercise Cardiac Load and Autonomic Nervous System Recovery during In-Season Training: The Impact on Speed Deterioration in American Football Athletes. J. Funct. Morphol. Kinesiol. 2023, 8, 134. [Google Scholar] [CrossRef]
- Ajdaraga, E.; Gusev, M. Analysis of Sampling Frequency and Resolution in ECG Signals. In Proceedings of the 2017 25th Telecommunication Forum (TELFOR), Belgrade, Serbia, 21–22 November 2017; pp. 1–4. [Google Scholar]
- Elgendi, M. Fast QRS Detection with an Optimized Knowledge-Based Method: Evaluation on 11 Standard ECG Databases. PLoS ONE 2013, 8, e73557. [Google Scholar] [CrossRef]
- Rahman, S.; Karmakar, C.; Natgunanathan, I.; Yearwood, J.; Palaniswami, M. Robustness of Electrocardiogram Signal Quality Indices. J. R. Soc. Interface 2022, 19, 20220012. [Google Scholar] [CrossRef]
- Ernst, G. Hidden Signals-The History and Methods of Heart Rate Variability. Front. Public Health 2017, 5, 265. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Cronin, K.A.; Scott, S.; Firth, A.U.; Sung, H.; Henley, S.J.; Sherman, R.L.; Siegel, R.L.; Anderson, R.N.; Kohler, B.A.; Benard, V.B.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. Cancer 2022, 128, 4251–4284. [Google Scholar] [CrossRef]
- Hong, Y.; Zhang, L.; Liu, N.; Xu, X.; Liu, D.; Tu, J. The Central Nervous Mechanism of Stress-Promoting Cancer Progression. Int. J. Mol. Sci. 2022, 23, 12653. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, Q.; Lu, Z.; Wang, L.; Ding, L.; Xia, L.; Zhang, H.; Wang, M.; Chen, Y.; Li, G. Role of the Nervous System in Cancers: A Review. Cell Death Discov. 2021, 7, 76. [Google Scholar] [CrossRef]
- Kleiger, R.E.; Miller, J.P.; Bigger, J.T.J.; Moss, A.J. Decreased Heart Rate Variability and Its Association with Increased Mortality after Acute Myocardial Infarction. Am. J. Cardiol. 1987, 59, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, I.; Goldkorn, R.; Shlomo, N.; Einhorn, M.; Levitan, J.; Kuperstein, R.; Klempfner, R.; Johnson, B. Heart Rate Variability for Risk Assessment of Myocardial Ischemia in Patients Without Known Coronary Artery Disease: The HRV-DETECT (Heart Rate Variability for the Detection of Myocardial Ischemia) Study. J. Am. Heart Assoc. 2019, 8, e014540. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, S.; Gast, K.B.; de Mutsert, R.; Swenne, C.A.; Jukema, J.W.; Middeldorp, S.; Rosendaal, F.R.; Dekkers, O.M. Heart Rate Variability and First Cardiovascular Event in Populations without Known Cardiovascular Disease: Meta-Analysis and Dose–Response Meta-Regression. EP Eur. 2013, 15, 742–749. [Google Scholar] [CrossRef]
- Trivedi, G.Y.; Saboo, B.; Singh, R.B.; Maheshwari, A.; Sharma, K.; Verma, N. Can Decreased Heart Rate Variability Be a Marker of Autonomic Dysfunction, Metabolic Syndrome and Diabetes? J. Diabetol. 2019, 10, 48–56. [Google Scholar] [CrossRef]
- Bettermann, H.; Kröz, M.; Girke, M.; Heckmann, C. Heart Rate Dynamics and Cardiorespiratory Coordination in Diabetic and Breast Cancer Patients. Clin. Physiol. 2001, 21, 411–420. [Google Scholar] [CrossRef]
- Selye, H. Stress and the General Adaptation Syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef]
- Hall, I.J.; Tangka, F.K.L.; Sabatino, S.A.; Thompson, T.D.; Graubard, B.I.; Breen, N. Patterns and Trends in Cancer Screening in the United States. Prev. Chronic. Dis. 2018, 15, E97. [Google Scholar] [CrossRef]
- Kamineni, A.; Doria-Rose, V.P.; Chubak, J.; Inadomi, J.M.; Corley, D.A.; Haas, J.S.; Kobrin, S.C.; Winer, R.L.; Elston Lafata, J.; Beaber, E.F.; et al. Evaluation of Harms Reporting in U.S. Cancer Screening Guidelines. Ann. Intern. Med. 2022, 175, 1582–1590. [Google Scholar] [CrossRef]
- National Opinion Research Center at the University of Chicago Only 14% of Cancers Are Detected through a Preventive Screening Test; Chicago, IL, USA. 2022. Available online: https://cancerdetection.norc.org/geojson/PCDS%20Technical%20Report.pdf (accessed on 3 January 2024).
| Cancer Cases (n = 399) | Non-Cancer Cases (n = 399) | Test for Homogeneity ** | |
|---|---|---|---|
| Mean (SD) or % | Mean (SD) or % | ||
| Sex (% Male) | 50.4 | 55.1 | p < 0.00001 |
| Age (years) | 68.1 ± 13.2 | 58.6 ± 15.7 | p < 0.00001 |
| 18–29 | 1.6 | 4.0 | p < 0.00001 |
| 30–39 | 2.5 | 9.5 | p < 0.00001 |
| 40–49 | 6.3 | 11.3 | p < 0.00001 |
| 50–59 | 8.5 | 26.1 | p < 0.00001 |
| 60–69 | 27.1 | 20.1 | p < 0.00001 |
| 70–79 | 38.4 | 22.1 | p < 0.00001 |
| ≥80 | 15.5 | 7.0 | p < 0.00001 |
| Race/Ethnicity | |||
| Non-Hispanic White | 33.3 | 42.1 | p < 0.00001 |
| Non-Hispanic Black | 5.8 | 7.0 | p < 0.00001 |
| Hispanic | 59.4 | 49.1 | p < 0.00001 |
| Other | 0.3 | 0.5 | p < 0.00001 |
| Body Mass Index (kg/m2) | 27.5 ± 5.8 | 27.1 ± 5.3 | p < 0.0012 |
| BMI Classification (%) | |||
| Normal Weight | 29.3 | 30.3 | p < 0.00001 |
| Overweight | 38.4 | 38.9 | p < 0.00001 |
| Obese | 27.3 | 24.8 | p < 0.00001 |
| Comorbidities (%) | |||
| Cardiovascular | 74.7 | 55.4 | p < 0.00001 |
| Respiratory | 36.3 | 29.8 | p < 0.00001 |
| Endocrine | 47.6 | 41.4 | p < 0.00001 |
| Neurological | 33.3 | 31.3 | p < 0.00001 |
| Other | 57.6 | 56.6 | p < 0.00001 |
| Cancer Location (n, %) | 399 | 0 | |
| Breast | 18.1 | ----- | ----- |
| Gastrointestinal | 15.0 | ----- | ----- |
| Genitourinary | 27.1 | ----- | ----- |
| Respiratory | 25.1 | ----- | ----- |
| Other | 14.8 | ----- | ----- |
| Stage I | Stage II | Stage III | Stage IV | |
|---|---|---|---|---|
| Heart Rate (bpm) | ||||
| All | 69.9 ± 5.5 | 82.4 ± 5.9 | 81.3 ± 5.3 | 88.1 ± 3.5 |
| Breast a,d,e | 67.9 ± 6.1 | 81.3 ± 5.3 | 82.5 ± 3.8 | 87.4 ± 2.3 |
| Gastrointestinal a,d,e | 71.5 ± 4.2 | 85.1 ± 8.1 | 80.9 ± 4.9 | 87.8 ± 3.2 |
| Genitourinary a,c,d,e,f | 69.8 ± 6.5 | 82.2 ± 4.8 | 81.9 ± 5.3 | 90.0 ± 3.9 |
| Respiratory a,c,d,e | 70.1 ± 5.2 | 82.7 ± 6.5 | 80.7 ± 3.6 | 87.4 ± 3.6 |
| Other a,b,d,e | 70.9 ± 3.6 | 81.3 ±4.8 | 80.3 ± 4.1 | 86.6 ± 2.6 |
| RR Interval (ms) | ||||
| All | 863.5 ± 78.1 | 731.3 ± 48.6 | 740.0 ± 40.7 | 682. ± 26.1 |
| Breast a,d,e | 890.7 ± 90.9 | 740.3 ± 47.8 | 728.9 ± 33.5 | 686.8 ± 17.9 |
| Gastrointestinal a,d,e | 842.4 ± 52.7 | 710.8 ± 62.5 | 744.8 ± 47.4 | 683.7 ± 24.7 |
| Genitourinary a,c,d,e,f | 867.7 ± 95.4 | 732.4 ± 41.2 | 735.8 ± 48.2 | 667.5 ± 28.4 |
| Respiratory a,c,d,e | 860.8 ± 74.5 | 729.8 ± 52.2 | 744.7 ± 33.5 | 687.7 ± 27.4 |
| Other a,b,d,e | 848.5 ± 44.4 | 740.6 ± 40.8 | 748.9 ± 38.1 | 693.4 ± 20.8 |
| rMSSD (ms) | ||||
| All | 40.0 ± 5.5 | 26.4 ± 5.5 | 15.9 ± 2.9 | 9.4 ± 2.7 |
| Breast a,b,d,e,f | 39.4 ± 6.4 | 26.6 ± 5.2 | 14.9 ± 2.9 | 9.6 ± 1.8 |
| Gastrointestinal a,b,d,e,f | 40.4 ± 5.2 | 29.2 ± 3.4 | 15.9 ± 1.9 | 9.7 ± 2.7 |
| Genitourinary a,b,c,d,e,f | 40.8 ± 5.8 | 26.7 ± 4.4 | 16.1 ± 2.9 | 9.2 ± 2.5 |
| Respiratory a,b,c,d,e,f | 38.2 ± 4.9 | 25.4 ± 5.5 | 15.4 ± 3.3 | 9.4 ± 2.2 |
| Other a,c,d,e,f | 41.9 ± 5.1 | 24.4 ± 8.4 | 17.4 ± 2.7 | 9.5 ± 4.3 |
| SDNN (ms) | ||||
| All | 43.8 ± 5.8 | 30.1 ± 5.9 | 20.5 ± 2.8 | 14.7 ± 2.5 |
| Breast a,d,e,f | 44.2 ± 6.6 | 27.7 ± 6.3 | 20.4 ± 3.3 | 14.7 ± 2.1 |
| Gastrointestinal a,b,d,e,f | 44.9 ± 3.9 | 34.9 ± 3.5 | 20.6 ± 14.7 | 15.2 ± 2.2 |
| Genitourinary a,b,d,e,f | 44.3 ± 5.2 | 31.4 ± 6.7 | 20.2 ± 2.5 | 14.4 ± 2.4 |
| Respiratory a,b,d,e,f | 43.2 ± 6.2 | 28.8 ± 5.1 | 20.8 ± 2.9 | 15.1 ± 2.4 |
| Other a,b,d,e,f | 42.3 ± 7.0 | 27.8 ± 4.7 | 20.8 ± 5.1 | 13.9 ± 3.3 |
| pNN50 | ||||
| All | 33.9 ± 6.0 | 18.2 ± 5.8 | 14.5 ± 4.9 | 10.4 ± 3.6 |
| Breast a,d,e | 32.9 ± 6.1 | 18.4 ± 5.1 | 16.1 ± 4.9 | 12.0 ± 3.8 |
| Gastrointestinal a,d,e | 34.3 ± 7.5 | 15.8 ± 5.5 | 14.7 ± 5.3 | 10.2 ± 3.6 |
| Genitourinary a,d,e,f | 34.3 ± 5.4 | 19.0 ± 5.7 | 13.6 ± 4.6 | 9.8 ± 3.2 |
| Respiratory a,d,e,f | 34.5 ± 6.5 | 17.6 ± 6.3 | 14.4 ± 5.2 | 10.4 ± 3.9 |
| Other a,d,e,f | 32.8 ± 4.7 | 19.9 ± 6.2 | 13.9 ± 5.1 | 9.5 ± 3.6 |
| Stage I | Stage II | Stage III | Stage IV | |||||
|---|---|---|---|---|---|---|---|---|
| Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value | Mean Difference (95% CI) | p-Value | |
| Heart Rate (bpm) | ||||||||
| Non-Cancer vs. | ||||||||
| Breast | 3.2 (−1.9, 8.4) | 0.81 | −10.2 (−15.8, −4.7) | <0.0001 | −11.3 (−16.3, −6.3) | <0.0001 | −16.3 (−22.2, −10.4) | <0.0001 |
| Gastrointestinal | −0.3 (5.9, 5.2) | 0.99 | −13.9 (−20.2, −7.6) | <0.0001 | −9.7 (−15.1, −4.3) | <0.0001 | −16.7 (−23.2, −10.2) | <0.0001 |
| Genitourinary | 1.3 (−2.9, 5.6) | 0.99 | −11.0 (−15.8, −6.3) | <0.0001 | −10.7 (−14.9, −6.5) | <0.0001 | −18.9 (−23.8, −13.9) | <0.0001 |
| Respiratory | 1.0 (−3.4, 5.5) | 0.99 | −11.5 (−16.4, −6.5) | <0.0001 | −9.5 (−13.8, −5.3) | <0.0001 | −16.2 (−21.4, −11.1) | <0.0001 |
| Other | 0.3 (−5.4, 5.9) | 0.99 | −10.1 (−16.4, −3.8) | <0.0001 | −9.2 (−14.7, −3.6) | <0.0001 | −15.4 (−21.9, −8.9) | <0.0001 |
| RR Intervals (ms) | ||||||||
| Non-Cancer vs. | ||||||||
| Breast | −36.4 (−97.1, 24.1) | 0.85 | 113.9 (48.6, 179.2) | <0.0001 | 125.2 (67.2, 183.2) | <0.0001 | 167.5 (98.3, 236.6) | <0.0001 |
| Gastrointestinal | 11.8 (−53.5, 77.1) | 0.99 | 143.4 (69.5, 217.4) | <0.0001 | 109.4 (45.8, 173.0) | <0.0001 | 170.5 (93.7, 247.3) | <0.0001 |
| Genitourinary | −13.5 (−64.1, 37.1) | 0.99 | 121.8 (65.9, 177.6) | <0.0001 | 118.4 (69.2, 167.6) | <0.0001 | 186.7 (128.6, 244.7) | <0.0001 |
| Respiratory | −6.5 (−58.7, 45.6) | 0.99 | 124.4 (66.3, 182.4) | <0.0001 | 109.5 (58.9, 160.1) | <0.0001 | 166.4 (105.8, 227.1) | <0.0001 |
| Other | 5.7 (−61.5, 72.8) | 0.99 | 113.6 (39.6, 187.5) | <0.0001 | 105.4 (40.1, 170.6) | <0.0001 | 160.8 (83.9, 237.6) | <0.0001 |
| rMSSD (ms) | ||||||||
| Non-Cancer vs. | ||||||||
| Breast | 6.1 (1.0, 11.3) | 0.003 | 18.9 (13.9, 24.0) | <0.0001 | 30.7 (25.9, 35.4) | <0.0001 | 35.9 (30.4, 41.5) | <0.0001 |
| Gastrointestinal | 5.2 (−0.6, 10.9) | 0.15 | 16.4 (10.9, 21.8) | <0.0001 | 29.6 (24.2, 34.8) | <0.0001 | 35.8 (29.9, 41.8) | <0.0001 |
| Genitourinary | 4.8 (0.4, 9.1) | 0.01 | 18.8 (14.6, 23.0) | <0.0001 | 29.5 (25.5, 33.5) | <0.0001 | 36.4 (31.7, 41.1) | <0.0001 |
| Respiratory | 7.2 (2.7, 11.7) | <0.0001 | 20.2 (15.9, 24.5) | <0.0001 | 30.1 (25.9, 34.3) | <0.0001 | 36.2 (31.4, 40.9) | <0.0001 |
| Other | 3.7 (−2.1, 9.4) | 0.78 | 21.2 (15.6, 26.8) | <0.0001 | 28.1 (22.7, 33.5) | <0.0001 | 36.1 (30.1, 42.0) | <0.0001 |
| SDNN (ms) | ||||||||
| Non-Cancer vs. | ||||||||
| Breast | 18.8 (12.6, 25.0) | <0.0001 | 35.3 (28.6, 41.9) | <0.0001 | 42.6 (36.1, 49.1) | <0.0001 | 48.3 (41.9, 54.7) | <0.0001 |
| Gastrointestinal | 18.1 (11.2, 24.9) | <0.0001 | 28.2 (20.9, 35.5) | <0.0001 | 42.5 (35.2, 49.8) | <0.0001 | 47.8 (40.9, 54.7) | <0.0001 |
| Genitourinary | 18.8 (13.6, 23.9) | <0.0001 | 31.6 (25.9, 37.3) | <0.0001 | 42.9 (37.5, 48.3) | <0.0001 | 48.7 (43.3, 54.1) | <0.0001 |
| Respiratory | 19.9 (14.4, 25.3) | <0.0001 | 34.2 (28.4, 40.1) | <0.0001 | 42.2 (36.5, 47.9) | <0.0001 | 47.9 (42.4, 53.4) | <0.0001 |
| Other | 20.8 (13.7, 27.9) | <0.0001 | 35.2 (27.9, 42.5) | <0.0001 | 42.3 (34.9, 49.6) | <0.0001 | 49.1 (42.1, 56.2) | <0.0001 |
| pNN50 | ||||||||
| Non-Cancer vs. | ||||||||
| Breast | 0.7 (−4.2, 5.6) | 0.99 | 15.3 (9.9, 20.6) | <0.0001 | 17.5 (12.9, 22.3) | <0.0001 | 21.7 (16.1, 27.3) | <0.0001 |
| Gastrointestinal | −0.6 (−6.0, 4.9) | 0.99 | 17.9 (12.1, 23.7) | <0.0001 | 19.0 (13.9, 24.2) | <0.0001 | 23.5 (17.2, 29.7) | <0.0001 |
| Genitourinary | −0.6 (−4.7, 3.5) | 0.99 | 14.6 (10.1, 19.2) | <0.0001 | 20.1 (16.1, 24.1) | <0.0001 | 23.9 (19.1, 28.6) | <0.0001 |
| Respiratory | 0.9 (−5.2, 3.4) | 0.99 | 16.0 (11.4, 20.7) | <0.0001 | 19.2 (15.1, 23.4) | <0.0001 | 23.3 (18.3, 28.2) | <0.0001 |
| Other | 0.9 (−4.7, 6.5) | 0.99 | 13.8 (8.0, 19.6) | <0.0001 | 19.7 (14.4, 25.0) | <0.0001 | 24.1 (17.9, 30.4) | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben-David, K.; Wittels, H.L.; Wishon, M.J.; Lee, S.J.; McDonald, S.M.; Howard Wittels, S. Tracking Cancer: Exploring Heart Rate Variability Patterns by Cancer Location and Progression. Cancers 2024, 16, 962. https://doi.org/10.3390/cancers16050962
Ben-David K, Wittels HL, Wishon MJ, Lee SJ, McDonald SM, Howard Wittels S. Tracking Cancer: Exploring Heart Rate Variability Patterns by Cancer Location and Progression. Cancers. 2024; 16(5):962. https://doi.org/10.3390/cancers16050962
Chicago/Turabian StyleBen-David, Kfir, Harrison L. Wittels, Michael J. Wishon, Stephen J. Lee, Samantha M. McDonald, and S. Howard Wittels. 2024. "Tracking Cancer: Exploring Heart Rate Variability Patterns by Cancer Location and Progression" Cancers 16, no. 5: 962. https://doi.org/10.3390/cancers16050962
APA StyleBen-David, K., Wittels, H. L., Wishon, M. J., Lee, S. J., McDonald, S. M., & Howard Wittels, S. (2024). Tracking Cancer: Exploring Heart Rate Variability Patterns by Cancer Location and Progression. Cancers, 16(5), 962. https://doi.org/10.3390/cancers16050962





