Predictive Value of Magnetic Resonance Imaging in Risk Stratification and Molecular Classification of Endometrial Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
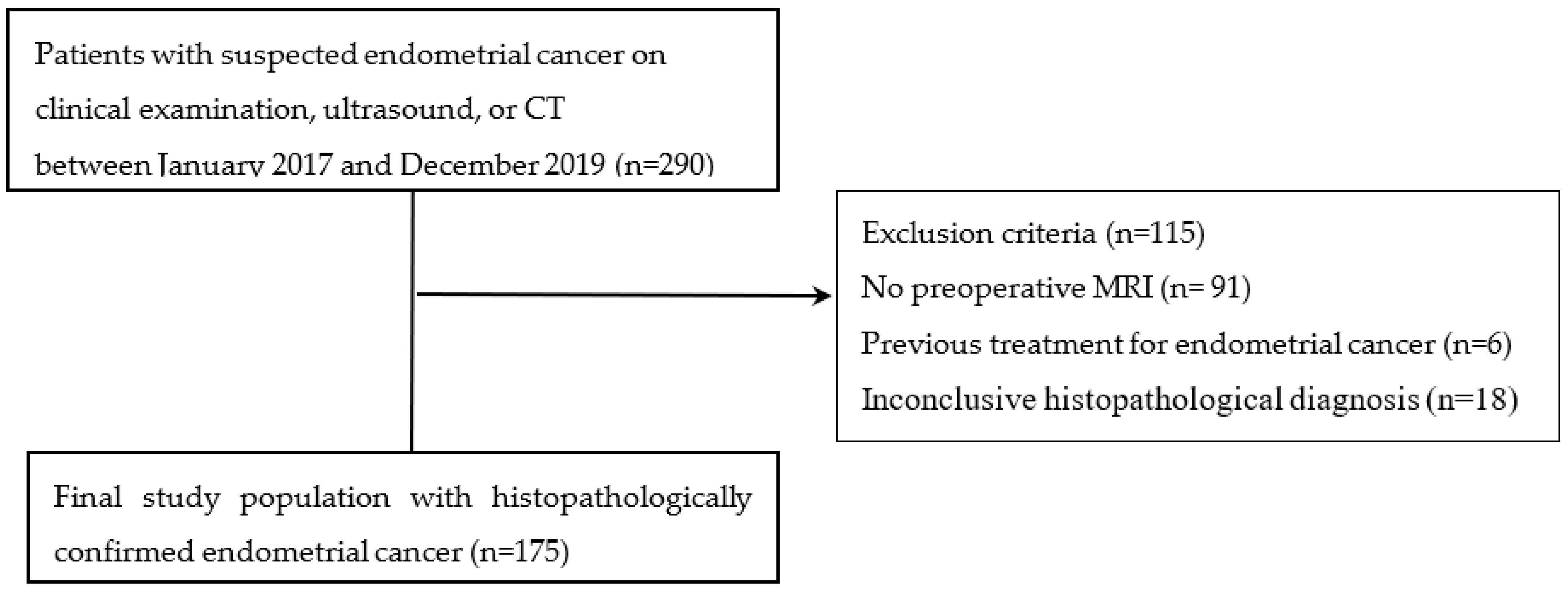
2.1. Study Population
2.2. Imaging Techniques
2.3. Image Analysis and Interpretation
2.4. Histopathological Analysis
2.5. Statistical Analysis
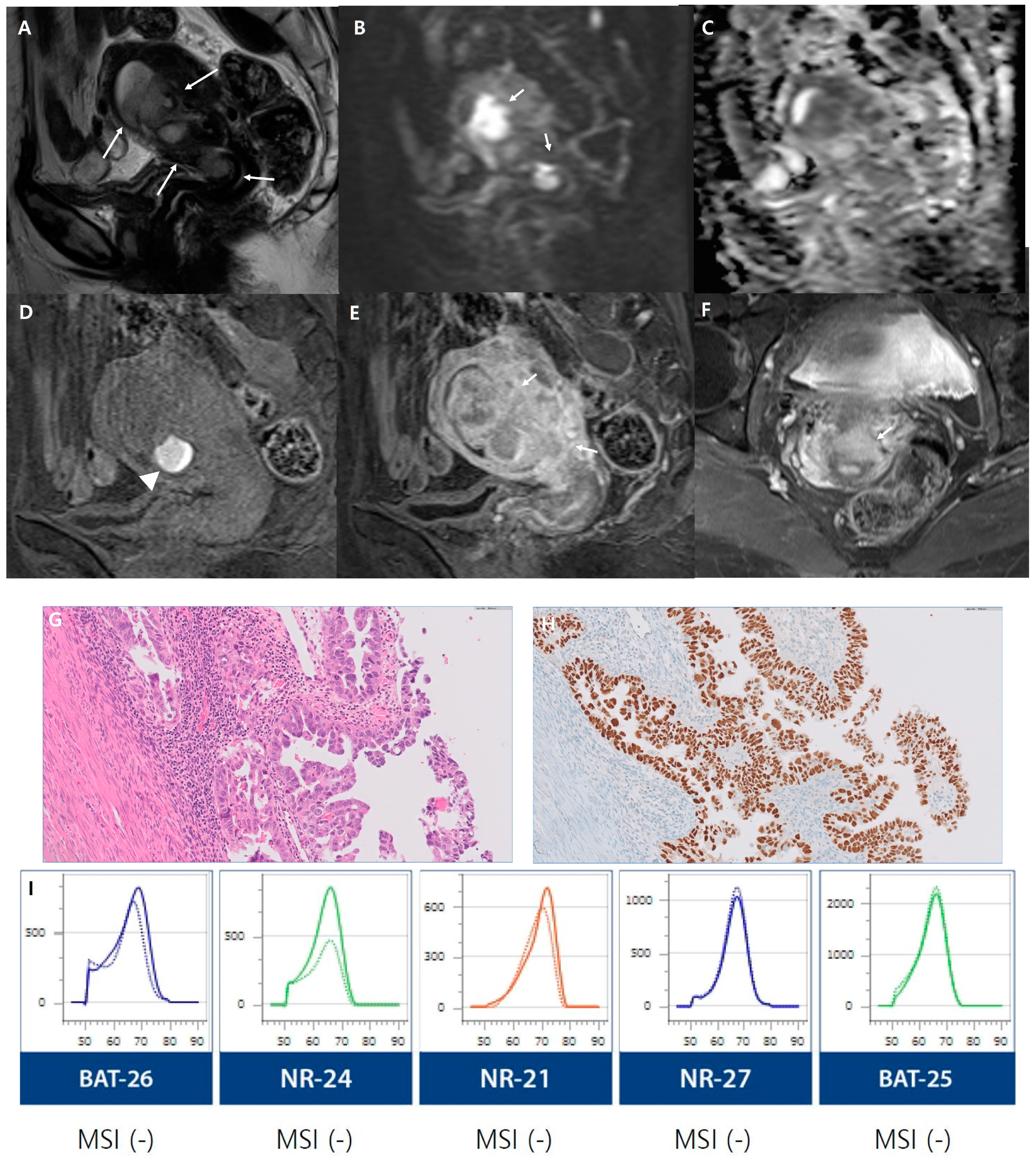
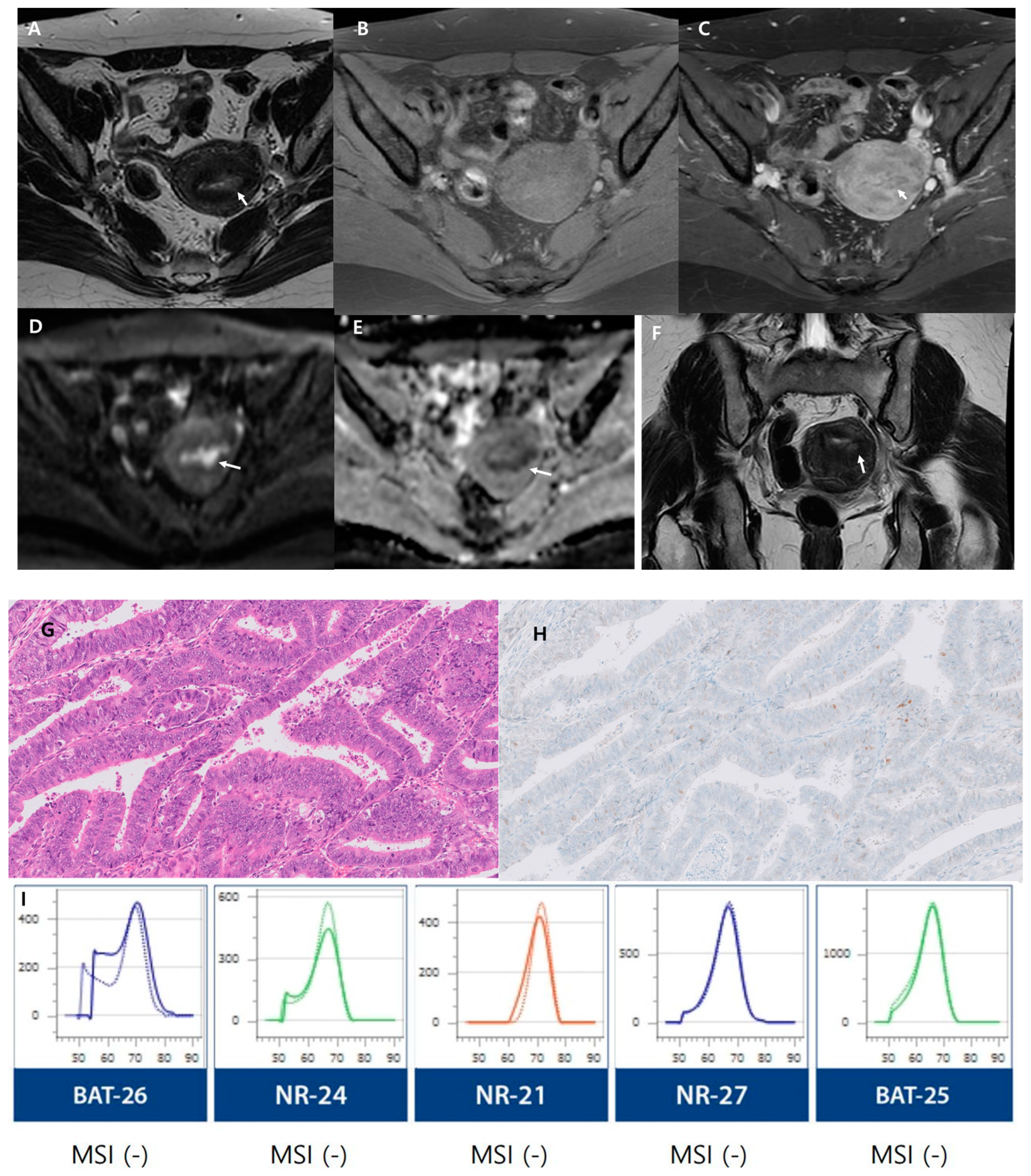
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Constantine, G.D.; Kessler, G.; Graham, S.; Goldstein, S.R. Increased Incidence of Endometrial Cancer Following the Women’s Health Initiative: An Assessment of Risk Factors. J. Womens Health 2019, 28, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Arthur, R.S.; Kabat, G.C.; Kim, M.Y.; Wild, R.A.; Shadyab, A.H.; Wactawski-Wende, J.; Ho, G.Y.F.; Reeves, K.W.; Kuller, L.H.; Luo, J.; et al. Metabolic syndrome and risk of endometrial cancer in postmenopausal women: A prospective study. Cancer Causes Control 2019, 30, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Friberg, E.; Orsini, N.; Mantzoros, C.S.; Wolk, A. Diabetes mellitus and risk of endometrial cancer: A meta-analysis. Diabetologia 2007, 50, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Soslow, R.A.; Tornos, C.; Park, K.J.; Malpica, A.; Matias-Guiu, X.; Oliva, E.; Parkash, V.; Carlson, J.; McCluggage, W.G.; Gilks, C.B. Endometrial Carcinoma Diagnosis: Use of FIGO Grading and Genomic Subcategories in Clinical Practice: Recommendations of the International Society of Gynecological Pathologists. Int. J. Gynecol. Pathol. 2019, 38 (Suppl. 1), S64–S74. [Google Scholar] [CrossRef]
- Cree, I.A.; White, V.A.; Indave, B.I.; Lokuhetty, D. Revising the WHO classification: Female genital tract tumours. Histopathology 2020, 76, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Bokhman, J.V. Two pathogenetic types of endometrial carcinoma. Gynecol. Oncol. 1983, 15, 10–17. [Google Scholar] [CrossRef]
- Oaknin, A.; Bosse, T.J.; Creutzberg, C.L.; Giornelli, G.; Harter, P.; Joly, F.; Lorusso, D.; Marth, C.; Makker, V.; Mirza, M.R.; et al. Endometrial cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2022, 33, 860–877. [Google Scholar] [CrossRef]
- Jamieson, A.; Huvila, J.; Chiu, D.; Thompson, E.F.; Scott, S.; Salvador, S.; Vicus, D.; Helpman, L.; Gotlieb, W.; Kean, S.; et al. Grade and Estrogen Receptor Expression Identify a Subset of No Specific Molecular Profile Endometrial Carcinomas at a Very Low Risk of Disease-Specific Death. Mod. Pathol. 2023, 36, 100085. [Google Scholar] [CrossRef]
- Bellone, S.; Centritto, F.; Black, J.; Schwab, C.; English, D.; Cocco, E.; Lopez, S.; Bonazzoli, E.; Predolini, F.; Ferrari, F.; et al. Polymerase ε (POLE) ultra-mutated tumors induce robust tumor-specific CD4+ T cell responses in endometrial cancer patients. Gynecol. Oncol. 2015, 138, 11–17. [Google Scholar] [CrossRef]
- Chang, Z.; Talukdar, S.; Mullany, S.A.; Winterhoff, B. Molecular characterization of endometrial cancer and therapeutic implications. Curr. Opin. Obstet. Gynecol. 2019, 31, 24–30. [Google Scholar] [CrossRef]
- Drakes, M.L.; Czerlanis, C.M.; Stiff, P.J. Immune Checkpoint Blockade in Gynecologic Cancers: State of Affairs. Cancers 2020, 12, 3301. [Google Scholar] [CrossRef] [PubMed]
- Howitt, B.E.; Shukla, S.A.; Sholl, L.M.; Ritterhouse, L.L.; Watkins, J.C.; Rodig, S.; Stover, E.; Strickland, K.C.; D’Andrea, A.D.; Wu, C.J.; et al. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015, 1, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Vermij, L.; Jobsen, J.J.; León-Castillo, A.; Brinkhuis, M.; Roothaan, S.; Powell, M.E.; de Boer, S.M.; Khaw, P.; Mileshkin, L.R.; Fyles, A.; et al. Prognostic refinement of NSMP high-risk endometrial cancers using oestrogen receptor immunohistochemistry. Br. J. Cancer 2023, 128, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Marnitz, S.; Walter, T.; Schömig-Markiefka, B.; Engler, T.; Kommoss, S.; Brucker, S.Y. A Modern Approach to Endometrial Carcinoma: Will Molecular Classification Improve Precision Medicine in the Future? Cancers 2020, 12, 2577. [Google Scholar] [CrossRef] [PubMed]
- Berek, J.S.; Matias-Guiu, X.; Creutzberg, C.; Fotopoulou, C.; Gaffney, D.; Kehoe, S.; Lindemann, K.; Mutch, D.; Concin, N. FIGO staging of endometrial cancer: 2023. J. Gynecol. Oncol. 2023, 34, e85. [Google Scholar] [CrossRef]
- Freeman, S.J.; Aly, A.M.; Kataoka, M.Y.; Addley, H.C.; Reinhold, C.; Sala, E. The revised FIGO staging system for uterine malignancies: Implications for MR imaging. Radiographics 2012, 32, 1805–1827. [Google Scholar] [CrossRef] [PubMed]
- Restaino, S.; Paglietti, C.; Arcieri, M.; Biasioli, A.; Della Martina, M.; Mariuzzi, L.; Andreetta, C.; Titone, F.; Bogani, G.; Raimondo, D.; et al. Management of Patients Diagnosed with Endometrial Cancer: Comparison of Guidelines. Cancers 2023, 15, 1091. [Google Scholar] [CrossRef]
- Tamai, K.; Koyama, T.; Saga, T.; Umeoka, S.; Mikami, Y.; Fujii, S.; Togashi, K. Diffusion-weighted MR imaging of uterine endometrial cancer. J. Magn. Reson. Imaging 2007, 26, 682–687. [Google Scholar] [CrossRef]
- Beddy, P.; Moyle, P.; Kataoka, M.; Yamamoto, A.K.; Joubert, I.; Lomas, D.; Crawford, R.; Sala, E. Evaluation of depth of myometrial invasion and overall staging in endometrial cancer: Comparison of diffusion-weighted and dynamic contrast-enhanced MR imaging. Radiology 2012, 262, 530–537. [Google Scholar] [CrossRef]
- Takeuchi, M.; Matsuzaki, K.; Nishitani, H. Diffusion-weighted magnetic resonance imaging of endometrial cancer: Differentiation from benign endometrial lesions and preoperative assessment of myometrial invasion. Acta Radiol. 2009, 50, 947–953. [Google Scholar] [CrossRef]
- Yan, B.C.; Li, Y.; Ma, F.H.; Feng, F.; Sun, M.H.; Lin, G.W.; Zhang, G.F.; Qiang, J.W. Preoperative Assessment for High-Risk Endometrial Cancer by Developing an MRI- and Clinical-Based Radiomics Nomogram: A Multicenter Study. J. Magn. Reson. Imaging 2020, 52, 1872–1882. [Google Scholar] [CrossRef]
- Jacob, H.; Dybvik, J.A.; Ytre-Hauge, S.; Fasmer, K.E.; Hoivik, E.A.; Trovik, J.; Krakstad, C.; Haldorsen, I.S. An MRI-Based Radiomic Prognostic Index Predicts Poor Outcome and Specific Genetic Alterations in Endometrial Cancer. J. Clin. Med. 2021, 10, 538. [Google Scholar] [CrossRef]
- Celli, V.; Guerreri, M.; Pernazza, A.; Cuccu, I.; Palaia, I.; Tomao, F.; Di Donato, V.; Pricolo, P.; Ercolani, G.; Ciulla, S.; et al. MRI- and Histologic-Molecular-Based Radio-Genomics Nomogram for Preoperative Assessment of Risk Classes in Endometrial Cancer. Cancers 2022, 14, 5881. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 16–41. [Google Scholar] [CrossRef]
- Park, S.B.; Moon, M.H.; Sung, C.K.; Oh, S.; Lee, Y.H. Dynamic contrast-enhanced MR imaging of endometrial cancer: Optimizing the imaging delay for tumour-myometrium contrast. Eur. Radiol. 2014, 24, 2795–2799. [Google Scholar] [CrossRef]
- Manfredi, R.; Mirk, P.; Maresca, G.; Margariti, P.A.; Testa, A.; Zannoni, G.F.; Giordano, D.; Scambia, G.; Marano, P. Local-regional staging of endometrial carcinoma: Role of MR imaging in surgical planning. Radiology 2004, 231, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Maheshwari, E.; Nougaret, S.; Stein, E.B.; Rauch, G.M.; Hwang, K.P.; Stafford, R.J.; Klopp, A.H.; Soliman, P.T.; Maturen, K.E.; Rockall, A.G.; et al. Update on MRI in Evaluation and Treatment of Endometrial Cancer. Radiographics 2022, 42, 2112–2130. [Google Scholar] [CrossRef] [PubMed]
- McMahon, C.J.; Rofsky, N.M.; Pedrosa, I. Lymphatic metastases from pelvic tumors: Anatomic classification, characterization, and staging. Radiology 2010, 254, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Kwon, Y.; Kim, H.; Kim, H.; Min, B.S.; Park, Y.; Kim, T.I.; Hong, S.P.; Kim, W.K. Microsatellite instability test using peptide nucleic acid probe-mediated melting point analysis: A comparison study. BMC Cancer 2018, 18, 1218. [Google Scholar] [CrossRef] [PubMed]
- Rafaniello-Raviele, P.; Betella, I.; Rappa, A.; Vacirca, D.; Tolva, G.; Guerrieri-Gonzaga, A.; Bertario, L.; Barberis, M.; Bonanni, B.; Marabelli, M. Microsatellite instability evaluation: Which test to use for endometrial cancer? J. Clin. Pathol. 2023, 76, 29–33. [Google Scholar] [CrossRef]
- Gilson, P.; Levy, J.; Rouyer, M.; Demange, J.; Husson, M.; Bonnet, C.; Salleron, J.; Leroux, A.; Merlin, J.L.; Harlé, A. Evaluation of 3 molecular-based assays for microsatellite instability detection in formalin-fixed tissues of patients with endometrial and colorectal cancers. Sci. Rep. 2020, 10, 16386. [Google Scholar] [CrossRef]
- Libera, L.; Sahnane, N.; Pepe, F.; Pisapia, P.; De Luca, C.; Russo, G.; Parente, P.; Covelli, C.; Chiaravalli, A.M.; Sessa, F.; et al. Critical aspects of microsatellite instability testing in endometrial cancer: A comparison study. Hum. Pathol. 2022, 128, 134–140. [Google Scholar] [CrossRef]
- Malapelle, U.; Parente, P.; Pepe, F.; De Luca, C.; Pisapia, P.; Sgariglia, R.; Nacchio, M.; Gragnano, G.; Russo, G.; Conticelli, F.; et al. Evaluation of Micro Satellite Instability and Mismatch Repair Status in Different Solid Tumors: A Multicenter Analysis in a Real World Setting. Cells 2021, 10, 1878. [Google Scholar] [CrossRef]
- Jin, X.; Shen, C.; Yang, X.; Yu, Y.; Wang, J.; Che, X. Association of Tumor Size With Myometrial Invasion, Lymphovascular Space Invasion, Lymph Node Metastasis, and Recurrence in Endometrial Cancer: A Meta-Analysis of 40 Studies With 53,276 Patients. Front. Oncol. 2022, 12, 881850. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Yue, S.; Liu, J.; Qiu, Z.; Xie, L.; Huang, X.; Li, S.; Hu, L.; Wu, J. Association of Tumor Size With Prognosis in Patients With Resectable Endometrial Cancer: A SEER Database Analysis. Front. Oncol. 2022, 12, 887157. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Mumtaz, M.; Naqvi, Z.; Farooqui, R.; Shah, S.A. Assessing Tumor Size by MRI and Pathology in Type I Endometrial Carcinoma to Predict Lymph Node Metastasis. Cureus 2022, 14, e23135. [Google Scholar] [CrossRef] [PubMed]
- Nougaret, S.; Reinhold, C.; Alsharif, S.S.; Addley, H.; Arceneau, J.; Molinari, N.; Guiu, B.; Sala, E. Endometrial Cancer: Combined MR Volumetry and Diffusion-weighted Imaging for Assessment of Myometrial and Lymphovascular Invasion and Tumor Grade. Radiology 2015, 276, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.L.; Lukovic, J.; Laframboise, S.; Ferguson, S.E.; Han, K. Brachy-ing Unresectable Endometrial Cancers with Magnetic Resonance Guidance. Cureus 2018, 10, e2274. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Dobrotwir, A.; McNally, O.; Abu-Rustum, N.R.; Narayan, K. Role of imaging in the routine management of endometrial cancer. Int. J. Gynaecol. Obstet. 2018, 143 (Suppl. 2), 109–117. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Chiappa, V.; Lopez, S.; Salvatore, C.; Interlenghi, M.; D’Oria, O.; Giannini, A.; Leone Roberti Maggiore, U.; Chiarello, G.; Palladino, S.; et al. Radiomics and Molecular Classification in Endometrial Cancer (The ROME Study): A Step Forward to a Simplified Precision Medicine. Healthcare 2022, 10, 2464. [Google Scholar] [CrossRef]
- Li, Y.F.; Ren, Q.; Sun, C.H.; Li, L.; Lian, H.D.; Sun, R.X.; Su, X.; Yu, H. Efficacy and mechanism of anti-vascular endothelial growth factor drugs for diabetic macular edema patients. World J. Diabetes 2022, 13, 532–542. [Google Scholar] [CrossRef]
- Fadare, O.; Parkash, V. p53 aberrations in low grade endometrioid carcinoma of the endometrium with nodal metastases: Possible insights on pathogenesis discerned from immunohistochemistry. Diagn. Pathol. 2017, 12, 81. [Google Scholar] [CrossRef]
- Leslie, K.K.; Filiaci, V.L.; Mallen, A.R.; Thiel, K.W.; Devor, E.J.; Moxley, K.; Richardson, D.; Mutch, D.; Secord, A.A.; Tewari, K.S.; et al. Mutated p53 portends improvement in outcomes when bevacizumab is combined with chemotherapy in advanced/recurrent endometrial cancer: An NRG Oncology study. Gynecol. Oncol. 2021, 161, 113–121. [Google Scholar] [CrossRef]
- Thiel, K.W.; Devor, E.J.; Filiaci, V.L.; Mutch, D.; Moxley, K.; Alvarez Secord, A.; Tewari, K.S.; McDonald, M.E.; Mathews, C.; Cosgrove, C.; et al. TP53 Sequencing and p53 Immunohistochemistry Predict Outcomes When Bevacizumab Is Added to Frontline Chemotherapy in Endometrial Cancer: An NRG Oncology/Gynecologic Oncology Group Study. J. Clin. Oncol. 2022, 40, 3289–3300. [Google Scholar] [CrossRef]
- Wang, H.; Yan, R.; Li, Z.; Wang, B.; Jin, X.; Guo, Z.; Liu, W.; Zhang, M.; Wang, K.; Guo, J.; et al. Quantitative dynamic contrast-enhanced parameters and intravoxel incoherent motion facilitate the prediction of TP53 status and risk stratification of early-stage endometrial carcinoma. Radiol. Oncol. 2023, 57, 257–269. [Google Scholar] [CrossRef]
- Shifeng, T.; Yue, W.; Wen, Z.; Lihua, C.; Nan, W.; Liangjie, L.; Ailian, L. The value of multimodal functional magnetic resonance imaging in differentiating p53abn from p53wt endometrial carcinoma. Acta Radiol. 2023, 64. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Al-Khafaji, J.F.; Class, C.A.; Wei, W.; Ramalingam, P.; Wakkaa, H.; Soliman, P.T.; Frumovitz, M.; Iyer, R.B.; Bhosale, P.R. Can MRI help assess aggressiveness of endometrial cancer? Clin. Radiol. 2018, 73, 833.e11–833.e18. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.; Ramalingam, P.; Ma, J.; Iyer, R.; Soliman, P.; Frumovitz, M.; Kundra, V. Can reduced field-of-view diffusion sequence help assess microsatellite instability in FIGO stage 1 endometrial cancer? J. Magn. Reason. Imaging 2017, 45, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Barth, M.; Breuer, F.; Koopmans, P.J.; Norris, D.G.; Poser, B.A. Simultaneous multislice (SMS) imaging techniques. Magn. Reson. Med. 2016, 75, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Ohno, Y.; Yamamoto, K.; Murayama, K.; Ikedo, M.; Yui, M.; Hanamatsu, S.; Tanaka, Y.; Obama, Y.; Ikeda, H.; et al. Deep Learning Reconstruction of Diffusion-weighted MRI Improves Image Quality for Prostatic Imaging. Radiology 2022, 303, 373–381. [Google Scholar] [CrossRef] [PubMed]
| Step | Procedure | Details | Scan Time | Phase |
|---|---|---|---|---|
| 1 | Patient Preparation | -Fasting for 3 h;
-Administration of antispasmodic drug intravenously. | NA | NA |
| 2 | Baseline Scans | -Conducting initial MRI scans before contrast injection. | NA | NA |
| 3 | Contrast Administration | -Administering 0.2 mmol/kg gadoteridol;
-Automated power injector at a rate of 1.0 mL/s followed by a flush within 30 mL of 0.9% sterile saline. | NA | NA |
| 4 | Multiphase 3D fat-saturated contrast-enhanced T1-weighted imaging | -Performing imaging every 30 sec for 4.5 min in the sagittal plane without breath-holding. | 0–30 s 60–90 s 120–150 s 180–210 s 240–270 s | Early phase (Subendometrial enhancement assessment) Equilibrium phase (Maximal tumor-to-myometrium contrast) Delayed phase (Cervical stromal invasion assessment) |
| 5 | Postcontrast T1-weighted imaging | -Conducting scans 5 min after contrast material administration in axial and sagittal planes with parameters similar to unenhanced T1-weighted imaging. | NA | NA |
| Imaging Factor | Kappa (95% Confidence Interval) |
|---|---|
| Growth pattern | 0.81 (0.72, 0.9) |
| SI on T2WI | 0.74 (0.63, 0.86) |
| Heterogeneous SI on T2WI | 0.91 (0.84, 0.99) |
| SI on CET1 | 0.86 (0.74, 0.98) |
| Heterogeneous SI on CET1 | 0.92 (0.86, 0.99) |
| SI on DWI | 0.90 (0.81, 0.99) |
| Heterogeneous SI on DWI | 0.95 (0.88, 1.00) |
| Deep myometrial invasion | 0.94 (0.88, 1.00) |
| Cervical stromal involvement | 0.97 (0.91, 1.00) |
| Extrauterine extension | 1.00 (1.00, 1.00) |
| Rectal or bladder invasion | 1.00 (1.00, 1.00) |
| Abnormal ascites | 0.66 (0.05, 1.00) |
| Peritoneal dissemination | 1.00 (1.00, 1.00) |
| Lymphadenopathy | 0.83 (0.65, 1.00) |
| Age, mean (range) | 55.12 (27–84) |
| Postmenopausal, n (%) | 108 (61.7%) |
| FIGO Stage (2018), n (%) | |
| I | 131 (74.86) |
| IA | 111 (63.43) |
| IB | 20 (11.43) |
| II | 11 (6.29) |
| III | 24 (13.71) |
| IIIA | 7 (4) |
| IIIB | 2 (1.14) |
| IIIC1 | 5 (2.86) |
| IIIC2 | 10 (5.71) |
| IV | 9 (5.14) |
| IVA | 0 (0) |
| IVB | 9 (5.14) |
| Endometrial cancer subtype, n (%) | |
| Endometrioid adenocarcinoma | 150 (85.71) |
| Grade 1 | 81 (46.29) |
| Grade 2 | 48 (27.43) |
| Grade 3 | 21 (12.00) |
| Mucinous carcinoma | 3 (1.71) |
| Serous carcinoma | 8 (4.57) |
| Clear-cell carcinoma | 4 (2.29) |
| Carcinosarcoma | 10 (5.71) |
| Lymphovascular space invasion, n (%) | |
| Positive | 39 (22.29%) |
| Negative | 136 (77.71%) |
| Recurrence, n (%) | 20 (11.4%) |
| Locoregional | 8 (40%) |
| Non-locoregional | 12 (60%) |
| Low-Risk Group | Non-Low-Risk Group | p-Value | ||||
|---|---|---|---|---|---|---|
| Low | Intermediate | High-Intermediate | High | Metastatic | ||
| (n = 90) | (n = 7) | (n = 19) | (n = 46) | (n = 13) | ||
| Maximum tumor diameter(cm) | <0.001 | |||||
| Median (IQR) | 2 (1.3, 3) | 3.1 (2.7, 4.7) | 3.6 (2.1, 5.2) | 4 (2.5, 5.7) | 5.1 (2.3, 7.5) | |
| Growth pattern | 0.185 | |||||
| Infiltrative | 37 (48.1) | 6 (85.7) | 8 (42.1) | 28 (63.6) | 6 (50) | |
| Expansile | 40 (52) | 1 (14.3) | 11 (57.9) | 16 (36.4) | 6 (50) | |
| SI on T2WI | 0.802 | |||||
| Hypo or iso | 54 (70.1) | 5 (71.4) | 15 (79) | 30 (68.2) | 6 (50) | |
| Hyper | 23 (29.9) | 2 (28.6) | 4 (21.1) | 14 (31.8) | 6 (50) | |
| Heterogeneous SI on T2WI | 0.193 | |||||
| No | 63 (81.8) | 6 (85.7) | 13 (68.4) | 32 (72.7) | 9 (75) | |
| Yes | 14 (18.2) | 1 (14.3) | 6 (31.6) | 12 (27.3) | 3 (25) | |
| SI on CET1 | 0.697 | |||||
| Hypo or iso | 66 (85.7) | 7 (100) | 18 (94.7) | 37 (84.1) | 10 (83.3) | |
| Hyper | 11 (14.3) | - | 1 (5.3) | 7 (15.9) | 2 (16.7) | |
| Heterogeneous SI on CET1 | 0.142 | |||||
| No | 58 (75.3) | 4 (57.1) | 13 (68.4) | 29 (65.9) | 7 (58.3) | |
| Yes | 19 (24.7) | 3 (42.9) | 6 (31.6) | 15 (34.1) | 5 (41.7) | |
| SI on DWI | 0.003 | |||||
| Hypo or iso | 22 (29.3) | 1 (14.3) | 1 (5.9) | 5 (11.6) | 1 (8.3) | |
| Hyper | 53 (70.7) | 6 (85.7) | 16 (94.1) | 38 (88.4) | 11 (91.7) | |
| Heterogeneous SI on DWI | 0.003 | |||||
| No | 66 (88) | 6 (85.7) | 11 (64.7) | 31 (72.1) | 6 (50) | |
| Yes | 9 (12) | 1 (14.3) | 6 (35.3) | 12 (27.9) | 6 (50) | |
| ADC value | 0.419 | |||||
| Median (IQR) | 811.2 (719.1, 946.4) | 812.6 (761, 895.5) | 731.8 (668.8, 822.9) | 893.3 (776.7, 960.7) | 898.7 (783.8, 1125.1) | |
| Deep myometrial invasion | <0.001 | |||||
| No | 86 (95.6) | 3 (42.9) | 11 (57.9) | 20 (43.5) | 7 (53.9) | |
| Yes | 4 (4.4) | 4 (57.1) | 8 (42.1) | 26 (56.5) | 6 (46.2) | |
| Cervical stromal involvement | <0.001 | <0.001 | ||||
| No | 90 (100) | 6 (85.7) | 17 (89.5) | 36 (78.3) | 8 (61.5) | |
| Yes | - | 1 (14.3) | 2 (10.5) | 10 (21.7) | 5 (38.5) | |
| Extrauterine extension | 0.002 | |||||
| No | 89 (98.9) | 7 (100) | 18 (94.7) | 40 (87) | 9 (69.2) | |
| Yes | 1 (1.1) | - | 1 (5.3) | 6 (13) | 4 (30.8) | |
| Rectal or bladder invasion | 0.113 † | |||||
| No | 90 (100) | 7 (100) | 18 (94.7) | 46 (100) | 11 (84.6) | |
| Yes | - | - | 1 (5.3) | - | 2 (15.4) | |
| Abnormal ascites | 0.486 † | |||||
| No | 90 (100) | 7 (100) | 19 (100) | 45 (97.8) | 13 (100) | |
| Yes | - | - | - | 1 (2.2) | - | |
| Peritoneal dissemination | 0.235 † | |||||
| No | 90 (100) | 7 (100) | 19 (100) | 46 (100) | 11 (84.6) | |
| Yes | - | - | - | 2 (15.4) | ||
| Lymphadenopathy | 0.003 † | |||||
| No | 90 (100) | 7 (100) | 18 (94.7) | 42 (91.3) | 10 (76.9) | |
| Yes | - | - | 1 (5.3) | 4 (8.7) | 3 (23.1) | |
| Low-Risk Group | Non-Low-Risk Group | p-Value | ||||
|---|---|---|---|---|---|---|
| Low | Intermediate | High-Intermediate | High | Metastatic | ||
| (n = 90) | (n = 7) | (n = 19) | (n = 46) | (n = 13) | ||
| Recurrence | <0.001 | |||||
| No | 90 (100) | 6 (85.7) | 18 (94.7) | 35 (76.1) | 6 (46.2) | |
| Yes | - | 1 (14.3) | 1 (5.3) | 11 (23.9) | 7 (53.9) | |
| Stage concordance | <0.001 | |||||
| Concordance | 85 (94.4) | 5 (71.4) | 14 (73.7) | 26 (56.5) | 6 (46.2) | |
| Discordance | 5 (5.6) | 2 (28.6) | 5 (26.3) | 20 (43.5) | 7 (53.9) | |
| P53 Wild | p53 Mutant | ||
|---|---|---|---|
| (n = 62) | (n = 24) | p-Value | |
| Maximum tumor diameter (cm) | 0.077 | ||
| Median (IQR) | 2.6 (1.5, 3.9) | 4 (1.8, 5.3) | |
| Growth pattern | 0.995 | ||
| Infiltrative | 30 (56.6) | 13 (56.5) | |
| Expansile | 23 (43.4) | 10 (43.5) | |
| SI on T2WI | 0.572 | ||
| Hypo or iso | 38 (71.7) | 15 (65.2) | |
| Hyper | 15 (28.3) | 8 (34.8) | |
| Heterogeneous SI on T2WI | 0.362 | ||
| No | 42 (79.3) | 16 (69.6) | |
| Yes | 11 (20.8) | 7 (30.4) | |
| SI on CET1 | 0.195 † | ||
| Hypo or iso | 46 (86.8) | 17 (73.9) | |
| Hyper | 7 (13.2) | 6 (26.1) | |
| Heterogeneous SI on CET1 | 0.552 | ||
| No | 36 (67.9) | 14 (60.9) | |
| Yes | 17 (32.1) | 9 (39.1) | |
| SI on DWI | 0.669 | ||
| Hypo or iso | 11 (21.6) | 6 (26.1) | |
| Hyper | 40 (78.4) | 17 (73.9) | |
| Heterogeneous SI on DWI | >0.999 † | ||
| No | 41 (80.4) | 18 (78.3) | |
| Yes | 10 (19.6) | 5 (21.7) | |
| ADC value | 0.182 | ||
| Median (IQR) | 800.7 (712.8, 903.7) | 843.1 (777.6, 951.3) | |
| Deep myometrial invasion | 0.752 | ||
| No | 46 (74.2) | 17 (70.8) | |
| Yes | 16 (25.8) | 7 (29.2) | |
| Cervical stromal involvement | 0.491 † | ||
| No | 55 (88.7) | 20 (83.3) | |
| Yes | 7 (11.3) | 4 (16.7) | |
| Extrauterine extension | 0.670 † | ||
| No | 58 (93.6) | 22 (91.7) | |
| Yes | 4 (6.5) | 2 (8.3) | |
| Rectal or bladder invasion | >0.999 † | ||
| No | 60 (96.8) | 24 (100) | |
| Yes | 2 (3.2) | - | |
| Abnormal ascites | - | ||
| No | 62 (100) | 24 (100) | |
| Yes | - | - | |
| Peritoneal dissemination | >0.999 † | ||
| No | 61 (98.4) | 24 (100) | |
| Yes | 1 (1.6) | - | |
| Lymphadenopathy | >0.999 † | ||
| No | 60 (96.8) | 24 (100) | |
| Yes | 2 (3.2) | - | |
| MSS | MSI | ||
|---|---|---|---|
| (n = 28) | (n = 8) | p-Value | |
| Maximum tumor diameter (cm) | 0.848 | ||
| Median (IQR) | 3.3 (1.7, 4.7) | 2.8 (2.3, 4.7) | |
| Growth pattern | 0.175 † | ||
| Infiltrative | 12 (44.4) | 5 (83.3) | |
| Expansile | 15 (55.6) | 1 (16.7) | |
| SI on T2 | 0.640 † | ||
| Hypo or iso | 18 (66.7) | 5 (83.3) | |
| Hyper | 9 (33.3) | 1 (16.7) | |
| Heterogeneous SI on T2 | 0.156 † | ||
| No | 18 (66.7) | 6 (100) | |
| Yes | 9 (33.3) | - | |
| SI on CET1 | >0.999 † | ||
| Hypo or iso | 23 (85.2) | 6 (100) | |
| Hyper | 4 (14.8) | - | |
| Heterogeneous SI on CET1 | 0.027 † | ||
| No | 13 (48.2) | 6 (100) | |
| Yes | 14 (51.9) | - | |
| SI on DWI | >0.999 † | ||
| Hypo or iso | 3 (11.5) | - | |
| Hyper | 23 (88.5) | 6 (100) | |
| Heterogeneous SI on DWI | 0.565 † | ||
| No | 20 (76.9) | 6 (100) | |
| Yes | 6 (23.1) | - | |
| ADC value | 0.469 | ||
| Median (IQR) | 822.9 (758.5, 958.9) | 784.5 (712.8, 812.6) | |
| Deep myometrial invasion | 0.384 † | ||
| No | 22 (78.6) | 5 (62.5) | |
| Yes | 6 (21.4) | 3 (37.5) | |
| Cervical stromal involvement | >0.999 † | ||
| No | 24 (85.7) | 7 (87.5) | |
| Yes | 4 (14.3) | 1 (12.5) | |
| Extrauterine extension | 0.400 † | ||
| No | 27 (96.4) | 7 (87.5) | |
| Yes | 1 (3.6) | 1 (12.5) | |
| Rectal or bladder invasion | - | ||
| No | 28 (100) | 8 (100) | |
| Yes | - | - | |
| Abnormal ascites | - | ||
| No | 28 (100) | 8 (100) | |
| Yes | - | - | |
| Peritoneal dissemination | - | ||
| No | 28 (100) | 8 (100) | |
| Yes | - | - | |
| Lymphadenopathy | - | ||
| No | 28 (100) | 8 (100) | |
| Yes | - | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, H.; Rha, S.E.; Kim, H.; Kang, J.; Shin, Y.R. Predictive Value of Magnetic Resonance Imaging in Risk Stratification and Molecular Classification of Endometrial Cancer. Cancers 2024, 16, 921. https://doi.org/10.3390/cancers16050921
Bae H, Rha SE, Kim H, Kang J, Shin YR. Predictive Value of Magnetic Resonance Imaging in Risk Stratification and Molecular Classification of Endometrial Cancer. Cancers. 2024; 16(5):921. https://doi.org/10.3390/cancers16050921
Chicago/Turabian StyleBae, Hanna, Sung Eun Rha, Hokun Kim, Jun Kang, and Yu Ri Shin. 2024. "Predictive Value of Magnetic Resonance Imaging in Risk Stratification and Molecular Classification of Endometrial Cancer" Cancers 16, no. 5: 921. https://doi.org/10.3390/cancers16050921
APA StyleBae, H., Rha, S. E., Kim, H., Kang, J., & Shin, Y. R. (2024). Predictive Value of Magnetic Resonance Imaging in Risk Stratification and Molecular Classification of Endometrial Cancer. Cancers, 16(5), 921. https://doi.org/10.3390/cancers16050921






