Liver Transplantation for Hepatocellular Carcinoma beyond the Milan Criteria: A Specific Role for Living Donor Liver Transplantation after Neoadjuvant Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
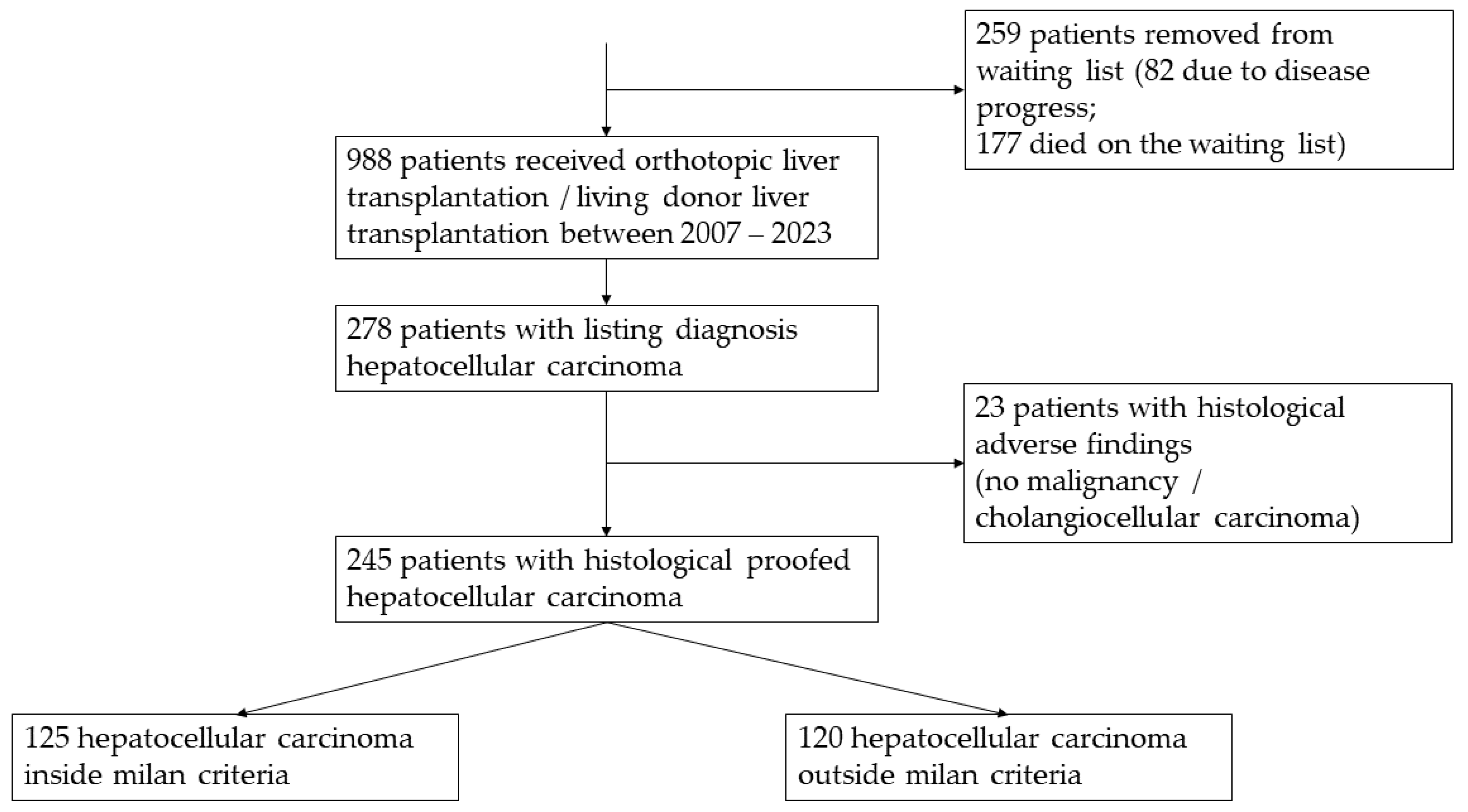
2.1. Patient Selection
2.2. Outcome Measures
2.3. Statistical Analysis
3. Results
3.1. Epidemiology
3.2. Overall Survival Rate Inside and Outside the MC
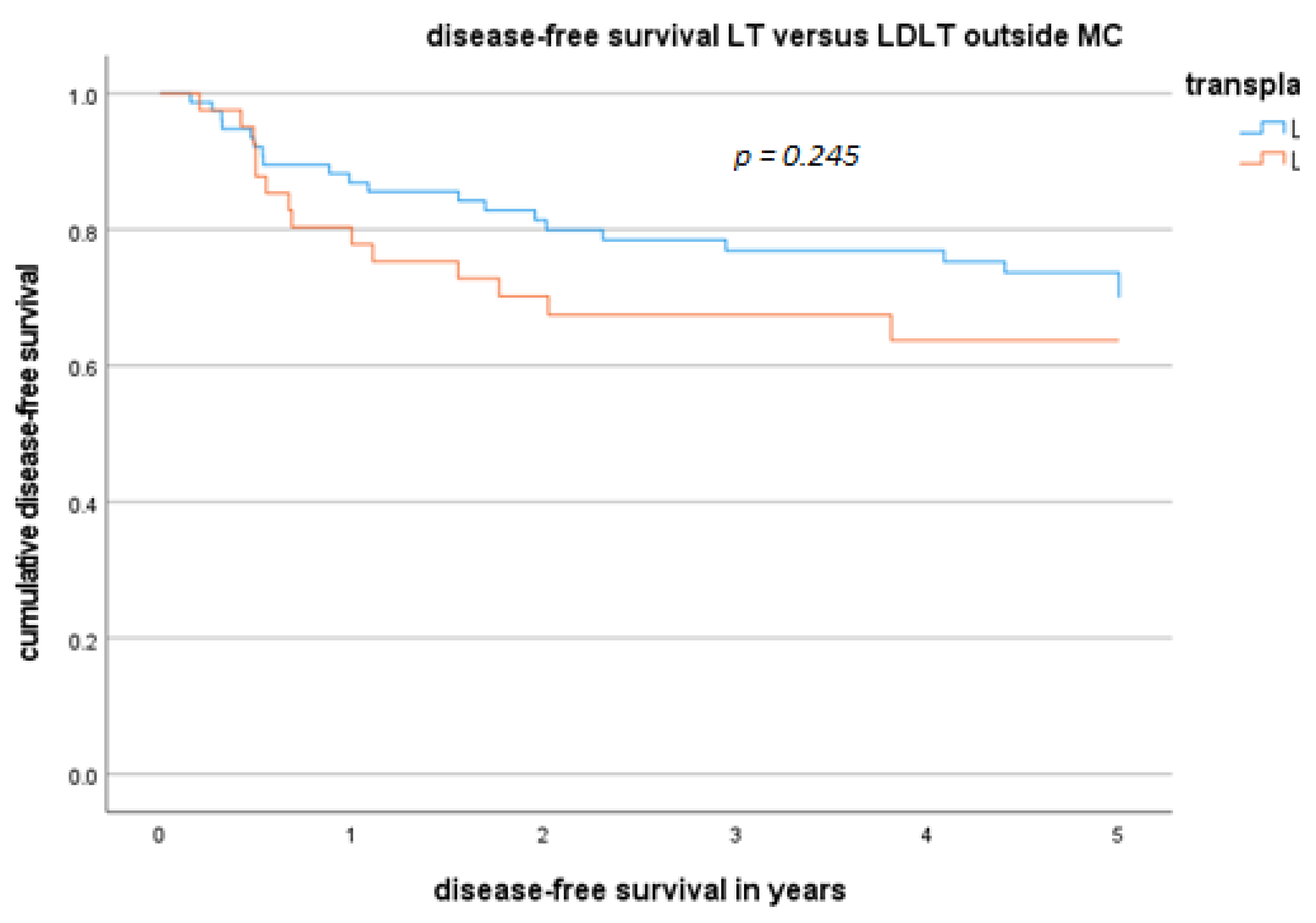
3.3. Disease-Free Survival Rate Outside the MC and Factors Related to Disease-Free Survival Outside the MC
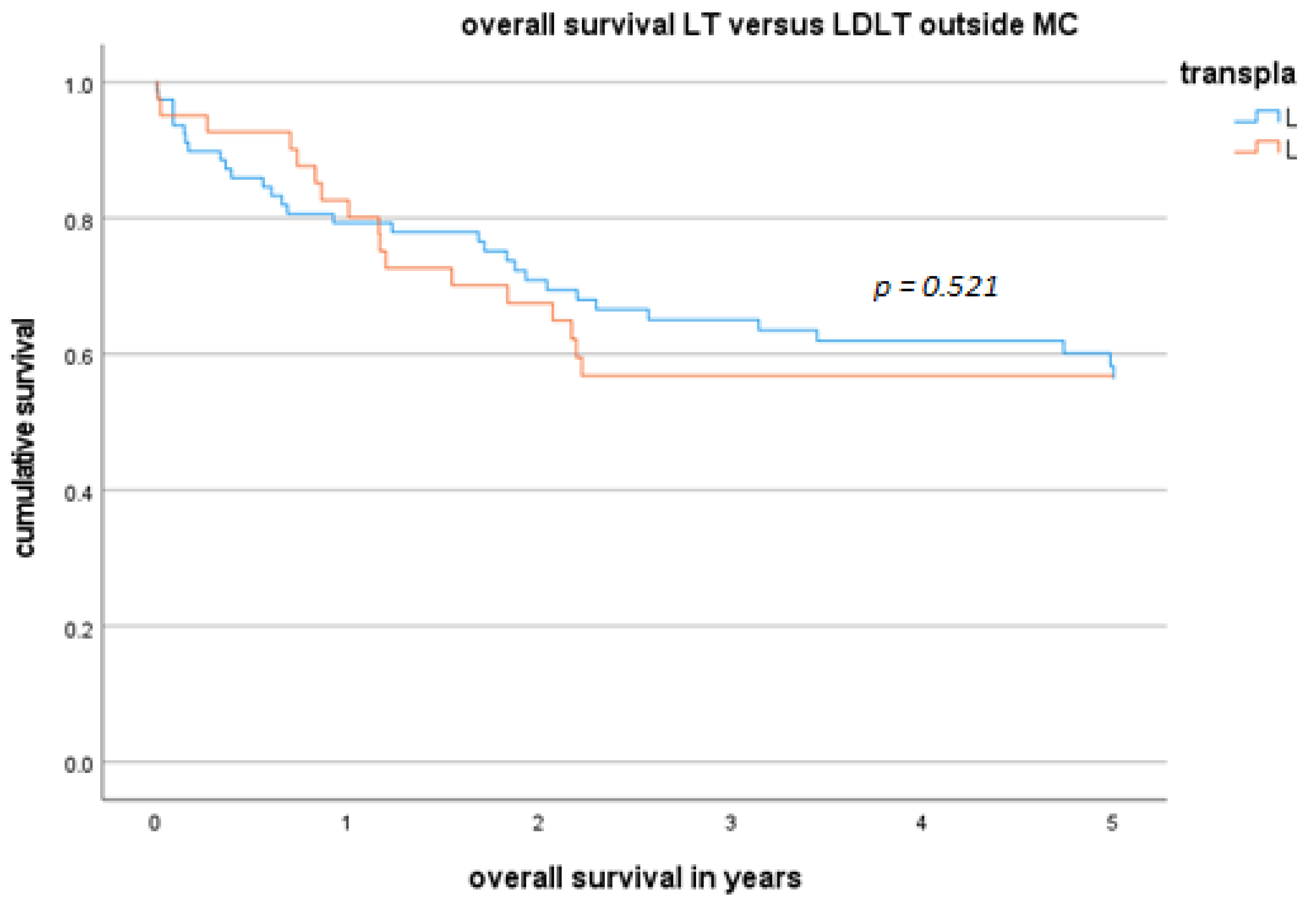
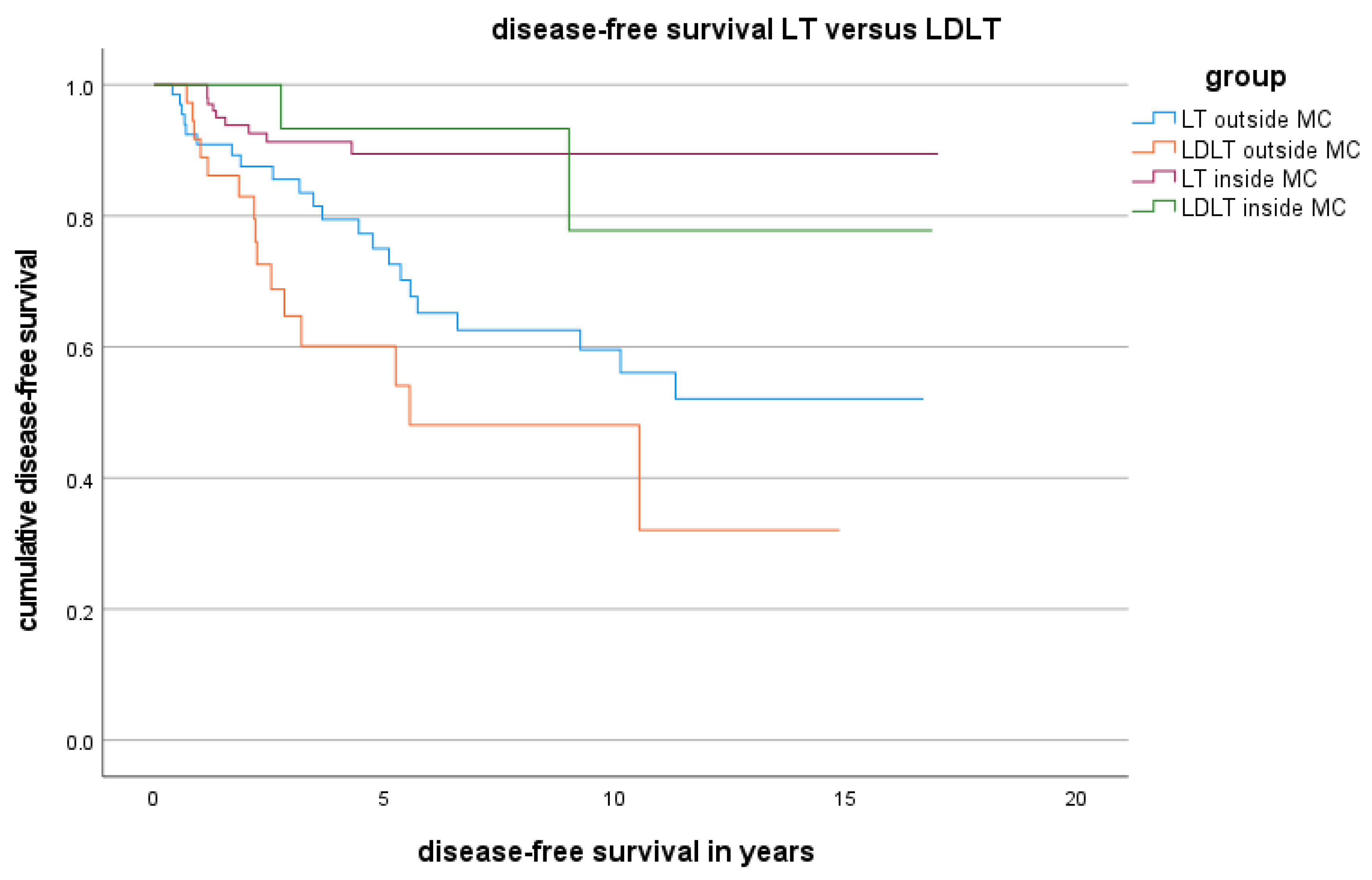
3.4. DFS and OS for Patients Who Underwent LDLT or LT Outside the MC
3.5. MELD Score, Tumour Morphology and Bridging Response Outside the MC
3.6. Results According to Different Classifications beyond the Milan Grade
3.7. HCC Waitlist Dynamics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Da, B.L.; Suchman, K.I.; Lau, L.; Rabiee, A.; He, A.R.; Shetty, K.; Yu, H.; Wong, L.L.; Amdur, R.L.; Crawford, J.M.; et al. Pathogenesis to Management of Hepatocellular Carcinoma. Genes Cancer 2022, 13, 72–87. [Google Scholar] [CrossRef]
- Dopazo, C.; Søreide, K.; Rangelova, E.; Mieog, S.; Carrion-Alvarez, L.; Diaz-Nieto, R.; Primavesi, F.; Stättner, S. Hepatocellular Carcinoma. Eur. J. Surg. Oncol. 2024, 50, 107313. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, V.; Llovet, J.M.; Miceli, R.; Bhoori, S.; Schiavo, M.; Mariani, L.; Camerini, T.; Roayaie, S.; Schwartz, M.E.; Grazi, G.L.; et al. Predicting Survival after Liver Transplantation in Patients with Hepatocellular Carcinoma beyond the Milan Criteria: A Retrospective, Exploratory Analysis. Lancet Oncol. 2009, 10, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Shehta, A.; Lee, J.-M.; Suh, K.-S.; Kim, H.-C.; Hong, S.K.; Cho, J.-H.; Yi, N.-J.; Lee, K.-W. Bridging and Downstaging Role of Trans-Arterial Radio-Embolization for Expected Small Remnant Volume before Liver Resection for Hepatocellular Carcinoma. Ann. Hepato-Biliary-Pancreat. Surg. 2020, 24, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Hui, K.M.; Shi, M.; Reau, N.; Aloman, C. Differential Expression of Hepatic Cancer Stemness and Hypoxia Markers in Residual Cancer after Locoregional Therapies for Hepatocellular Carcinoma. Hepatol. Commun. 2022, 6, 3247–3259. [Google Scholar] [CrossRef] [PubMed]
- Ettorre, G.M.; Laurenzi, A. Other “bridge” therapies for liver transplantation: RFA, TACE, and TARE. In Liver Transplantation and Hepatobiliary Surgery, Interplay of Technical and Theoretical Aspects; Springer: Berlin/Heidelberg, Germany, 2019; pp. 183–191. [Google Scholar] [CrossRef]
- Gao, Q.; Anwar, I.J.; Abraham, N.; Barbas, A.S. Liver Transplantation for Hepatocellular Carcinoma after Downstaging or Bridging Therapy with Immune Checkpoint Inhibitors. Cancers 2021, 13, 6307. [Google Scholar] [CrossRef] [PubMed]
- Zori, A.G.; Ismael, M.N.; Limaye, A.R.; Firpi, R.; Morelli, G.; Soldevila-Pico, C.; Suman, A.; Vogel, J.D.; Lazarowicz, M.; Geller, B.S.; et al. Locoregional Therapy Protocols With and Without Radioembolization for Hepatocellular Carcinoma as Bridge to Liver Transplantation. Am. J. Clin. Oncol. 2020, 43, 325–333. [Google Scholar] [CrossRef]
- Makary, M.S.; Bozer, J.; Miller, E.D.; Diaz, D.A.; Rikabi, A. Long-Term Clinical Outcomes of Yttrium-90 Transarterial Radioembolization for Hepatocellular Carcinoma: A 5-Year Institutional Experience. Acad. Radiol. 2023, in press. [CrossRef]
- Zhang, J.; Hu, C.; Xie, X.; Qi, L.; Li, C.; Li, S. Immune Checkpoint Inhibitors in HBV-Caused Hepatocellular Carcinoma Therapy. Vaccines 2023, 11, 614. [Google Scholar] [CrossRef]
- Wassmer, C.-H.; Hajji, S.E.; Papazarkadas, X.; Compagnon, P.; Tabrizian, P.; Lacotte, S.; Toso, C. Immunotherapy and Liver Transplantation: A Narrative Review of Basic and Clinical Data. Cancers 2023, 15, 4574. [Google Scholar] [CrossRef] [PubMed]
- Wehrenberg-Klee, E.; Goyal, L.; Dugan, M.; Zhu, A.X.; Ganguli, S. Y-90 Radioembolization Combined with a PD-1 Inhibitor for Advanced Hepatocellular Carcinoma. Cardiovasc. Interv. Radiol. 2018, 41, 1799–1802. [Google Scholar] [CrossRef]
- Yao, F.Y.; Xiao, L.; Bass, N.M.; Kerlan, R.; Ascher, N.L.; Roberts, J.P. Liver Transplantation for Hepatocellular Carcinoma: Validation of the UCSF-Expanded Criteria Based on Preoperative Imaging. Am. J. Transplant. 2007, 7, 2587–2596. [Google Scholar] [CrossRef] [PubMed]
- Sapisochin, G.; Goldaracena, N.; Laurence, J.M.; Dib, M.; Barbas, A.; Ghanekar, A.; Cleary, S.P.; Lilly, L.; Cattral, M.S.; Marquez, M.; et al. The Extended Toronto Criteria for Liver Transplantation in Patients with Hepatocellular Carcinoma: A Prospective Validation Study. Hepatology 2016, 64, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Takishima, T.; Haruki, K.; Taniai, T.; Furukawa, K.; Horiuchi, T.; Onda, S.; Yanagaki, M.; Shirai, Y.; Hamura, R.; Ikegami, T. The Japanese 5-5-500 Rule Predicts Prognosis of Hepatocellular Carcinoma After Hepatic Resection. Anticancer Res. 2023, 43, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Reig, M.; Forner, A.; Rimola, J.; Ferrer-Fàbrega, J.; Burrel, M.; Garcia-Criado, Á.; Kelley, R.K.; Galle, P.R.; Mazzaferro, V.; Salem, R.; et al. BCLC Strategy for Prognosis Prediction and Treatment Recommendation: The 2022 Update. J. Hepatol. 2022, 76, 681–693. [Google Scholar] [CrossRef]
- Lubel, J.S.; Roberts, S.K.; Strasser, S.I.; Shackel, N. Australian Recommendations for the Management of Hepatocellular Carcinoma. Med. J. Aust. 2021, 215, 334–334.e1. [Google Scholar] [CrossRef]
- Seehofer, D.; Petrowsky, H.; Schneeberger, S.; Vibert, E.; Ricke, J.; Sapisochin, G.; Nault, J.-C.; Berg, T. Patient Selection for Downstaging of Hepatocellular Carcinoma Prior to Liver Transplantation—Adjusting the Odds? Transpl. Int. 2022, 35, 10333. [Google Scholar] [CrossRef]
- DSO. Jahresbericht der Deutschen Stiftung für Organspende 2021. Available online: https://dso.de/BerichteTransplantationszentren/Grafiken%20D%202021%20Leber.pdf (accessed on 1 September 2023).
- Goldaracena, N.; Gorgen, A.; Doyle, A.; Hansen, B.E.; Tomiyama, K.; Zhang, W.; Ghanekar, A.; Lilly, L.; Cattral, M.; Galvin, Z.; et al. Live Donor Liver Transplantation for Patients with Hepatocellular Carcinoma Offers Increased Survival vs. Deceased Donation. J. Hepatol. 2019, 70, 666–673. [Google Scholar] [CrossRef]
- Nadalin, S.; Capobianco, I.; Panaro, F.; Francesco, F.D.; Troisi, R.; Sainz-Barriga, M.; Muiesan, P.; Königsrainer, A.; Testa, G. Living Donor Liver Transplantation in Europe. Hepatobiliary Surg. Nutr. 2016, 5, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Malinchoc, M.; Kamath, P.S.; Gordon, F.D.; Peine, C.J.; Rank, J.; Borg, P.C.J. ter. A Model to Predict Poor Survival in Patients Undergoing Transjugular Intrahepatic Portosystemic Shunts. Hepatology 2000, 31, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Lencioni, R.; Llovet, J. Modified RECIST (MRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 052–060. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Meyer, T.; Sapisochin, G.; Salem, R.; Saborowski, A. Hepatocellular Carcinoma. Lancet 2022, 400, 1345–1362. [Google Scholar] [CrossRef] [PubMed]
- Hameed, B.; Mehta, N.; Sapisochin, G.; Roberts, J.P.; Yao, F.Y. Alpha-fetoprotein Level > 1000 Ng/ML as an Exclusion Criterion for Liver Transplantation in Patients with Hepatocellular Carcinoma Meeting the Milan Criteria. Liver Transplant. 2014, 20, 945–951. [Google Scholar] [CrossRef]
- Feng, L.-H.; Zhu, Y.-Y.; Zhou, J.-M.; Wang, M.; Wang, L.; Xu, W.-Q.; Zhang, T.; Mao, A.-R.; Cong, W.-M.; Dong, H.; et al. A Practical Risk Classification of Early Recurrence in Hepatocellular Carcinoma Patients with Microvascular Invasion after Hepatectomy: A Decision Tree Analysis. Ann. Surg. Oncol. 2023, 30, 363–372. [Google Scholar] [CrossRef]
- Toso, C.; Meeberg, G.; Hernandez-Alejandro, R.; Dufour, J.; Marotta, P.; Majno, P.; Kneteman, N.M. Total Tumor Volume and Alpha-fetoprotein for Selection of Transplant Candidates with Hepatocellular Carcinoma: A Prospective Validation. Hepatology 2015, 62, 158–165. [Google Scholar] [CrossRef]
- Bhangui, P.; Vibert, E.; Majno, P.; Salloum, C.; Andreani, P.; Zocrato, J.; Ichai, P.; Saliba, F.; Adam, R.; Castaing, D.; et al. Intention-to-treat Analysis of Liver Transplantation for Hepatocellular Carcinoma: Living versus Deceased Donor Transplantation. Hepatology 2011, 53, 1570–1579. [Google Scholar] [CrossRef]
- Yao, F.Y.; Ferrell, L.; Bass, N.M.; Watson, J.J.; Bacchetti, P.; Venook, A.; Ascher, N.L.; Roberts, J.P. Liver Transplantation for Hepatocellular Carcinoma: Expansion of the Tumor Size Limits Does Not Adversely Impact Survival. Hepatology 2001, 33, 1394–1403. [Google Scholar] [CrossRef]
- Sotiropoulos, G.C.; Lang, H.; Nadalin, S.; Neuhäuser, M.; Molmenti, E.P.; Baba, H.A.; Paul, A.; Saner, F.H.; Weber, F.; Hilgard, P.; et al. Liver Transplantation for Hepatocellular Carcinoma: University Hospital Essen Experience and Metaanalysis of Prognostic Factors. J. Am. Coll. Surg. 2007, 205, 661–675. [Google Scholar] [CrossRef]
- Vakili, K.; Pomposelli, J.J.; Cheah, Y.L.; Akoad, M.; Lewis, W.D.; Khettry, U.; Gordon, F.; Khwaja, K.; Jenkins, R.; Pomfret, E.A. Living Donor Liver Transplantation for Hepatocellular Carcinoma: Increased Recurrence but Improved Survival. Liver Transplant. 2009, 15, 1861–1866. [Google Scholar] [CrossRef]
- Sandro, S.D.; Slim, A.O.; Giacomoni, A.; Lauterio, A.; Mangoni, I.; Aseni, P.; Pirotta, V.; Aldumour, A.; Mihaylov, P.; Carlis, L.D. Living Donor Liver Transplantation for Hepatocellular Carcinoma: Long-Term Results Compared With Deceased Donor Liver Transplantation. Transplant. Proc. 2009, 41, 1283–1285. [Google Scholar] [CrossRef]
- Sandhu, L.; Sandroussi, C.; Guba, M.; Selzner, M.; Ghanekar, A.; Cattral, M.S.; McGilvray, I.D.; Levy, G.; Greig, P.D.; Renner, E.L.; et al. Living Donor Liver Transplantation versus Deceased Donor Liver Transplantation for Hepatocellular Carcinoma: Comparable Survival and Recurrence. Liver Transplant. 2012, 18, 315–322. [Google Scholar] [CrossRef]
- Park, M.-S.; Lee, K.-W.; Suh, S.-W.; You, T.; Choi, Y.; Kim, H.; Hong, G.; Yi, N.-J.; Kwon, C.-H.D.; Joh, J.-W.; et al. Living-Donor Liver Transplantation Associated With Higher Incidence of Hepatocellular Carcinoma Recurrence Than Deceased-Donor Liver Transplantation. Transplant. J. 2014, 97, 71–77. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, S.; Joh, J.; Suh, K.; Kim, D. Liver Transplantation for Adult Patients with Hepatocellular Carcinoma in Korea: Comparison between Cadaveric Donor and Living Donor Liver Transplantations. Liver Transplant. 2005, 11, 1265–1272. [Google Scholar] [CrossRef]
- Sandri, G.B.L.; Rayar, M.; Qi, X.; Lucatelli, P. Liver Transplant for Patients Outside Milan Criteria. Transl. Gastroenterol. Hepatol. 2018, 3, 81. [Google Scholar] [CrossRef]
- Ramos, F.; Castellanos, M.; las Heras, N.D.; Escalante, F.; Fernandez-Ferrero, S.; Vidal, M.J.; Villalobos, M.L. ECOG Performance Status Shows a Stronger Association with Treatment Tolerability Than Some Multidimensional Scales in Elderly Patients Diagnosed with Hematological Malignancies. Blood 2020, 136 (Suppl. 1), 15–16. [Google Scholar] [CrossRef]
- Martino, M.D.; Lai, Q.; Lucatelli, P.; Damato, E.; Calabrese, A.; Masci, G.M.; Parisse, S.; Sedati, P.; Merli, M.; Mennini, G.; et al. Comparison of Up-to-Seven Criteria with Milan Criteria for Liver Transplantation in Patients with HCC. Trends Transplant. 2021, 14, 5. [Google Scholar] [CrossRef]
- Rauchfuß, F.; Dondorf, F.; Fahrner, R.; Tautenhahn, H.-M.; Ardelt, M.; Settmacher, U. Searching the Ideal Hepatocellular Carcinoma Patient for Liver Transplantation: Are the Toronto Criteria a Step in the Right Direction? Hepatobiliary Surg. Nutr. 2017, 6, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.-S.; Xu, X.; Wu, J.; Chen, J.; Wang, W.-L.; Zhang, M.; Liang, T.-B.; Wu, L.-M. Liver Transplantation for Hepatocellular Carcinoma: Hangzhou Experiences. Transplantation 2008, 85, 1726–1732. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Yan, L.-N. Staging of Hepatocellular Carcinoma. Hepatobiliary Pancreat. Dis. Int. HBPD INT 2003, 2, 491–495. [Google Scholar]
- Yap, A.Q.; Chen, C.-L.; Yong, C.-C.; Kuo, F.-Y.; Wang, S.-H.; Lin, C.-C.; Liu, Y.-W.; Lin, T.-L.; Li, W.-F.; Millan, C.A.; et al. Clinicopathological Factors Impact the Survival Outcome Following the Resection of Combined Hepatocellular Carcinoma and Cholangiocarcinoma. Surg. Oncol. 2013, 22, 55–60. [Google Scholar] [CrossRef]
- Bhatti, A.B.H.; Naqvi, W.; Khan, N.Y.; Zia, H.H.; Dar, F.S.; Khan, Z.A.; Rana, A. Living Donor Liver Transplantation for Advanced Hepatocellular Carcinoma Including Macrovascular Invasion. J. Cancer Res. Clin. Oncol. 2022, 148, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-C.; Chen, C.-L. Living Donor Liver Transplantation for Hepatocellular Carcinoma Achieves Better Outcomes. Hepatobiliary Surg. Nutr. 2016, 5, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, A.B.H.; Waheed, A.; Khan, N.A. Living Donor Liver Transplantation for Hepatocellular Carcinoma: Appraisal of the United Network for Organ Sharing Modified TNM Staging. Front. Surg. 2021, 7, 622170. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.C.L.; Ng, K.K.C.; Fung, J.Y.Y.; Chan, A.A.C.; Cheung, T.-T.; Chok, K.S.H.; Dai, J.W.C.; Lo, C.-M. Long-Term Survival Outcome Between Living Donor and Deceased Donor Liver Transplant for Hepatocellular Carcinoma: Intention-to-Treat and Propensity Score Matching Analyses. Ann. Surg. Oncol. 2019, 26, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Lei, J.Y.; Wang, W.T.; Yan, L.N. Hangzhou Criteria for Liver Transplantation in Hepatocellular Carcinoma: A Single-Center Experience. Eur. J. Gastroenterol. Hepatol. 2014, 26, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Ivanics, T.; Claasen, M.P.; Samstein, B.; Emond, J.C.; Fox, A.N.; Pomfret, E.; Pomposelli, J.; Tabrizian, P.; Florman, S.S.; Mehta, N.; et al. Living Donor Liver Transplantation (LDLT) for Hepatocellular Carcinoma (HCC) within and Outside Traditional Selection Criteria: A Multicentric North American Experience. Ann. Surg. 2023, 279, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, X.; Ling, Q.; Wu, J.; Zheng, S. Role of Pittsburgh Modified TNM Criteria in Prognosis Prediction of Liver Transplantation for Hepatocellular Carcinoma. Chin. Med. J. 2007, 120, 2200–2203. [Google Scholar] [CrossRef]
- Pons, F.; Varela, M.; Llovet, J.M. Staging Systems in Hepatocellular Carcinoma. HPB 2005, 7, 35–41. [Google Scholar] [CrossRef]
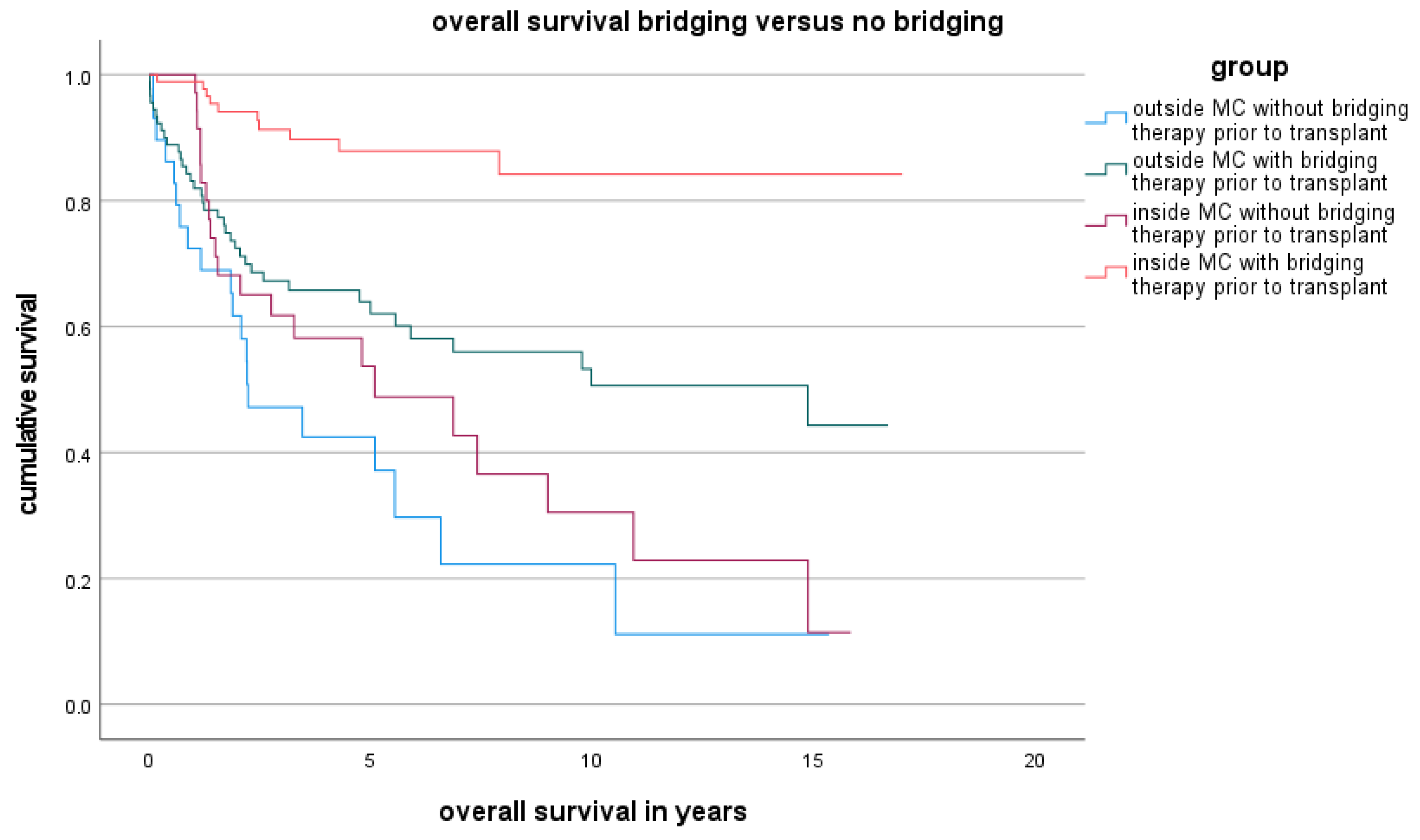
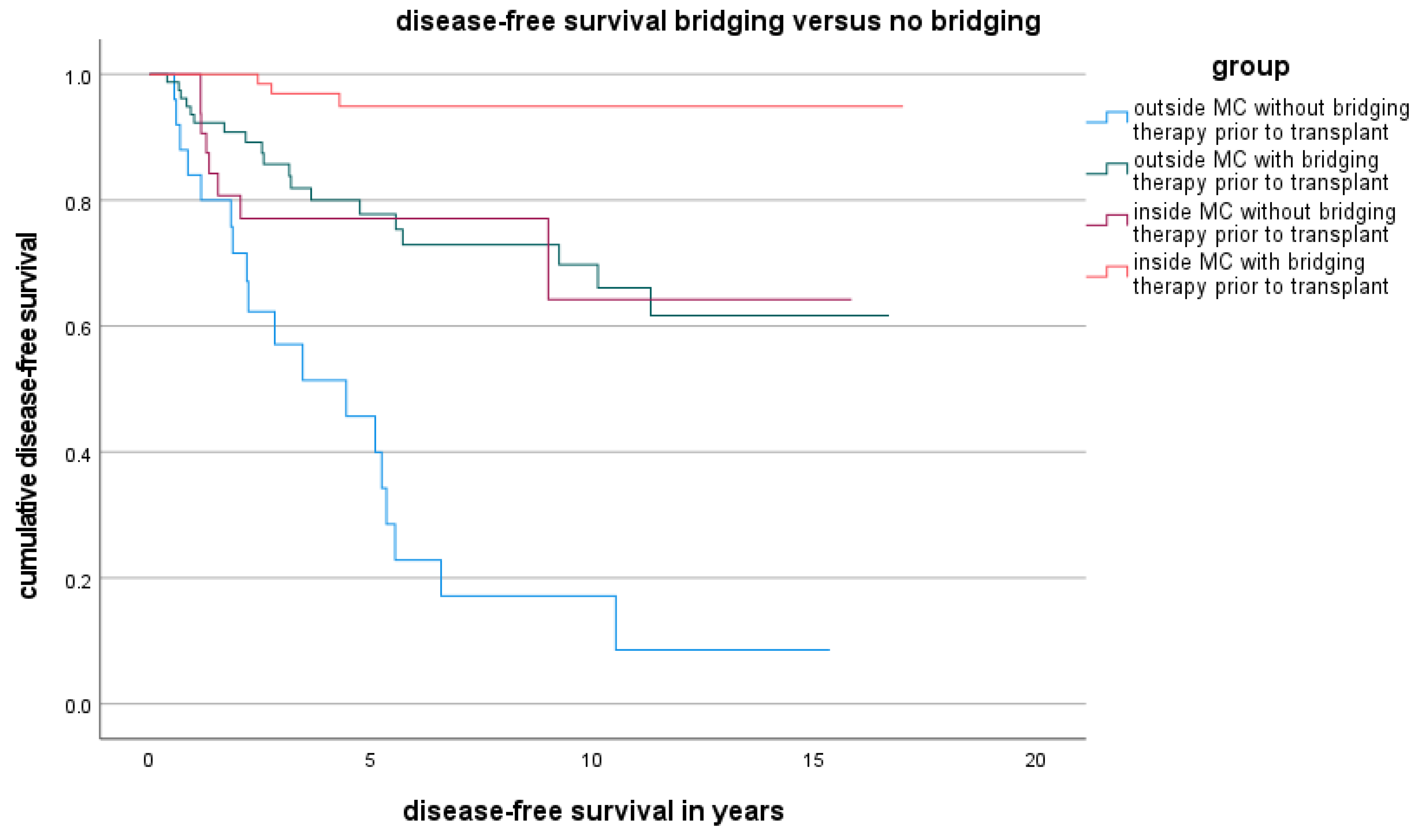
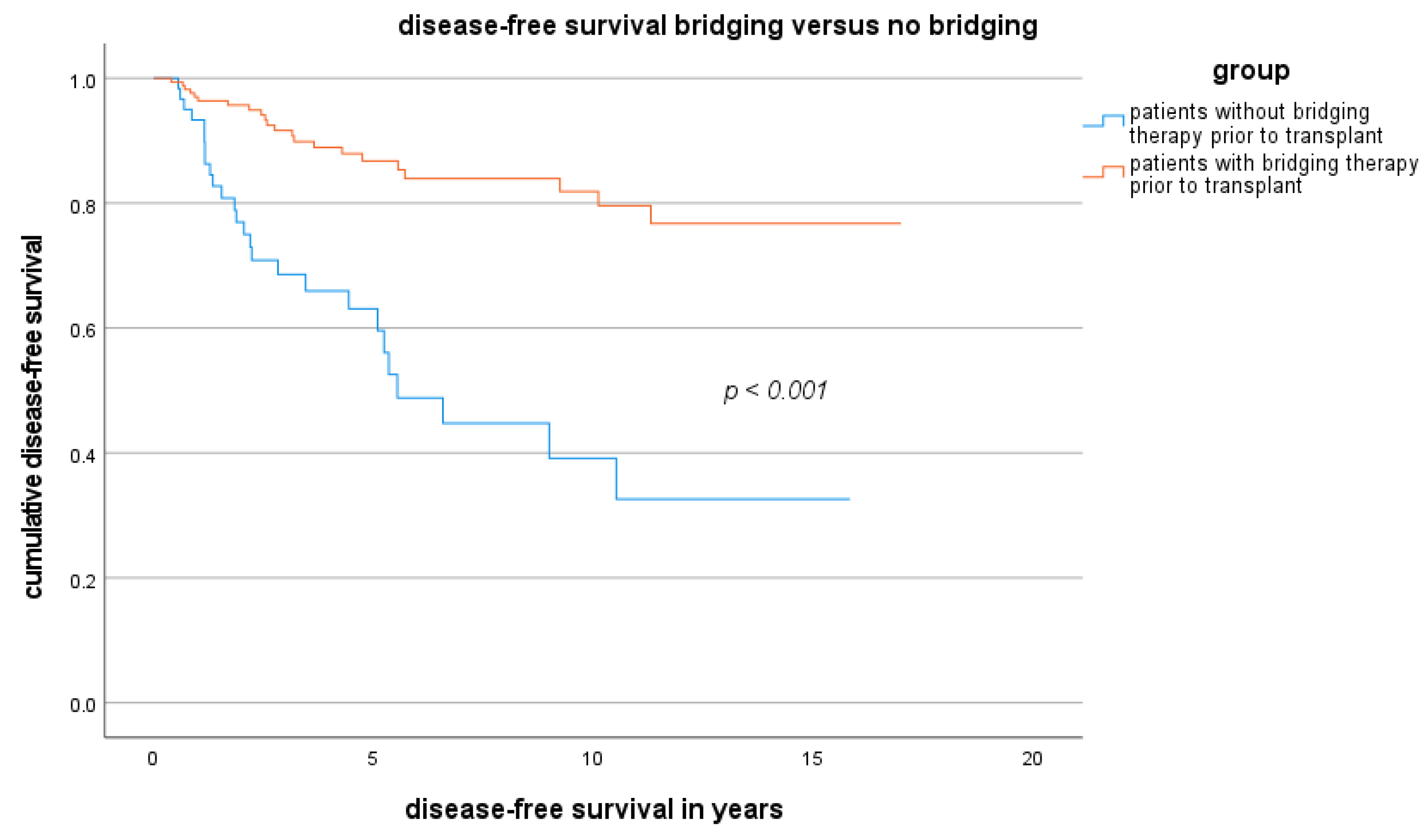
| LT (n = 106) | LDLT (n = 19) | p Value | |
|---|---|---|---|
| Preoperative factors | |||
| Age (median/range) | 62 (40–70) | 57 (23–69) | 0.0001 |
| Sex (male/female) | 90/16 | 18/1 | 0.25 |
| Cirrhosis (CPT A/B/C), n | 27/66/10 | 6/7/5 | 0.73 |
| No cirrhosis | 2 | 2 | 0.05 |
| Laboratory data (median, range) | |||
| labMELD | 11 (6–40) | 16 (6–24) | 0.58 |
| SE-MELD | 28 (22–39) | 25 (22–31) | 0.26 |
| Preoperative AFP (mg/dL) | 11.3 (1.0–1240) | 12.9 (1.7–5675) | 0.82 |
| Bridging therapy (%) | 67.9 | 47.4 | 0.22 |
| Partial response and downstaging (%) | 76.5 | 68.8 | 0.72 |
| Vital tumour residue after bridging (%) | 15.4 | 13.1 | 0.67 |
| Pathological findings (n) | |||
| Histological type (GX/G1/G2/G3) | 54/29/21/2 | 10/5/3/1 | 0.96 |
| Lymphatic permeation | 2 | 1 | 0.38 |
| Microvascular invasion | 9 | 3 | 0.32 |
| Perineural invasion | 4 | 1 | 0.76 |
| UICC T1/T2/T3/T4 | 50/42/14/0 | 8/8/3/0 | 0.66 |
| UICC N0/N1/N2 | 106/0/0 | 19/0/0 | |
| Postoperative course | |||
| Overall survival in d | 1882 | 2526 | 0.30 |
| 1 year survival (%) | 99.1 | 100 | 0.67 |
| 5 year survival (%) | 80.1 | 78.8 | 0.74 |
| LT (n = 79) | LDLT (n = 39) | p Value | |
|---|---|---|---|
| Preoperative factors | |||
| Age (median/range) | 63 (44–71) | 59 (23–71) | <0.0001 |
| Sex (male/female) | 69/10 | 34/5 | 0.98 |
| Cirrhosis (CPT A/B/C), n | 28/32/19 | 22/14/3 | 0.0001 |
| Laboratory data (median, range) | |||
| labMELD | 12 (6–40) | 10 (6–31) | 0.005 |
| Preoperative AFP (mg/dL) | 14.45 (2.3–161974) | 15.4 (1.9–28284) | 0.93 |
| Bridging therapy (%) | 79.7 | 66.7 | 0.12 |
| Partial response and downstaging (%) | 70.4 | 53.8 | 0.24 |
| Vital tumour residue after bridging (%) | 29.6 | 18.8 | 0.25 |
| Pathological findings (n) | |||
| Histological type (GX/G1/G2/G3) | 44/4/27/4 | 11/5/20/3 | 0.01 |
| Lymphatic invasion | 3 | 5 | 0.07 |
| Microvascular invasion | 18 | 9 | 0.42 |
| Perineural invasion | 3 | 0 | 0.22 |
| UICC T1/T2/T3/T4 | 13/42/19/1 | 10/16/10/1 | 0.76 |
| UICC N0/N1/N2 | 76/3/0 | 39/0/0 | 0.22 |
| Largest tumour diameter | 48 (48.4/17–220) | 52 (60/30–220) | 0.07 |
| Number of tumours | 2.5 (4.2/1–25) | 3 (4/1–20) | 0.84 |
| Postoperative course | |||
| Overall survival in d | 1964 | 1313 | 0.06 |
| 1 y survival (%) | 75.9 | 84.2 | 0.85 |
| 5 y survival (%) | 56.4 | 40 | 0.84 |
| Disease-free survival in d | 2705 | 1604 | 0.006 |
| Recurrence (%) | 27.8 | 38.5 | 0.25 |
| Initial recurrence site | |||
| Peritoneum | 6 | 3 | 0.96 |
| Local (Liver) | 10 | 6 | 0.71 |
| Lung | 12 | 10 | 0.22 |
| Lymph node | 5 | 5 | 0.27 |
| Bones | 5 | 6 | 0.14 |
| Adrenal gland | 4 | 2 | 0.96 |
| Factors | Disease-Free Survival | |||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Male (vs. female) | 1.12 (0.45–2.57) | 0.744 | ||
| Complication (grade ≥ 3) | 2.10 (0.30–15.3) | 0.371 | ||
| Largest tumour diameter > 50 mm (vs. <50 mm) | 0.79 (0.40–1.89) | 0.492 | ||
| >3 tumours (vs. <3 tumours) | 0.81 (0.33–1.39) | 0.566 | ||
| Tumour differentiation G1 or G2 (vs. G3) | 6.34 (2.51–17.2) | 0.0002 | 4.47 (0.99–19.1) | 0.0324 |
| Microvascular invasion (V1 vs. V0) | 8.21 (3.11–17.85) | <0.0001 | 1.89 (1.12–6.47) | 0.169 |
| The N1, 2 (vs. N0) | 3.39 (1.41–8.8) | 0.08 | ||
| Lymphatic permeation | 1.84 (0.54–3.23) | 0.226 | ||
| Bridging (vs. no bridging) | 7.50 (3.46–12.44) | 0.0001 | 2.67 (1.86–6.11) | 0.01 |
| Downstaging (vs. no downstaging) | 2.21 (0.76–4.21) | 0.652 | ||
| AFP > 1000 ng/mL (vs. <1000 ng/mL) | 4.19 (0.24–14.73) | 0.09 | ||
| labMELD > 20 (vs. <20) | 1.43 (0.73–4.10) | 0.429 | ||
| Preoperative ICU-stay (vs. no hospitality) | 2.13 (0.47–3.56) | 0.584 | ||
| Waiting time > 1 y (vs. <1 y) | 2.42 (0.75–4.83) | 0.08 | ||
| LDLT (vs. LT) | 1.28 (0.55–2.61) | 0.12 | ||
| MILAN | UCSF | “5-5-500”-rule | BCLC | Toronto | “Up-to-Seven” | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inside Milan | Outside Milan | Inside UCSF | Outside UCSF | Inside 5-5- 500 | Outside 5-5-500 | BCLC A | BCLC B | BCLC C | Inside Toronto | Outside Toronto | Inside UTSC | Outside UTSC | |
| Total (n =) | 125 | 120 | 47 | 73 | 40 | 80 | 25 | 64 | 31 | 104 | 16 | 45 | 75 |
| OS in d | 1979.9 | 1748.8 | 1646.5 | 1798.75 | 1304.7 | 1958.2 | 1880.8 | 1744.5 | 1618.7 | 1776 | 1508.9 | 1524.9 | 1869.7 |
| 5 y survival (%) | 79.9% | 51% | 60.9% | 46.4% | 53% | 50.8% | 64% | 52.4% | 40% | 54.4% | 33.3% | 63.2% | 47.5% |
| 1 y survival (%) | 99.5% | 78.64% | 88.4% | 76.1% | 79% | 80.8% | 88% | 82% | 70% | 80.4% | 73.3% | 90.5% | 75.8% |
| DFS in d | 2951.5 | 2341.1 | 2098.3 | 2504.5 | 1784.9 | 2618.2 | 2639.2 | 2432.2 | 1909.9 | 2464 | 1537.1 | 1992.8 | 2549 |
| recurrence (%) | 11.2% | 31.3% | 19.1% | 37.5% | 12.5% | 40% | 12% | 29.7% | 48.4% | 26% | 62.5% | 13.3% | 41.3% |
| LT (n =) | 106 | 79 | 33 | 46 | 24 | 55 | 13 | 46 | 20 | 70 | 9 | 30 | 49 |
| LDLT (n =) | 19 | 39 | 14 | 27 | 16 | 25 | 12 | 18 | 11 | 34 | 7 | 15 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohland, O.; Freye, L.; Schwenk, L.; Ali-Deeb, A.; Ardelt, M.; Bauschke, A.; Settmacher, U.; Rauchfuß, F.; Dondorf, F. Liver Transplantation for Hepatocellular Carcinoma beyond the Milan Criteria: A Specific Role for Living Donor Liver Transplantation after Neoadjuvant Therapy. Cancers 2024, 16, 920. https://doi.org/10.3390/cancers16050920
Rohland O, Freye L, Schwenk L, Ali-Deeb A, Ardelt M, Bauschke A, Settmacher U, Rauchfuß F, Dondorf F. Liver Transplantation for Hepatocellular Carcinoma beyond the Milan Criteria: A Specific Role for Living Donor Liver Transplantation after Neoadjuvant Therapy. Cancers. 2024; 16(5):920. https://doi.org/10.3390/cancers16050920
Chicago/Turabian StyleRohland, Oliver, Lea Freye, Laura Schwenk, Aladdin Ali-Deeb, Michael Ardelt, Astrid Bauschke, Utz Settmacher, Falk Rauchfuß, and Felix Dondorf. 2024. "Liver Transplantation for Hepatocellular Carcinoma beyond the Milan Criteria: A Specific Role for Living Donor Liver Transplantation after Neoadjuvant Therapy" Cancers 16, no. 5: 920. https://doi.org/10.3390/cancers16050920
APA StyleRohland, O., Freye, L., Schwenk, L., Ali-Deeb, A., Ardelt, M., Bauschke, A., Settmacher, U., Rauchfuß, F., & Dondorf, F. (2024). Liver Transplantation for Hepatocellular Carcinoma beyond the Milan Criteria: A Specific Role for Living Donor Liver Transplantation after Neoadjuvant Therapy. Cancers, 16(5), 920. https://doi.org/10.3390/cancers16050920





