Long Noncoding RNA RP11-278A23.1, a Potential Modulator of p53 Tumor Suppression, Contributes to Colorectal Cancer Progression
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Analysis of Gene Expression Datasets
2.2. Cell Culture
2.3. siRNAs and Expression Vectors
2.4. RNA Extraction and Reverse Transcription–Quantitative PCR (RT–qPCR)
2.5. Gel Electrophoresis of PCR Products
2.6. Cell Growth Assay
2.7. Quantification of Apoptosis Induction
2.8. Antibodies
2.9. Western Blot Analysis
2.10. Subcellular Fractionation
2.11. RNA Sequencing (RNA-seq) Analysis
2.12. Colony Formation Assay
3. Results
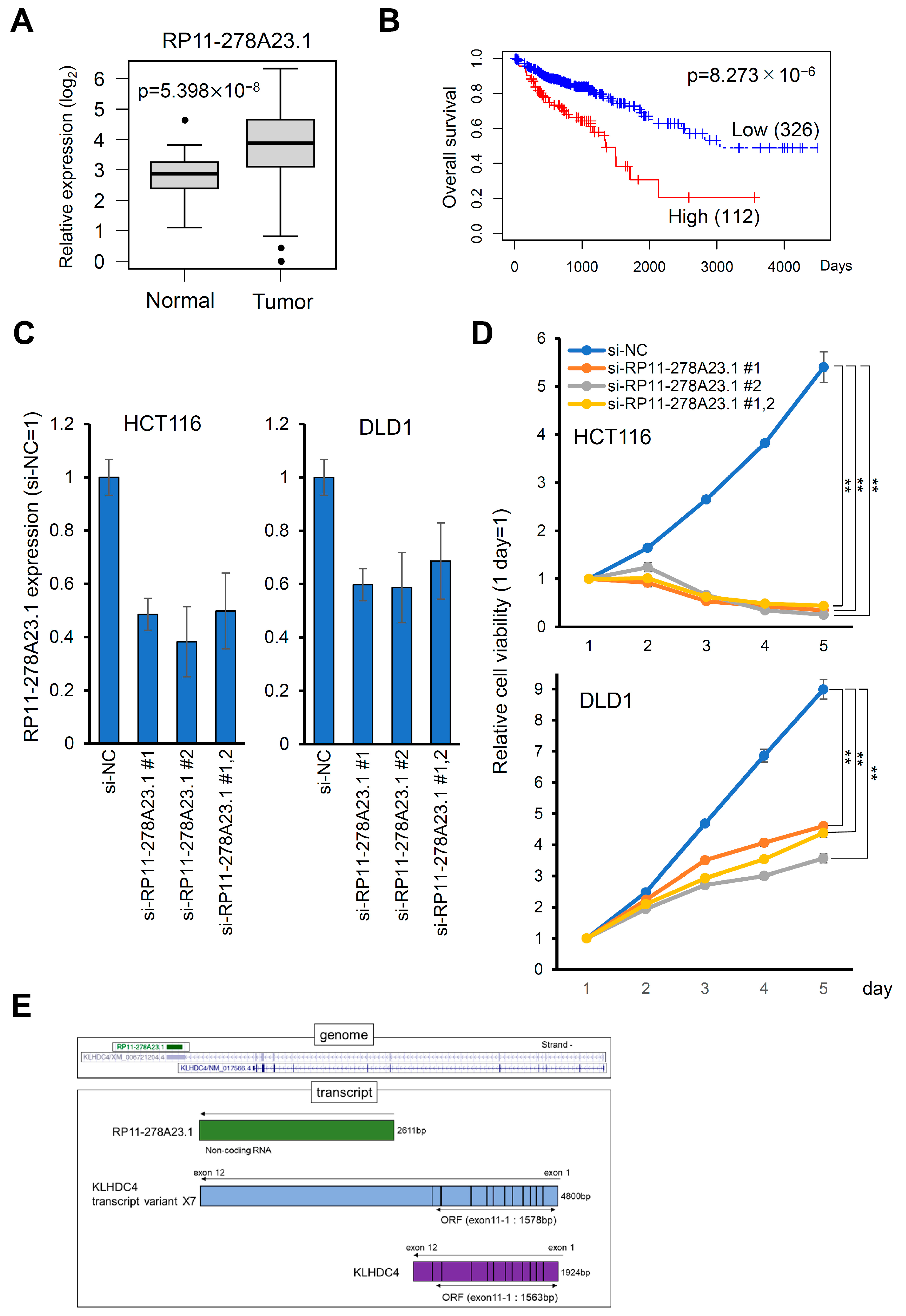
3.1. High Expression of lncRNA RP11-278A23.1 Is Correlated with Poor Prognosis in Colorectal Cancers
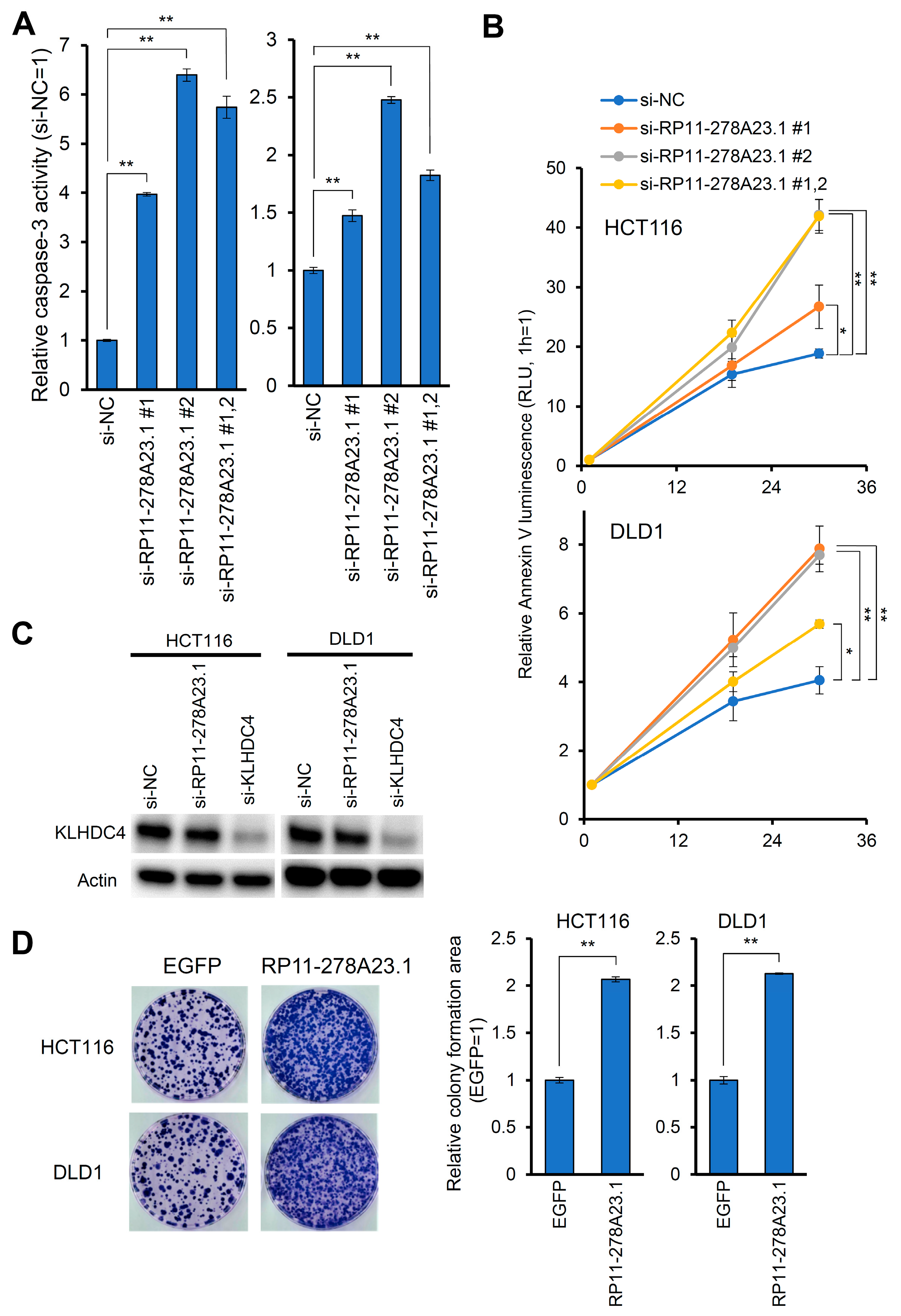
3.2. Knockdown of RP11-278A23.1 Suppresses Cell Growth and Induces Apoptosis
3.3. RP11-278A23.1 Function Is Independent of the KLHDC4 Protein
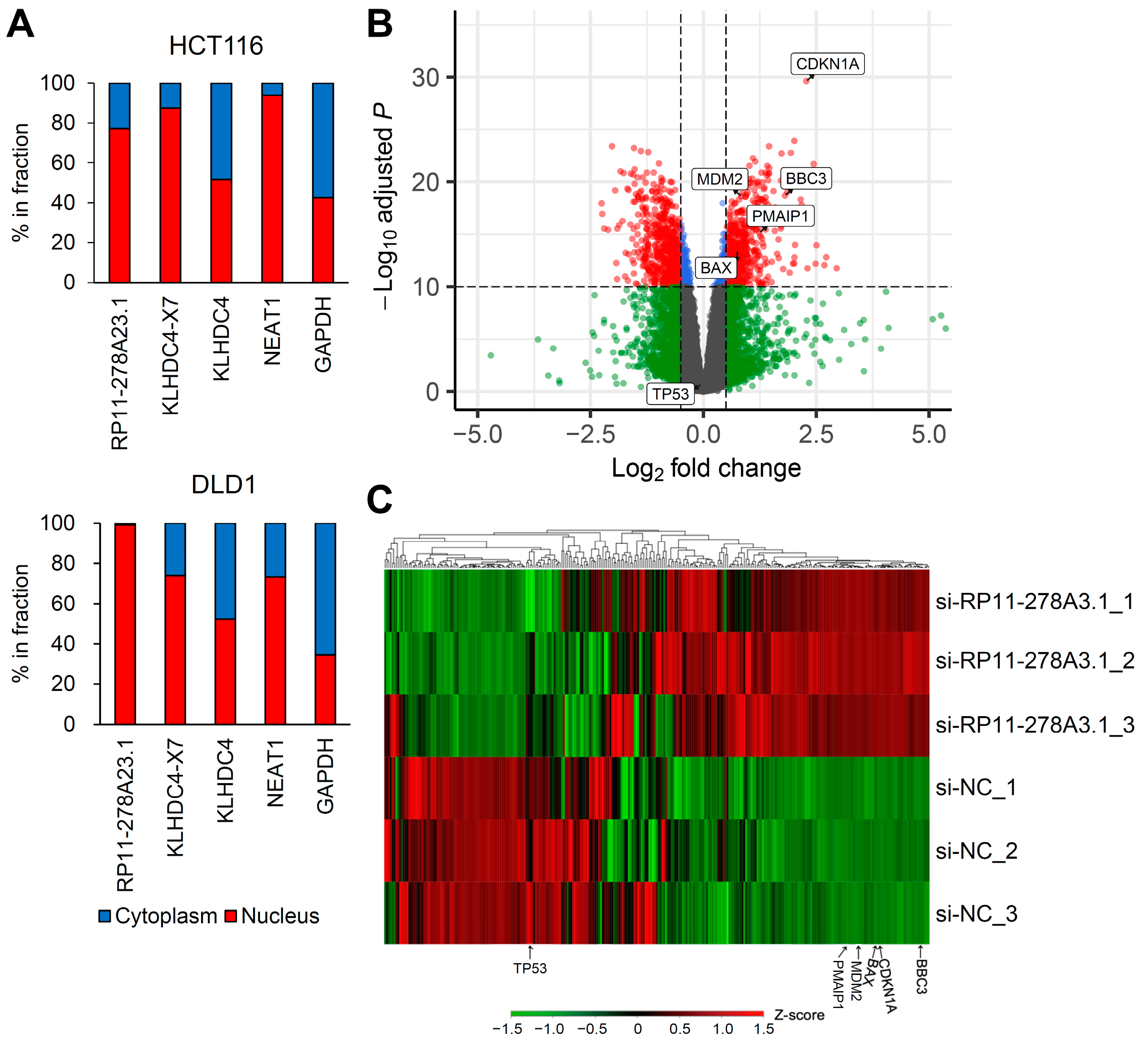
3.4. RP11-278A23.1 and KLHDC4-X7 Localize to the Nucleus
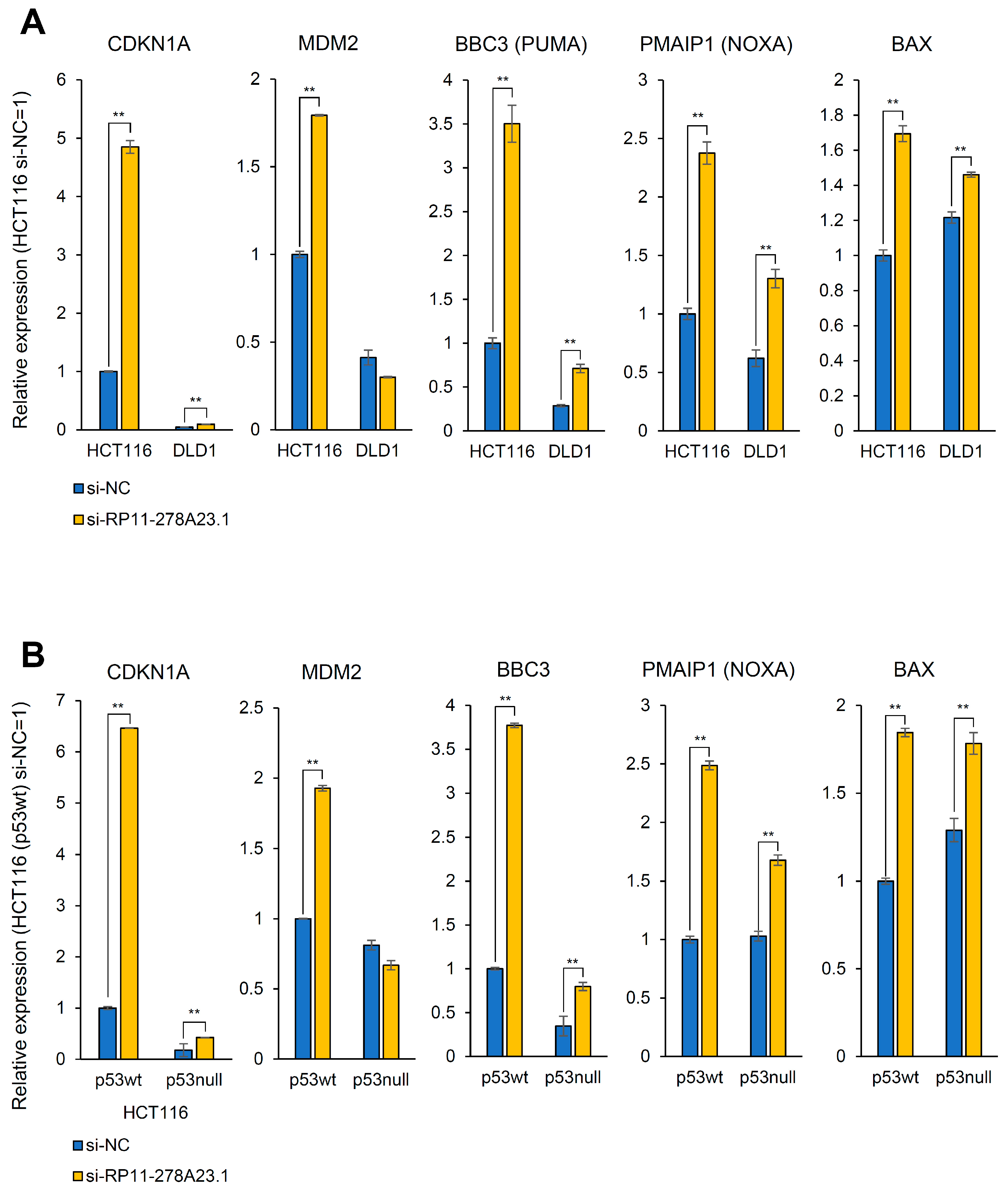
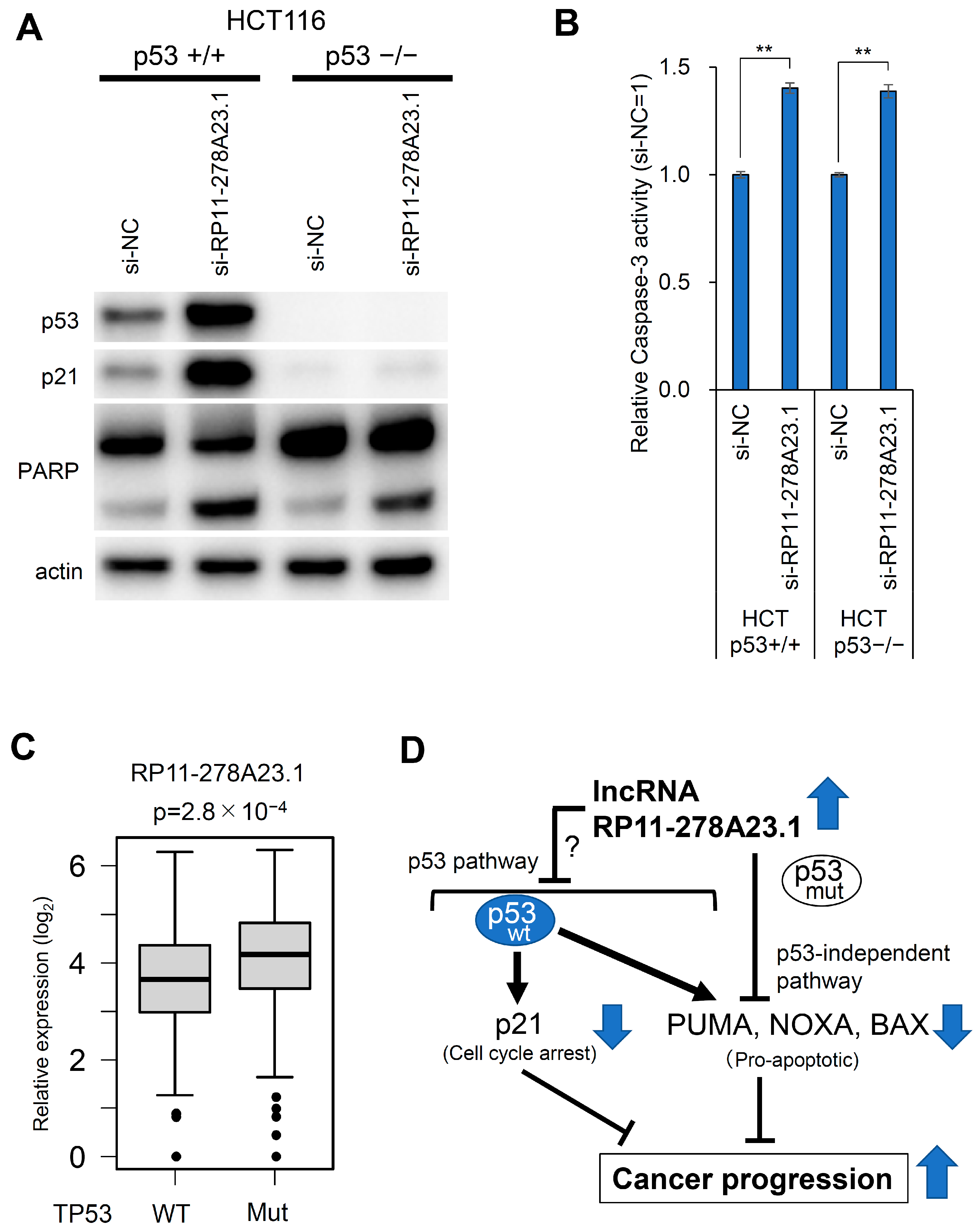
3.5. RP11-278A23.1 Knockdown in p53-Wild-Type Colorectal Cancer Cells Alters p53 Target Gene Expression
3.6. RP11-278A23.1 Also Affects p53-Independent Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Human Genome Sequencing Consortium. Finishing the euchromatic sequence of the human genome. Nature 2004, 431, 931–945. [Google Scholar] [CrossRef]
- Consortium, E.P. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Gomes, C.P.C.; Agg, B.; Andova, A.; Arslan, S.; Baker, A.; Bartekova, M.; Beis, D.; Betsou, F.; Wettinger, S.B.; Bugarski, B.; et al. Catalyzing Transcriptomics Research in Cardiovascular Disease: The CardioRNA COST Action CA17129. Noncoding RNA 2019, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Sweta, S.; Dudnakova, T.; Sudheer, S.; Baker, A.H.; Bhushan, R. Importance of Long Non-coding RNAs in the Development and Disease of Skeletal Muscle and Cardiovascular Lineages. Front. Cell Dev. Biol. 2019, 7, 228. [Google Scholar] [CrossRef] [PubMed]
- Idogawa, M.; Ohashi, T.; Sasaki, Y.; Nakase, H.; Tokino, T. Long non-coding RNA NEAT1 is a transcriptional target of p53 and modulates p53-induced transactivation and tumor-suppressor function. Int. J. Cancer 2017, 140, 2785–2791. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, X. Long non-coding RNA signature in colorectal cancer: Research progression and clinical application. Cancer Cell Int. 2023, 23, 28. [Google Scholar] [CrossRef]
- Chen, S.; Shen, X. Long noncoding RNAs: Functions and mechanisms in colon cancer. Mol. Cancer 2020, 19, 167. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Chen, X.; Niu, X.H.; Peng, X.F. LncRNAs in colorectal cancer: Biomarkers to therapeutic targets. Clin. Chim. Acta 2023, 543, 117305. [Google Scholar] [CrossRef]
- Siddiqui, H.; Al-Ghafari, A.; Choudhry, H.; Al Doghaither, H. Roles of long non-coding RNAs in colorectal cancer tumorigenesis: A Review. Mol. Clin. Oncol. 2019, 11, 167–172. [Google Scholar] [CrossRef]
- Kogo, R.; Shimamura, T.; Mimori, K.; Kawahara, K.; Imoto, S.; Sudo, T.; Tanaka, F.; Shibata, K.; Suzuki, A.; Komune, S.; et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011, 71, 6320–6326. [Google Scholar] [CrossRef]
- Gupta, R.A.; Shah, N.; Wang, K.C.; Kim, J.; Horlings, H.M.; Wong, D.J.; Tsai, M.C.; Hung, T.; Argani, P.; Rinn, J.L.; et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature 2010, 464, 1071–1076. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Chen, X.; Liu, J.; Gu, H.; Fan, R.; Ge, H. Long non-coding RNA HOTAIR knockdown enhances radiosensitivity through regulating microRNA-93/ATG12 axis in colorectal cancer. Cell Death Dis. 2020, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Tufail, M. HOTAIR in colorectal cancer: Structure, function, and therapeutic potential. Med. Oncol. 2023, 40, 259. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.W.; Jin, J.; Wu, X.Y.; Ren, Q.L.; Farzaneh, M. MALAT1-related signaling pathways in colorectal cancer. Cancer Cell Int. 2022, 22, 126. [Google Scholar] [CrossRef]
- Ji, Q.; Zhang, L.; Liu, X.; Zhou, L.; Wang, W.; Han, Z.; Sui, H.; Tang, Y.; Wang, Y.; Liu, N.; et al. Long non-coding RNA MALAT1 promotes tumour growth and metastasis in colorectal cancer through binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2 complex. Br. J. Cancer 2014, 111, 736–748. [Google Scholar] [CrossRef]
- Xu, C.; Yang, M.; Tian, J.; Wang, X.; Li, Z. MALAT-1: A long non-coding RNA and its important 3’ end functional motif in colorectal cancer metastasis. Int. J. Oncol. 2011, 39, 169–175. [Google Scholar] [CrossRef]
- Tian, W.; Du, Y.; Ma, Y.; Gu, L.; Zhou, J.; Deng, D. MALAT1-miR663a negative feedback loop in colon cancer cell functions through direct miRNA-lncRNA binding. Cell Death Dis. 2018, 9, 857. [Google Scholar] [CrossRef]
- Ozawa, T.; Matsuyama, T.; Toiyama, Y.; Takahashi, N.; Ishikawa, T.; Uetake, H.; Yamada, Y.; Kusunoki, M.; Calin, G.; Goel, A. CCAT1 and CCAT2 long noncoding RNAs, located within the 8q.24.21 ‘gene desert’, serve as important prognostic biomarkers in colorectal cancer. Ann. Oncol. 2017, 28, 1882–1888. [Google Scholar] [CrossRef]
- McCleland, M.L.; Mesh, K.; Lorenzana, E.; Chopra, V.S.; Segal, E.; Watanabe, C.; Haley, B.; Mayba, O.; Yaylaoglu, M.; Gnad, F.; et al. CCAT1 is an enhancer-templated RNA that predicts BET sensitivity in colorectal cancer. J. Clin. Investig. 2016, 126, 639–652. [Google Scholar] [CrossRef]
- Ling, H.; Spizzo, R.; Atlasi, Y.; Nicoloso, M.; Shimizu, M.; Redis, R.S.; Nishida, N.; Gafa, R.; Song, J.; Guo, Z.; et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013, 23, 1446–1461. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.F.; Yin, Q.F.; Chen, T.; Zhang, Y.; Zhang, X.O.; Wu, Z.; Zhang, S.; Wang, H.B.; Ge, J.; Lu, X.; et al. Human colorectal cancer-specific CCAT1-L lncRNA regulates long-range chromatin interactions at the MYC locus. Cell Res. 2014, 24, 513–531. [Google Scholar] [CrossRef]
- Idogawa, M.; Nakase, H.; Sasaki, Y.; Tokino, T. Prognostic Effect of Long Noncoding RNA NEAT1 Expression Depends on p53 Mutation Status in Cancer. J. Oncol. 2019, 2019, 4368068. [Google Scholar] [CrossRef]
- Suzuki, N.; Idogawa, M.; Tange, S.; Ohashi, T.; Sasaki, Y.; Nakase, H.; Tokino, T. p53-induced ARVCF modulates the splicing landscape and supports the tumor suppressive function of p53. Oncogene 2020, 39, 2202–2211. [Google Scholar] [CrossRef]
- Lian, Y.F.; Yuan, J.; Cui, Q.; Feng, Q.S.; Xu, M.; Bei, J.X.; Zeng, Y.X.; Feng, L. Upregulation of KLHDC4 Predicts a Poor Prognosis in Human Nasopharyngeal Carcinoma. PLoS ONE 2016, 11, e0152820. [Google Scholar] [CrossRef]
- Derrien, T.; Johnson, R.; Bussotti, G.; Tanzer, A.; Djebali, S.; Tilgner, H.; Guernec, G.; Martin, D.; Merkel, A.; Knowles, D.G.; et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012, 22, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M. Census and evaluation of p53 target genes. Oncogene 2017, 36, 3943–3956. [Google Scholar] [CrossRef]
- Harper, J.W.; Adami, G.R.; Wei, N.; Keyomarsi, K.; Elledge, S.J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 75, 805–816. [Google Scholar] [CrossRef]
- Tripathi, V.; Ellis, J.D.; Shen, Z.; Song, D.Y.; Pan, Q.; Watt, A.T.; Freier, S.M.; Bennett, C.F.; Sharma, A.; Bubulya, P.A.; et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell 2010, 39, 925–938. [Google Scholar] [CrossRef]
- Yu, Y.; Nangia-Makker, P.; Farhana, L.; Majumdar, A.P.N. A novel mechanism of lncRNA and miRNA interaction: CCAT2 regulates miR-145 expression by suppressing its maturation process in colon cancer cells. Mol. Cancer 2017, 16, 155. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.Z.; Chu, B.F.; Zhang, Y.; Weng, M.Z.; Qin, Y.Y.; Gong, W.; Quan, Z.W. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015, 6, e1583. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamikokura, M.; Tange, S.; Nakase, H.; Tokino, T.; Idogawa, M. Long Noncoding RNA RP11-278A23.1, a Potential Modulator of p53 Tumor Suppression, Contributes to Colorectal Cancer Progression. Cancers 2024, 16, 882. https://doi.org/10.3390/cancers16050882
Kamikokura M, Tange S, Nakase H, Tokino T, Idogawa M. Long Noncoding RNA RP11-278A23.1, a Potential Modulator of p53 Tumor Suppression, Contributes to Colorectal Cancer Progression. Cancers. 2024; 16(5):882. https://doi.org/10.3390/cancers16050882
Chicago/Turabian StyleKamikokura, Masayo, Shoichiro Tange, Hiroshi Nakase, Takashi Tokino, and Masashi Idogawa. 2024. "Long Noncoding RNA RP11-278A23.1, a Potential Modulator of p53 Tumor Suppression, Contributes to Colorectal Cancer Progression" Cancers 16, no. 5: 882. https://doi.org/10.3390/cancers16050882
APA StyleKamikokura, M., Tange, S., Nakase, H., Tokino, T., & Idogawa, M. (2024). Long Noncoding RNA RP11-278A23.1, a Potential Modulator of p53 Tumor Suppression, Contributes to Colorectal Cancer Progression. Cancers, 16(5), 882. https://doi.org/10.3390/cancers16050882










