Safety of Boron Neutron Capture Therapy with Borofalan(10B) and Its Efficacy on Recurrent Head and Neck Cancer: Real-World Outcomes from Nationwide Post-Marketing Surveillance
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Data Collection
2.3. Treatments
2.4. Assessments
2.5. Statistical Analysis
3. Results
3.1. Patient Disposition and Baseline Characteristics
3.2. Status of BNCT Implementation
3.3. Safety
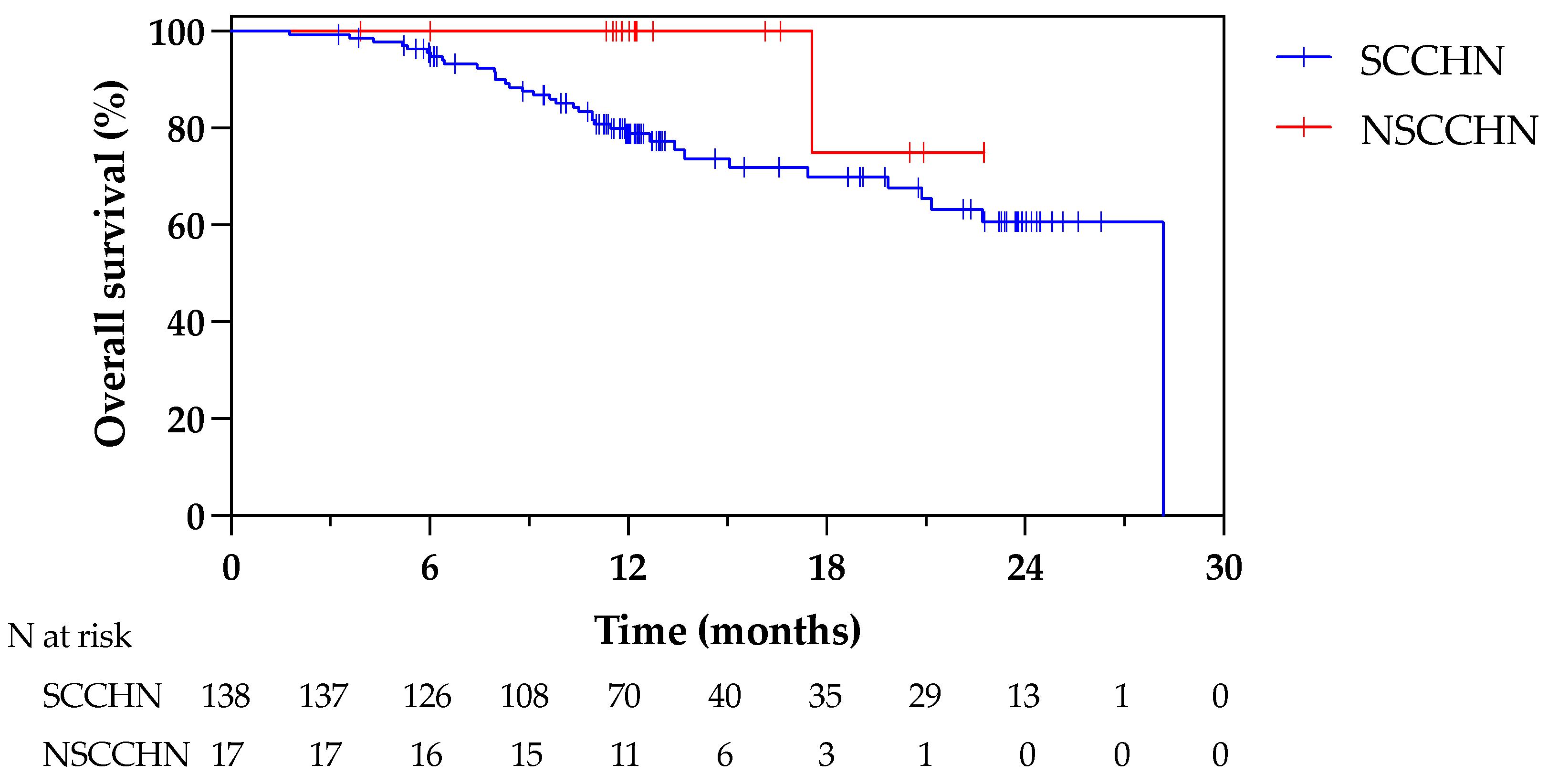
3.4. Efficacy on LA/LR-HNC
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauerwein, W.A.; Wittig, A.; Moss, R.; Nakagawa, Y. Neutron Capture Therapy: Principles and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Barth, R.F.; Grecula, J.C. Boron neutron capture therapy at the crossroads—Where do we go from here? Appl. Radiat. Isot. 2020, 160, 109029. [Google Scholar] [CrossRef] [PubMed]
- Kiyanagi, Y. Accelerator-based neutron source for boron neutron capture therapy. Ther. Radiol. Oncol. 2018, 2, 55. [Google Scholar] [CrossRef]
- Tanaka, H.; Sakurai, Y.; Suzuki, M.; Masunaga, S.; Kinashi, Y.; Kashino, G.; Liu, Y.; Mitsumoto, T.; Yajima, S.; Tsutsui, H.; et al. Characteristics comparison between a cyclotron-based neutron source and KUR-HWNIF for boron neutron capture therapy. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2009, 267, 1970–1977. [Google Scholar] [CrossRef]
- Tanaka, H.; Sakurai, Y.; Suzuki, M.; Takata, T.; Masunaga, S.; Kinashi, Y.; Kashino, G.; Liu, Y.; Mitsumoto, T.; Yajima, S.; et al. Improvement of dose distribution in phantom by using epithermal neutron source based on the Be(p,n) reaction using a 30 MeV proton cyclotron accelerator. Appl. Radiat. Isot. 2009, 67, S258–S261. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Sakurai, Y.; Suzuki, M.; Masunaga, S.; Mitsumoto, T.; Fujita, K.; Kashino, G.; Kinashi, Y.; Liu, Y.; Takada, M.; et al. Experimental verification of beam characteristics for cyclotron-based epithermal neutron source (C-BENS). Appl. Radiat. Isot. 2011, 69, 1642–1645. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, A.; Asano, T.; Hirose, K.; Igaki, H.; Kawabata, S.; Kumada, H. Initiatives Toward Clinical Boron Neutron Capture Therapy in Japan. Cancer Biother. Radiopharm. 2023, 38, 201–207. [Google Scholar] [CrossRef]
- Gormley, M.; Creaney, G.; Schache, A.; Ingarfield, K.; Conway, D.I. Reviewing the epidemiology of head and neck cancer: Definitions, trends and risk factors. Br. Dent. J. 2022, 233, 780–786. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Head and Neck Cancers. NCCN Guidelines Version 1.2024. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 20 December 2023).
- Hirose, K.; Konno, A.; Hiratsuka, J.; Yoshimoto, S.; Kato, T.; Ono, K.; Otsuki, N.; Hatazawa, J.; Tanaka, H.; Takayama, K.; et al. Boron neutron capture therapy using cyclotron-based epithermal neutron source and borofalan ((10)B) for recurrent or locally advanced head and neck cancer (JHN002): An open-label phase II trial. Radiother. Oncol. 2021, 155, 182–187. [Google Scholar] [CrossRef]
- Hirose, K.; Sato, M.; Kato, T.; Takayama, K.; Suzuki, M.; Yamaguchi, H.; Seto, I.; Kikuchi, Y.; Murakami, M.; Takai, Y. Profile analysis of adverse events after boron neutron capture therapy for head and neck cancer: A sub-analysis of the JHN002 study. J. Radiat. Res. 2022, 63, 393–401. [Google Scholar] [CrossRef]
- White, D.R.; Griffith, I.J.; Wilson, I.J. Report 46. J. Int. Comm. Radiat. Units Meas. 1992, 24. [Google Scholar] [CrossRef]
- Aihara, T.; Hiratsuka, J.; Ishikawa, H.; Kumada, H.; Ohnishi, K.; Kamitani, N.; Suzuki, M.; Sakurai, H.; Harada, T. Fatal carotid blowout syndrome after BNCT for head and neck cancers. Appl. Radiat. Isot. 2015, 106, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Awan, M.J.; Nedzi, L.; Wang, D.; Tumati, V.; Sumer, B.; Xie, X.J.; Smith, I.; Truelson, J.; Hughes, R.; Myers, L.L.; et al. Final results of a multi-institutional phase II trial of reirradiation with concurrent weekly cisplatin and cetuximab for recurrent or second primary squamous cell carcinoma of the head and neck. Ann. Oncol. 2018, 29, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Vargo, J.A.; Ferris, R.L.; Ohr, J.; Clump, D.A.; Davis, K.S.; Duvvuri, U.; Kim, S.; Johnson, J.T.; Bauman, J.E.; Gibson, M.K.; et al. A prospective phase 2 trial of reirradiation with stereotactic body radiation therapy plus cetuximab in patients with previously irradiated recurrent squamous cell carcinoma of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Vormittag, L.; Lemaire, C.; Radonjic, D.; Kornek, G.; Selzer, E. Re-irradiation combined with capecitabine in locally recurrent squamous cell carcinoma of the head and neck. A prospective phase II trial. Strahlenther Onkol. 2012, 188, 235–242. [Google Scholar] [CrossRef]
- Berger, B.; Belka, C.; Weinmann, M.; Bamberg, M.; Budach, W.; Hehr, T. Reirradiation with alternating docetaxel-based chemotherapy for recurrent head and neck squamous cell carcinoma: Update of a single-center prospective phase II protocol. Strahlenther Onkol. 2010, 186, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Spencer, S.A.; Harris, J.; Wheeler, R.H.; Machtay, M.; Schultz, C.; Spanos, W.; Rotman, M.; Meredith, R.; Ang, K.K. Final report of RTOG 9610, a multi-institutional trial of reirradiation and chemotherapy for unresectable recurrent squamous cell carcinoma of the head and neck. Head Neck 2008, 30, 281–288. [Google Scholar] [CrossRef]
- Langer, C.J.; Harris, J.; Horwitz, E.M.; Nicolaou, N.; Kies, M.; Curran, W.; Wong, S.; Ang, K. Phase II study of low-dose paclitaxel and cisplatin in combination with split-course concomitant twice-daily reirradiation in recurrent squamous cell carcinoma of the head and neck: Results of Radiation Therapy Oncology Group Protocol 9911. J. Clin. Oncol. 2007, 25, 4800–4805. [Google Scholar] [CrossRef]
- Langendijk, J.A.; Kasperts, N.; Leemans, C.R.; Doornaert, P.; Slotman, B.J. A phase II study of primary reirradiation in squamous cell carcinoma of head and neck. Radiother. Oncol. 2006, 78, 306–312. [Google Scholar] [CrossRef]
- Spencer, S.A.; Harris, J.; Wheeler, R.H.; Machtay, M.; Schultz, C.; Spanos, W.; Rotman, M.; Meredith, R. RTOG 96-10: Reirradiation with concurrent hydroxyurea and 5-fluorouracil in patients with squamous cell cancer of the head and neck. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 1299–1304. [Google Scholar] [CrossRef]
- Harrington, K.J.; Cohen, E.E.W.; Denis Soulieres, J.D.; Licitra, L.F.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.H.; Mach, N.; Mehra, R.; Burtness, B.; et al. Pembrolizumab (pembro) for recurrent head and neck squamous cell carcinoma (HNSCC): Post hoc analyses of phase 3 KEYNOTE-040 prior radiation treatment (RT) and disease state. J. Clin. Oncol. 2019, 37 (Suppl. S15), 6026. [Google Scholar] [CrossRef]
- Cognetti, D.M.; Johnson, J.M.; Curry, J.M.; Kochuparambil, S.T.; McDonald, D.; Mott, F.; Fidler, M.J.; Stenson, K.; Vasan, N.R.; Razaq, M.A.; et al. Phase 1/2a, open-label, multicenter study of RM-1929 photoimmunotherapy in patients with locoregional, recurrent head and neck squamous cell carcinoma. Head Neck 2021, 43, 3875–3887. [Google Scholar] [CrossRef]
- Hayashi, K.; Koto, M.; Ikawa, H.; Hagiwara, Y.; Tsuji, H.; Ogawa, K.; Kamada, T. Feasibility of Re-irradiation using carbon ions for recurrent head and neck malignancies after carbon-ion radiotherapy. Radiother. Oncol. 2019, 136, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Nakamura, T.; Mitsudo, K.; Kimura, K.; Yamaguchi, H.; Ono, T.; Azami, Y.; Takayama, K.; Hirose, K.; Yabuuchi, T.; et al. Re-irradiation using proton beam therapy combined with weekly intra-arterial chemotherapy for recurrent oral cancer. Asia Pac. J. Clin. Oncol. 2017, 13, e394–e401. [Google Scholar] [CrossRef] [PubMed]
- Romesser, P.B.; Cahlon, O.; Scher, E.D.; Hug, E.B.; Sine, K.; DeSelm, C.; Fox, J.L.; Mah, D.; Garg, M.K.; Han-Chih Chang, J.; et al. Proton Beam Reirradiation for Recurrent Head and Neck Cancer: Multi-institutional Report on Feasibility and Early Outcomes. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Phan, J.; Sio, T.T.; Nguyen, T.P.; Takiar, V.; Gunn, G.B.; Garden, A.S.; Rosenthal, D.I.; Fuller, C.D.; Morrison, W.H.; Beadle, B.; et al. Reirradiation of Head and Neck Cancers With Proton Therapy: Outcomes and Analyses. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 30–41. [Google Scholar] [CrossRef]
- McDonald, M.W.; Zolali-Meybodi, O.; Lehnert, S.J.; Estabrook, N.C.; Liu, Y.; Cohen-Gadol, A.A.; Moore, M.G. Reirradiation of Recurrent and Second Primary Head and Neck Cancer With Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 808–819. [Google Scholar] [CrossRef]

| Period | CRF Issued, n | CRF Collected and Fixed, n | Collection Rate (%) |
|---|---|---|---|
| Within 7 days | 179 | 179 | 100 |
| 6 months | 198 | 177 | 99.4 |
| 1 year | 156 | 142 | 100 |
| 2 years | 128 | 76 | 64.4 |
| 3 years | 51 | 11 | 22.9 |
| Characteristic | All (n = 162) | SCCHN (n = 144) | NSCCHN (n = 17) |
|---|---|---|---|
| Median age, years (range) | 68 (38–89) | 68 (38–89) | 71 (39–87) |
| >65, n (%) | 93 (57.4) | 81 (56.3) | 11 (64.7) |
| Sex, n (%) | |||
| Male | 114 (70.4) | 107 (74.3) | 7 (41.2) |
| Female | 48 (29.6) | 37 (25.7) | 10 (58.8) |
| ECOG-PS, n (%) | |||
| 0 | 78 (48.2) | 69 (47.9) | 9 (52.9) |
| 1 | 78 (48.2) | 70 (48.6) | 8 (47.1) |
| 2 | 5 (3.1) | 5 (3.5) | 0 |
| Unknown | 1 (0.6) | 0 | 0 |
| Tumor location, n (%) | |||
| Cervical lymph node | 52 (29.2) | 49 (30.8) | 3 (16.7) |
| Hypopharynx | 24 (13.5) | 24 (15.1) | 0 |
| Oral (excluding tongue) | 20 (11.2) | 19 (12.0) | 1 (5.6) |
| Oropharynx | 18 (10.1) | 18 (11.3) | 0 |
| Larynx | 11 (6.2) | 11 (6.9) | 0 |
| External auditory canal | 10 (5.6) | 10 (6.3) | 0 |
| Maxillary sinus | 6 (3.4) | 6 (3.8) | 0 |
| Nasopharynx | 5 (2.8) | 2 (1.3) | 3 (16.7) |
| Maxilla | 5 (2.8) | 4 (2.5) | 1 (5.7) |
| Parotid gland | 5 (2.8) | 1 (0.6) | 4 (22.2) |
| Orbit | 4 (2.3) | 3 (1.9) | 1 (5.7) |
| Parapharyngeal space | 3 (1.7) | 2 (1.3) | 1 (5.7) |
| Tongue | 3 (1.7) | 3 (1.9) | 0 |
| Brain | 1 (0.6) | NA | NA |
| Others | 11 * (6.2) | 7 ** (4.4) | 4 *** (22.2) |
| TNM classification, n (%) | |||
| T1–2 | 48 (30.3) | 43 (29.9) | 5 (29.4) |
| T3–4 | 72 (45.6) | 63 (43.8) | 9 (52.9) |
| N1–2 | 33 (20.5) | 31 (21.5) | 2 (11.8) |
| N3 | 19 (11.8) | 18 (12.5) | 1 (5.9) |
| Prior systemic therapy, n (%) | 128 (79.0) | 120 (83.3) | 8 (47.1) |
| Prior radiation therapy, n (%) | 151 (93.2) | 136 (94.4) | 14 (82.4) |
| Median cumulative dose, Gy (range) | 70 (24–130) | 70 (24–130) | 68 (48–90) |
| Duration from last irradiation | |||
| <6 months, n (%) | 20 (13.3) | 19 (14.0) | 1 (7.1) |
| ≥6 months, n (%) | 128 (84.8) | 114 (83.8) | 13 (92.9) |
| Unknown | 3 (2.0) | 3 (2.2) | 0 |
| Parameter | All (N = 160) | SCCHN (n = 143) | NSCCHN (n = 17) |
|---|---|---|---|
| 10B conc. after 2-h infusion, ppm (range) | 31.3 (20.9–46.5) | 31.1 (21.0–46.5) | 32.3 (20.9–40.7) |
| Completion of planned irradiation, n (%) | |||
| Completed | 155 (96.9) | 138 (96.5) | 17 (100) |
| Interrupted | 5 (3.1) | 5 (3.5) | 0 |
| Actual charge amount of accelerator, C (interquartile range) | 2.44 (2.05–2.95) | 2.45 (2.08–3.03) | 2.29 (2.03–2.59) |
| Prescribed dose of mucosa, n (%) | |||
| <12 Gy-Eq | 14 (8.8) | 11 (7.7) | 3 (17.7) |
| 12 Gy-Eq | 60 (37.5) | 53 (37.1) | 7 (41.2) |
| >12 Gy-Eq | 86 (53.8) | 79 (55.2) | 7 (41.2) |
| Tumor dose, Gy-Eq (interquartile range) | |||
| Dmax | 46.9 (38.9–56.1) | 46.4 (38.7–55.8) | 50.3 (42.7–71.7) |
| Dmin | 26.9 (22.4–32.2) | 26.9 (22.2–31.7) | 25.5 (24.1–37.8) |
| Dmean | 38.7 (32.3–45.3) | 38.4 (32.4–44) | 44.6 (31.2–57.4) |
| Acute TRAE (N = 162) | Grade 1 and 2 n (%) | Grade 3 n (%) | Grade 4 n (%) |
|---|---|---|---|
| Hyperamylasemia *1 | 26 (16.1) | 63 (38.9) | 47 (29.0) |
| Stomatitis *2 | 66 (40.7) | 17 (10.5) | 0 |
| Sialoadenitis *3 | 81 (50.0) | 1 (0.6) | 0 |
| Alopecia | 80 (49.4) | 0 | 0 |
| Decreased appetite *4 | 53 (32.7) | 4 (2.5) | 0 |
| Nausea | 48 (29.6) | 2 (1.2) | 0 |
| Taste disorder | 39 (24.1) | 0 | 0 |
| Crystalluria | 33 (20.4) | 0 | 0 |
| Pharyngeal inflammation *5 | 28 (17.3) | 2 (1.2) | 0 |
| Thirst *6 | 24 (15) | 0 | 0 |
| Dysphagia | 15 (9.3) | 8 (4.9) | 0 |
| Malaise | 15 (9.3) | 1 (0.6) | 0 |
| Vomiting | 16 (9.9) | 0 | 0 |
| Conjunctivitis | 14 (8.6) | 0 | 0 |
| Tumour pain *7 | 14 (8.6) | 0 | 0 |
| Pyrexia | 13 (8.0) | 0 | 0 |
| Facial oedema *8 | 12 (7.4) | 0 | 0 |
| Dermatitis *9 | 7 (4.3) | 1 (0.6) | 0 |
| Pneumonia aspiration | 0 | 4 (2.5) | 0 |
| Renal impairment *10 | 1 (0.6) | 2 (1.2) | 0 |
| Anaemia *11 | 1 (0.6) | 2 (1.2) | 0 |
| Dehydration | 0 | 2 (1.2) | 0 |
| Diarrhoea | 1 (0.6) | 1 (0.6) | 0 |
| Skin disorder *12 | 1 (0.6) | 1 (0.6) | 0 |
| Septic shock | 0 | 0 | 1 (0.6) |
| Anaphylactic shock | 0 | 0 | 1 (0.6) |
| Tracheal stenosis | 0 | 0 | 1 (0.6) |
| Obstructive airways disorder | 0 | 0 | 1 (0.6) |
| Cellulitis | 0 | 1 (0.6) | 0 |
| Acinetobacter infection (site unknown) | 0 | 1 (0.6) | 0 |
| Tumour necrosis | 0 | 1 (0.6) | 0 |
| Malnutrition | 0 | 1 (0.6) | 0 |
| Disorientation | 0 | 1 (0.6) | 0 |
| Hypertension | 0 | 1 (0.6) | 0 |
| Laryngeal necrosis | 0 | 1 (0.6) | 0 |
| Mucosal ulceration | 0 | 1 (0.6) | 0 |
| Late TRAE (N = 157) | Grade 1 and 2 n (%) | Grade 3 n (%) | Grade 4 n (%) |
|---|---|---|---|
| Dysphagia | 4 (2.6) | 3 (1.9) | 0 |
| Thirst *1 | 4 (2.6) | 0 | 0 |
| Skin disorder *2 | 1 (0.6) | 2 (1.3) | 0 |
| Soft tissue infection | 0 | 2 (1.3) | 0 |
| Hyperamylasemia | 0 | 2 (1.3) | 0 |
| Papilloedema | 0 | 2 (1.3) | 0 |
| Visual acuity reduced | 2 (1.3) | 0 | 0 |
| Radiation necrosis (site unknown) | 2 (1.3) | 0 | 0 |
| Decreased appetite *3 | 2 (1.3) | 0 | 0 |
| Pneumonia aspiration | 0 | 0 | 1 (0.6) |
| Abscess bacterial (site unknown) | 0 | 1 (0.6) | 0 |
| Brain abscess | 0 | 1 (0.6) | 0 |
| Infected neoplasm | 0 | 1 (0.6) | 0 |
| Cranial nerve paralysis | 0 | 1 (0.6) | 0 |
| Cataract | 0 | 1 (0.6) | 0 |
| Pneumonitis | 0 | 1 (0.6) | 0 |
| Oroantral fistula | 0 | 1 (0.6) | 0 |
| Ulcer haemorrhage | 0 | 1 (0.6) | 0 |
| Radiation retinopathy | 0 | 1 (0.6) | 0 |
| Osteoradionecrosis | 0 | 1 (0.6) | 0 |
| SCCHN (n = 137) | NSCCHN (n = 17) | |
|---|---|---|
| ORR, % (95% CI) | 72.3 (64.0–79.6) | 64.7 (38.3–85.8) |
| Best overall response | ||
| CR, n (%) | 63 (46.0) | 8 (47.1) |
| PR, n (%) | 36 (26.3) | 3 (17.7) |
| SD, n (%) | 31 (22.6) | 5 (29.4) |
| PD, n (%) | 6 (4.4) | 0 |
| NE, n (%) | 1 (0.7) | 1 (5.9) |
| Without N (n = 91) | With N (n = 46) | |
|---|---|---|
| ORR, % (95% CI) | 75.8 (65.7–84.2) | 65.2 (49.8–78.6) |
| Best overall response | ||
| CR, n (%) | 46 (50.6) | 17 (36.7) |
| PR, n (%) | 23 (25.3) | 13 (28.3) |
| SD, n (%) | 17 (18.7) | 14 (30.4) |
| PD, n (%) | 5 (5.2) | 1 (2.2) |
| NE, n (%) | 0 | 1 (2.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sato, M.; Hirose, K.; Takeno, S.; Aihara, T.; Nihei, K.; Takai, Y.; Hayashi, T.; Bando, K.; Kimura, H.; Tsurumi, K.; et al. Safety of Boron Neutron Capture Therapy with Borofalan(10B) and Its Efficacy on Recurrent Head and Neck Cancer: Real-World Outcomes from Nationwide Post-Marketing Surveillance. Cancers 2024, 16, 869. https://doi.org/10.3390/cancers16050869
Sato M, Hirose K, Takeno S, Aihara T, Nihei K, Takai Y, Hayashi T, Bando K, Kimura H, Tsurumi K, et al. Safety of Boron Neutron Capture Therapy with Borofalan(10B) and Its Efficacy on Recurrent Head and Neck Cancer: Real-World Outcomes from Nationwide Post-Marketing Surveillance. Cancers. 2024; 16(5):869. https://doi.org/10.3390/cancers16050869
Chicago/Turabian StyleSato, Mariko, Katsumi Hirose, Satoshi Takeno, Teruhito Aihara, Keiji Nihei, Yoshihiro Takai, Toshimitsu Hayashi, Kosuke Bando, Hitomi Kimura, Keisuke Tsurumi, and et al. 2024. "Safety of Boron Neutron Capture Therapy with Borofalan(10B) and Its Efficacy on Recurrent Head and Neck Cancer: Real-World Outcomes from Nationwide Post-Marketing Surveillance" Cancers 16, no. 5: 869. https://doi.org/10.3390/cancers16050869
APA StyleSato, M., Hirose, K., Takeno, S., Aihara, T., Nihei, K., Takai, Y., Hayashi, T., Bando, K., Kimura, H., Tsurumi, K., & Ono, K. (2024). Safety of Boron Neutron Capture Therapy with Borofalan(10B) and Its Efficacy on Recurrent Head and Neck Cancer: Real-World Outcomes from Nationwide Post-Marketing Surveillance. Cancers, 16(5), 869. https://doi.org/10.3390/cancers16050869





