Maintain Efficacy and Spare Toxicity: Traditional and New Radiation-Based Conditioning Regimens in Hematopoietic Stem Cell Transplantation
Abstract
Simple Summary
Abstract
1. Introduction
2. Total Body Irradiation
2.1. Myeloablative Total Body Irradiation
| Study Design | Conditioning Regimen | Number of Patients | OS | DFS | RI | NRM | aGVHD | cGVHD | |
|---|---|---|---|---|---|---|---|---|---|
| Bunin et al. [13] | Randomized prospective trial | TBI or Bu + Cy-Etoposide | 22 vs. 21 | 67 vs. 47 | 58 vs. 29 | 32 vs. 43 | 9 vs. 24 | 25 | 9 |
| Peter et al. [20] | Randomized prospective trial | Etoposide-TBI (12 Gy) vs. Flu-thiotepa-Bu/Treo | 212 vs. 201 | ALL | 91 vs. 75 | 86 vs. 58 | 12 vs. 33 | 2 vs. 9 | 37 vs. 29 |
| Jamy et al. [24] | Prospective trial | Flu-TBI (12 Gy) | 19 | 68 | 63 | 7 | 31 | 26 | 21 |
| Zhang et al. [27] | Prospective trial | Cy-TBI (9 Gy) vs. Bu-Cy | 273 vs. 272 | 79 vs. 76 | 70 vs. 69 | 18 vs. 20 | 11 vs. 11 | 28 vs. 31 | 29 vs. 31 |
| Granados et al. [15] | Retrospective multicenter cohort study | TBI-based vs. Bu-based regimens | 114 vs. 42 | NA | 43 vs. 22 | 47 vs. 71 | 17 vs. 22 | 17 vs. 12 | 4 vs. 0 |
| Kiehl et al. [16] | Retrospective multicenter cohort study | TBI (10–13.5 Gy)-based vs. Bu-based regimens | 221 | 34 | 29 | 29 | 45 | 30 | NA |
| Marks et al. [17] | Retrospective multicenter cohort study | Cy-TBI (<13 Gy) vs. Cy-TBI (>13 Gy) vs. etoposide-TBI (<13 Gy) vs. etoposide-TBI (>13 Gy) | 217 vs. 81 vs. 53 vs. 151 | 74 vs. 74 vs. 71 vs. 80 | 68 vs. 69 vs. 67 vs. 79 | 23 vs. 16 vs. 9 vs. 12 | 9 vs. 13 vs. 23 vs. 9 | 29 vs. 24 vs. 30 vs. 25 | 23 vs. 23 vs. 19 vs. 34 |
| Cahu et al. [19] | Retrospective multicenter cohort study | TBI-based vs. Bu-based regimens | 523 vs. 78 | 47 vs. 28 | 44 vs. 25 | 33 | 25 | 40 vs. 27 | 44 vs. 30 |
| Mitsuhashi et al. [3] | Retrospective multicenter cohort study | Cy-TBI vs. p.o. Bu-Cy vs. i.v. Bu-Cy | 2028 vs. 60 vs. 42 | 69 vs. 56 vs. 71 | 62 vs. 54 vs. 47 | 20 vs. 21 vs. 24 | 18 vs. 24 vs. 20 | 40 vs. 37 vs. 33 | 37 vs. 31 vs. 40 |
| Kebriaei et al. [4] | Retrospective multicenter cohort study | TBI-based (9–12 Gy or 13–16 Gy) vs. Bu-based regimens | 819 vs. 299 | 53 vs. 57 | 48 vs. 45 | 28 vs. 37 | 25 vs. 19 | 12 vs. 47 | 55 vs. 49 |
| Eder et al. [5] | Retrospective multicenter cohort study | Cy-TBI vs. thiotepa-based regimens | 540 vs. 180 | 49 vs. 46 | 39 vs. 33 | 36 vs. 43 | 24 vs. 23 | 25 vs. 22 | 45 vs. 43 |
| Pavlu et al. [26] | Retrospective multicenter cohort study | MAC (TBI 8–14 Gy) or RIC (TBI < 6 Gy) | 86 | 36 | 28 | 51 | 20 | 33 | 32 |
| Dholaria et al. [21] | Retrospective multicenter cohort study | TBI vs. CT-based regimens | 188 vs. 239 | 51 vs. 57 | 45 vs. 37 | NA | 21 vs. 31 | 38 vs. 19 | 34 vs. 17 |
| Solomon et al. [23] | Retrospective multicenter cohort study | Flu-TBI (12 Gy) | 82 | 85 | 78 | 15 | 7 | 52 | 37 |
| Swoboda et al. [22] | Retrospective multicenter cohort study | Flu-TBI vs. thiotepa-Bu-Flu | 117 vs. 119 | 60 vs. 58 | 50 vs. 52 | 19 vs. 30 | 31 vs. 17 | 38 vs. 30 | 25 vs. 28 |
2.2. Reduced-Intensity Total Body Irradiation
3. TBI-Associated Late Toxicities
4. Total Marrow and Total Lymphoid Irradiation
4.1. Planning
4.2. Prescription Dose and Fractionation
4.3. Treatment Delivery
4.4. TMI/TMLI Indications and Current Role
- (a)
- Dose escalation of TMI/TMLI to improve disease control in high-risk patients who have a poor outcome with standard HSCT protocols.
- (b)
- Integration of TMI/TMLI in reduced-intensity conditioning regimens to improve disease control without increasing the toxicity profile
- (c)
- Addition of TMI/TMLI in the conditioning regimen of haploidentical HSCT to reduce GvHD.
- (d)
- Investigation of TMI/TMLI in standard-risk patients as an alternative to standard TBI.
- (e)
- TLI as nonmyeloablative conditioning
4.5. Ongoing Trials
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Carreras, E.; Dufour, C.; Mohty, M.; Kröger, N. (Eds.) The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies, 7th ed.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Wong, J.Y.C.; Filippi, A.R.; Scorsetti, M.; Hui, S.; Muren, L.P.; Mancosu, P. Total marrow and total lymphoid irradiation in bone marrow transplantation for acute leukaemia. Lancet Oncol. 2020, 21, e477–e487. [Google Scholar] [CrossRef]
- Mitsuhashi, K.; Kako, S.; Shigematsu, A.; Atsuta, Y.; Doki, N.; Fukuda, T.; Kanamori, H.; Onizuka, M.; Takahashi, S.; Ozawa, Y.; et al. Adult Acute Lymphoblastic Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Comparison of Cyclophosphamide Combined with Total Body Irradiation, Oral Busulfan, or Intravenous Busulfan for Allogeneic Hematopoietic Cell Transplantation in Adults with Acute Lymphoblastic Leukemia. Biol. Blood Marrow Transplant. 2016, 22, 2194–2200. [Google Scholar]
- Kebriaei, P.; Anasetti, C.; Zhang, M.J.; Wang, H.L.; Aldoss, I.; De Lima, M.; Khoury, H.J.; Sandmaier, B.M.; Horowitz, M.M.; Artz, A.; et al. Acute Leukemia Committee of the CIBMTR. Intravenous Busulfan Compared with Total Body Irradiation Pretransplant Conditioning for Adults with Acute Lymphoblastic Leukemia. Biol. Blood Marrow Transplant. 2018, 24, 726–733. [Google Scholar] [CrossRef]
- Eder, S.; Canaani, J.; Beohou, E.; Labopin, M.; Sanz, J.; Arcese, W.; Or, R.; Finke, J.; Cortelezzi, A.; Beelen, D.; et al. Thiotepa-based conditioning versus total body irradiation as myeloablative conditioning prior to allogeneic stem cell transplantation for acute lymphoblastic leukemia: A matched-pair analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Am. J. Hematol. 2017, 92, 997–1003. [Google Scholar] [PubMed]
- Sabloff, M.; Tisseverasinghe, S.; Babadagli, M.E.; Samant, R. Total Body Irradiation for Hematopoietic Stem Cell Transplantation: What Can We Agree on? Curr. Oncol. 2021, 28, 903–917. [Google Scholar] [CrossRef]
- Chen, C.I.; Abraham, R.; Tsang, R.; Crump, M.; Keating, A.; Stewart, A.K. Radiation-associated pneumonitis following autologous stem cell transplantation: Predictive factors, disease characteristics and treatment outcomes. Bone Marrow Transplant. 2001, 27, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.C.; Filippi, A.R.; Dabaja, B.S.; Yahalom, J.; Specht, L. Total Body Irradiation: Guidelines from the International Lymphoma Radiation Oncology Group (ILROG). Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 521–529. [Google Scholar] [CrossRef]
- Giebel, S.; Miszczyk, L.; Slosarek, K.; Moukhtari, L.; Ciceri, F.; Esteve, J.; Gorin, N.C.; Labopin, M.; Nagler, A.; Schmid, C.; et al. Extreme heterogeneity of myeloablative total body irradiation techniques in clinical practice: A survey of the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer 2014, 120, 2760–2765. [Google Scholar] [CrossRef] [PubMed]
- Shank, B.; O’Reilly, R.J.; Cunningham, I.; Kernan, N.; Yaholom, J.; Brochstein, J.; Castro-Malaspina, H.; Kutcher, G.J.; Mohan, R.; Bonfiglio, P. Total body irradiation for bone marrow transplantation: The Memorial Sloan-Kettering Cancer Center experience. Radiother. Oncol. 1990, 18 (Suppl. S1), 68–81. [Google Scholar] [CrossRef]
- De Filipp, Z.; Advani, A.S.; Bachanova, V.; Cassaday, R.D.; Deangelo, D.J.; Kebriaei, P.; Rowe, J.M.; Seftel, M.D.; Stock, W.; Tallman, M.S.; et al. Hematopoietic Cell Transplantation in the Treatment of Adult Acute Lymphoblastic Leukemia: Updated 2019 Evidence-Based Review from the American Society for Transplantation and Cellular Therapy. Biol. Blood Marrow Transplant. 2019, 25, 2113–2123. [Google Scholar] [CrossRef] [PubMed]
- Giebel, S.; Marks, D.I.; Boissel, N.; Baron, F.; Chiaretti, S.; Ciceri, F.; Cornelissen, J.J.; Doubek, M.; Esteve, J.; Fielding, A.; et al. Hematopoietic stem cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first remission: A position statement of the European Working Group for Adult Acute Lymphoblastic Leukemia (EWALL) and the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplant. 2019, 54, 798–809. [Google Scholar]
- Bunin, N.; Aplenc, R.; Kamani, N.; Shaw, K.; Cnaan, A.; Simms, S. Randomized trial of busulfan vs total body irradiation containing conditioning regimens for children with acute lymphoblastic leukemia: A Pediatric Blood and Marrow Transplant Consortium study. Bone Marrow Transplant. 2003, 32, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.M.; Ramsay, N.K.; Klein, J.P.; Weisdorf, D.J.; Bolwell, B.; Cahn, J.Y.; Camitta, B.M.; Gale, R.P.; Giralt, S.; Heilmann, C.; et al. Comparison of preparative regimens in transplants for children with acute lymphoblastic leukemia. J. Clin. Oncol. 2000, 18, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Granados, E.; De La Cámara, R.; Madero, L.; Díaz, M.A.; Martín-Regueira, P.; Steegmann, J.L.; Arranz, R.; Figuera, A.; Fernández-Rañada, J.M. Hematopoietic cell transplantation in acute lymphoblastic leukemia: Better long term event-free survival with conditioning regimens containing total body irradiation. Haematologica 2000, 85, 1060–1067. [Google Scholar] [PubMed]
- Kiehl, M.G.; Kraut, L.; Schwerdtfeger, R.; Hertenstein, B.; Remberger, M.; Kroeger, N.; Stelljes, M.; Bornhaeuser, M.; Martin, H.; Scheid, C.; et al. Outcome of allogeneic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: No difference in related compared with unrelated transplant in first complete remission. J. Clin. Oncol. 2004, 22, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.I.; Forman, S.J.; Blume, K.G.; Pérez, W.S.; Weisdorf, D.J.; Keating, A.; Gale, R.P.; Cairo, M.S.; Copelan, E.A.; Horan, J.T.; et al. A comparison of cyclophosphamide and total body irradiation with etoposide and total body irradiation as conditioning regimens for patients undergoing sibling allografting for acute lymphoblastic leukemia in first or second complete remission. Biol. Blood Marrow Transplant. 2006, 12, 438–453. [Google Scholar] [CrossRef] [PubMed]
- Giebel, S.; Labopin, M.; Socié, G.; Beelen, D.; Browne, P.; Volin, L.; Kyrcz-Krzemien, S.; Yakoub-Agha, I.; Aljurf, M.; Wu, D.; et al. Improving results of allogeneic hematopoietic cell transplantation for adults with acute lymphoblastic leukemia in first complete remission: An analysis from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica 2017, 102, 139–149. [Google Scholar] [CrossRef]
- Cahu, X.; Labopin, M.; Giebel, S.; Aljurf, M.; Kyrcz-Krzemien, S.; Socié, G.; Eder, M.; Bonifazi, F.; Bunjes, D.; Vigouroux, S.; et al. Acute Leukemia Working Party of EBMT. Impact of conditioning with TBI in adult patients with T-cell ALL who receive a myeloablative allogeneic stem cell transplantation: A report from the acute leukemia working party of EBMT. Bone Marrow Transplant. 2016, 51, 351–357. [Google Scholar] [CrossRef]
- Peters, C.; Dalle, J.H.; Locatelli, F.; Poetschger, U.; Sedlacek, P.; Buechner, J.; Shaw, P.J.; Staciuk, R.; Ifversen, M.; Pichler, H.; et al. Total Body Irradiation or Chemotherapy Conditioning in Childhood ALL: A Multinational, Randomized, Noninferiority Phase III Study. J. Clin. Oncol. 2021, 39, 295–307. [Google Scholar] [CrossRef]
- Dholaria, B.; Labopin, M.; Angelucci, E.; Tischer, J.; Arat, M.; Ciceri, F.; Gulbas, Z.; Ozdogu, H.; Sica, S.; Diez-Martin, J.; et al. Outcomes of Total Body Irradiation- Versus Chemotherapy-Based Myeloablative Conditioning Regimen in Haploidentical Hematopoietic Cell Transplantation with Post-Transplant Cyclophosphamide for Acute Lymphoblastic Leukemia: ALWP of the EBMT Study. Blood 2019, 134 (Suppl. S1), 320. [Google Scholar]
- Swoboda, R.; Labopin, M.; Giebel, S.; Angelucci, E.; Arat, M.; Aljurf, M.; Sica, S.; Pavlu, J.; Socié, G.; Bernasconi, P.; et al. Total body irradiation plus fludarabine versus thiotepa busulfan plus fludarabine as a myeloablative conditioning for adults with acute lymphoblastic leukemia treated with haploidentical hematopoietic cell transplantation A study by the Acute Leukemia Working Party of the EBMT. Bone Marrow Transplant. 2022, 57, 399–406. [Google Scholar] [PubMed]
- Solomon, S.R.; Solh, M.; Zhang, X.; Morris, L.E.; Holland, H.K.; Bashey, A. Fludarabine and Total-Body Irradiation Conditioning before Ablative Haploidentical Transplantation: Long-Term Safety and Efficacy. Biol. Blood Marrow Transplant. 2019, 25, 2211–2216. [Google Scholar] [CrossRef]
- Jamy, O.H.; Salzman, D.E.; Stasi, A.D.; Innis-Shelton, R.; Luciano, C.; Lamb, L.S.; Mineishi, S.; Saad, A. A phase II study of myeloablative allogeneic hematopoietic stem cell transplantation (aHSCT) for acute lymphoblastic leukemia (ALL) in older patients using fludarabine and total body irradiation (Flu/TBI). J. Clin. Oncol. 2019, 37 (Suppl. S15), TPS7069-TPS. [Google Scholar] [CrossRef]
- Pavlů, J.; Labopin, M.; Niittyvuopio, R.; Socié, G.; Yakoub-Agha, I.; Wu, D.; Remenyi, P.; Passweg, J.; Beelen, D.W.; Aljurf, M.; et al. Measurable residual disease at myeloablative allogeneic transplantation in adults with acute lymphoblastic leukemia: A retrospective registry study on 2780 patients from the acute leukemia working party of the EBMT. J. Hematol. Oncol. 2019, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Pavlů, J.; Labopin, M.; Zoellner, A.K.; Sakellari, I.; Stelljes, M.; Finke, J.; Fanin, R.; Stuhler, G.; Afanasyev, B.V.; Bloor, A.J.; et al. Allogeneic hematopoietic cell transplantation for primary refractory acute lymphoblastic leukemia: A report from the Acute Leukemia Working Party of the EBMT. Cancer 2017, 123, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fan, Z.; Huang, F.; Han, L.; Xu, Y.; Xu, N.; Deng, L.; Wang, S.; Lin, D.; Luo, X.; et al. Busulfan Plus Cyclophosphamide Versus Total Body Irradiation Plus Cyclophosphamide for Adults Acute B Lymphoblastic Leukemia: An Open-Label, Multicenter, Phase III Trial. J. Clin. Oncol. 2023, 41, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Weisser, M.; Schleuning, M.; Ledderose, G.; Rolf, B.; Schnittger, S.; Schoch, C.; Schwerdtfeger, R.; Kolb, H.J. Reduced-intensity conditioning using TBI (8 Gy), fludarabine, cyclophosphamide and ATG in elderly CML patients provides excellent results especially when performed in the early course of the disease. Bone Marrow Transplant. 2004, 34, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, P.A.; Niederwieser, D.; Shizuru, J.A.; Sandmaier, B.M.; Molina, A.J.; Maloney, D.G.; Chauncey, T.R.; Gooley, T.A.; Hegenbart, U.; Nash, R.A.; et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: Replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood 2001, 97, 3390–3400. [Google Scholar] [CrossRef]
- Kornblit, B.; Maloney, D.G.; Storb, R.; Storek, J.; Hari, P.; Vucinic, V.; Maziarz, R.T.; Chauncey, T.R.; Pulsipher, M.A.; Bruno, B.; et al. Fludarabine and 2-Gy TBI is superior to 2 Gy TBI as conditioning for HLA-matched related hematopoietic cell transplantation: A phase III randomized trial. Biol. Blood Marrow Transplant. 2013, 19, 1340–1347. [Google Scholar] [CrossRef][Green Version]
- Storb, R.; Gyurkocza, B.; Storer, B.E.; Sorror, M.L.; Blume, K.; Niederwieser, D.; Chauncey, T.R.; Pulsipher, M.A.; Petersen, F.B.; Sahebi, F.; et al. Graft-versus-host disease and graft-versus-tumor effects after allogeneic hematopoietic cell transplantation. J. Clin. Oncol. 2013, 31, 1530–1538. [Google Scholar] [CrossRef]
- Cooper, J.P.; Storer, B.E.; Granot, N.; Gyurkocza, B.; Sorror, M.L.; Chauncey, T.R.; Shizuru, J.; Franke, G.N.; Maris, M.B.; Boyer, M.; et al. Allogeneic hematopoietic cell transplantation with non-myeloablative conditioning for patients with hematologic malignancies: Improved outcomes over two decades. Haematologica 2021, 106, 1599–1607. [Google Scholar] [CrossRef]
- Krakow, E.F.; Gyurkocza, B.; Storer, B.E.; Chauncey, T.R.; McCune, J.S.; Radich, J.P.; Bouvier, M.E.; Estey, E.H.; Storb, R.; Maloney, D.G.; et al. Phase I/II multisite trial of optimally dosed clofarabine and low-dose TBI for hematopoietic cell transplantation in acute myeloid leukemia. Am. J. Hematol. 2020, 95, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Gyurkocza, B.; Storb, R.; Storer, B.E.; Chauncey, T.R.; Lange, T.; Shizuru, J.A.; Langston, A.A.; Pulsipher, M.A.; Bredeson, C.N.; Maziarz, R.T.; et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in patients with acute myeloid leukemia. J. Clin. Oncol. 2010, 28, 2859–2867. [Google Scholar] [CrossRef]
- Hegenbart, U.; Niederwieser, D.; Sandmaier, B.M.; Maris, M.B.; Shizuru, J.A.; Greinix, H.; Cordonnier, C.; Rio, B.; Gratwohl, A.; Lange, T.; et al. Treatment for acute myelogenous leukemia by low-dose, total-body, irradiation-based conditioning and hematopoietic cell transplantation from related and unrelated donors. J. Clin. Oncol. 2006, 24, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Sorror, M.L.; Sandmaier, B.M.; Storer, B.E.; Franke, G.N.; Laport, G.G.; Chauncey, T.R.; Agura, E.; Maziarz, R.T.; Langston, A.; Hari, P.; et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA 2011, 306, 1874–1883. [Google Scholar] [CrossRef] [PubMed]
- Monaco, F.; Scott, B.L.; Chauncey, T.R.; Petersen, F.B.; Storer, B.E.; Baron, F.; Flowers, M.E.; Deeg, H.J.; Maloney, D.G.; Storb, R.; et al. Total body irradiation dose escalation decreases risk of progression and graft rejection after hematopoietic cell transplantation for myelodysplastic syndromes or myeloproliferative neoplasms. Haematologica 2019, 104, 1221–1229. [Google Scholar] [CrossRef]
- Deeg, H.J.; Stevens, E.A.; Salit, R.B.; Ermoian, R.P.; Fang, M.; Gyurkocza, B.; Sorror, M.L.; Fatobene, G.; Baumgart, J.; Burroughs, L.M.; et al. Transplant Conditioning with Treosulfan/Fludarabine with or without Total Body Irradiation: A Randomized Phase II Trial in Patients with Myelodysplastic Syndrome and Acute Myeloid Leukemia. Biol. Blood Marrow Transplant. 2018, 24, 956–963. [Google Scholar] [CrossRef]
- Alkhaldi, H.; Goloubeva, O.; Rapoport, A.P.; Dahiya, S.; Pang, Y.; Ali, M.M.; Hardy, N.M.; Mohindra, P.; Bukhari, A.; Lutfi, F.; et al. Outcomes of Busulfan, Fludarabine, and 400 cGy Total Body Irradiation Compared with Busulfan and Fludarabine Reduced-Intensity Conditioning Regimens for Allogeneic Stem Cell Transplantation in Adult Patients With Hematologic Diseases: A Single-Center Experience. Transplant. Proc. 2023, 55, 214–224. [Google Scholar]
- Freyer, C.W.; Babushok, D.V.; Frey, N.V.; Gill, S.I.; Loren, A.W.; Luger, S.M.; Maity, A.; Martin, M.E.; Plastaras, J.P.; Porter, D.L.; et al. Low-Dose Total Body Irradiation Added to Fludarabine and Busulfan Reduced-Intensity Conditioning Reduces Graft Failure in Patients with Myelofibrosis. Transplant. Cell Ther. 2022, 28, 590–596. [Google Scholar] [CrossRef]
- Bornhäuser, M.; Kienast, J.; Trenschel, R.; Burchert, A.; Hegenbart, U.; Stadler, M.; Baurmann, H.; Schäfer-Eckart, K.; Holler, E.; Kröger, N.; et al. Reduced-intensity conditioning versus standard conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: A prospective, open-label randomised phase 3 trial. Lancet Oncol. 2012, 13, 1035–1044. [Google Scholar] [CrossRef]
- Fasslrinner, F.; Schetelig, J.; Burchert, A.; Kramer, M.; Trenschel, R.; Hegenbart, U.; Stadler, M.; Schäfer-Eckart, K.; Bätzel, M.; Eich, H.; et al. Long-term efficacy of reduced-intensity versus myeloablative conditioning before allogeneic haemopoietic cell transplantation in patients with acute myeloid leukaemia in first complete remission: Retrospective follow-up of an open-label, randomised phase 3 trial. Lancet Haematol. 2018, 5, e161–e169. [Google Scholar]
- Spyridonidis, A.; Labopin, M.; Savani, B.; Giebel, S.; Schmid, C.; Peric, Z.; Bug, G.; Schönland, S.; Kröger, N.; Stelljes, M.; et al. Retrospective Comparison between 12-Gray and 8-Gray Total Body Irradiation (TBI) before Allogeneic Hematopoietic Cell Transplantation in Patients with Acute Lymphoblastic Leukemia in First Complete Remission. Blood 2021, 138 (Suppl. S1), 1783. [Google Scholar] [CrossRef]
- Giaccone, L.; Felicetti, F.; Butera, S.; Faraci, D.; Cerrano, M.; Dionisi Vici, M.; Brunello, L.; Fortunati, N.; Brignardello, E.; Bruno, B. Optimal Delivery of Follow-Up Care After Allogeneic Hematopoietic Stem-Cell Transplant: Improving Patient Outcomes with a Multidisciplinary Approach. J. Blood Med. 2020, 11, 141–162. [Google Scholar] [CrossRef] [PubMed]
- Bruno, B.; Nash, R.A.; Wallace, P.M.; Gass, M.J.; Thompson, J.; Storb, R.; McSweeney, P.A. CD34+ selected bone marrow grafts are radioprotective and establish mixed chimerism in dogs given high dose total body irradiation. Transplantation 1999, 68, 338–344. [Google Scholar] [CrossRef]
- Holmqvist, A.S.; Chen, Y.; Hageman, L.; Landier, W.; Wu, J.; Francisco, L.F.; Ross, E.S.; Balas, N.A.; Bosworth, A.; Te, H.S.; et al. Severe, life-threatening, and fatal chronic health conditions after allogeneic blood or marrow transplantation in childhood. Cancer 2023, 129, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, O.; Kubota, C.; Hinohara, T.; Hattori, K.; Yabe, H.; Yabe, M.; Kato, S. Growth and growth hormone secretion in children after bone marrow transplantation. Acta Paediatr. Jpn. 1993, 35, 22–26. [Google Scholar] [CrossRef]
- Dandoy, C.E.; Davies, S.M.; Woo Ahn, K.; He, Y.; Kolb, A.E.; Levine, J.; Bo-Subait, S.; Abdel-Azim, H.; Bhatt, N.; Chewning, J.; et al. Comparison of total body irradiation versus non-total body irradiation containing regimens for de novo acute myeloid leukemia in children. Haematologica 2021, 106, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Rovelli, A.; Bakker, B.; Uderzo, C.; Van Lint, M.T.; Esperou, H.; Gaiero, A.; Leiper, A.D.; Dopfer, R.; Cahn, J.Y.; et al. Final height of patients who underwent bone marrow transplantation for hematological disorders during childhood: A study by the Working Party for Late Effects-EBMT. Blood 1999, 93, 4109–4115. [Google Scholar]
- Felicetti, F.; Manicone, R.; Corrias, A.; Manieri, C.; Biasin, E.; Bini, I.; Boccuzzi, G.; Brignardello, E. Endocrine late effects after total body irradiation in patients who received hematopoietic cell transplantation during childhood: A retrospective study from a single institution. J. Cancer Res. Clin. Oncol. 2011, 137, 1343–1348. [Google Scholar] [CrossRef]
- Wong, J.Y.; Liu, A.; Schultheiss, T.; Popplewell, L.; Stein, A.; Rosenthal, J.; Essensten, M.; Forman, S.; Somlo, G. Targeted total marrow irradiation using three-dimensional image-guided tomographic intensity-modulated radiation therapy: An alternative to standard total body irradiation. Biol. Blood Marrow Transplant. 2006, 12, 306–315. [Google Scholar] [CrossRef]
- Oudin, C.; Auquier, P.; Bertrand, Y.; Chastagner, P.; Kanold, J.; Poirée, M.; Thouvenin, S.; Ducassou, S.; Plantaz, D.; Tabone, M.D.; et al. Late thyroid complications in survivors of childhood acute leukemia. An L.E.A. study. Haematologica 2016, 101, 747–756. [Google Scholar] [CrossRef]
- Sanders, J.E.; Hoffmeister, P.A.; Woolfrey, A.E.; Carpenter, P.A.; Storer, B.E.; Storb, R.F.; Appelbaum, F.R. Thyroid function following hematopoietic cell transplantation in children: 30 years’ experience. Blood 2009, 113, 306–308. [Google Scholar] [CrossRef]
- Jung, Y.J.; Jeon, Y.J.; Cho, W.K.; Lee, J.W.; Chung, N.G.; Jung, M.H.; Cho, B.; Suh, B.K. Risk factors for short term thyroid dysfunction after hematopoietic stem cell transplantation in children. Korean J. Pediatr. 2013, 56, 298–303. [Google Scholar] [CrossRef]
- Madden, L.M.; Ngwube, A.I.; Shenoy, S.; Druley, T.E.; Hayashi, R.J. Late toxicity of a novel allogeneic stem cell transplant using single fraction total body irradiation for hematologic malignancies in children. J. Pediatr. Hematol. Oncol. 2015, 37, e94–e101. [Google Scholar] [CrossRef][Green Version]
- Toubert, M.E.; Socié, G.; Gluckman, E.; Aractingi, S.; Espérou, H.; Devergie, A.; Ribaud, P.; Parquet, N.; Schlageter, M.H.; Beressi, J.P.; et al. Short- and long-term follow-up of thyroid dysfunction after allogeneic bone marrow transplantation without the use of preparative total body irradiation. Br. J. Haematol. 1997, 98, 453–457. [Google Scholar] [CrossRef]
- Felicetti, F.; Gatti, F.; Faraci, D.; Rosso, D.; Zavattaro, M.; Fortunati, N.; Marinelli, L.; Leone, S.; Gill, J.; Dionisi-Vici, M.; et al. Impact of allogeneic stem cell transplantation on thyroid function. J. Endocrinol. Investig. 2023, 46, 1825–1834. [Google Scholar] [CrossRef]
- Wei, C.; Thyagiarajan, M.S.; Hunt, L.P.; Shield, J.P.; Stevens, M.C.; Crowne, E.C. Reduced insulin sensitivity in childhood survivors of haematopoietic stem cell transplantation is associated with lipodystropic and sarcopenic phenotypes. Pediatr. Blood Cancer 2015, 62, 1992–1999. [Google Scholar] [CrossRef]
- Pophali, P.A.; Klotz, J.K.; Ito, S.; Jain, N.A.; Koklanaris, E.; Le, R.Q.; Hourigan, C.S.; Savani, B.N.; Chawla, K.; Shanbhag, S.; et al. Male survivors of allogeneic hematopoietic stem cell transplantation have a long term persisting risk of cardiovascular events. Exp. Hematol. 2014, 42, 83–89. [Google Scholar] [CrossRef]
- Vadmand, A.C.; Nissen, A.A.; Mathiesen, S.; Soerum, M.E.; Gerbek, T.; Fridh, M.K.; Sørensen, K.; Hartmann, B.; Holst, J.J.; Müller, K. Metabolic Dysregulation in Adult Survivors of Pediatric Hematopoietic Stem Cell Transplantation: The Role of Incretins. J. Clin. Endocrinol. Metab. 2023, 108, 453–462. [Google Scholar] [CrossRef]
- Chow, E.J.; Baker, K.S.; Lee, S.J.; Flowers, M.E.; Cushing-Haugen, K.L.; Inamoto, Y.; Khera, N.; Leisenring, W.M.; Syrjala, K.L.; Martin, P.J. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J. Clin. Oncol. 2014, 32, 191–198. [Google Scholar] [CrossRef]
- Friedman, D.N.; Hilden, P.; Moskowitz, C.S.; Suzuki, M.; Boulad, F.; Kernan, N.A.; Wolden, S.L.; Oeffinger, K.C.; Sklar, C.A. Cardiovascular Risk Factors in Survivors of Childhood Hematopoietic Cell Transplantation Treated with Total Body Irradiation: A Longitudinal Analysis. Biol. Blood Marrow Transplant. 2017, 23, 475–482. [Google Scholar] [CrossRef]
- Griffith, M.L.; Savani, B.N.; Boord, J.B. Dyslipidemia after allogeneic hematopoietic stem cell transplantation: Evaluation and management. Blood 2010, 116, 1197–1204. [Google Scholar] [CrossRef]
- Marini, B.L.; Choi, S.W.; Byersdorfer, C.A.; Cronin, S.; Frame, D.G. Treatment of dyslipidemia in allogeneic hematopoietic stem cell transplant patients. Biol. Blood Marrow Transplant. 2015, 21, 809–820. [Google Scholar] [CrossRef]
- Felicetti, F.; Fortunati, N.; Brignardello, E. Cancer survivors: An expanding population with an increased cardiometabolic risk. Diabetes Res. Clin. Pract. 2018, 143, 432–442. [Google Scholar] [CrossRef]
- Paix, A.; Antoni, D.; Waissi, W.; Ledoux, M.P.; Bilger, K.; Fornecker, L.; Noel, G. Total body irradiation in allogeneic bone marrow transplantation conditioning regimens: A review. Crit. Rev. Oncol. Hematol. 2018, 123, 138–148. [Google Scholar] [CrossRef]
- Huang, T.T.; Hudson, M.M.; Stokes, D.C.; Krasin, M.J.; Spunt, S.L.; Ness, K.K. Pulmonary outcomes in survivors of childhood cancer: A systematic review. Chest 2011, 140, 881–901. [Google Scholar] [CrossRef]
- Limper, A.H. Chemotherapy-induced lung disease. Clin. Chest Med. 2004, 25, 53–64. [Google Scholar] [CrossRef]
- Shalitin, S.; Pertman, L.; Yackobovitch-Gavan, M.; Yaniv, I.; Lebenthal, Y.; Phillip, M.; Stein, J. Endocrine and Metabolic Disturbances in Survivors of Hematopoietic Stem Cell Transplantation in Childhood and Adolescence. Horm. Res. Paediatr. 2018, 89, 108–121. [Google Scholar] [CrossRef]
- Sanders, J.E.; Buckner, C.D.; Leonard, J.M.; Sullivan, K.M.; Witherspoon, R.P.; Deeg, H.J.; Storb, R.; Thomas, E.D. Late effects on gonadal function of cyclophosphamide, total-body irradiation, and marrow transplantation. Transplantation 1983, 36, 252–255. [Google Scholar] [CrossRef]
- Sanders, J.E.; Buckner, C.D.; Amos, D.; Levy, W.; Appelbaum, F.R.; Doney, K.; Storb, R.; Sullivan, K.M.; Witherspoon, R.P.; Thomas, E.D. Ovarian function following marrow transplantation for aplastic anemia or leukemia. J. Clin. Oncol. 1988, 6, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Sanders, J.E.; Hawley, J.; Levy, W.; Gooley, T.; Buckner, C.D.; Deeg, H.J.; Doney, K.; Storb, R.; Sullivan, K.; Witherspoon, R.; et al. Pregnancies following high-dose cyclophosphamide with or without high-dose busulfan or total-body irradiation and bone marrow transplantation. Blood 1996, 87, 3045–3052. [Google Scholar] [CrossRef]
- Meistrich, M.L. Effects of chemotherapy and radiotherapy on spermatogenesis in humans. Fertil. Steril. 2013, 100, 1180–1186. [Google Scholar] [CrossRef]
- Wallace, W.H.; Thomson, A.B.; Kelsey, T.W. The radiosensitivity of the human oocyte. Hum. Reprod. 2003, 18, 117–121. [Google Scholar] [CrossRef]
- Felicetti, F.; Castiglione, A.; Biasin, E.; Fortunati, N.; Dionisi-Vici, M.; Matarazzo, P.; Ciccone, G.; Fagioli, F.; Brignardello, E. Effects of treatments on gonadal function in long-term survivors of pediatric hematologic malignancies: A cohort study. Pediatr. Blood Cancer 2020, 67, e28709. [Google Scholar] [CrossRef]
- Gurney, J.G.; Ness, K.K.; Rosenthal, J.; Forman, S.J.; Bhatia, S.; Baker, K.S. Visual, auditory, sensory, and motor impairments in long-term survivors of hematopoietic stem cell transplantation performed in childhood: Results from the Bone Marrow Transplant Survivor study. Cancer 2006, 106, 1402–1408. [Google Scholar] [CrossRef]
- Inamoto, Y.; Lee, S.J. Late effects of blood and marrow transplantation. Haematologica 2017, 102, 614–625. [Google Scholar] [CrossRef]
- Ferry, C.; Gemayel, G.; Rocha, V.; Labopin, M.; Esperou, H.; Robin, M.; De Latour, R.P.; Ribaud, P.; Devergie, A.; Leblanc, T.; et al. Long-term outcomes after allogeneic stem cell transplantation for children with hematological malignancies. Bone Marrow Transplant. 2007, 40, 219–224. [Google Scholar] [CrossRef]
- Ness, K.K.; Kirkland, J.L.; Gramatges, M.M.; Wang, Z.; Kundu, M.; McCastlain, K.; Li-Harms, X.; Zhang, J.; Tchkonia, T.; Pluijm, S.M.F.; et al. Premature Physiologic Aging as a Paradigm for Understanding Increased Risk of Adverse Health Across the Lifespan of Survivors of Childhood Cancer. J. Clin. Oncol. 2018, 36, 2206–2215. [Google Scholar] [CrossRef]
- Van Atteveld, J.E.; De Winter, D.T.C.; Pluimakers, V.G.; Fiocco, M.; Nievelstein, R.A.J.; Hobbelink, M.G.G.; Kremer, L.C.M.; Grootenhuis, M.A.; Maurice-Stam, H.; Tissing, W.J.E.; et al. Frailty and sarcopenia within the earliest national Dutch childhood cancer survivor cohort (DCCSS-LATER): A cross-sectional study. Lancet Healthy Longev. 2023, 4, e155–e165. [Google Scholar] [CrossRef]
- Felicetti, F.; Cento, A.S.; Fornengo, P.; Cassader, M.; Mastrocola, R.; D’Ascenzo, F.; Settanni, F.; Benso, A.; Arvat, E.; Collino, M.; et al. Advanced glycation end products and chronic inflammation in adult survivors of childhood leukemia treated with hematopoietic stem cell transplantation. Pediatr. Blood Cancer 2020, 67, e28106. [Google Scholar] [CrossRef]
- Bhatia, S.; Louie, A.D.; Bhatia, R.; O’Donnell, M.R.; Fung, H.; Kashyap, A.; Krishnan, A.; Molina, A.; Nademanee, A.; Niland, J.C.; et al. Solid cancers after bone marrow transplantation. J. Clin. Oncol. 2001, 19, 464–471. [Google Scholar] [CrossRef]
- Hasegawa, W.; Pond, G.R.; Rifkind, J.T.; Messner, H.A.; Lau, A.; Daly, A.S.; Kiss, T.L.; Kotchetkova, N.; Galal, A.; Lipton, J.H. Long-term follow-up of secondary malignancies in adults after allogeneic bone marrow transplantation. Bone Marrow Transplant. 2005, 35, 51–55. [Google Scholar] [CrossRef]
- Pole, J.D.; Darmawikarta, D.; Gassas, A.; Ali, M.; Egler, M.; Greenberg, M.L.; Doyle, J.; Nathan, P.C.; Schechter, T. Subsequent malignant neoplasms in pediatric cancer patients treated with and without hematopoietic SCT. Bone Marrow Transplant. 2015, 50, 721–726. [Google Scholar] [CrossRef][Green Version]
- Brignardello, E.; Felicetti, F.; Castiglione, A.; Gallo, M.; Maletta, F.; Isolato, G.; Biasin, E.; Fagioli, F.; Corrias, A.; Palestini, N. Ultrasound surveillance for radiation-induced thyroid carcinoma in adult survivors of childhood cancer. Eur. J. Cancer 2016, 55, 74–80. [Google Scholar] [CrossRef]
- Friedman, D.L.; Rovo, A.; Leisenring, W.; Locasciulli, A.; Flowers, M.E.; Tichelli, A.; Sanders, J.E.; Deeg, H.J.; Socie, G.; FHCRC; et al. Increased risk of breast cancer among survivors of allogeneic hematopoietic cell transplantation: A report from the FHCRC and the EBMT-Late Effect Working Party. Blood 2008, 111, 939–944. [Google Scholar] [CrossRef]
- Baker, K.S.; Leisenring, W.M.; Goodman, P.J.; Ermoian, R.P.; Flowers, M.E.; Schoch, G.; Storb, R.; Sandmaier, B.M.; Deeg, H.J. Total body irradiation dose and risk of subsequent neoplasms following allogeneic hematopoietic cell transplantation. Blood 2019, 133, 2790–2799. [Google Scholar] [CrossRef]
- Socié, G.; Curtis, R.E.; Deeg, H.J.; Sobocinski, K.A.; Filipovich, A.H.; Travis, L.B.; Sullivan, K.M.; Rowlings, P.A.; Kingma, D.W.; Banks, P.M.; et al. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J. Clin. Oncol. 2000, 18, 348–357. [Google Scholar] [CrossRef]
- Hui, S.K.; Das, R.K.; Thomadsen, B.; Henderson, D. CT-based analysis of dose homogeneity in total body irradiation using lateral beam. J. Appl. Clin. Med. Phys. 2004, 5, 71–79. [Google Scholar]
- Esiashvili, N.; Lu, X.; Ulin, K.; Laurie, F.; Kessel, S.; Kalapurakal, J.A.; Merchant, T.E.; Followill, D.S.; Sathiaseelan, V.; Schmitter, M.K.; et al. Higher Reported Lung Dose Received During Total Body Irradiation for Allogeneic Hematopoietic Stem Cell Transplantation in Children with Acute Lymphoblastic Leukemia Is Associated With Inferior Survival: A Report from the Children’s Oncology Group. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 513–521. [Google Scholar] [CrossRef]
- Mohty, M.; Malard, F.; Savani, B.N. High-dose total body irradiation and myeloablative conditioning before allogeneic hematopoietic cell transplantation: Time to rethink? Biol. Blood Marrow Transplant. 2015, 21, 620–624. [Google Scholar] [CrossRef]
- Liu, A.; Han, C.; Neylon, J. Implementation of Targeted Total Body Irradiation as a Bone Marrow Transplant Conditioning Regimen: A Review; Total Marrow Irradiation; Wong, J., Hui, S., Eds.; Springer Nature: New York, NY, USA, 2020; pp. 29–45. [Google Scholar]
- Wong, J.Y.C.; Liu, A.; Han, C.; Dandapani, S.; Schultheiss, T.; Palmer, J.; Yang, D.; Somlo, G.; Salhotra, A.; Hui, S.; et al. Total marrow irradiation (TMI): Addressing an unmet need in hematopoietic cell transplantation—A single institution experience review. Front. Oncol. 2022, 12, 1003908. [Google Scholar] [CrossRef]
- Wilkie, J.R.; Tiryaki, H.; Smith, B.D.; Roeske, J.C.; Radosevich, J.A.; Aydogan, B. Feasibility study for linac-based intensity modulated total marrow irradiation. Med. Phys. 2008, 35, 5609–5618. [Google Scholar] [CrossRef] [PubMed]
- Mancosu, P.; Navarria, P.; Castagna, L.; Reggiori, G.; Sarina, B.; Tomatis, S.; Alongi, F.; Nicolini, G.; Fogliata, A.; Cozzi, L.; et al. Interplay effects between dose distribution quality and positioning accuracy in total marrow irradiation with volumetric modulated arc therapy. Med. Phys. 2013, 40, 111713. [Google Scholar] [CrossRef] [PubMed]
- Corvò, R.; Zeverino, M.; Vagge, S.; Agostinelli, S.; Barra, S.; Taccini, G.; Van Lint, M.T.; Frassoni, F.; Bacigalupo, A. Helical tomotherapy targeting total bone marrow after total body irradiation for patients with relapsed acute leukemia undergoing an allogeneic stem cell transplant. Radiother. Oncol. 2011, 98, 382–386. [Google Scholar] [CrossRef]
- Cherpak, A.J.; Monajemi, T.; Chytyk-Praznik, K.; Mulroy, L. Energy-dependent OAR sparing and dose conformity for total marrow irradiation of obese patients. J. Appl. Clin. Med. Phys. 2018, 19, 532–538. [Google Scholar] [CrossRef]
- Stein, A.; Palmer, J.; Tsai, N.C.; Al Malki, M.M.; Aldoss, I.; Ali, H.; Aribi, A.; Farol, L.; Karanes, C.; Khaled, S.; et al. Phase I Trial of Total Marrow and Lymphoid Irradiation Transplantation Conditioning in Patients with Relapsed/Refractory Acute Leukemia. Biol. Blood Marrow Transplant. 2017, 23, 618–624. [Google Scholar] [CrossRef]
- Stein, A. Dose Escalation of Total Marrow and Lymphoid Irradiation in Advanced Acute Leukemia; Total Marrow Irradiation; Springer: Berlin/Heidelberg, Germany, 2020; pp. 69–75. [Google Scholar]
- Hui, S.; Brunstein, C.; Takahashi, Y.; DeFor, T.; Holtan, S.G.; Bachanova, V.; Wilke, C.; Zuro, D.; Ustun, C.; Weisdorf, D.; et al. Dose Escalation of Total Marrow Irradiation in High-Risk Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2017, 23, 1110–1116. [Google Scholar] [CrossRef]
- Bao, Z.; Zhao, H.; Wang, D.; Gong, J.; Zhong, Y.; Xiong, Y.; Deng, D.; Xie, C.; Liu, A.; Wang, X.; et al. Feasibility of a novel dose fractionation strategy in TMI/TMLI. Radiat. Oncol. 2018, 13, 248. [Google Scholar] [CrossRef]
- Schultheiss, T.E.; Wong, J.; Liu, A.; Olivera, G.; Somlo, G. Image-guided total marrow and total lymphatic irradiation using helical tomotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 1259–1267. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.; Rosenthal, J.; Liu, A.; Schultheiss, T.; Forman, S.; Somlo, G. Image-guided total-marrow irradiation using helical tomotherapy in patients with multiple myeloma and acute leukemia undergoing hematopoietic cell transplantation. Int. J. Radiat. Oncol. Biol. Phys. 2009, 73, 273–279. [Google Scholar] [CrossRef]
- Rosenthal, J.; Wong, J.; Stein, A.; Qian, D.; Hitt, D.; Naeem, H.; Dagis, A.; Thomas, S.H.; Forman, S. Phase 1/2 trial of total marrow and lymph node irradiation to augment reduced-intensity transplantation for advanced hematologic malignancies. Blood 2011, 117, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.; Yang, D.; Frankel, P.; Liu, A.; Han, C.; Del Vecchio, B.; Schultheiss, T.; Cheng, J.; Li, R.; Kim, D.; et al. Radiation-Related Toxicities Using Organ Sparing Total Marrow Irradiation Transplant Conditioning Regimens. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.; Forman, S.; Somlo, G.; Rosenthal, J.; Liu, A.; Schultheiss, T.; Radany, E.; Palmer, J.; Stein, A. Dose escalation of total marrow irradiation with concurrent chemotherapy in patients with advanced acute leukemia undergoing allogeneic hematopoietic cell transplantation. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Deeg, H.J.; Sandmaier, B.M. Who is fit for allogeneic transplantation? Blood 2010, 116, 4762–4770. [Google Scholar] [CrossRef] [PubMed]
- Gyurkocza, B.; Sandmaier, B.M. Conditioning regimens for hematopoietic cell transplantation: One size does not fit all. Blood. 2014, 124, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.L.; Pasquini, M.C.; Logan, B.R.; Wu, J.; Devine, S.M.; Porter, D.L.; Maziarz, R.T.; Warlick, E.D.; Fernandez, H.F.; Alyea, E.P.; et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J. Clin. Oncol. 2017, 35, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Hahn, T.; Wilding, G.E.; Groman, A.; Hutson, A.; Zhang, Y.; Khan, U.; Liu, H.; Ross, M.; Bambach, B.; et al. Reduced-Intensity Conditioning with Fludarabine, Melphalan, and Total Body Irradiation for Allogeneic Hematopoietic Cell Transplantation: The Effect of Increasing Melphalan Dose on Underlying Disease and Toxicity. Biol. Blood Marrow Transplant. 2019, 25, 689–698. [Google Scholar] [CrossRef]
- Jensen, L.G.; Stiller, T.; Wong, J.Y.C.; Palmer, J.; Stein, A.; Rosenthal, J. Total Marrow Lymphoid Irradiation/Fludarabine/ Melphalan Conditioning for Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 301–307. [Google Scholar] [CrossRef]
- Wong, J.Y.C. Total Marrow Irradiation: Redefining the Role of Radiotherapy in Bone Marrow Transplantation; Total Marrow Irradiation; Wong, J.Y.C., Hui, S.K., Eds.; Springer Nature: New York, NY, USA, 2020; pp. 1–28. [Google Scholar]
- Lowsky, R.; Takahashi, T.; Liu, Y.P.; Dejbakhsh-Jones, S.; Grumet, F.C.; Shizuru, J.A.; Laport, G.G.; Stockerl-Goldstein, K.E.; Johnston, L.J.; Hoppe, R.T.; et al. Protective conditioning for acute graft-versus-host disease. New Engl. J. Med. 2005, 353, 1321–1331. [Google Scholar] [CrossRef]
- Kohrt, H.E.; Turnbull, B.B.; Heydari, K.; Shizuru, J.A.; Laport, G.G.; Miklos, D.B.; Johnston, L.J.; Arai, S.; Weng, W.K.; Hoppe, R.T.; et al. TLI and ATG conditioning with low risk of graft-versus-host disease retains antitumor reactions after allogeneic hematopoietic cell transplantation from related and unrelated donors. Blood 2009, 114, 1099–1109. [Google Scholar] [CrossRef]
- Messina, G.; Giaccone, L.; Festuccia, M.; Irrera, G.; Scortechini, I.; Sorasio, R.; Gigli, F.; Passera, R.; Cavattoni, I.; Filippi, A.R.; et al. Gruppo Italiano Trapianti di Midollo. Multicenter experience using total lymphoid irradiation and antithymocyte globulin as conditioning for allografting in hematological malignancies. Biol. Blood Marrow Transplant. 2012, 18, 1600–1607. [Google Scholar] [CrossRef]
- Benjamin, J.; Chhabra, S.; Kohrt, H.E.; Lavori, P.; Laport, G.G.; Arai, S.; Johnston, L.; Miklos, D.B.; Shizuru, J.A.; Weng, W.K.; et al. Total lymphoid irradiation-antithymocyte globulin conditioning and allogeneic transplantation for patients with myelodysplastic syndromes and myeloproliferative neoplasms. Biol. Blood Marrow Transplant. 2014, 20, 837–843. [Google Scholar] [CrossRef]
- Baron, F.; Zachée, P.; Maertens, J.; Kerre, T.; Ory, A.; Seidel, L.; Graux, C.; Lewalle, P.; Van Gelder, M.; Theunissen, K.; et al. Non-myeloablative allogeneic hematopoietic cell transplantation following fludarabine plus 2 Gy TBI or ATG plus 8 Gy TLI: A phase II randomized study from the Belgian Hematological Society. J. Hematol. Oncol. 2015, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Spinner, M.A.; Kennedy, V.E.; Tamaresis, J.S.; Lavori, P.W.; Arai, S.; Johnston, L.J.; Meyer, E.H.; Miklos, D.B.; Muffly, L.S.; Negrin, R.S.; et al. Nonmyeloablative TLI-ATG conditioning for allogeneic transplantation: Mature follow-up from a large single-center cohort. Blood Adv. 2019, 3, 2454–2464. [Google Scholar] [CrossRef] [PubMed]
- Brochstein, J.A.; Kernan, N.A.; Groshen, S.; Cirrincione, C.; Shank, B.; Emanuel, D.; Laver, J.; O’Reilly, R.J. Allogeneic bone marrow transplantation after hyperfractionated total-body irradiation and cyclophosphamide in children with acute leukemia. New Engl. J. Med. 1987, 317, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
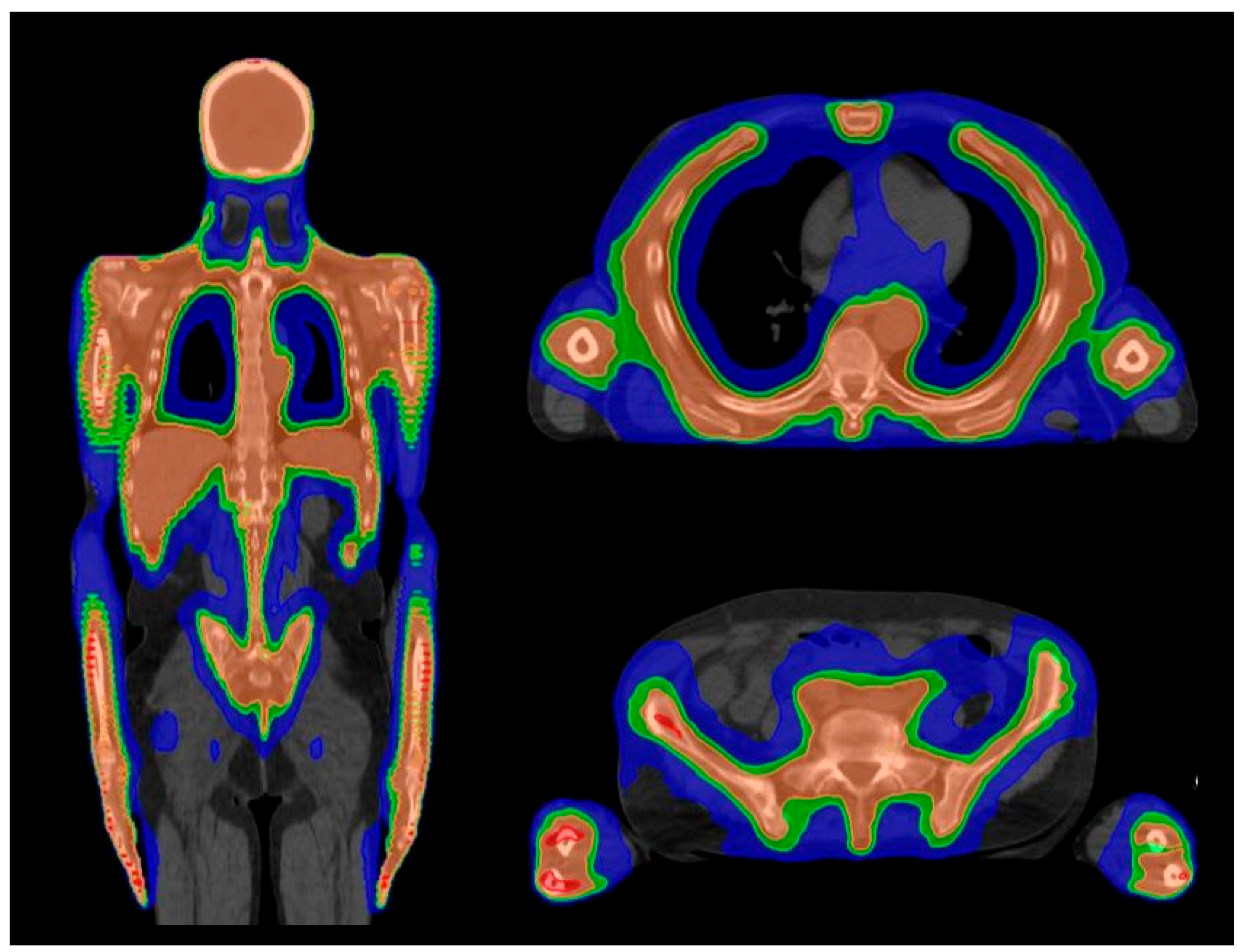
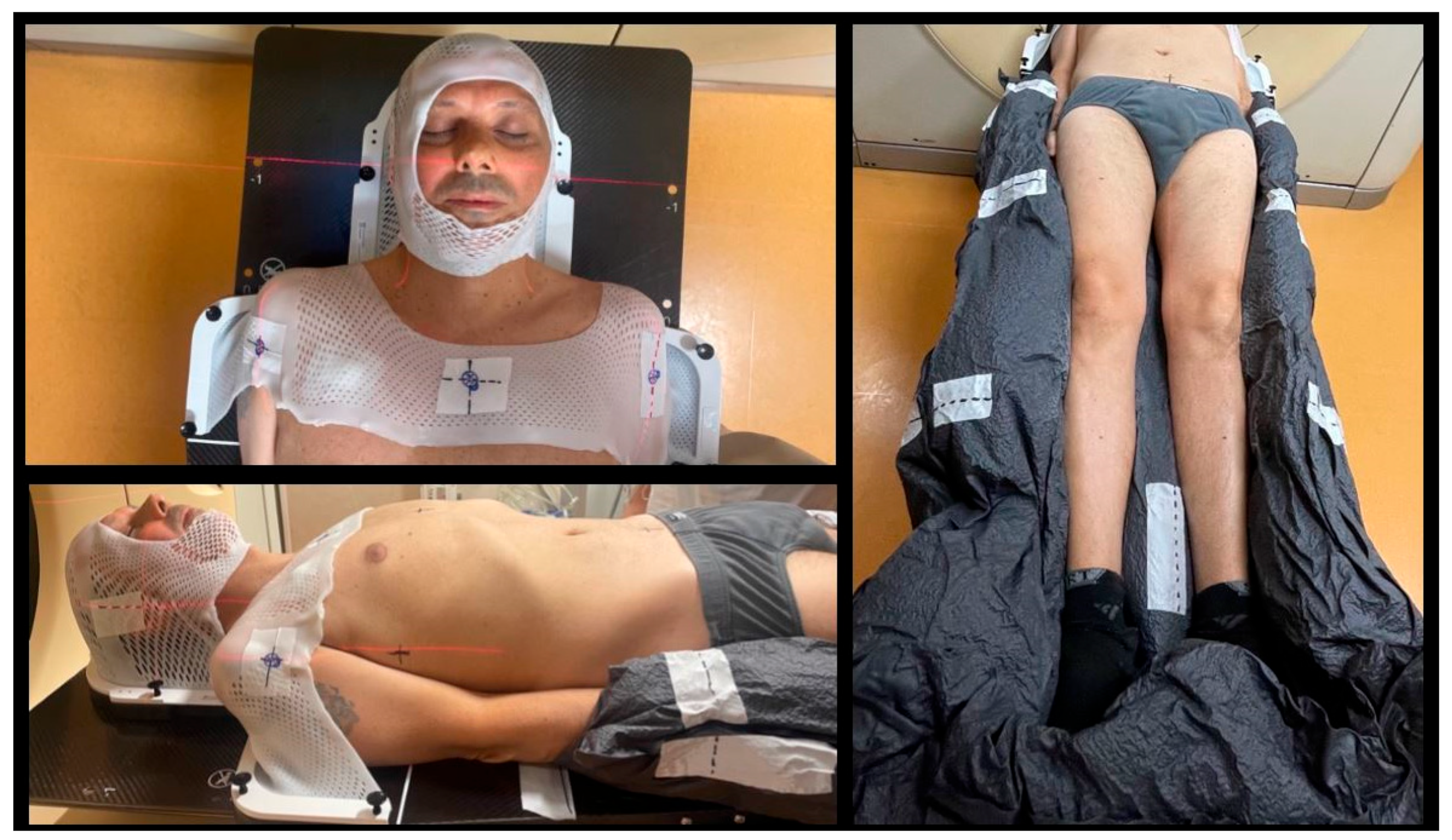
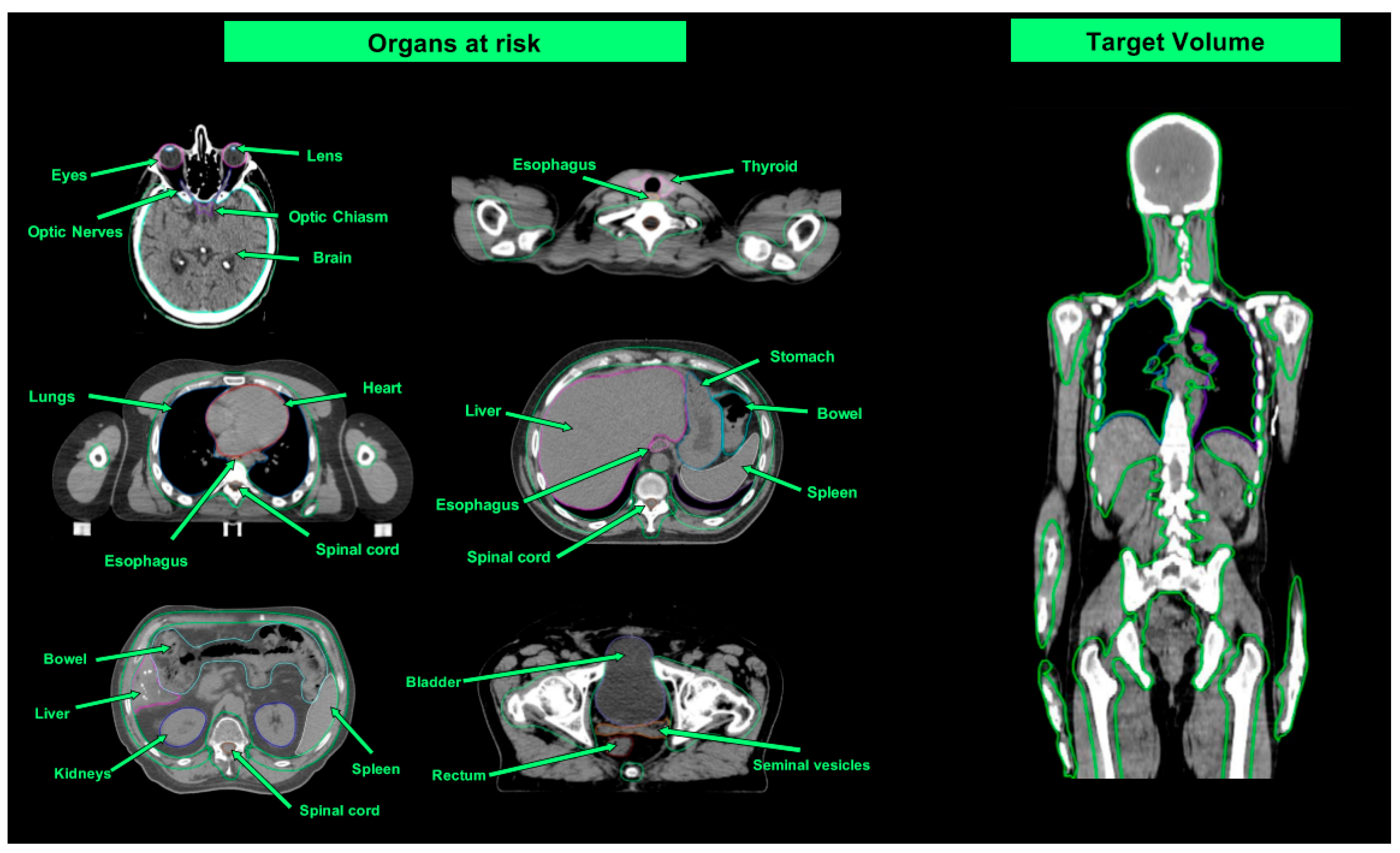
| Organ at Risk | TBI Median Doses (Gy) | Studies Evaluating TMI/TMLI Median Doses (Gy) | ||
|---|---|---|---|---|
| Wong et al. [51] (TBI 12 Gy) | Wong et al. [51] (TMI/TMLI 12 Gy) | Wong et al. [51] (TMI/TMLI 20 Gy) | Our Case (TMLI 12 Gy) | |
| Brain | 12.0 | 4.0 | 7.9 | - |
| Lens | 11.3 | 1.5 | 1.9 | 1.7 |
| Eyes | 11.3 | 6.6 | 7.0 | 5.7 |
| Optic nerves | 12.4 | - | - | - |
| Oral cavity | 11.8 | 3.9 | 4.8 | 8.5 |
| Parotids | 11.8 | 3.9 | 4.8 | 9 |
| Thyroid | 12.1 | 3.7 | 4.9 | 3.9 |
| Esophagus | 12.4 | 3.9 | 5.6 | 11.7 |
| Breasts | 11.5 | 6.9 | 8.7 | - |
| Lungs | 8.9 | 4.3 | 6.8 | 7.7 |
| Heart | 12.1 | 6.2 | 6.4 | 6.1 |
| Stomach | 12.2 | 3.1 | 5.0 | 5.5 |
| Small Intestine | 12.5 | - | - | 5.7 |
| Liver | 12.3 | 6.0 | 8.7 | - |
| Kidneys | 12.2 | 5.6 | 8.7 | 5 |
| Bladder | 12.4 | 7.0 | 7.4 | 6 |
| Rectum | 12.6 | - | - | 5.9 |
| Registration Number | Study Design | Type of HCT | Disease Type | RT Targets | TMI/TMLI Dose (Gy) | Chemotherapy Regimen | Estimated Enrollment |
|---|---|---|---|---|---|---|---|
| NCT02094794 | Phase II | Allogeneic | AML and AML | Bone, spleen, nodes (full dose) Liver, brain (12 Gy) | 20 (2 Gy fractions/BID) | Cy 100 mg/kg VP-16 60 mg/kg | 87 |
| NCT03467386 | Phase I | Allogeneic | AML | Bone, spleen, nodes (full dose) Liver, brain (12 Gy) | 18–20 (2 Gy fractions/BID) | P-T Cy 50 mg/m2/d × 2 | 24 |
| NCT02446964 | Phase I | Allogeneic Haploidentical | AML, ALL and MDS | Bone, spleen, nodes (full dose) Liver (12 Gy) Testes, brain (only ALL pts) | 12–20 (1.5–2 Gy fractions/BID) | FLU 25 mg/m2/d × 5 Cy 14.5 mg/kg/d × 2 P-T Cy 50 mg/kg/d × 2 | 24 |
| NCT03494569 | Phase I | Allogeneic Haploidentical | Unfit or >55 years AML, ALL and MDS | Bone, spleen, nodes (full dose) Testes (only ALL pts) | 12–20 (1.5–2 Gy fractions/BID) | FLU 30 mg/m2/d × 3 Mel 100 mg/m2 P-T Cy 50 mg/d × 2 | 36 |
| NCT04262843 | Phase II | Allogeneic Haploidentical | AML, ALL and MDS | TMLI | 20 (2 Gy fractions/BID) | FLU (doses unknown) P-T Cy (doses unknown) | 70 |
| NCT03121014 | Phase II | Allogeneic | High-risk AML and MDS | Bone | 9 (1.5 Gy fractions/BID) | FLU 40 mg/m2/d × 4 BU 4800 uM/min | 38 |
| NCT02333162 | Phase I | Allogeneic | Second HCT AML, ALL and MDS | Bone | N.A. | FLU + Mel | 30 |
| NCT03408210 | NA | Allogeneic | AML, ALL and MDS | Total body or TMLI | TBI 10 Gy or TMLI 12–20 Gy | Cy 60 mg/kg/d × 2 | 191 |
| NCT02122081 | Pilot | Allogeneic | Unfit or >50 years | Bone | 12 (2 Gy fractions/BID) | Cy (doses unknown) | 45 |
| NCT03262220 | NA | Allogeneic | Unfit, age 40–80 Hematologic Malignancies | Bone | 12 (4 Gy/die) | Variable schemes | 87 |
| NCT05139004 | Phase I | Allogeneic | High-risk AML, ALL and MDS | Bone | TMLI 12 Gy | 90Y-DOTA-anti-CD25 + FLU + Mel (doses unknown) | 30 |
| NCT00112827 | Phase II | Autologous (tandem) | MM | Bone | 16 (2 Gy fractions/BID) | Mel 200 mg/m2 for 1st auto-HCT | 54 |
| NCT02043847 | Phase I | Autologous | MM relapsed/refractory | Bone | 3–9 (3 Gy/fractions/die) | Mel 200 mg/m2 | 12 |
| NCT00800059 | Phase I/II | Autologous | MM | Bone | 14–28 (2 Gy fractions/die) | N.A. | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dogliotti, I.; Levis, M.; Martin, A.; Bartoncini, S.; Felicetti, F.; Cavallin, C.; Maffini, E.; Cerrano, M.; Bruno, B.; Ricardi, U.; et al. Maintain Efficacy and Spare Toxicity: Traditional and New Radiation-Based Conditioning Regimens in Hematopoietic Stem Cell Transplantation. Cancers 2024, 16, 865. https://doi.org/10.3390/cancers16050865
Dogliotti I, Levis M, Martin A, Bartoncini S, Felicetti F, Cavallin C, Maffini E, Cerrano M, Bruno B, Ricardi U, et al. Maintain Efficacy and Spare Toxicity: Traditional and New Radiation-Based Conditioning Regimens in Hematopoietic Stem Cell Transplantation. Cancers. 2024; 16(5):865. https://doi.org/10.3390/cancers16050865
Chicago/Turabian StyleDogliotti, Irene, Mario Levis, Aurora Martin, Sara Bartoncini, Francesco Felicetti, Chiara Cavallin, Enrico Maffini, Marco Cerrano, Benedetto Bruno, Umberto Ricardi, and et al. 2024. "Maintain Efficacy and Spare Toxicity: Traditional and New Radiation-Based Conditioning Regimens in Hematopoietic Stem Cell Transplantation" Cancers 16, no. 5: 865. https://doi.org/10.3390/cancers16050865
APA StyleDogliotti, I., Levis, M., Martin, A., Bartoncini, S., Felicetti, F., Cavallin, C., Maffini, E., Cerrano, M., Bruno, B., Ricardi, U., & Giaccone, L. (2024). Maintain Efficacy and Spare Toxicity: Traditional and New Radiation-Based Conditioning Regimens in Hematopoietic Stem Cell Transplantation. Cancers, 16(5), 865. https://doi.org/10.3390/cancers16050865






